Abstract
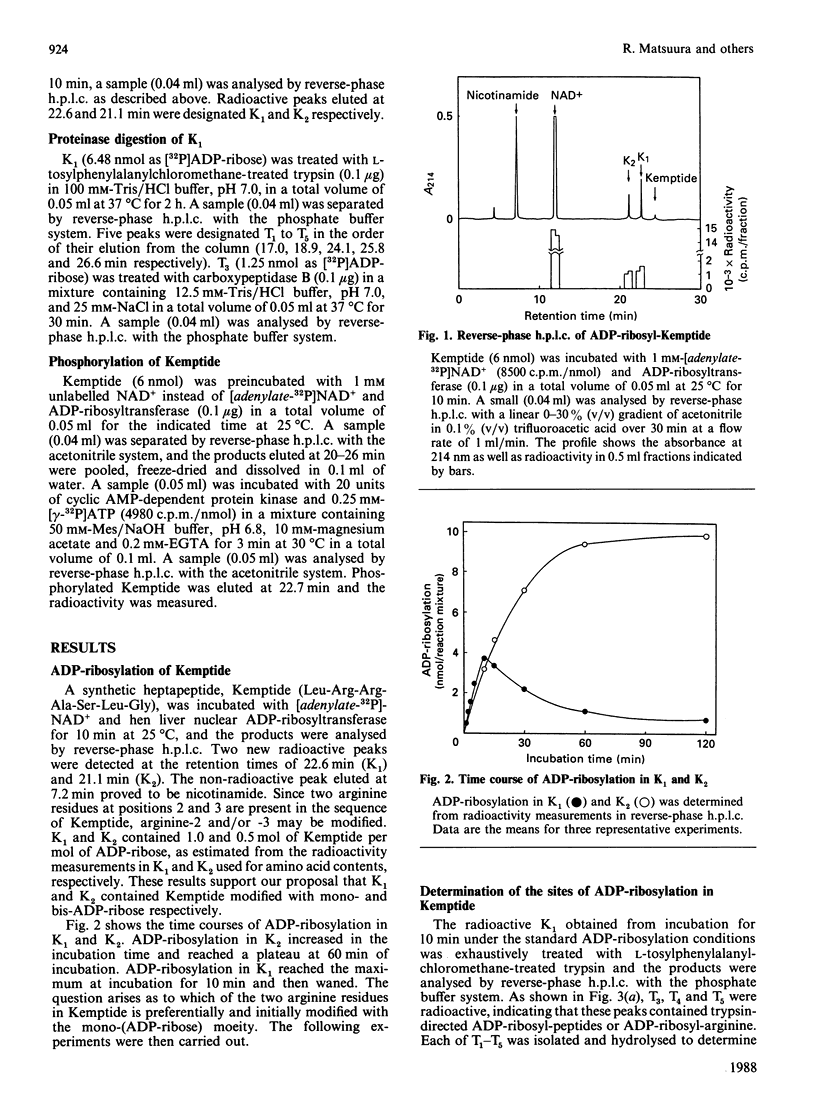
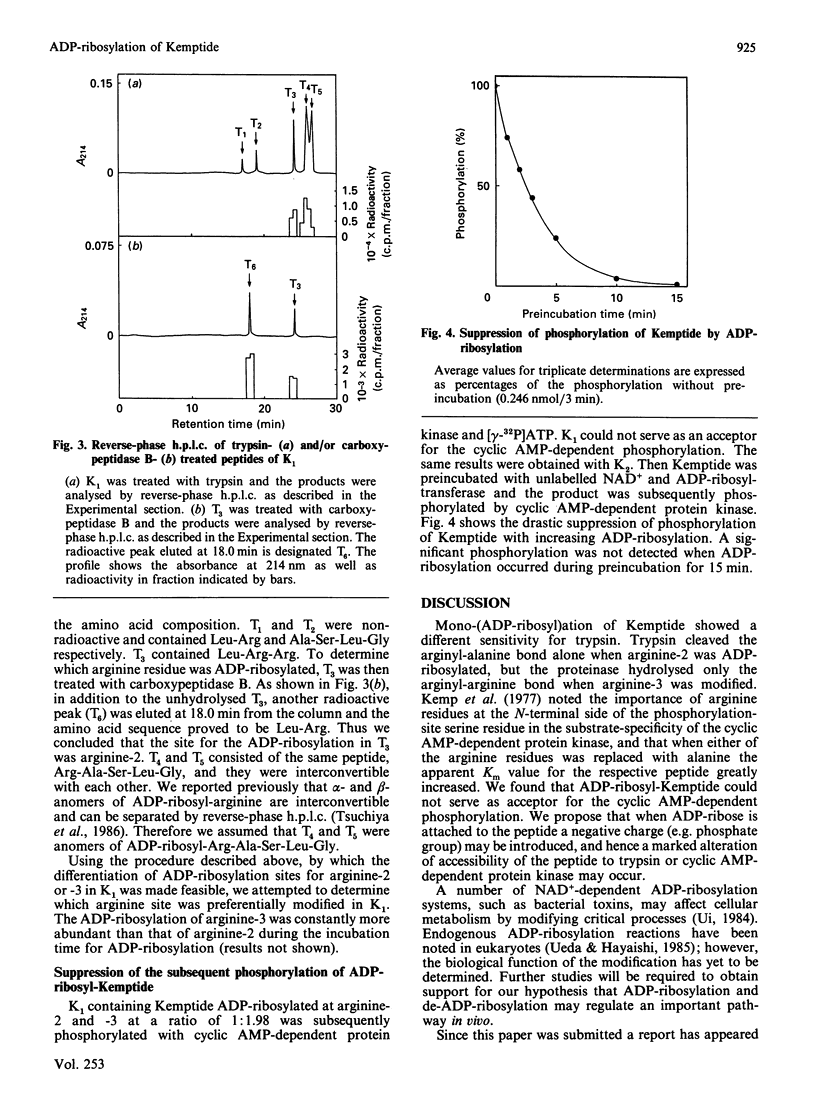
Hen liver nuclear ADP-ribosyltransferase modified the synthetic heptapeptide Kemptide (Leu-Arg-Arg-Ala-Ser-Leu-Gly) at arginine-2 and/or arginine-3. Trypsin treatment of ADP-ribosyl-Kemptide revealed that the ADP-ribosylation of arginine-3 was constantly more abundant than that of arginine-2. ADP-ribosylation of Kemptide suppressed the subsequent phosphorylation by cyclic AMP-dependent protein kinase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berglund L., Ljungström O., Engström L. Studies on the cyclic 3':5'-AMP-stimulated pig liver protein kinase reaction with pyruvate kinase as substrate. J Biol Chem. 1977 Jan 25;252(2):613–619. [PubMed] [Google Scholar]
- Kemp B. E., Graves D. J., Benjamini E., Krebs E. G. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J Biol Chem. 1977 Jul 25;252(14):4888–4894. [PubMed] [Google Scholar]
- Kharadia S. V., Graves D. J. Relationship of phosphorylation and ADP-ribosylation using a synthetic peptide as a model substrate. J Biol Chem. 1987 Dec 25;262(36):17379–17383. [PubMed] [Google Scholar]
- Matsuura R., Tanigawa Y., Tsuchiya M., Mishima K., Yoshimura Y., Shimoyama M. ADP-ribosylation suppresses phosphorylation of the L-type pyruvate kinase. Biochim Biophys Acta. 1988 Apr 2;969(1):57–65. doi: 10.1016/0167-4889(88)90088-2. [DOI] [PubMed] [Google Scholar]
- Moss J., Stanley S. J., Watkins P. A. Isolation and properties of an NAD- and guanidine-dependent ADP-ribosyltransferase from turkey erythrocytes. J Biol Chem. 1980 Jun 25;255(12):5838–5840. [PubMed] [Google Scholar]
- Tanigawa Y., Tsuchiya M., Imai Y., Shimoyama M. ADP-ribosylation regulates the phosphorylation of histones by the catalytic subunit of cyclic AMP-dependent protein kinase. FEBS Lett. 1983 Aug 22;160(1-2):217–220. doi: 10.1016/0014-5793(83)80970-3. [DOI] [PubMed] [Google Scholar]
- Tanigawa Y., Tsuchiya M., Imai Y., Shimoyama M. ADP-ribosyltransferase from hen liver nuclei. Purification and characterization. J Biol Chem. 1984 Feb 10;259(3):2022–2029. [PubMed] [Google Scholar]
- Tsuchiya M., Tanigawa Y., Mishima K., Shimoyama M. Determination of ADP-ribosyl arginine anomers by reverse-phase high-performance liquid chromatography. Anal Biochem. 1986 Sep;157(2):381–384. doi: 10.1016/0003-2697(86)90641-x. [DOI] [PubMed] [Google Scholar]
- Tsuchiya M., Tanigawa Y., Ushiroyama T., Matsuura R., Shimoyama M. ADP-ribosylation of phosphorylase kinase and block of phosphate incorporation into the enzyme. Eur J Biochem. 1985 Feb 15;147(1):33–40. doi: 10.1111/j.1432-1033.1985.tb08714.x. [DOI] [PubMed] [Google Scholar]
- Ueda K., Hayaishi O. ADP-ribosylation. Annu Rev Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]


