Abstract
Breast cancer (BC) is the most prominent cancer amongst women, but fortunately, early diagnosis and advances in multimodality treatments have improved patient survivability. Cancer survivors, however, experience increased biological ageing which may accelerate other co-morbidities. Exercise intervention is a promising clinical adjuvant approach to improve BC patients’ physiological function, recovery from treatment, and quality of life. However, the effects of combined aerobic and strength exercise training on biological ageing in BC patients have not been studied. The Breast Cancer Exercise Intervention (BREXINT) Pilot Study will evaluate the effects of a 24-week combined aerobic and strength exercise intervention against usual care in 50 BC patients’ post-treatment randomised to either group. The primary outcomes include changes in cardiorespiratory fitness, muscle strength, cancer-related symptoms, and rate of biological ageing following exercise intervention period. The secondary outcomes include habitual physical activity measured with tri-axial accelerometery and supporting questionnaires, including physical activity, food diary, and quality of life questionnaires. This study will identify the effects of combined aerobic exercise strength training on biological ageing in BC patients from Singapore. Results from this study could further support the implementation of regular exercise programmes as routine care for cancer patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-024-01145-9.
Keywords: Exercise intervention, Breast cancer, Cardiorespiratory fitness, Muscle strength, Biological ageing
Introduction
Breast cancer (BC) is the most prevalent cancer in women in Singapore with 29.7% of all cancers diagnosed and the leading cause of cancer death in this demographic at 17.2% [1]. This disease also leads the years of life lost (YLL) in 2017, accounting for 31.8% of total YLL—an indicator of premature mortality [2]. BC patients have better prognostic outcomes with the emergence of better diagnostic tools and treatment options [3–5] which can vary depending on the molecular subtype and stage of disease progression [6, 7]. Increased BC survivorship is associated with patients suffering from other adverse effects such as cancer-related fatigue [8, 9]. In a recent meta-analysis, the authors reported that BC patients treated with anthracycline (AC)-based chemotherapy present cardiotoxicity, lower ventricular ejection fraction (LVEF), and increased prevalence of heart failure [10, 11]. Such cardiovascular complications result in a higher risk of death than BC alone especially in older patients [12]. These side effects are exacerbated by poor cardiorespiratory fitness and sarcopenia—low skeletal mass and strength [11, 13, 14].
These adverse side effects of cancer and chemotherapy are mimicked in the process of biological ageing which reflects the accumulative tissue and cellular damage over time [15, 16]. Chronological ageing inevitably increases the risk of cancer [17]. Conversely, there is growing consensus that cancer and its treatments accelerate the ageing process [5, 16, 18]. The St. Jude Lifetime (SJLIFE) Cohort Study has shown that cancer survivors develop co-morbidities earlier than cancer-free patients. It was reported that by 45 years of age, childhood cancer survivors have double the burden of disease compared to the general population [19]. This may be explained by overlapping hallmarks such as genomic instability, telomere attrition, epigenetic alteration, and chronic inflammation [5, 20]. Insufficient participation in regular exercise may further propagate the physical and cognitive decline of patients, as exercise has been demonstrated to be beneficial as supportive care in cancer patients [21–23].
Exercise training modulates cardiorespiratory fitness and sarcopenia in breast cancer
The benefits of aerobic exercise for BC therapy are well-established. A seminal study found that BC patients have 27% lower VO2peak—the gold standard for cardiorespiratory fitness and predictor of survival, than healthy, age-matched women leading sedentary lifestyles [24]. Similarly, another study identified that VO2peak of BC patients initiating chemotherapy could achieve ~ 96% of expected VO2peak whereas post-adjuvant (4 weeks) chemotherapy patients were only able to reach ~ 63% of expected VO2peak [25]. Fortunately, regular aerobic exercise training improved BC patients’ VO2peak which mitigates cardiotoxicity during chemotherapy [11]. Adoption of aerobic exercise after adjuvant therapy also improves treatment outcomes, mitigates inflammation, and decreases recurrence rates [11, 26–29].
The loss of skeletal muscle mass—sarcopenia—is also an important prognostic factor for BC patients. Sarcopenic BC patients have a 71% greater risk of mortality than non-sarcopenic patients [30] and were also twice as likely as those without the condition, to present with high-grade chemotherapy which can promote systemic inflammation and impair insulin sensitivity [31, 32]. Furthermore, increased circulating inflammatory mediators such as IL (interleukin)-1β, IL-2, IL-6, IL-8, and C-reactive protein (CRP) may drive the loss of muscle mass and strength, whilst increased CRP, fibrinogen, tumour necrosis factor (TNF)-α and IL-6 drive BC progression [33–35]. Meneses-Echávez et al. reported in a meta-analysis that combined aerobic and strength exercise training decreases systemic TNF-α, IL-2, IL-6, and IL-8 in BC patients, suggesting that incorporating strength exercise can be efficacious in modulating the pro-inflammatory milieu and preserving muscle strength [36]. Khosravi et al. also report that combined aerobic and resistance exercise training programmes for cancer patients led to a decline in pro-inflammatory markers [37].
Both aerobic and resistance exercise training programmes also alleviated cancer-related fatigue and improved weight loss, muscle strength and quality of life (QoL) even after 1 year [38, 39]. However, there is a need to evaluate the lower physical capacity of BC patients due to cancer burden or treatment to optimise exercise protocols [11, 38].
Biological ageing is accelerated in cancer
Biological ageing is the time-dependent rate of decline and damage in different organ systems with a heterogenous expression in the general population. Biological ageing is accelerated in cancer; a cancer patient is likely to present with a higher biological age relative to her chronological age [15, 16]. Likewise, cancer induces gradual cellular dysfunction, whilst current treatment methods such as chemotherapy compound the damage through its cytotoxic mechanisms [40]. Patients with a history of, or with ongoing cancer presented with physical (lower functional mobility and loss of muscle strength) and cognitive impairments at an earlier chronological age, which subsequently impeded their abilities to perform physical activities [16, 41]. As mentioned previously, these impairments could culminate in death as well [12].
Ageing is also characterized by increased cellular senescence, which functions to suppress tumour formation but consequently contributes to low-grade chronic inflammation. Senescent stromal cells that exhibit this phenotype are senescent-associated secretory phenotypes (SASP) where they release inflammatory mediators including nuclear factor (NF)-κβ and pro-inflammatory cytokines such as IL-6, IL-8, and TNF-α [16]. As described earlier, the mechanisms implicated in biological ageing are similar to cancer and exercise shows promising benefits in decreasing the expression of mediators involved in cell senescence. Initial evidence shows a significant reduction in cyclin-dependent kinase inhibitors p16 and p21, other mediators of the SASP, and inflammatory markers such as Interferon (IFN)-γ and TNF-α [42].
Therefore, assessing blood biomarkers throughout the exercise intervention could shed light on the systemic effects of a combined exercise training programme on biological ageing.
Global adoption of exercise is medicine (EIM) initiative
Together with benefits on cardiorespiratory fitness, muscle strength, immune markers, and cell senescence, exercise is promising as supportive therapy for cancer care and is well-accepted by the international oncology community. The American Cancer Society, the National Comprehensive Cancer Network, and the Clinical Oncology Society of Australia have established position statements and guidelines for clinicians to prescribe exercise for cancer patients [43–46]. Yet, referral to cancer rehabilitation programmes is not part of standard care in many countries, including Singapore, and analyses of cancer survivors reveal low adherence to an active lifestyle [46]. Clinicians and scholars have recently pushed for strength and aerobic conditioning to be the basic level of cancer rehabilitation [47].
Challenges in Singapore’s cancer survivorship model
Despite increased recognition of EIM by clinicians globally, hospitals in Singapore have not adopted exercise as a core component of their cancer rehabilitation programmes. At the first cancer supportive and survivorship care forum in 2016 [48], Singapore oncologists met to address the inadequacy of the prevailing healthcare model in cancer survivorship. The observations and consensus amongst the clinicians were as follows:
A focus on cancer surveillance with scant attention given to the rehabilitation needs of survivors, including exercise and other psychological needs,
No community-based programmes for cancer survivors to incorporate healthy lifestyle behaviours and self-monitoring, and
The absence of research infrastructure to address relevant issues in cancer survivorship.
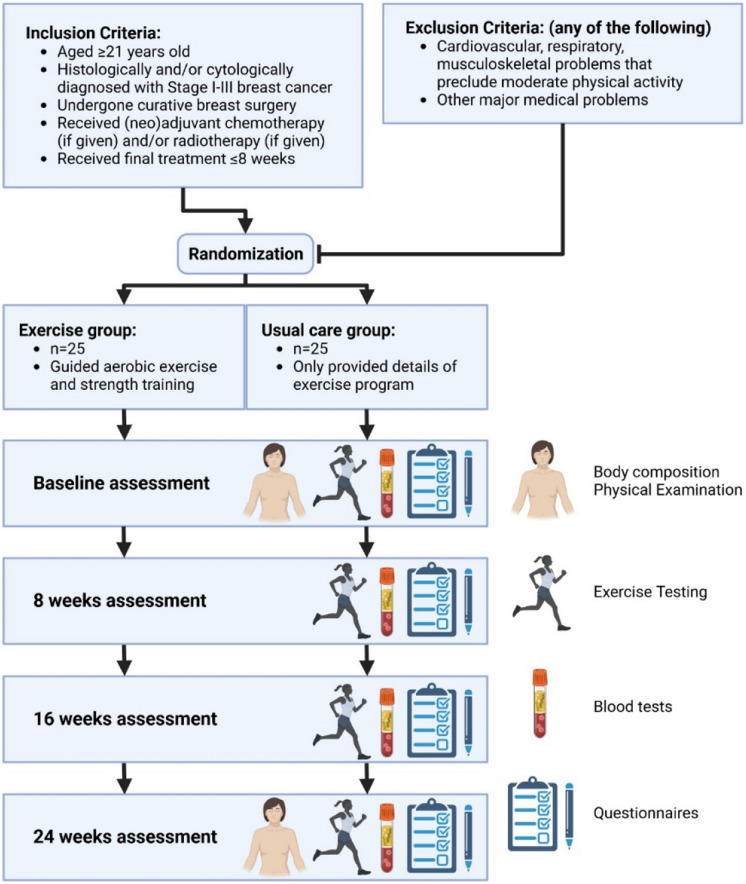
The Breast Cancer Exercise Intervention (BREXINT) Pilot Study is the first research study in Singapore to comprehensively assess VO2 parameters in routine submaximal cardiopulmonary exercise testing (CPET), during a 6-month cardiorespiratory fitness and strength training programme in BC patients. The aims of the BREXINT Pilot Study include (1) evaluation of the efficacy of exercise training in the reduction of biological age, determined by the DNA methylation status in BC patients; (2) evaluation of the effect of exercise training on cardiovascular fitness in BC patients; (3) investigating whether exercise training is associated with reduced adiposity and improved muscle mass in BC patients; and (4) analysis of the changes in immune and metabolic blood markers in BC patients undergoing exercise training (Fig. 1).
Fig. 1.
Diagram for the BREXINT Pilot Study design (created with BioRender.com)
Our intended research parameters of cardiorespiratory fitness, muscle strength and skeletal muscle index (SMI) will be evaluated using muscle strength analysis and Dual-energy X-ray absorptiometry (DEXA), to determine the association with treatment prognosis. Specific hallmarks of biological ageing will also be used to identify potential mechanisms ameliorated by exercise in ageing and BC. Our research will evaluate patient outcomes from an Asian population, which is an understudied population, compared with most research that has been conducted in Caucasian populations. These results could identify the merits of implementing exercise into standard care for BC patients.
Methodology
Study design
The study is a prospective, randomised clinical trial that will involve a 24-week exercise intervention programme. An exercise group will undergo guided exercise sessions comprising aerobic exercise and strength training at the Singapore Cancer Society (SCS) Rehabilitation Centre whilst a usual care (UC) group will be provided information on the exercise programme. Participants will be assessed at baseline before the exercise intervention, 8 weeks, 16 weeks and 24 weeks at the end of the exercise intervention. The various assessments will be elaborated the following sections. This study has been approved by NUS IRB (NUS-IRB-2022–700), SingHealth CIRB (2020–2451), and A*STAR IRB (2022–079) and registered at clinicaltrials.gov (NCT05957068).
Patient recruitment
Up to 50 women will be recruited and randomised into the exercise (n = 25) or UC group (n = 25). The patients will include women aged ≥ 21 years of Chinese, Malay and Indian ethnicity and histologically and/or cytologically diagnosed with stage I-III breast cancer. They will be enrolled within 8 weeks of curative breast cancer surgery or the final session of chemotherapy or radiotherapy (if given). The patients must also understand and voluntarily sign the informed consent forms before study-specific procedures can commence.
Patients will be excluded should they have cardiovascular, respiratory, or musculoskeletal issues that prevent them from carrying out moderate-intensity physical activity and other major medical problems deemed unsuitable for enrolment by the investigator.
Group assignment
Patients will be randomised to the exercise intervention or UC group by BC stage (early or locally advanced), BC status (oestrogen receptor (ER)/progesterone receptor (PR)/HER2), age (< 50 or ≥ 50 years), menopausal status, body fat/muscle ratio, level of physical activity in the year before study entry and receipt of chemotherapy.
Anthropometric measurements
Height and weight (avamech B1000, Avalanche Mechatronics, Singapore) (to the nearest 0.1 kg) will be measured at baseline and 24 weeks.
Vital signs
Body temperature, pulse rate and blood pressure (Braun Pro 6000 Dock, Welch Allyn Inc., USA) will be measured at baseline and 24 weeks.
Performance status
Eastern Cooperative Oncology Group (ECOG) performance status will be assessed at baseline and 24 weeks. Subjects will proceed as long as ECOG score ranges from 0 to 2. The treating physician will judge whether the exercise intervention subjects continue with the exercise programme if ECOG deteriorates or if medical conditions arise that preclude continuing participation.
Physical examination
Physical examination of the patients’ will also be performed at baseline and 24 weeks. This includes general appearance, skin, neck, eyes, ears, nose, throat, lungs, heart, abdomen, back, lymph nodes, extremities and neurological system. Any significant findings present before and after signing consent will be recorded.
Updates of medical history
Medical history and medications taken by the patients will be recorded at baseline and 24 weeks. Development of new conditions (e.g. diabetes, hypertension, and hypercholesterolemia), and menopausal status will be recorded.
Body composition
Patients will undergo DEXA scans (Horizon A, Hologic, USA) at baseline and 24 weeks to quantify skeletal muscle and adipose tissue mass.
Blood collection
Antecubital blood will be collected at baseline, 8, 16 and 24 weeks to assess for haematological, biochemical, inflammation, metabolic and ageing biomarkers as specified in Table 1.
Table 1.
Blood parameters and biomarkers are to be tested on blood samples from baseline, 8, 16, and 24-week timepoints
| Haematology & chemistry | Inflammation | Metabolism | Biological age |
|---|---|---|---|
|
• Haemoglobin • Platelets • White blood cell counts • Creatinine • Urea • Sodium • Potassium chloride • Bicarbonate • Glucose • Albumin • Total bilirubin • Alkaline phosphatase • Alanine transaminase (ALT) • Aspartate transaminase (AST) • Lipid profile • HbA1c • Insulin |
• High-mobility group box protein (HMBG)-1 • IL-1β • MCP-1 • IL-6 • IFN- γ • IL-10 • IL-12 • C-reactive protein (CRP) • TNF-α |
• Adiponectin • Leptin • Fibroblast growth factor 21 (FGF-21) • Growth differentiation factors (GDF)-11, 15 |
• DNA methylation • P16INK4A |
Laboratory tests will be done to measure haematological, biochemical, immunological, inflammation and metabolism parameters. Buffy coats will also be extracted for DNA methylation assays. The haematological, chemistry, and DNA methylation assays will be used to generate the biological age using validated epigenetic clocks such as DNAm PhenoAge [49] and GrimAge [50] to evaluate the effects of exercise on ageing. Protein biomarkers of inflammation and metabolism will also be quantified to evaluate if concentrations are modified by exercise (Procarta Plex, Luminex, Thermo Fisher, USA). Circulating peripheral blood mononuclear cells (PBMC) will be obtained via gradient centrifugation (Ficoll-paque, Cytiva, USA) to determine p16INK4A (common senescence marker) mRNA expression in various immune cell subsets. The transcriptomic profile of the inflammatory and metabolic analytes will also be quantified in PBMCs (QuantigenePlex, Luminex, ThermoFisher, USA).
Exercise training protocol
The exercise group will perform aerobic exercise training comprising of treadmill walking for 3 days per week for 24 weeks (total of 72 sessions), for 20 min each at 50% corresponding to age-predicted maximal heart rate (MHR) (220 age in years), eventually progressing to 75% of MHR. The strength training programme will comprise a series of circuit resistance exercises targeting the major muscle groups. The patients will perform 3 sets of 8–10 repetitions and increase to 4 sets of 8–10 repetitions. The exercises and progression are outlined in the supplementary material. The exercise training will be supervised by cancer exercise trainers at each session. The UC group will only be provided with details about the exercise intervention programme.
Exercise testing
Both groups will have their cardiorespiratory fitness (submaximal VO2) and muscle strength assessed at baseline, 8, 16 and 24 weeks.
Cardiorespiratory fitness will be determined during CPET with a metabolic cart (Metalyzer 3B, Cortex Biophysik, Germany) using indirect calorimetry with continuous heart rate monitoring (H10, Polar Electro, Singapore). Specifically, physiological parameters will be determined during the graded exercise test, which progressively increases the intensity whilst simultaneously measuring the corresponding ventilation (VE), oxygen (O2), and carbon dioxide (CO2) concentrations in the inhaled and exhaled air respectively. Taking into consideration that the BC patients may be deconditioned from treatment and physical inactivity, a modified Balke protocol will be adhered to—adapted from Burnett et al. [51]. Patients will start with a 5-min rest to obtain baseline measurements before moving to the treadmill for warm-up. The warm-up portion will comprise 1 min at 3.2 km/h at 0% incline, 1 min at 4.3 km/h at 0% incline and finally 1 min at 4.8 km/h at 1% incline. Subsequently, the test portion begins with increasing the incline by 1% every 1 min. The test stops if the patient does not feel well or once 85% of MHR is attained. Patients will then continue to cool down for 5 min at 3.2 km/h at 0% incline. Finally, recovery parameters will be taken by allowing the patients to sit down for 10 min on a chair whilst continuing to measure cardiopulmonary parameters. Blood pressure will be measured before and after each session to monitor the patient’s well-being.
Muscle strength will be assessed using the 10-repetition maximum (10-RM) test using the following exercises: (i) chest press, (ii) latissimus pull-down, (iii) 2-arm curl, and (iv) leg press.
Routine physical activity level
The patients from both groups will be issued accelerometers (Fibion Inc., Finland) to wear in the front thigh pocket of their trousers for 7 days of monitoring of habitual physical activity at baseline, 8, 16, and 24 weeks. This device measures tri-axial acceleration with minute-to-minute resolution.
The patients will also record their diets in a 3-day food diary at baseline, 8, 16, and 24 weeks. They will be briefed on how to record the information, which will include the type and quantity of food consumed over 2 weekdays and 1 weekend consecutively.
Data from the accelerometers and food diary will provide an estimate of the patient’s energy balance (energy expenditure-energy intake).
Quality of life survey
The patients will complete a QoL survey at baseline, 8, 16 and 24 weeks. The survey will follow the European Organization Research and Treatment of Cancer Quality of Life Questionnaire (EORTCQLQ-C30) which is a cancer-specific measure of health-related indices and QoL that is validated in the Singapore population [52].
Primary and secondary outcomes
The primary outcomes include changes in cardiorespiratory fitness, muscle strength, cancer-related symptoms, and rate of biological ageing following exercise intervention. In addition, effects on adipo-metabolic, inflammatory, ageing parameters, and adherence to digital physical activity trackers will be investigated. The secondary outcomes include data gathered from supporting questionnaires, including physical activity, dietary journals, and QoL.
Statistical analyses
Sample sizes were established by taking reference from a similar exercise intervention pilot study in breast cancer [53]. In this 4-month study, 15 subjects were randomised to exercise intervention and 13 subjects to the control groups, with ~ 3.5 mL/kg/min improvement in VO2max, showing a large effect size (Cohen’s d = 1.1). In our study, each group will have 25 subjects to have 90% power to detect significant differences at P < 0.05 in VO2peak changes. Patients will be matched for age, tumour stage, and body mass index for each group. Student’s t-test will be used to test for differences in our primary and secondary outcomes.
Safety assessments
The exercises are submaximal; hence no adverse events are expected for the duration of the exercise intervention. Nonetheless, all serious adverse events (SAEs) will be documented and reported to the Principal Investigators (PIs) within 24 hr who will then inform NUS-IRB and CIRB.
Discussion
BC is the leading cancer in Singapore women [1]. Currently available therapies have improved patient survivability [3, 4]. As such, patients require additional care for life after cancer or to improve the prognosis for patients still undergoing treatment. Exercise training is a viable adjuvant therapy as it reduces adverse chemotherapy symptoms. The purported mechanisms may involve inflammatory and senescence mediators [36, 42]. Conversely, exercise training enhances cardiorespiratory fitness [11, 26–29], muscle strength [36], and QoL [11]. Similar studies have involved 16-week exercise programmes comprising aerobic and resistance exercise training. The main benefits for BC patients were a significant enhancement to VO2peak, muscle strength, reduced side effects from primary cancer treatments, and improved QoL. These benefits were also preserved after 12 months [11, 21, 38]. Our study will determine whether exercise training improved cardiorespiratory fitness, skeletal muscle composition, cancer-related symptoms, and QoL in Asian women with BC.
Exercise is known to improve hallmarks of biological ageing, such as extending telomere lengths [54]. An accelerated pace of biological ageing also resembles the side effects of cancer, which are potentially due to overlapping hallmarks that accumulate cellular and tissue damage [5, 20]. Interestingly, there are some molecular similarities between these pathways which share a greater expression of inflammatory mediators, such as IL-6, IL-8, and TNF-α [16, 36]. A meta-analysis from Meneses-Echávez et al. also reveals some conflicting data that exercise training does not bring about significant changes, but these discrepancies may be due to the timing of blood collection, or not accounting for advanced cancer states [36]. Our study can identify the effects of exercise training on inflammatory markers due to cancer and ageing. In addition, we will evaluate ageing parameters by profiling transcriptomic and proteomic changes in PBMCs and plasma. Specific protein biomarkers such as adiponectin could also provide insights into the metabolic profile of BC patients. These data may allow us to identify molecular pathways that are implicated during ageing and in BC.
Finally, we will determine patients’ adherence to exercise guidelines using physical activity trackers once the programme concludes. A recent review of digital activity trackers used in cancer patients showed improved levels of more than 70% adherence and more time spent on exercise, which improved QoL, depression, and fitness [55]. With growing numbers of studies on exercise interventions in cancer, there is still a need to identify its effect on biological ageing as there are unresolved, converging mechanisms. This study will provide valuable information from an Asian population and pave the way forward for population-specific interventions.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
The BREXINT Pilot Study is supported by grants from the NUS Yong Loo Lin School of Medicine, Healthy Longevity Translational Research Program (HLTRP/2022/PS-03), and National Cancer Centre Research Fund (NCCRF-YR2019-JUL-PG4).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elaine Hsuen Lim, Email: elaine.lim.hsuen@singhealth.com.sg.
Jorming Goh, Email: jorming@nus.edu.sg.
References
- 1.Health Promotion Board, Singapore. Singapore Cancer Registry Annual Report 2020. Available at: https://nrdo.gov.sg/docs/librariesprovider3/default-document-library/scr-2020-annual-report_web-release.pdf. Accessed 19 Dec 2023.
- 2.Epidemiology & Disease Control Division, Ministry of Health, Singapore; Institute for Health Metrics and Evaluation. The Burden of Disease in Singapore, 1990–2017: an overview of the Global Burden of Disease Study 2017 results. Seattle, WA: IHME; 2019.
- 3.Botteri E, et al. Improved prognosis of young patients with breast cancer undergoing breast-conserving surgery. Br J Surg. 2017;104(13):1802–10. [DOI] [PubMed] [Google Scholar]
- 4.Litton JK, Burstein HJ, Turner NC. Molecular testing in breast cancer. Am Soc Clin Oncol Educ Book. 2019;39:e1–7. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia R, et al. Do cancer and cancer treatments accelerate aging? Curr Oncol Rep. 2022;24(11):1401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moo TA, et al. Overview of breast cancer therapy. PET Clin. 2018;13(3):339–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park M, et al. Breast cancer metastasis: mechanisms and therapeutic implications. Int J Mol Sci. 2022;23(12):6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodai BI, Tuso P. Breast cancer survivorship: a comprehensive review of long-term medical issues and lifestyle recommendations. Perm J. 2015;19(2):48–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ewertz M, Jensen AB. Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncol. 2011;50(2):187–93. [DOI] [PubMed] [Google Scholar]
- 10.Giordano SH, et al. Congestive heart failure (CHF) in older women treated with anthracycline (A) chemotherapy (C). J Clin Oncol. 2006;24(18_suppl):521–521. [DOI] [PubMed] [Google Scholar]
- 11.Maginador G, et al. Aerobic exercise-induced changes in cardiorespiratory fitness in breast cancer patients receiving chemotherapy: a systematic review and meta-analysis. Cancers (Basel). 2020;12(8):2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Möhl A, et al. The impact of cardiovascular disease on all-cause and cancer mortality: results from a 16-year follow-up of a German breast cancer case–control study. Breast Cancer Res. 2023;25(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L-K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21(3):300-307.e2. [DOI] [PubMed] [Google Scholar]
- 14.Morlino D, et al. Prevalence of sarcopenia in women with breast cancer. Nutrients. 2022;14(9):1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maltoni R, et al. Chronological age or biological age: what drives the choice of adjuvant treatment in elderly breast cancer patients? Translational Oncology. 2022;15(1): 101300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll JE, Bower JE, Ganz PA. Cancer-related accelerated ageing and biobehavioural modifiers: a framework for research and clinical care. Nat Rev Clin Oncol. 2022;19(3):173–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22(17):R741–52. [DOI] [PubMed] [Google Scholar]
- 18.Henderson TO, Ness KK, Cohen HJ. Accelerated aging among cancer survivors: from pediatrics to geriatrics. Am Soc Clin Oncol Educ Book. 2014;34:e423–30. [DOI] [PubMed] [Google Scholar]
- 19.Bhakta N, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017;390(10112):2569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.López-Otín C, et al. Meta-hallmarks of aging and cancer. Cell Metab. 2023;35(1):12–35. [DOI] [PubMed] [Google Scholar]
- 21.Dieli-Conwright CM, et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: a randomized controlled trial. Breast Cancer Res. 2018;20(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odynets T, Briskin Y, Todorova V. Effects of different exercise interventions on quality of life in breast cancer patients: a randomized controlled trial. Integr Cancer Ther. 2019;18:1534735419880598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poier D, et al. Influence of a multimodal and multimodal-aerobic therapy concept on health-related quality of life in breast cancer survivors. Integr Cancer Ther. 2019;18:1534735418820447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones LW, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30(20):2530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klassen O, et al. Cardiorespiratory fitness in breast cancer patients undergoing adjuvant therapy. Acta Oncol. 2014;53(10):1356–65. [DOI] [PubMed] [Google Scholar]
- 26.Zaorsky NG, et al. Exercise therapy and radiation therapy for cancer: a systematic review. Int J Radiat Oncol Biol Phys. 2021;110(4):973–83. [DOI] [PubMed] [Google Scholar]
- 27.Holmes MD, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–86. [DOI] [PubMed] [Google Scholar]
- 28.Holick CN, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(2):379–86. [DOI] [PubMed] [Google Scholar]
- 29.Tsuji K, Matsuoka YJ, Ochi E. High-intensity interval training in breast cancer survivors: a systematic review. BMC Cancer. 2021;21(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang XM, et al. Sarcopenia as a predictor of mortality in women with breast cancer: a meta-analysis and systematic review. BMC Cancer. 2020;20(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodpaster BH, et al. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46(10):1579–85. [DOI] [PubMed] [Google Scholar]
- 32.Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. 2017;35:200–21. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, et al. Can exercise-induced modulation of the tumor physiologic microenvironment improve antitumor immunity? Cancer Res. 2019;79(10):2447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson D, et al. Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res Rev. 2017;36:1–10. [DOI] [PubMed] [Google Scholar]
- 35.Iwase T, et al. Body composition and breast cancer risk and treatment: mechanisms and impact. Breast Cancer Res Treat. 2021;186(2):273–83. [DOI] [PubMed] [Google Scholar]
- 36.Meneses-Echávez JF, et al. The effect of exercise training on mediators of inflammation in breast cancer survivors: a systematic review with meta-analysis. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1009–17. [DOI] [PubMed] [Google Scholar]
- 37.Khosravi N, et al. Exercise training, circulating cytokine levels and immune function in cancer survivors: a meta-analysis. Brain Behav Immun. 2019;81:92–104. [DOI] [PubMed] [Google Scholar]
- 38.Mijwel S, et al. High-intensity exercise during chemotherapy induces beneficial effects 12 months into breast cancer survivorship. J Cancer Surviv. 2019;13(2):244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milne HM, et al. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2008;108(2):279–88. [DOI] [PubMed] [Google Scholar]
- 40.Muhandiramge J, et al. The acceleration of ageing in older patients with cancer. J Geriatr Oncol. 2021;12(3):343–51. [DOI] [PubMed] [Google Scholar]
- 41.Mandelblatt JS, et al. Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know? Semin Oncol. 2013;40(6):709–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Englund DA, et al. Exercise reduces circulating biomarkers of cellular senescence in humans. Aging Cell. 2021;20(7): e13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rock CL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–74. [DOI] [PubMed] [Google Scholar]
- 44.Nyrop KA, et al. Randomized controlled trial of a home-based walking program to reduce moderate to severe aromatase inhibitor-associated arthralgia in breast cancer survivors. Oncologist. 2017;22(10):1238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cormie P, et al. Exercise as part of routine cancer care. Lancet Oncol. 2018;19(9): e432. [DOI] [PubMed] [Google Scholar]
- 46.Schmitz KH, et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69(6):468–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alfano CM, Cheville AL, Mustian K. Developing high-quality cancer rehabilitation programs: a timely need. Am Soc Clin Oncol Educ Book. 2016;35:241–9. [DOI] [PubMed] [Google Scholar]
- 48.Loh KW, et al. Cancer supportive and survivorship care in singapore: current challenges and future outlook. J Glob Oncol. 2018;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levine ME, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10(4):573–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu AT, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019;11(2):303–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burnett D, et al. Cardiorespiratory fitness in breast cancer survivors. Springerplus. 2013;2(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo N, et al. The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30): Validation of English version in Singapore. Qual Life Res. 2005;14(4):1181–6. [DOI] [PubMed] [Google Scholar]
- 53.Rogers LQ, et al. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integr Cancer Ther. 2013;12(4):323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puterman E, et al. Aerobic exercise lengthens telomeres and reduces stress in family caregivers: a randomized controlled trial - Curt Richter Award Paper 2018. Psychoneuroendocrinology. 2018;98:245–52. [DOI] [PubMed] [Google Scholar]
- 55.Schaffer K, et al. Systematic review of randomized controlled trials of exercise interventions using digital activity trackers in patients with cancer. J Natl Compr Canc Netw. 2019;17(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.