Abstract
Dementia with Lewy bodies (DLB) and Alzheimer’s disease (AD) are often associated with depressive symptoms from the prodromal stage. The aim of the present study was to investigate the neuroanatomical correlates of depression in prodromal to mild DLB patients compared with AD patients. Eighty-three DLB patients, 37 AD patients, and 18 healthy volunteers were enrolled in this study. Depression was evaluated with the Mini International Neuropsychiatric Interview (MINI), French version 5.0.0. T1-weighted three-dimensional anatomical images were acquired for all participants. Regression and comparison analyses were conducted using a whole-brain voxel-based morphometry (VBM) approach on the grey matter volume (GMV). DLB patients presented a significantly higher mean MINI score than AD patients (p = 0.004), 30.1% of DLB patients had clinical depression, and 56.6% had a history of depression, while 0% of AD patients had clinical depression and 29.7% had a history of depression. VBM regression analyses revealed negative correlations between the MINI score and the GMV of right prefrontal regions in DLB patients (p < 0.001, uncorrected). Comparison analyses between DLB patients taking and those not taking an antidepressant mainly highlighted a decreased GMV in the bilateral middle/inferior temporal gyrus (p < 0.001, uncorrected) in treated DLB patients. In line with the literature, our behavioral analyses revealed higher depression scores in DLB patients than in AD patients. We also showed that depressive symptoms in DLB are associated with decreased GMV in right prefrontal regions. Treated DLB patients with long-standing depression would be more likely to experience GMV loss in the bilateral middle/inferior temporal cortex. These findings should be taken into account when managing DLB patients.
Keywords: Dementia with Lewy bodies, Alzheimer’s disease, Depression, Prefrontal cortex, VBM, Antidepressant
Introduction
Dementia with Lewy bodies (DLB) is the second most common form of neurodegenerative disease after Alzheimer’s disease (AD). DLB is one of the synucleinopathies, diseases that are characterized by a diffuse aggregation of abnormal α-synuclein, forming Lewy bodies. A diagnosis of probable DLB can be made if, in addition to cognitive impairment, at least two of the following clinical core features are present: fluctuating cognition with pronounced variations in attention and alertness, recurrent visual hallucinations, spontaneous parkinsonian features, and rapid eye movement sleep behaviour disorder (RBD) [1, 2]. Additionally, depression has been reported to be a supportive clinical feature of DLB [1, 2]. According to the DSM-5, major depressive disorder (MDD) or clinical depression is defined by a depressed mood and/or a loss of interest or pleasure, in association with other symptoms such as significant weight changes or sleep disorders. Furthermore, subclinical depression can be defined by the presence of depressive symptoms without the standard diagnostic criteria being met [3]. Many studies have actually demonstrated depressive symptoms from the prodromal stage of DLB [4–6] and DLB may sometimes begin with a psychiatric onset characterized by “predominant psychiatric symptoms that typically correspond to late-onset MDD” [2]. Depression as a prodromal symptom could affect between 34% [7] and 55% of DLB patients [8], and over one-third of DLB patients would even experience depressive symptoms in the 5 years prior to diagnosis [4]. Interestingly, a Japanese study reported that 14% of people aged over 50 hospitalized for depression had prodromal or demented DLB [9].
Depression has also long been known to be a frequent symptom of AD [10, 11], but it seems that depressive symptoms are more frequent and severe in DLB patients than in AD patients [12]. DLB would be associated with a higher risk of developing depression [13] and with a higher frequency of MDD [14] compared to AD. Moreover, several studies have demonstrated higher Geriatric Depression Scale (GDS) scores in DLB patients than in AD patients, both in prodromal [15] and in dementia stages [16, 17].
Clinical or subclinical depression is a complex condition involving many brain regions and networks. Neuroimaging studies investigating depression tend to show heterogeneous results, which appear to be quite dependent on clinical and demographic factors, such as the medication, the onset age, the illness duration, and the comorbidities. Despite this relative inconsistency, the existing voxel-based morphometry (VBM) studies and meta-analyses have revealed structural abnormalities in multiple fronto-striatal-limbic regions, such as the prefrontal and cingulate cortices, the hippocampus, the insula, and the amygdala, in both patients with MDD [18–28] and patients with late-life depression (LLD) [29–33] when compared to healthy controls.
It is interesting to note that structural neuroimaging studies comparing DLB patients with healthy subjects reported a reduced volume in some of the brain regions mentioned above. In particular, prodromal DLB is associated with a loss of grey matter volume (GMV) in the bilateral insula and the anterior cingulate, as shown by a VBM study conducted by our team [34]. An insular cortical thinning has also been demonstrated in two structural neuroimaging studies comparing DLB patients and controls [35, 36]. Finally, the prodromal DLB criteria [2] also highlighted the occurrence of grey matter atrophy in some frontal structures.
However, the existing literature on the neuroanatomical substrates of clinical or subclinical depression in DLB remains unclear and limited. There are essentially post-mortem studies highlighting the likely involvement of the dopaminergic, serotoninergic, and noradrenergic systems in depressive symptoms in DLB. A loss of substantia nigra dopaminergic neurons [37, 38], rostral raphe serotoninergic neurons [39], and locus cœruleus noradrenergic neurons [40] is primarily reported in DLB patients. Only one magnetic resonance imaging (MRI) surface-based morphometric study shows that depression in mild AD and Lewy body disease (LBD) would be associated with cortical thinning in prefrontal and temporal areas [41]. However, it should be noted that that study only compared a group of depressed mild AD and LBD patients with a group of non-depressed mild AD and LBD patients and did not offer a comparison between the two diseases or a differentiation of DLB and Parkinson’s disease (PD) patients in the LBD group.
Concerning AD, the existing VBM studies suggest associations between depression and GMV reductions mainly in temporal [42–44], frontal [44, 45], and insular regions [44].
The aim of our study was to shed light on the specific underlying structural mechanisms of depressive symptoms in DLB patients in comparison to AD patients and healthy controls using a whole-brain VBM approach. Based on the fact that the insula, the anterior cingulate, and the frontal cortex have been identified as structurally impacted in both depression and prodromal DLB [2, 18–36], we supposed that depressive symptoms of DLB patients might be related to GMV reductions in these areas. Furthermore, we hypothesized that depression in AD patients would rather be related to GMV reductions in fronto-temporo-insular regions, as suggested in the literature.
As mentioned above, medication can influence neuroimaging results. Thus, we also planned to explore the impact of antidepressant treatment on GMV in DLB patients using comparison analyses between treated and non-treated DLB patients.
Methodology
Population
Eighty-three DLB patients, 37 AD patients, and 18 healthy control subjects (HCS) matched in terms of age, gender, and educational level were included in this research from the larger cohort study AlphaLewyMA (https://clinicaltrials.gov/ct2/show/NCT01876459). All participants were recruited from the tertiary memory clinic of Strasbourg University Hospital, France. Diagnoses were made by a multidisciplinary team (i.e., geriatricians, neurologists, psychiatrists, and neuropsychologists) who performed a clinical and neurologic examination. DLB patients met the revised DLB consensus criteria [1, 2], while AD patients met the Dubois criteria [46]. Fluctuations were assessed with the Mayo Clinic Fluctuations scale [47] and hallucinations using the Parkinson’s disease-associated psychotic symptoms questionnaire [48]. Features of parkinsonism were measured with the Unified Parkinson’s Disease Rating Scale (UPDRS, part 3) [49]: akinesia, rigidity, and tremor at rest. Rapid eye movement sleep behavior disorder (RBD) was evaluated using a sleep questionnaire on RBD from the publication by Gjerstad [50] simplified into two questions for the patient and the caregiver: one concerning movements during sleep and the other concerning vivid dreams and nightmares. Patients also had to be in the prodromal or mild stages of the disease. We operationalized this aspect by means of four questions concerning patient autonomy (Instrumental Activities of Daily Living [IADL] score). Patients who had difficulties in at least one of the following dimensions: medication, finances, telephone, and transportation, were considered to have dementia. Patients who had cognitive impairment but no difficulties in these dimensions were considered to be at the prodromal stage. Additionally, we selected only patients with a Mini Mental State Examination (MMSE) [51] score ≥ 20. This is the cut-off used to differentiate between patients with mild dementia (MMSE score ≥ 20) and those with moderate dementia (MMSE score < 20). Please note that our two groups of patients were matched in terms of the respective proportions of prodromal and dementia patients (χ2 = 0.346, p = 0.557).
Exclusion criteria for all participants included: contraindication to MRI; history of alcohol/substance abuse; sensory or motor deficits; relevant neurological or psychiatric comorbidities; or the presence of other severe or unstable medical illnesses. In addition, some participants were not included in the analyses due to a lack of behavioural or imaging data. All participants gave written informed consent for the study according to the Declaration of Helsinki, and the study was approved by the local ethics committee of East France (IV).
Assessment of depression
The Mini International Neuropsychiatric Interview, French version 5.0.0 (MINI) [52] was used to assess the presence of depressive symptoms, as already done in several studies on elderly subjects [53–56]. The MINI is a structured diagnostic interview exploring the main neuropsychiatric disorders, based on the DSM-5 criteria. In the present study, we focused on the MINI depression screening score, measured by means of nine questions. The patient is considered to have major depression if their score is ≥ 5/9; we considered a patient to have depressive symptoms if their score was ≥ 2. Additionally, we noted the presence or absence of a personal history of depression for each participant based on the information collected by our clinicians.
Neuroimaging study
We used VBM to investigate the neuroanatomical correlates of depression in 73 of the 83 DLB patients and in the 37 AD patients. Ten DLB patients were excluded from the VBM analyses because of unusable MRI images (i.e., excessive artefacts). Each of the 73 DLB and 37 AD patients underwent a high-resolution anatomical MRI scan. T1-weighted three-dimensional anatomical images were obtained using a 3 T MRI scanner (Verio 32-channel Tim Siemens scanner; Siemens, Erlangen, Germany) using a volumetric magnetization-prepared rapid acquisition with gradient-echo (MPRAGE) sequence (FOV = 256 × 256 mm2, image matrix = 256 × 256, slice thickness = 1 mm, repetition time = 1900 ms, echo time = 2.52 ms, flip angle = 9°).
VBM analyses included image pre-processing and statistical analyses. These steps were carried out using the SPM12 software package (Wellcome Department of Imaging Neuroscience, London; http://www.fil.ion.ucl.ac.uk/) running on Matlab R2023a (MathWorks, Natick, MA, USA). Anatomical MRI images were spatially pre-processed using standard procedures [57]. All T1-weighted structural images were first segmented, bias-corrected, and spatially normalized to the Montreal Neurological Institute (MNI) space using an extension of the unified segmentation procedure [58] that includes six classes of tissue. The DARTEL registration toolbox was then used to build a study-specific template and bring all the segmentation images into alignment. The VBM analysis was done on modulated grey matter (GM) images; that is, the GM value in each voxel was multiplied by the Jacobian determinant derived from the spatial normalization. This procedure preserves the total amount of GM from the original images. These modulated GM images were smoothed with a Gaussian kernel (full-width at half-maximum [FWHM]: 8 mm).
Statistical analyses
Behavioural analyses
All statistical behavioural analyses were performed using JASP software (https://jasp-stats.org). We applied an ANOVA to compare intergroup differences between DLB, AD, and HCS in terms of educational level, while non-parametric Kruskal–Wallis tests and Dunn post hoc tests were used for age, MMSE score, hallucinations, fluctuations, akinesia, rigidity, and RBD. To compare intergroup differences in terms of depression, we used Mann–Whitney U tests and Cohen’s d to evaluate effect size. The proportion of personal histories of depression was evaluated with a χ2 test. We also used the Spearman correlation coefficient to investigate the monotonic relationships between the MINI score and the DLB clinical criteria corresponding to quantitative variables (fluctuations, hallucinations, akinesia, rigidity, and RBD).
DLB patients were divided into two subgroups for comparison analysis between patients with and those without depression: depressed DLB (dDLB; MINI score ≥ 2) patients and non-depressed DLB (ndDLB; MINI score < 2) patients. The dDLB group corresponds to DLB patients with clinical or subclinical depression. We decided to compare this group with a non-depressed group to visualize the relationships between current depression and GMV.
DLB patients were also divided into two other subgroups for comparison analysis between patients with and those without antidepressant treatment: treated DLB (tDLB; use of antidepressants) patients and non-treated DLB (ntDLB; non-use of any kind of antidepressants) patients. This analysis was conducted to assess the effect of antidepressants on GMV. According to the oldest data we were able to retrieve from medical records, it should be noted that tDLB patients had been taking their treatment for an average of at least 8 years. Then, we think that this group represents quite well patients with long-standing depression. Based on this assumption, we hope to use these comparative analyses to assess the neurotoxicity of depression in terms of GMV.
We used a student t-test to compare dDLB and ndDLB patients as well as tDLB and ntDLB patients in terms of age, educational level, and MMSE score. For categorical measures (gender, handedness, stage of disease, tremor, pharmacological treatment), χ2 tests were applied.
VBM analyses
Statistical correlations between local GMV and depression scores were investigated using the general linear model (GLM). Raw scores on the MINI were tested for both DLB and AD patients. Multiple regression analyses were conducted on the whole brain in each group successively. Educational level (EL), total intracranial volume (TIV), and antidepressant treatment were considered as covariates in the model.
Two GLM models were also constructed to assess GMV differences between dDLB and ndDLB patients and between tDLB and ntDLB patients. We used a two-tailed t test, adjusted for TIV, EL, and antidepressants. We also added handedness as a covariate for the comparison between dDLB and ndDLB patients, and age as a covariate for the comparison between tDLB and ntDLB patients, because there were significant differences between the two groups. The results were first analysed using a statistical threshold of p < 0.05, FDR-corrected. When no correlations were found using FDR correction, we considered a less stringent statistical threshold of p < 0.001, uncorrected. In this case, a minimum cluster spatial extent of 50 voxels was used to avoid irrelevant and isolated detections whenever possible. The XjView software package (http://www.alivelearn.net/xjview/) was used to visualize the results and report the brain regions involved in the detected clusters.
Results
Behavioural results
Clinical characteristics
Table 1 presents the demographic and clinical characteristics of DLB patients, AD patients, and HCS. Our three groups were matched in terms of age, gender, handedness, and educational level. However, HCS had a significantly higher mean MMSE score than DLB and AD patients, and DLB patients had a significantly higher mean MMSE score than AD patients. Regarding clinical symptoms, the presence of DLB core features (fluctuations, hallucinations, akinesia, rigidity, and RBD) was significantly higher in DLB patients compared to AD patients and HCS, except for tremor.
Table 1.
Demographic and clinical characteristics of dementia with Lewy bodies (DLB) patients, Alzheimer’s disease (AD) patients, and healthy control subjects (HCS)
| Group | |||||
|---|---|---|---|---|---|
| Characteristics | DLB (n = 83) | AD (n = 37) | HCS (n = 18) | Statistic test, p | Dunn post hoc test |
| Age (years) a | 71.18 (9.57) | 74.12 (7.61) | 67.86 (7.93) | H = 5.798, p = 0.055 | |
| Gender (M/F) | 33/50 | 18/19 | 9/9 | χ2 = 1.181, p = 0.554 | |
| Handedness (R/L) f | 74/9 | 31/5 | 18/0 | χ2 = 2.613, p = 0.271 | |
| EL (years) a | 12.06 (4.05) | 12.19 (3.80) | 13.67 (2.38) | F = 1.337, p = 0.266 | |
| Stage of disease (pro/dem) | 54/29 | 22/15 | χ2 = 0.346, p = 0.557 | ||
| MMSE score a,f | 26.16 (2.72) | 24.68 (2.56) | 29.06 (0.96) | H = 32.105, p < 0.001 | HCS > DLB, AD; DLB > AD |
| Hallucinations (/9) a,b,f | 1.92 (2.06) | 0.33 (0.79) | 0.11 (0.32) | H = 38.556, p < 0.001 | DLB > HCS, AD |
| Fluctuations c,f,g | 8/24/18/23/10 | 20/11/3/1/1 | 12/5/1/0/0 | H = 46.406, p < 0.001 | DLB > HCS, AD |
| Akinesia d,f,g | 30/45/8/0/0 | 33/3/0/0/0 | 17/1/0/0/0 | H = 42.202, p < 0.001 | DLB > HCS, AD |
| Rigidity d,f,g | 34/43/6/0/0 | 30/5/1/0/0 | 16/2/0/0/0 | H = 25.502, p < 0.001 | DLB > HCS, AD |
| Tremor d,f,g | 76/7/0/0/0 | 36/0/0/0/0 | 16/2/0/0/0 | χ2 = 3.606, p = 0.165 | |
| RBD e,f,h | 28/23/31 | 30/3/3 | 12/5/1 | H = 26.348, p < 0.001 | DLB > HCS, AD |
| Antidepressant (0/1) i | 58/25 | 26/11 | χ2 = 0.002, p = 0.966 | ||
| Benzodiazepine (0/1) i | 67/16 | 32/5 | χ2 = 0.589, p = 0.443 | ||
| Antipsychotic (0/1) i | 75/8 | 36/1 | χ2 = 1.775, p = 0.183 | ||
AD Alzheimer’s disease, DLB dementia with Lewy bodies, HCS healthy control subjects, EL educational level, pro prodromal stage, dem mild dementia stage, MMSE mini-mental state examination, RBD rapid eye movement behaviour disorder
Significant p values (p < 0.05) are in boldface type
aValues are mean (SD)
bAccording to [48]
cAccording to [47]
dAccording to the unified Parkinson’s disease rating scale [49]
eAccording to [50]
fData missing for 1 patient
gRating from 0 to 4 (0/1/2/3/4)
hRating from 0 to 2 (0/1/2)
iAnalysis performed on DLB and AD groups only
Regarding pharmacological treatments, 30.1% of DLB patients and 29.7% of AD patients were taking an antidepressant, 19.3% of DLB patients and 13.5% of AD patients were taking benzodiazepines, while 9.6% of DLB patients and 2.7% of AD patients were receiving antipsychotic treatment. None of our control subjects were receiving any of the above medications (Table 1).
Table 2 presents the demographic and clinical characteristics of depressed DLB (dDLB) patients and non-depressed DLB (ndDLB) patients. This separation was carried out as part of the imaging analyses in DLB patients. The two groups of dDLB and ndDLB patients were matched in terms of age, gender, educational level, stage of the disease, and MMSE scores. However, the proportion of left-handed patients was significantly higher in the ndDLB group.
Table 2.
Demographic and clinical characteristics of depressed DLB (dDLB) patients and non-depressed DLB (ndDLB) patients
| Group | |||
|---|---|---|---|
| Characteristics | dDLB (n = 28) | ndDLB (n = 45) | Statistic test, p |
| Age a | 69.74 (9.6) | 71.49 (9.96) | t = -0.739, p = 0.462 |
| Gender (M/F) | 11/17 | 18/27 | χ2 = 0.004, p = 0.952 |
| Handedness (R/L) | 28/0 | 38/7 | χ2 = 4.818, p = 0.028 |
| EL (years) a | 11.61 (4.57) | 12.16 (3.7) | t = -0.562, p = 0.576 |
| Stage of disease (pro/dem) | 19/9 | 31/14 | χ2 = 0.009, p = 0.926 |
| MMSE score a | 25.75 (2.84) | 26.4 (2.75) | t = -0.970, p = 0.335 |
| MINI score a | 6.25 (1.94) | 0 (0) | |
dDLB depressed dementia with Lewy bodies, ndDLB non-depressed dementia with Lewy bodies, EL educational level, pro prodromal stage, dem mild dementia stage, MMSE mini-mental state examination, MINI Mini International Neuropsychiatric Interview French version 5.0.0
Significant p values (p < 0.05) are in boldface type
aValues are mean (SD)
Table 3 shows the demographic and clinical characteristics of antidepressant-treated DLB (tDLB) patients and non-treated DLB (ntDLB) patients. This separation was carried out as part of the imaging analyses in DLB patients. The two groups of tDLB and ntDLB patients were matched in terms of gender, handedness, educational level, stage of disease, MMSE score, and MINI score. However, the mean age of the ntDLB group was significantly higher than that of the tDLB group.
Table 3.
Demographic and clinical characteristics of treated DLB (tDLB) patients and non-treated DLB (ntDLB) patients
| Group | |||
|---|---|---|---|
| Characteristics | tDLB (n = 24) | ntDLB (n = 49) | Statistic test, p |
| Age a | 67.1 (8.78) | 72.66 (9.82) | t = 2.369, p = 0.021 |
| Gender (M/F) | 8/16 | 21/28 | χ2 = 0.610, p = 0.435 |
| Handedness (R/L) | 22/2 | 44/5 | χ2 = 0.065, p = 0.799 |
| EL (years) a | 11.83 (4.16) | 12.0 (4.0) | t = 0.165, p = 0.87 |
| Stage of disease (pro/dem) | 16/8 | 34/15 | χ2 = 0.628, p = 0.730 |
| MMSE score a | 26.04 (2.82) | 26.20 (2.79) | t = 0.233, p = 0.817 |
| MINI score a | 3.08 (3.56) | 2.06 (3.12) | t = -1.255, p = 0.214 |
tDLB treated dementia with Lewy bodies, ntDLB non-treated dementia with Lewy bodies, EL educational level, pro prodromal stage, dem mild dementia stage, MMSE mini-mental state examination, MINI Mini International Neuropsychiatric Interview French version 5.0.0
Significant p values (p < 0.05) are in boldface type
aValues are mean (SD)
Treated DLB patients had been taking their treatment for an average of at least 8 years, according to the oldest data we were able to retrieve from medical records. Moreover, most of them (22/24, 91.7%) had a personal history of depression. Thus, the treated DLB group reflects DLB patients with long-standing/chronic depression.
Depression
DLB patients presented a significantly higher mean MINI score than AD patients (p = 0.004) (Table 4). Moreover, 30.1% of DLB patients (25 out of 83) could be considered to have major depression, while none of the AD patients had major depression (MINI score ≥ 5). Thirty-six percent (36.1%) of DLB patients (30 out of 83) had depressive symptoms (MINI score ≥ 2), compared with 13.5% of AD patients (5 out of 37). The proportion of patients with a personal history of depression was significantly different between DLB and AD patients (p = 0.006) (Table 4). The proportion of patients with a personal history of depression was almost twice as high in DLB patients (56.6%) compared to AD patients (29.7%). Please note that the HCS group was not included in the above analyses because all the HCS had a MINI score of 0 and none of them had a personal history of depression.
Table 4.
Comparisons between DLB and AD patients—Mann Whitney test
| Group | ||||
|---|---|---|---|---|
| Characteristics | DLB (n = 83) | AD (n = 37) | Statistic test, p | Cohen’s d |
| MINI score a,b | 2.25 (3.27) | 0.35 (1) | U = 1125.0, p = 0.004 | d =-0.267 |
| Personal history of depression (no/yes) c | 36/47 | 26/11 | χ2 = 7.414, p = 0.006 | |
DLB dementia with Lewy bodies, AD Alzheimer’s disease, MINI Mini International Neuropsychiatric Interview French version 5.0.0
Significant p values (p < 0.05) are in boldface type. Effect size was calculated using Cohen’s d for significant main effect
aValues are mean (SD)
bData missing for 1 patient
cIncluding two patients with a history of bipolarity
Statistical analysis (Spearman correlations) revealed positive correlations between the MINI score and the score for fluctuations (rho = 0.306, p = 0.005), hallucinations (rho = 0.280, p = 0.01), akinesia (rho = 0.367, p < 0.001), rigidity (rho = 0.258, p = 0.018), and RBD (rho = 0.298, p = 0.007).
Neuroimaging results
Regressions in DLB patients
Using p < 0.05, FDR corrected, we did not find any significant correlations between GMV and the MINI score in DLB patients.
With a less stringent threshold of p < 0.001, uncorrected, we found that higher MINI scores in DLB patients were associated with GMV loss in two regions of the right middle frontal gyrus (Brodmann area [BA] 10 and BA 46) (Fig. 1 and Table 5).
Fig. 1.
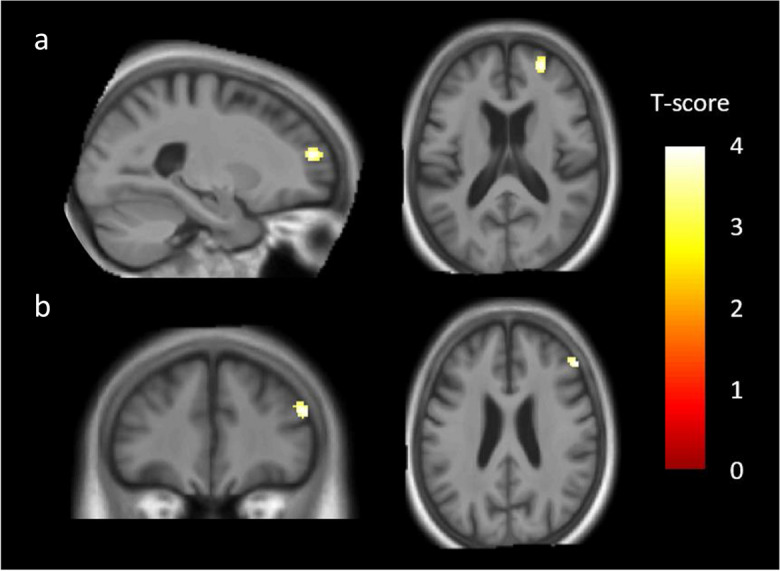
Brain regions correlated with the MINI (Mini International Neuropsychiatric Interview, French version 5.0.0) score in dementia with Lewy bodies (DLB) patients. Negative correlations were found between MINI scores and two clusters in the right middle frontal gyrus: BA 10 (a) and BA 46 (b). Results were significant at p < 0.001, uncorrected, with a cluster threshold of 50 voxels, and with total intracranial volume (TIV), educational level (EL), and antidepressant treatment as covariates
Table 5.
Voxel-based morphometry results for regression analyses (p < 0.001, uncorrected)
| Region | R/L | BA | k | T | MNI coordinates | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| DLB patients |
Middle frontal gyrus Middle frontal gyrus |
R R |
10 46 |
118 98 |
4.31 4.20 |
24.00 48.00 |
43.5 28.5 |
16.50 24.00 |
| AD patients | Temporal pole | R | 38 | 37 | 3.99 | 37.5 | 18.0 | -24.0 |
DLB dementia with Lewy bodies, AD Alzheimer’s disease, MINI Mini International Neuropsychiatric Interview French version 5.0.0, BA Brodmann area, k cluster extent, T t value for the cluster peak, MNI Montreal Neurological Institute
Regressions in AD patients
Using p < 0.05, FDR corrected, we did not find any significant correlations between GMV and the MINI score in AD patients.
With a threshold of p < 0.001, uncorrected, and a cluster threshold of 35 voxels, VBM analyses revealed a negative correlation between the MINI score and the right temporal pole (BA 38) (Fig. 2 and Table 5).
Fig. 2.
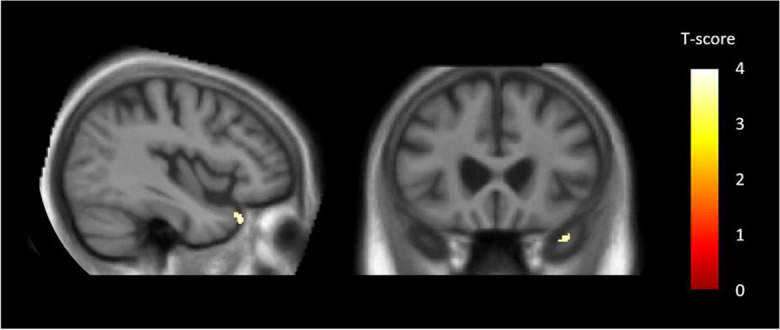
Brain regions correlated with the MINI (Mini International Neuropsychiatric Interview, French version 5.0.0) score in Alzheimer’s disease (AD) patients. Negative correlations were found between MINI scores and one cluster in the right temporal pole (BA 38). Results were significant at p < 0.001, uncorrected, with a cluster threshold of 35 voxels, and with total intracranial volume (TIV), educational level (EL), and antidepressant treatment as covariates
Comparison analyses between dDLB and ndDLB patients
Using p < 0.05, FDR corrected, we did not find any significant differences in GMV between dDLB and nDLB patients.
Using a less stringent threshold p < 0.001, uncorrected, dDLB patients showed decreased GMV in two regions of the right middle frontal gyrus (BA 10 and BA 46), and in the right posterior cingulate gyrus extending to the precuneus (BA 31, BA 7) compared to ndDLB patients (Fig. 3 and Table 6).
Fig. 3.
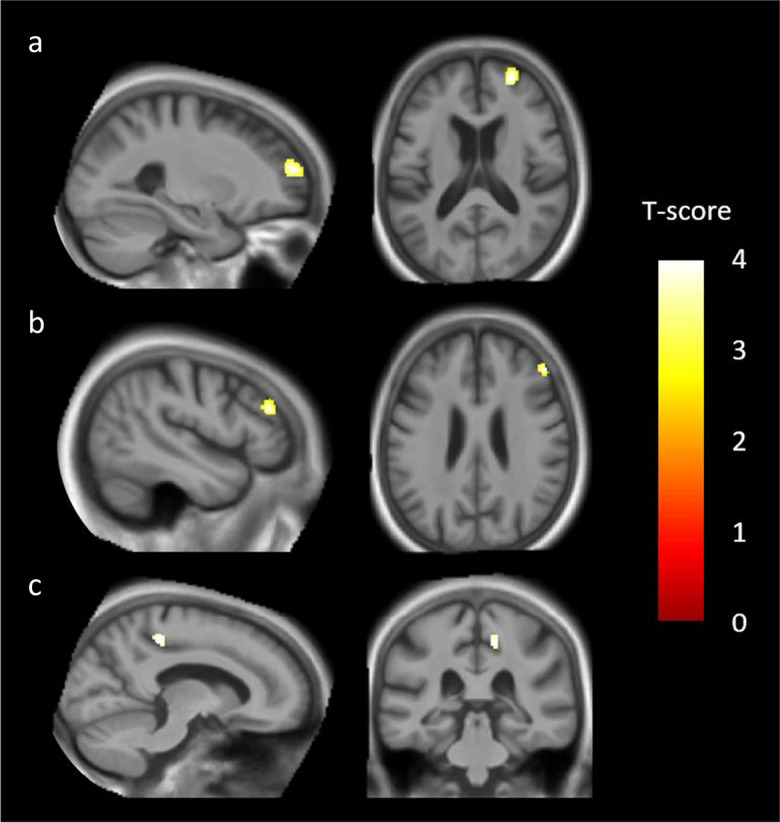
Comparison of grey matter volume (GMV) between depressed dementia with Lewy bodies (dDLB) patients and non-depressed dementia with Lewy bodies (ndDLB) patients. We found a significant GMV loss in four clusters, including the right middle frontal gyrus (BA 10) (a), the right middle frontal gyrus (BA 46) (b), and the posterior cingulate gyrus (BA 31) extending to the precuneus (BA 7) (c). Results were significant at p < 0.001, uncorrected, with a cluster threshold of 50 voxels, and with total intracranial volume (TIV), educational level (EL), antidepressant treatment, and handedness as covariates
Table 6.
Voxel-based morphometry results for group comparisons (p < 0.001, uncorrected)
| Region | R/L | BA | k | T | MNI coordinates | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| dDLB < ndDLB |
Middle frontal gyrus Middle frontal gyrus Posterior cingulate and precuneus |
R R R |
10 46 31/7 |
208 99 71 |
4.61 4.85 3.66 |
24.0 48.0 12.0 |
46.5 28.5 -36.0 |
16.5 24.0 43.5 |
| tDLB < ntDLB |
Middle/inferior temporal gyrus Middle/inferior temporal gyrus |
R L |
21/20 21/20 |
270 51 |
4.12 3.47 |
63.0 -63.0 |
-27.0 -34.5 |
-16.5 -18.0 |
| tDLB > ntDLB |
Cuneus/precuneus Supplementary motor area Superior/middle frontal gyrus Middle frontal gyrus Posterior cingulate Supplementary motor area |
L R/L R L R R |
19/7 6 6 10 31 6 |
455 260 309 186 79 85 |
5.28 4.54 3.64 4.33 4.24 4.35 |
-7.5 -1.5 22.5 -36.0 12.0 13.5 |
-84.0 -18.0 4.5 43.5 -37.5 -16.5 |
-27.0 64.5 49.5 9.0 34.5 66.0 |
DLB dementia with Lewy bodies, AD Alzheimer’s disease, BA Brodmann area, k cluster extent, T t value for the cluster peak, dDLB depressed dementia with Lewy bodies, ndDLB non-depressed dementia with Lewy bodies, tDLB treated dementia with Lewy bodies, ntDLB non-treated dementia with Lewy bodies
Comparison analyses between treated DLB patients and non-treated DLB patients
Using p < 0.05, FDR corrected, we did not find any significant differences in GMV between tDLB and ntDLB patients.
With a less stringent threshold of p < 0.001, uncorrected, comparison analyses between tDLB and ntDLB patients revealed lower GMV in the bilateral middle and inferior temporal gyrus (BA 21, BA 20, more prominent in the right hemisphere) in the tDLB group compared to the ntDLB group (Table 6).
Conversely, the tDLB group showed higher GMV in two larger clusters that included the left cuneus/precuneus (BA 19, BA 7) and the bilateral SMA (BA 6) compared to the ntDLB group (Fig. 4 and Table 6). Higher GMV was also found in four other clusters including the right superior and middle frontal gyrus (BA 6), the right SMA (BA 6), the left middle frontal gyrus (BA 10), and the right posterior cingulate (BA 31) in the tDLB group compared to the ntDLB group. (Fig. 4 and Table 6).
Fig. 4.
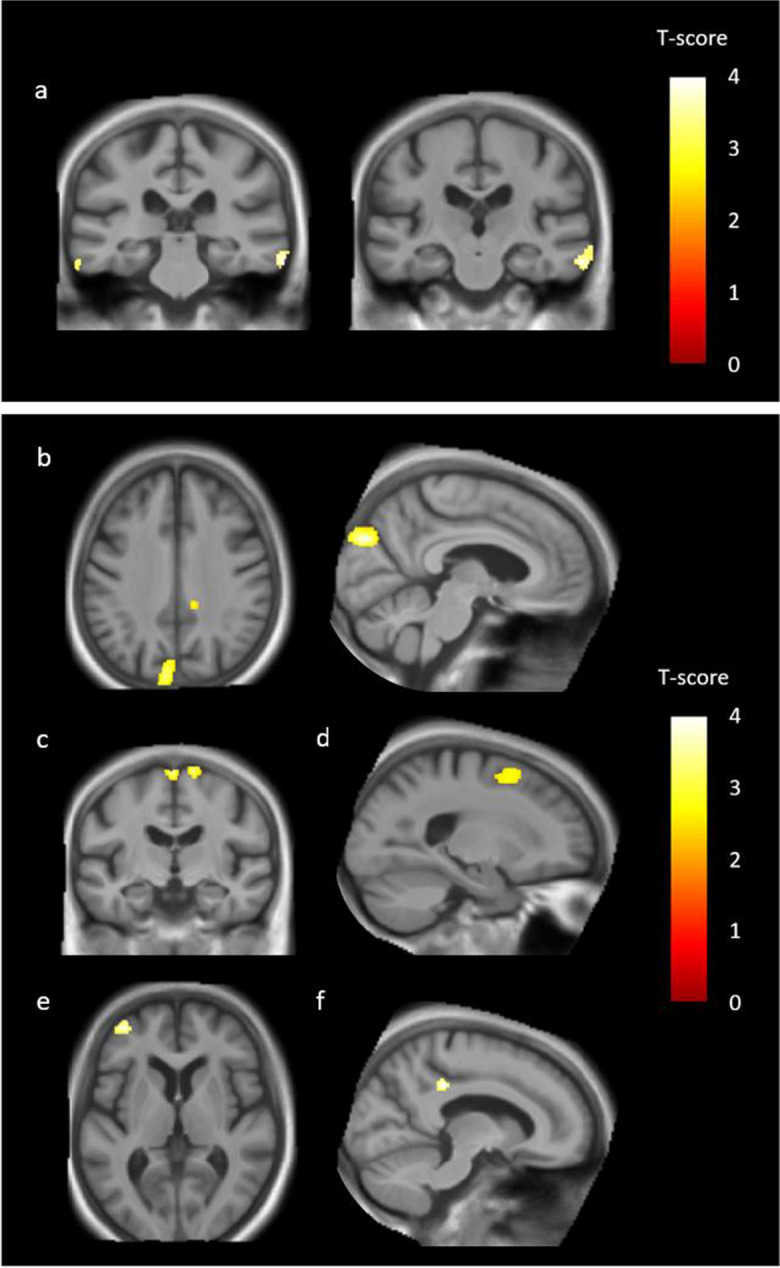
Comparison of grey matter volume (GMV) between treated dementia with Lewy bodies (tDLB) patients and non-treated dementia with Lewy bodies (ntDLB) patients. Top: regions showing significant GMV loss in tDLB patients compared to ntDLB patients, i.e., the bilateral middle/inferior temporal gyrus BA 21/20 (a). Bottom: regions showing significantly higher GMV in tDLB patients compared to ntDLB patients, i.e., the left cuneus (BA 19) extending to the precuneus (BA 7) (b), the bilateral supplementary motor area (BA 6) and the right supplementary motor area (BA 6) (c), the right superior/middle frontal gyrus (BA 6) (d), the left middle frontal gyrus (BA 10) (e), and the right posterior cingulate (BA 31) (f). Results were significant at p < 0.001, uncorrected, with a cluster threshold of 50 voxels, and with total intracranial volume (TIV), educational level (EL), and age as covariates
Common regions revealed in both the regression analysis in DLB patients and the comparison analysis between dDLB and ndDLB patients
Figure 5 shows the brain regions that overlap in both the regression analysis in DLB patients and the comparison analysis between dDLB and ndDLB patients. The two clusters in the right middle frontal gyrus (BA 46 and BA 10) are largely superposed in these two analyses.
Fig. 5.
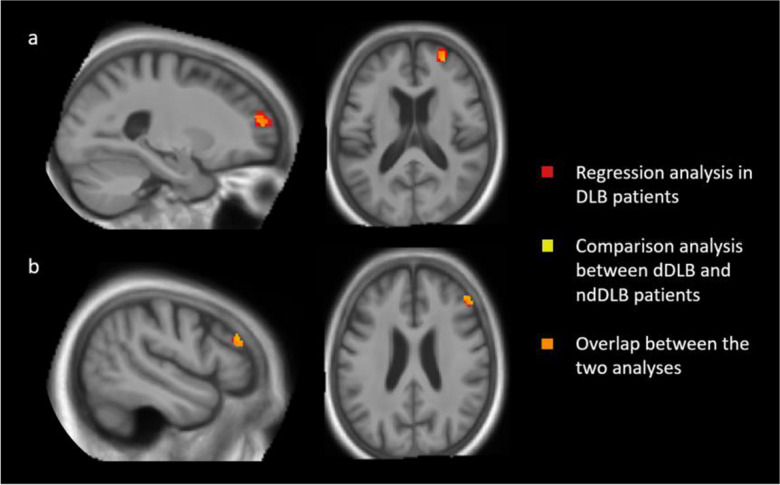
Overlap of the regression analysis in dementia with Lewy bodies (DLB) patients (Fig. 1), and the comparison analysis between depressed dementia with Lewy bodies (dDLB) patients and non-depressed dementia with Lewy bodies (ndDLB) patients (Fig. 3). The yellow colour represents the comparison analysis, the red color the regression analysis, and the orange colour represents voxels where the two analyses overlap. The two clusters in the right middle frontal gyrus, BA 10 (a) and BA 46 (b), are largely superposed in the two analyses
Discussion
The aim of the present study was to investigate the underlying structural mechanisms of depressive symptoms in DLB and AD patients by means of a whole-brain VBM approach, including regression and comparison analyses. At the behavioral level we demonstrated, as expected, higher MINI depression scores in DLB patients compared to AD patients, as well as a proportion of personal histories of depression almost twice as high in DLB patients (56.6%) compared to AD patients (29.7%). Furthermore, our VBM analysis on DLB and AD patients partially confirmed our initial hypotheses. Notably, we showed negative correlations between depression scores and decreased GMV in right prefrontal regions in DLB patients, and negative correlations between depression scores and GMV reductions in the right temporal pole in AD patients. Finally, the results of our additional comparison analyses on DLB patients receiving antidepressant treatment highlighted an increase in GMV mainly in left parieto-occipital regions and bilateral supplementary motor areas, as well as a decrease in GMV in bilateral middle temporal regions compared to non-treated DLB patients.
First, our behavioural results confirm the greater frequency and severity of depressive symptoms in DLB compared to AD, in line with the few existing studies [12, 14–17]. In particular, we showed that 30.1% of our DLB patients could be considered to have clinical depression and 36.1% to have depressive symptoms. These results are consistent with the study by Auning and colleagues [7], which demonstrated a rate of 34% of depression as a presenting symptom of DLB, and with a study conducted by our team [6], showing that 26.2% of prodromal DLB patients could be considered to have major depression.
Surprisingly, the prevalence and severity of depressive symptoms in our AD group were lower than expected. Only 13.5% of our AD patients had depressive symptoms, and none of them met the criteria for clinical depression, whereas two meta-analyses on this topic showed a prevalence of 22% for subclinical depression in AD [59] and 14.8% for clinical depression [60]. The small sample size of our AD group could partly explain this result, but we also assume that this may be linked to the stage of the disease, as we focused on a prodromal to mild AD population. Depressive symptoms would usually appear from the prodromal stage of DLB, and even years before diagnosis [4], whereas they would appear later in AD. This hypothesis is supported by a 5-year follow-up study demonstrating that DLB is associated with continuously increasing mean levels of depressive symptoms, whereas AD patients tend to show a delayed increase at later follow-up visits [61]. Interestingly, the global frequency of depressive symptoms in patients with DLB appears to be comparable to that of patients with PD, which has been evaluated at around 30.7% by a systematic review and meta-analysis [62]. High rates of depression have also been identified in patients with multiple system atrophy [8]. Therefore, synucleinopathies appear to be closely linked with depression, which is consistent with two studies demonstrating an association between depressive symptoms and α-synuclein burden [63, 64].
Furthermore, we showed that depression was correlated with several core features of DLB, particularly fluctuations, hallucinations, akinesia, rigidity, and RBD. Subjective poor sleep and daytime sleepiness [65] as well as autonomic dysfunctions and orthostatic hypotension [66] have also been associated with depressive symptoms in DLB in the literature. This suggests that there is a strong link between depressive symptoms and other DLB clinical characteristics, and therefore that depression is intrinsically linked to DLB.
In addition to being a frequent prodromal symptom of DLB and more generally a common symptom of synucleinopathies, depression also appears to be a risk factor for developing DLB, as suggested by a few studies [67, 68]. We actually showed that 56.6% of our DLB patients had a personal history of depression (versus 29.7% of our AD patients). A study by Boot and colleagues also demonstrated that having a personal history of depression increases sixfold the risk of developing DLB [69]. Thus, we believe that depression could be both a prodromal symptom of DLB and a major risk factor for developing the disease, as already suggested in a study by Ishiguro and colleagues on DLB [64] and a study by Bennett and Thomas [70] on dementia. An interesting point of view is proposed by Kessing and colleagues: early-onset depression before age 65 and recurrent depression may constitute long-term risk factors for the development of dementia, whereas the onset of more recent depressive symptoms may reflect a prodromal phase of dementia [71].
Beyond these interesting behavioural results, the second part of our study aimed to investigate the regions involved in DLB patients’ depressive symptoms. We were able to demonstrate a strong association between depressive symptoms and the volume of right dorsolateral (BA 46) and anterior (BA 10) prefrontal regions through both our regression analysis on DLB patients and our comparison analysis between dDLB and ndDLB patients. These results suggest that depressive symptoms in DLB patients are especially associated with a loss of GMV in the prefrontal cortex (PFC), an area that is typically involved in depression. A large number of VBM studies and meta-analyses have indeed reported the involvement of PFC in MDD [18, 22]. The surface-based morphometric study by Lebedev and colleagues also indicated a depression-associated cortical thinning in the dorsolateral prefrontal cortex (dlPFC) in a group of depressed LBD and AD patients, but in the left hemisphere [41]. Prefrontal areas are particularly involved in cognitive functions related to social, emotional, and motivational aspects of behavior [72]. The PFC has especially been associated with executive functions such as inhibition, working memory, shifting, and fluency [73]. A few functional magnetic resonance imaging (fMRI) studies have also demonstrated the involvement of the PFC in emotion regulation [74–76]. In particular, a systematic review on networks underpinning emotion [77] highlights the major involvement of the dlPFC in this capacity (among other fronto-limbic areas). According to Koenigs’ fMRI review on depression, damage to the dlPFC could especially lead to deficits in reappraisal/suppression of negative emotion [78]. Moreover, two other reviews investigating the consequences of PFC lesions indicated that damage to the dlPFC could also be linked to a loss of initiative, decreased motivation, reduced verbal output, and abulia [79, 80]. On the contrary, the most recent guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) [81] demonstrated a definite antidepressant efficacy of high-frequency rTMS of the left dlPFC and a probable antidepressant efficacy of low frequency rTMS of the right dlPFC in major depression. Taken together, these data indicate that prefrontal areas, and particularly the dlPFC, are key regions in the emergence of depressive symptoms, especially through their involvement in emotion regulation and motivation. Furthermore, it is worth noting that the prefrontal regions involved in depressive symptoms in DLB patients are all located in the right hemisphere, consistent with the hypothesis of general right hemispheric dominance for emotions, which is still widely accepted according to a recent review [82]. All in all, we suppose that GMV reductions in right prefrontal areas in DLB patients could, on one hand, lead to the development of emotional dysregulation through difficulties in reappraisal/suppression of negative emotion and, on the other hand, increase lack of initiation and pleasure in social and verbal activities. This hypothesis is supported by the review of Pizzagalli and Roberts, based on preclinical and clinical data, in which they demonstrated the functional involvement of PFC in the emergence and maintenance of anhedonic phenotypes, negative processing biases, and learned helplessness [83].
In addition to prefrontal involvement, we demonstrated a greater GMV loss in the right posterior cingulate cortex (PCC) and the precuneus in DLB patients with depressive symptoms when compared to DLB patients without depressive symptoms. These two regions are involved in self-related processing and social cognition [84–86]. Moreover, in their fMRI study on chronic MDD, Renner and colleagues demonstrated a decreased functional connectivity between the PCC and the precuneus following sad mood induction and suggested that this decrease might reflect a failure to exert cognitive control over negative memory retrieval [87]. These data indicate that the loss of GMV in the precuneus and PCC may be involved in the development of cognitive biases leading the subject to focus on negative stimuli. Besides, increased cortical thickness in the precuneus has been demonstrated in demented PD patients with depression [88]. The precuneus could be an important region in the emergence of depressive symptoms in DLB patients as well.
Although we demonstrated the involvement of frontal and cingulate areas in depressive symptoms in DLB, we were surprised to find no correlation between the depression scale and the GMV of the insula, contrary to our initial assumption. However, while we were unable to show a structural role for the insula in depressive symptoms in DLB, it is possible that this region has more of a functional role. Notably, the insular cortex is part of the default mode and salience functional networks that are known to be affected in both depression [89, 90] and DLB [91–93].
Furthermore, our study also highlights relevant imaging results regarding the impact of antidepressant treatment in DLB patients. Indeed, compared to our non-treated DLB group, our treated DLB group showed greater GMV loss in the bilateral middle/inferior temporal gyrus (BA 21, BA 20). It should be noted that, regarding this finding, we carried out additional retrospective behavioural analyses concerning the antidepressant treatment of our DLB patients. Patients in the treated DLB group had been receiving their treatment for 8 years on average, and the large majority (91.7%) had a personal history of depression. We therefore reasonably assumed that this treated DLB group reflected DLB patients with long-standing depression. Structural changes in those patients would then reflect the neurotoxicity of chronic depression in DLB. Besides, a VBM study on depression has already reported GMV reductions in the middle/inferior temporal gyrus when compared to controls [18]. We nevertheless cannot rule out the hypothesis that the loss of GMV in these areas could have been a direct consequence of the antidepressant treatment itself. However, we believe this to be unlikely in view of the well-known positive effect of antidepressants on brain structure. Indeed, according to a review by Videbech and colleagues, longitudinal MRI studies investigating the effect of antidepressants on brain volume generally demonstrated volume increases rather than structural damage [94].
In the present study, we demonstrated greater GMV volume in treated DLB patients compared to non-treated DLB patients in six clusters. The first and largest cluster was located in the left cuneus (BA 19/7), which is quite surprising since this area is not typically involved in emotion or mood disorders. However, the VBM study by Jung and colleagues revealed that the volume of the lingual gyrus highly predicted the antidepressant treatment effect [95]. Based on functional neuroimaging data, the authors suggested that antidepressant treatment shifted emotional processing bias for negative information towards a positive direction by modulating the functional activity in visual areas. Then, antidepressant treatment could first help to reduce negative processing biases in DLB patients through a specific action on the cuneus. Moreover, we also showed a greater GMV in three clusters in BA 6, particularly in the bilateral SMA, the right superior/middle frontal gyrus, and the right SMA in tDLB patients compared to ntDLB patients. Interestingly, lesions of the SMA have already been associated with an interruption of self-initiated movements [96] and with abulia [97]. One could therefore suppose that the GMV increase in these areas might conversely reflect greater behavioural activation and motivation in treated DLB patients. A significant GMV increase has also been found in the left middle frontal gyrus (BA 10) in tDLB patients compared to ntDLB patients. It is interesting to note that we highlighted above a loss of GMV in the right middle frontal gyrus (BA 10) in dDLB patients compared to ndDLB patients, and discussed the involvement of this region in emotion regulation and motivation [74, 77–80]. We therefore hypothesise that antidepressant treatment could provide a form of compensation through a positive effect on GMV in the contralateral BA 10, resulting in better emotion regulation and greater motivation in tDLB patients. Lastly, tDLB patients showed greater GMV in the right PCC compared to ntDLB patients. Interestingly, we mentioned above the involvement of this area in depressive symptoms in DLB patients. Overall, antidepressant treatment could have a pro-synaptic effect on specific regions associated with depressive symptoms in DLB and be especially effective in reducing negative processing biases, increasing motivation and behavioural activation, and improving emotion regulation in patients.
Taken together, these results could have interesting clinical implications. In addition to being a risk factor for cognitive impairment and having a negative impact on the quality of life of both patients and caregivers [98], we showed that depression can be associated with structural brain changes in DLB patients. Second, our results suggest that antidepressant treatment could have a beneficial effect not only on behaviour but also on GMV volume in those patients. However, although antidepressants are widely used in routine clinical practice, to our knowledge, there is only one randomized controlled-trial testing two types of antidepressants in DLB patients (risperidone and citalopram), but the results are inconclusive [99]. We therefore encourage the development of clinical trials using antidepressants in DLB patients, so that clinicians can be guided in their therapeutic choices when managing DLB patients with depressive symptoms.
Finally, concerning the imaging results of the AD group, our VBM analysis revealed a negative correlation between the MINI score and the GMV in the right temporal pole, meaning that the more AD patients are affected by depression, the less GMV they have in this region. Interestingly, decreased GMV in the temporal pole has already been reported in a few structural neuroimaging studies and meta-analyses on clinical depression [21, 24, 100] as well as in a VBM study on AD patients with depression [101]. An MRI study on patients with MDD even suggested a potential link between right temporal pole volume and the severity of depressive symptoms [102]. Through its connectivity with a number of sensory modalities (particularly visual and auditory), this region has been reported to be involved in socio-emotional processing [103–105]. The temporal pole would especially be involved in the interoceptive guidance of social interactions and sensitivity to markers of emotions [106]. Therefore, decreased GMV in the temporal pole might conceivably lead to a deficit to guide, process and/or respond to social interactions and emotional stimuli, and then to the development of depressive symptoms. But we cannot rule out the hypothesis that GMV reductions in this region might reflect the neurotoxic effects of prior depressive symptoms. Interestingly, in their VBM meta-analysis on MDD, Zackova and colleagues posit that GMV reductions in the temporal pole might lead to a reduced engagement in social activities and indirectly to the development of depressive symptoms or, on the contrary, that a poor social life would lead to temporal pole volume reductions and consequently to the development of depressive symptoms [21].
Note that we did not find any correlation between depression scores and frontal or insular GMV in AD patients, contrary to our initial hypothesis. Once again, we assume that this may have been because our AD patients were in the early stages of the disease. Indeed, we know that GMV reductions in frontal and insular cortex occurs at a late stage in typical AD, simultaneously with the progression of tauopathy through Braak stages [107]. It is thus possible that depressive symptoms worsen simultaneously with the evolution of the disease related atrophy.
This study has several limitations. First and foremost, none of our results were significant in FDR < 0.05. Although we chose to use a threshold of p < 0.001 with a spatial extent of 50 voxels in order to avoid irrelevant and/or isolated detections, the results we found will need to be replicated. Second, the depression scale we used (MINI) has been designed so that patients who answer “no” to the first two questions (looking at the two main depression criteria) are not asked the remaining seven items. Therefore, it is possible that our measure of depressive symptoms lacks sensitivity. Lastly, it should be noted that only 5 of our AD patients had depressive symptoms, and that the cluster highlighted by the regression analysis is below the 50-voxels threshold we originally set. Thus, although the obtained results are consistent with the literature, they should be taken with caution.
Conclusion
Using a whole-brain VBM approach, we were able to demonstrate an association between depressive symptoms in DLB and decreased GMV in right prefrontal regions involved in emotion regulation, initiation, and motivation. Reduced GMV in right frontal areas could thus lead to the development of depressive symptoms in DLB. On the contrary, we showed that treated DLB patients with long-standing depression would be more likely to experience GMV loss in the bilateral middle/inferior temporal cortex. GMV reductions in those regions could reflect the neurotoxic effects of chronic depressive symptoms in DLB patients. Finally, it should be noted that we demonstrated a positive effect of antidepressant treatment on the volume of several occipito-parietal and frontal regions in DLB patients.
While these structural imaging results provide us with a great deal of information, the fact remains that depression is essentially a functional disease, according to the widely accepted monoamine hypothesis. Notably, we discussed the functional impairment of the default mode and salience networks in depression and DLB. In addition, the dopaminergic system also appears to be significantly affected in both depression [108] and DLB [37, 109]. Therefore, further studies should investigate depression in DLB through fMRI methods, with a particular focus on these networks.
Acknowledgements
We are grateful to the patients and their relatives and to the healthy control subjects who took part in the present study. The authors also thank the medical doctors of the Memory Center of Strasbourg (CM2R), Pierre Anthony, and Catherine Martin-Hunyadi, the neuropsychologists, Timothée Albasser, Mathias Bilger, Laure Di Bitonto, Emmanuelle Epp-Ehrhard, Guillaume Jung, Jennifer Kemp, Catherine Kleitz, Jeanne Mérignac, Laetitia Monjoin, and Clélie Phillipps, the research clinical assistants, Lucie Rauch and Lea Sanna, as well as the secretary, Gabrielle Huck, for the essential collection of the clinical data. Finally, we would like to thank Vincent Gabriel and Alice Tisserand for their valuable help with image pre-processing and VBM analyses.
Author contributions
The present study was conceptualized, designed and coordinated by F.B., A.B., and M.Q. M.Q. conducted the behavioural and imaging analyses and wrote the article, while A.B. and F.B. played a major role in revising the manuscript. F.B., N.P., B.C., C.D., C.M., A.R., and B.S. were the medical doctors who carried out the clinical examinations and administered the depression scale, and L.S. was the clinical research assistant who took care of gathering information from medical records (i.e., treatments and medical history). P.LS. was in charge of the acquisition of MRI data, and M.M. was a great help with image pre-processing and VBM analyses. All authors read and approved the final manuscript.
Funding
This study was funded by Projet Hospitalier de Recherche Clinique (PHRC) inter-régional (IDRCB 2012-A00992-41).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor J-P, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies. Neurology. 2017;89:88–100. 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKeith IG, Ferman TJ, Thomas AJ, Blanc F, Boeve BF, Fujishiro H, et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology. 2020;94:743–55. 10.1212/WNL.0000000000009323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuijpers P, Koole SL, van Dijke A, Roca M, Li J, Reynolds CF. Psychotherapy for subclinical depression: meta-analysis. Br J Psychiatry. 2014;205:268–74. 10.1192/bjp.bp.113.138784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wyman-Chick KA, O’Keefe LR, Weintraub D, Armstrong MJ, Rosenbloom M, Martin PK, et al. Prodromal dementia with Lewy bodies: evolution of symptoms and predictors of dementia onset. J Geriatr Psychiatry Neurol. 2022;35:527–34. 10.1177/08919887211023586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Utsumi K, Fukatsu R, Hara Y, Takamaru Y, Yasumura S. Psychotic features among patients in the prodromal stage of dementia with lewy bodies during longitudinal observation. J Alzheimers Dis. 2021;83:1917–27. 10.3233/JAD-210416. [DOI] [PubMed] [Google Scholar]
- 6.Blanc F, Bouteloup V, Paquet C, Chupin M, Pasquier F, Gabelle A, et al. Prodromal characteristics of dementia with Lewy bodies: baseline results of the MEMENTO memory clinics nationwide cohort. Alzheimers Res Ther. 2022;14:96. 10.1186/s13195-022-01037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auning E, Rongve A, Fladby T, Booij J, Hortobágyi T, Siepel FJ, et al. Early and presenting symptoms of dementia with lewy bodies. Dement Geriatr Cogn Disord. 2011;32:202–8. 10.1159/000333072. [DOI] [PubMed] [Google Scholar]
- 8.Almeida L, Ahmed B, Walz R, De Jesus S, Patterson A, Martinez-Ramirez D, et al. Depressive symptoms are frequent in atypical parkinsonian disorders. Mov Disord Clin Pract. 2016;4:191–7. 10.1002/mdc3.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi S, Mizukami K, Yasuno F, Asada T. Depression associated with dementia with Lewy bodies (DLB) and the effect of somatotherapy. Psychogeriatrics. 2009;9:56–61. 10.1111/j.1479-8301.2009.00292.x. [DOI] [PubMed] [Google Scholar]
- 10.Fernández M, Gobartt AL, Balañá M. Behavioural symptoms in patients with Alzheimer’s disease and their association with cognitive impairment. BMC Neurol. 2010;10:87. 10.1186/1471-2377-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enache D, Winblad B, Aarsland D. Depression in dementia: epidemiology, mechanisms, and treatment. Curr Opin Psychiatry. 2011;24:461–72. 10.1097/YCO.0b013e32834bb9d4. [DOI] [PubMed] [Google Scholar]
- 12.Fritze F, Ehrt U, Sønnesyn H, Kurz M, Hortobágyi T, Nore SP, et al. Depression in mild dementia: associations with diagnosis, APOE genotype and clinical features. Int J Geriatr Psychiatry. 2011;26:1054–61. 10.1002/gps.2643. [DOI] [PubMed] [Google Scholar]
- 13.Andreasen P, Lönnroos E, von Euler-Chelpin MC. Prevalence of depression among older adults with dementia living in low- and middle-income countries: a cross-sectional study. Eur J Public Health. 2014;24:40–4. 10.1093/eurpub/ckt014. [DOI] [PubMed] [Google Scholar]
- 14.Chiu P-Y, Wang C-W, Tsai C-T, Li S-H, Lin C-L, Lai T-J. Depression in dementia with Lewy bodies: a comparison with Alzheimer’s disease. PLoS One. 2017;12:e0179399. 10.1371/journal.pone.0179399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Beek M, van Steenoven I, van der Zande JJ, Barkhof F, Teunissen CE, van der Flier WM, et al. Prodromal Dementia With Lewy Bodies: Clinical Characterization and Predictors of Progression. Mov Disord. 2020;35:859–67. 10.1002/mds.27997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakai K, Yamane Y, Yamamoto Y. Maeda K [Depression in dementia with Lewy bodies]. Seishin Shinkeigaku Zasshi. 2013;115:1127–34. [PubMed] [Google Scholar]
- 17.Yamane Y, Sakai K, Maeda K. Dementia with lewy bodies is associated with higher scores on the geriatric depression scale than is Alzheimer’s disease. Psychogeriatrics. 2011;11:157–65. 10.1111/j.1479-8301.2011.00368.x. [DOI] [PubMed] [Google Scholar]
- 18.Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM. Widespread reductions in gray matter volume in depression. Neuroimage Clin. 2013;3:332–9. 10.1016/j.nicl.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Hou Z, Yin Y, Xie C, Zhang H, Zhang H, et al. Decreased cortical thickness of left premotor cortex as a treatment predictor in major depressive disorder. Brain Imaging Behav. 2021;15:1420–6. 10.1007/s11682-020-00341-3. [DOI] [PubMed] [Google Scholar]
- 20.Kandilarova S, Stoyanov D, Sirakov N, Maes M, Specht K. Reduced grey matter volume in frontal and temporal areas in depression: contributions from voxel-based morphometry study. Acta Neuropsychiatr. 2019;31:252–7. 10.1017/neu.2019.20. [DOI] [PubMed] [Google Scholar]
- 21.Zacková MGRL, Jáni MGRM, Brázdil M, Nikolova YS, Marečková K. Cognitive impairment and depression: Meta-analysis of structural magnetic resonance imaging studies. Neuroimage Clin. 2021;32:102830. 10.1016/j.nicl.2021.102830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wise T, Radua J, Via E, Cardoner N, Abe O, Adams TM, et al. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol Psychiatry. 2017;22:1455–63. 10.1038/mp.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Wang Y, Niu C, Zhong S, Hu H, Chen P, et al. Common and distinct abnormal frontal-limbic system structural and functional patterns in patients with major depression and bipolar disorder. Neuroimage Clin. 2018;20:42–50. 10.1016/j.nicl.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abe O, Yamasue H, Kasai K, Yamada H, Aoki S, Inoue H, et al. Voxel-based analyses of gray/white matter volume and diffusion tensor data in major depression. Psychiatry Res. 2010;181:64–70. 10.1016/j.pscychresns.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Gray JP, Müller VI, Eickhoff SB, Fox PT. Multimodal abnormalities of brain structure and function in major depressive disorder: a meta-analysis of neuroimaging studies. Am J Psychiatry. 2020;177:422–34. 10.1176/appi.ajp.2019.19050560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng R, Zhang Y, Yang Z, Han S, Cheng J. Reduced brain gray matter volume in patients with first-episode major depressive disorder: a quantitative meta-analysis. Front Psychiatry. 2021;12:671348. 10.3389/fpsyt.2021.671348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng W, Chen Z, Yin L, Jia Z, Gong Q. Essential brain structural alterations in major depressive disorder: a voxel-wise meta-analysis on first episode, medication-naive patients. J Affect Disord. 2016;199:114–23. 10.1016/j.jad.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Li X, Cui Y, Liu K, Qu H, Lu Y, et al. Reduced gray matter volume in orbitofrontal cortex across schizophrenia, major depressive disorder, and bipolar disorder: a comparative imaging study. Front Neurosci. 2022;16:919272. 10.3389/fnins.2022.919272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim Y-K, Han K-M. Neural substrates for late-life depression: a selective review of structural neuroimaging studies. Prog Neuropsychopharmacol Biol Psychiatry. 2021;104:110010. 10.1016/j.pnpbp.2020.110010. [DOI] [PubMed] [Google Scholar]
- 30.Harada K, Matsuo K, Nakashima M, Hobara T, Higuchi N, Higuchi F, et al. Disrupted orbitomedial prefrontal limbic network in individuals with later-life depression. J Affect Disord. 2016;204:112–9. 10.1016/j.jad.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 31.Egger K, Schocke M, Weiss E, Auffinger S, Esterhammer R, Goebel G, et al. Pattern of brain atrophy in elderly patients with depression revealed by voxel-based morphometry. Psychiatry Res. 2008;164:237–44. 10.1016/j.pscychresns.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 32.Du M, Liu J, Chen Z, Huang X, Li J, Kuang W, et al. Brain grey matter volume alterations in late-life depression. J Psychiatry Neurosci. 2014;39:397–406. 10.1503/jpn.130275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang J-P, Lee T-W, Tsai S-J, Chen T-J, Yang C-H, Lirng J-F, et al. Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and voxel-based morphometry. J Geriatr Psychiatry Neurol. 2010;23:171–84. 10.1177/0891988710363713. [DOI] [PubMed] [Google Scholar]
- 34.Blanc F, Colloby SJ, Cretin B, de Sousa PL, Demuynck C, O’Brien JT, et al. Grey matter atrophy in prodromal stage of dementia with Lewy bodies and Alzheimer’s disease. Alzheimers Res Ther. 2016;8:31. 10.1186/s13195-016-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blanc F, Colloby SJ, Philippi N, de Pétigny X, Jung B, Demuynck C, et al. Cortical thickness in dementia with lewy bodies and Alzheimer’s disease: a comparison of prodromal and dementia stages. PLoS One. 2015;10:e0127396. 10.1371/journal.pone.0127396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roquet D, Noblet V, Anthony P, Philippi N, Demuynck C, Cretin B, et al. Insular atrophy at the prodromal stage of dementia with Lewy bodies: a VBM DARTEL study. Sci Rep. 2017;7:9437. 10.1038/s41598-017-08667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patterson L, Rushton SP, Attems J, Thomas AJ, Morris CM. Degeneration of dopaminergic circuitry influences depressive symptoms in Lewy body disorders. Brain Pathol. 2019;29:544–57. 10.1111/bpa.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saari L, Heiskanen L, Gardberg M, Kaasinen V. Depression and nigral neuron density in lewy body spectrum diseases. Ann Neurol. 2021;89:1046–50. 10.1002/ana.26046. [DOI] [PubMed] [Google Scholar]
- 39.Benarroch EE, Schmeichel AM, Sandroni P, Parisi JE, Low PA. Rostral raphe involvement in Lewy body dementia and multiple system atrophy. Acta Neuropathol. 2007;114:213–20. 10.1007/s00401-007-0260-3. [DOI] [PubMed] [Google Scholar]
- 40.Mizutani M, Sano T, Ohira M, Takao M. Neuropathological studies of serotonergic and noradrenergic systems in Lewy body disease patients with delusion or depression. Psychiatry Clin Neurosci. 2022;76:459–67. 10.1111/pcn.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lebedev AV, Beyer MK, Fritze F, Westman E, Ballard C, Aarsland D. Cortical changes associated with depression and antidepressant use in Alzheimer and Lewy body dementia: an MRI surface-based morphometric study. Am J Geriatr Psychiatry. 2014;22:4-13.e1. 10.1016/j.jagp.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Shimoda K, Kimura M, Yokota M, Okubo Y. Comparison of regional gray matter volume abnormalities in Alzheimer׳s disease and late life depression with hippocampal atrophy using VSRAD analysis: a voxel-based morphometry study. Psychiatry Res. 2015;232:71–5. 10.1016/j.pscychresns.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 43.Son JH, Han DH, Min KJ, Kee BS. Correlation between gray matter volume in the temporal lobe and depressive symptoms in patients with Alzheimer’s disease. Neurosci Lett. 2013;548:15–20. 10.1016/j.neulet.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 44.Wu Y, Wu X, Wei Q, Wang K, Tian Y. Differences in cerebral structure associated with depressive symptoms in the elderly with Alzheimer’s disease. Front Aging Neurosci. 2020;12:107. 10.3389/fnagi.2020.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu X, Meiberth D, Newport B, Jessen F. Anatomical correlates of the neuropsychiatric symptoms in Alzheimer’s disease. Curr Alzheimer Res. 2015;12:266–77. 10.2174/1567205012666150302154914. [DOI] [PubMed] [Google Scholar]
- 46.Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–46. 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 47.Ferman TJ, Smith GE, Boeve BF, Ivnik RJ, Petersen RC, Knopman D, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology. 2004;62:181–7. 10.1212/wnl.62.2.181. [DOI] [PubMed] [Google Scholar]
- 48.Fénelon G, Soulas T, Zenasni F, de Langavant LC. The changing face of Parkinson’s disease-associated psychosis: a cross-sectional study based on the new NINDS-NIMH criteria. Mov Disord. 2010;25:763–6. 10.1002/mds.22839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 2003;18:738–50. 10.1002/mds.10473 [DOI] [PubMed]
- 50.Gjerstad MD, Boeve B, Wentzel-Larsen T, Aarsland D, Larsen JP. Occurrence and clinical correlates of REM sleep behaviour disorder in patients with Parkinson’s disease over time. J Neurol Neurosurg Psychiatry. 2008;79:387–91. 10.1136/jnnp.2007.116830. [DOI] [PubMed] [Google Scholar]
- 51.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 52.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 53.Wahab S, Chua TY, Razali R, Mat Saher Z, Zamzam IH, Bujang MA. Suicidal behavior among elderly inpatients: its relation to functional disability and pain. Psychol Res Behav Manag. 2022;15:737–50. 10.2147/PRBM.S341768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin Y, Liyanage BN, Sun Y, Lu T, Zhu Z, Liao Y, et al. A deep learning-based model for detecting depression in senior population. Front Psychiatry. 2022;13:1016676. 10.3389/fpsyt.2022.1016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu M-E, Chang Y-H, Ku Y-C, Lee S-Y, Huang C-C, Chen S-L, et al. Executive functions in elderly men. Age (Dordr). 2012;34:59–66. 10.1007/s11357-011-9215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oh DJ, Han JW, Bae JB, Kim TH, Kwak KP, Kim BJ, et al. Executive dysfunction and risk of suicide in older adults: a population-based prospective cohort study. J Neurol Neurosurg Psychiatry. 2021;92:528–33. 10.1136/jnnp-2020-324390. [DOI] [PubMed] [Google Scholar]
- 57.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 58.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 59.Verdaguer ES, Stafford J, Tuijt R, Orgeta V. Minor and subthreshold depressive disorders in Alzheimer’s disease: a systematic review and meta-analysis of prevalence studies. J Affect Disord. 2020;263:728–34. 10.1016/j.jad.2019.11.053. [DOI] [PubMed] [Google Scholar]
- 60.Asmer MS, Kirkham J, Newton H, Ismail Z, Elbayoumi H, Leung RH, et al. Meta-analysis of the prevalence of major depressive disorder among older adults with dementia. J Clin Psychiatry. 2018;79:17r11772. 10.4088/JCP.17r11772. [DOI] [PubMed] [Google Scholar]
- 61.Römer B, Dalen I, Ballard C, Aarsland D. The course of depressive symptoms in Lewy body dementia and Alzheimer’s disease. J Affect Disord. 2023;333:459–67. 10.1016/j.jad.2023.04.076. [DOI] [PubMed] [Google Scholar]
- 62.Chendo I, Silva C, Duarte GS, Prada L, Vian J, Quintão A, et al. Frequency of depressive disorders in parkinson’s disease: a systematic review and meta-analysis. J Parkinsons Dis. 2022;12:1409–18. 10.3233/JPD-223207. [DOI] [PubMed] [Google Scholar]
- 63.Iritani S, Tsuchiya K, Arai T, Akiyama H, Ikeda K. An atypical autopsy case of Lewy body disease with clinically diagnosed major depression: a clinical, radiological and pathological study. Neuropathology. 2008;28:652–9. 10.1111/j.1440-1789.2008.00905.x. [DOI] [PubMed] [Google Scholar]
- 64.Ishiguro M, Baba H, Maeshima H, Shimano T, Inoue M, Ichikawa T, et al. Increased serum levels of α-synuclein in patients with major depressive disorder. Am J Geriatr Psychiatry. 2019;27:280–6. 10.1016/j.jagp.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 65.Elder GJ, Colloby SJ, Lett DJ, O’Brien JT, Anderson KN, Burn DJ, et al. Depressive symptoms are associated with daytime sleepiness and subjective sleep quality in dementia with Lewy bodies. Int J Geriatr Psychiatry. 2016;31:765–70. 10.1002/gps.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Del Pino R, Murueta-Goyena A, Acera M, Carmona-Abellan M, Tijero B, Lucas-Jiménez O, et al. Autonomic dysfunction is associated with neuropsychological impairment in Lewy body disease. J Neurol. 2020;267:1941–51. 10.1007/s00415-020-09783-7. [DOI] [PubMed] [Google Scholar]
- 67.Schaffert J, LoBue C, White CL, Wilmoth K, Didehbani N, Lacritz L, et al. Risk factors for earlier dementia onset in autopsy-confirmed Alzheimer’s disease, mixed Alzheimer’s with Lewy bodies, and pure Lewy body disease. Alzheimers Dement. 2020;16:524–30. 10.1002/alz.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kazmi H, Walker Z, Booij J, Khan F, Shah S, Sudre CH, et al. Late onset depression: dopaminergic deficit and clinical features of prodromal Parkinson’s disease: a cross-sectional study. J Neurol Neurosurg Psychiatry. 2021;92:158–64. 10.1136/jnnp-2020-324266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boot BP, Orr CF, Ahlskog JE, Ferman TJ, Roberts R, Pankratz VS, et al. Risk factors for dementia with Lewy bodies: a case-control study. Neurology. 2013;81:833–40. 10.1212/WNL.0b013e3182a2cbd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bennett S, Thomas AJ. Depression and dementia: cause, consequence or coincidence? Maturitas. 2014;79:184–90. 10.1016/j.maturitas.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 71.Kessing LV. Depression and the risk for dementia. Curr Opin Psychiatry. 2012;25:457–61. 10.1097/YCO.0b013e328356c368. [DOI] [PubMed] [Google Scholar]
- 72.Jones DT, Graff-Radford J. Executive dysfunction and the prefrontal cortex. Continuum (Minneap Minn). 2021;27:1586–601. 10.1212/CON.0000000000001009. [DOI] [PubMed] [Google Scholar]
- 73.Rabinovici GD, Stephens ML, Possin KL. Executive dysfunction. Continuum (Minneap Minn). 2015;21:646–59. 10.1212/01.CON.0000466658.05156.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berboth S, Windischberger C, Kohn N, Morawetz C. Test-retest reliability of emotion regulation networks using fMRI at ultra-high magnetic field. Neuroimage. 2021;232:117917. 10.1016/j.neuroimage.2021.117917. [DOI] [PubMed] [Google Scholar]
- 75.Golkar A, Lonsdorf TB, Olsson A, Lindstrom KM, Berrebi J, Fransson P, et al. Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS One. 2012;7:e48107. 10.1371/journal.pone.0048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–86. 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Underwood R, Tolmeijer E, Wibroe J, Peters E, Mason L. Networks underpinning emotion: a systematic review and synthesis of functional and effective connectivity. Neuroimage. 2021;243:118486. 10.1016/j.neuroimage.2021.118486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–43. 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pirau L, Lui F. Frontal Lobe Syndrome. StatPearls, Treasure Island (FL): StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 80.Szczepanski SM, Knight RT. Insights into human behavior from lesions to the prefrontal cortex. Neuron. 2014;83:1002–18. 10.1016/j.neuron.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lefaucheur J-P, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin Neurophysiol. 2020;131:474–528. 10.1016/j.clinph.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 82.Gainotti G. Emotions and the right hemisphere: can new data clarify old models? Neuroscientist. 2019;25:258–70. 10.1177/1073858418785342. [DOI] [PubMed] [Google Scholar]
- 83.Pizzagalli DA, Roberts AC. Prefrontal cortex and depression. Neuropsychopharmacology. 2022;47:225–46. 10.1038/s41386-021-01101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tisserand A, Philippi N, Botzung A, Blanc F. Me, myself and my insula: an oasis in the forefront of self-consciousness. Biology (Basel). 2023;12:599. 10.3390/biology12040599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn Sci. 2007;11:153–7. 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 86.Frewen P, Schroeter ML, Riva G, Cipresso P, Fairfield B, Padulo C, et al. Neuroimaging the consciousness of self: review, and conceptual-methodological framework. Neurosci Biobehav Rev. 2020;112:164–212. 10.1016/j.neubiorev.2020.01.023. [DOI] [PubMed] [Google Scholar]
- 87.Renner F, Siep N, Arntz A, van de Ven V, Peeters FPML, Quaedflieg CWEM, et al. Negative mood-induction modulates default mode network resting-state functional connectivity in chronic depression. J Affect Disord. 2017;208:590–6. 10.1016/j.jad.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 88.Zanigni S, Sambati L, Evangelisti S, Testa C, Calandra-Buonaura G, Manners DN, et al. Precuneal thickness and depression in parkinson disease. Neurodegener Dis. 2017;17:97–102. 10.1159/000450614. [DOI] [PubMed] [Google Scholar]
- 89.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiat. 2015;72:603–11. 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, et al. Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. Am J Psychiatry. 2018;175:1111–20. 10.1176/appi.ajp.2018.17101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lowther ER, O’Brien JT, Firbank MJ, Blamire AM. Lewy body compared with Alzheimer dementia is associated with decreased functional connectivity in resting state networks. Psychiatry Res. 2014;223:192–201. 10.1016/j.pscychresns.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 92.Pezzoli S, De Marco M, Zorzi G, Cagnin A, Venneri A. Functional brain connectivity patterns associated with visual hallucinations in dementia with Lewy bodies. J Alzheimers Dis Rep. 2021;5:311–20. 10.3233/ADR-200288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chabran E, Noblet V, Loureiro de Sousa P, Demuynck C, Philippi N, Mutter C, et al. Changes in gray matter volume and functional connectivity in dementia with Lewy bodies compared to Alzheimer’s disease and normal aging: implications for fluctuations. Alzheimers Res Ther 2020;12:9. 10.1186/s13195-019-0575-z. [DOI] [PMC free article] [PubMed]
- 94.Videbech P, Yttri J-E. The effect of antidepressants on brain volume. Ugeskr Laeger. 2019;181:V02190087. [PubMed] [Google Scholar]
- 95.Jung J, Kang J, Won E, Nam K, Lee M-S, Tae WS, et al. Impact of lingual gyrus volume on antidepressant response and neurocognitive functions in Major Depressive Disorder: a voxel-based morphometry study. J Affect Disord. 2014;169:179–87. 10.1016/j.jad.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 96.Thompson AE, Thompson PD. Frontal lobe motor syndromes. Handb Clin Neurol. 2023;196:443–55. 10.1016/B978-0-323-98817-9.00008-9. [DOI] [PubMed] [Google Scholar]
- 97.Das JM, Saadabadi A. Abulia. InStatPearls [Internet] 2023. StatPearls Publishing. [PubMed]
- 98.Jellinger KA. Depression in dementia with Lewy bodies: a critical update. J Neural Transm (Vienna). 2023;130:1207–18. 10.1007/s00702-023-02669-8. [DOI] [PubMed] [Google Scholar]
- 99.Culo S, Mulsant BH, Rosen J, Mazumdar S, Blakesley RE, Houck PR, et al. Treating neuropsychiatric symptoms in dementia with Lewy bodies: a randomized controlled-trial. Alzheimer Dis Assoc Disord. 2010;24:360–4. 10.1097/WAD.0b013e3181e6a4d7. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y-N, Li H, Shen Z-W, Xu C, Huang Y-J, Wu R-H. Healthy individuals vs patients with bipolar or unipolar depression in gray matter volume. World J Clin Cases. 2021;9:1304–17. 10.12998/wjcc.v9.i6.1304. [DOI] [PMC free article] [PubMed]
- 101.Mohamed Nour AEA, Jiao Y, Teng G-J. Alzheimer’s Disease Neuroimaging Initiative. Neuroanatomical associations of depression, anxiety and apathy neuropsychiatric symptoms in patients with Alzheimer’s disease. Acta Neurol Belg. 2021;121:1469–80. 10.1007/s13760-020-01349-8. [DOI] [PubMed] [Google Scholar]
- 102.Takahashi T, Yücel M, Lorenzetti V, Walterfang M, Kawasaki Y, Whittle S, et al. An MRI study of the superior temporal subregions in patients with current and past major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:98–103. 10.1016/j.pnpbp.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 103.Herlin B, Navarro V, Dupont S. The temporal pole: from anatomy to function-a literature appraisal. J Chem Neuroanat. 2021;113:101925. 10.1016/j.jchemneu.2021.101925. [DOI] [PubMed] [Google Scholar]
- 104.Wong C, Gallate J. The function of the anterior temporal lobe: a review of the empirical evidence. Brain Res. 2012;1449:94–116. 10.1016/j.brainres.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 105.Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–31. 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- 106.Mesulam MM. Temporopolar regions of the human brain. Brain. 2023;146:20–41. 10.1093/brain/awac339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–8. 10.1016/0197-4580(95)00021-6. (discussion 278-284). [DOI] [PubMed] [Google Scholar]
- 108.Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17:524–32. 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jreige M, Kurian GK, Perriraz J, Potheegadoo J, Bernasconi F, Stampacchia S, et al. The diagnostic performance of functional dopaminergic scintigraphic imaging in the diagnosis of dementia with Lewy bodies: an updated systematic review. Eur J Nucl Med Mol Imaging. 2023;50:1988–2035. 10.1007/s00259-023-06154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


