Abstract
Acute transverse myelitis (ATM) is a disease characterized by inflammation of the spinal cord and may have various causes. In the context of this work, the distinction between isolated ATM and initial manifestation of autoimmune-mediated diseases of the central nervous system such as multiple sclerosis (MS) is crucial. Hence, the aim of this work was to identify predictive factors associated with the conversion to definite MS in a collective of individuals after their initial episode of isolated ATM (no initial identified cause). In this retrospective data analysis from the Vienna MS Database, all patients from Jan. 1, 1999, to Dec. 31, 2019, with a diagnosis of isolated ATM (according to the criteria of the Transverse Myelitis Consortium Working Group) who underwent lumbar puncture were extracted. Electronic medical records were reviewed on the availability of clinical data including therapy and follow-up, laboratory results including cerebrospinal fluid (CSF) analysis, evoked potentials (EP) as well as magnetic resonance imaging data. Among 42 patients with the diagnosis of isolated ATM, 12 (29%) were subsequently diagnosed with MS over a median follow-up period of 7.7 years. Univariately, MS converters were younger (32 years [25–39] vs. 42 years [31–50], p = 0.032), had a lower CSF/serum albumin ratio (29 [24–35] vs 37 [27–52], p = 0.037), lower CSF total protein (4.5 [2.8–4.8] vs. 5.5 [3.4–8.5], p = 0.023) and a higher proportion of CSF-specific oligoclonal bands (OCB; 83% vs. 30%, p = 0.002). In the multivariate regression analysis, the presence of CSF-specific OCB emerged as the sole predictive factor of subsequent MS diagnosis (OR: 14.42, 95% CI 1.39 to 149.48, p = 0.03). In a collective of 42 patients with isolated ATM and an MS conversion rate of nearly 30%, the only but highly predictive factor were CSF-specific OCB. This emphasizes the significance of conducting timely CSF analysis in such patients and underscores the need for tailored monitoring and follow-up strategies in this specific group.
Keywords: Isolated acute transverse myelitis, Oligoclonal bands, Multiple sclerosis, Predictive factors
Subject terms: Immunology, Autoimmunity
Introduction
Isolated acute transverse myelitis (ATM) is characterized as an inflammatory myelopathy with an uncertain cause, according to the Transverse Myelitis Consortium Working Group (TMCWG). It is defined by the presence of bilateral (though not necessarily symmetric) signs or symptoms of myelopathy, a well-defined sensory level, evidence of inflammation based on cerebrospinal fluid (CSF) or magnetic resonance imaging (MRI) with contrast-enhancing lesions (CELs), and clinical progression reaching a nadir between 4 h and 21 days. Diagnosis of isolated ATM also necessitates the exclusion of specific alternative causes, including disease-associated acute transverse myelitis, and the absence of brain MRI abnormalities indicative of a disseminated process1,2. At this point it should be noted that in this paper we always refer to isolated and not idiopathic ATM, as this is seen as more accurate by the authors.
Epidemiological studies have reported the occurrence of isolated ATM with an age-standardized annual incidence of 24.6 per million and 6.2 per million for definite and possible isolated ATM, respectively3.
Pathobiological causes for isolated ATM include inflammatory diseases, in particular the spectrum of disseminated demyelinating diseases of the central nervous system with multiple sclerosis (MS) representing the most prevalent entity, followed by compressive, neoplastic, vascular, nutritional, or infectious causes. The diverse underlying etiologies pose a challenge in achieving precise diagnoses; nevertheless, ongoing progress enables us to provide an accurate diagnosis before classifying as isolated2.
Yet, isolated ATM continues to represent a subset of patients for whom no underlying pathobiology can be identified. Among them, a proportion will later be diagnosed with MS, though stratification for these patients remains limited.
Considering the potential implications for treatment decisions and long-term outcomes, it is crucial to not only conduct a thorough and challenging differential diagnosis when encountering isolated ATM and adhere to the proposed diagnostic criteria for isolated ATM, but also to identify definitive predictive factors that can help to determine the likelihood of future conversion to MS.
Hence, the aim of this work was to evaluate clinical and paraclinical predictive factors for potential later conversion to MS in a well characterized cohort of patients with a long follow-up, fulfilling the diagnostic criteria of isolated ATM.
Methods
Patients and definitions
For this retrospective cohort study, we initially screened in-house medical records retrospectively from Jan. 1, 1999, to Dec. 31, 2019 for all patients with available internal CSF data and diagnosis of isolated ATM (according to the criteria of the Transverse Myelitis Consortium Working Group)1.
Subsequently, we utilized the Vienna MS Database (VMSD) of the Department of Neurology, to extract clinical and follow-up data, which we confirmed and supplemented by individually reviewing patient charts4. Hence, data were obtained from 80 patients with documented isolated ATM and available CSF investigations as well as MRI data.
Clinical charts and patients’ files were reviewed using VMSD and individual medical records, and data retrieved, including demographic data, clinical data including symptoms, Expanded Disability Status Scale (EDSS), follow-up and received acute therapeutic management (intravenous high-dose methylprednisolone, plasma exchange/ Immuno-adsorption), and presence of an MRI of the spinal cord and brain. These were reviewed for other pathologies, presence of one or more spinal cord lesion(s) as well as presence of one or more brain lesion(s), in accordance with the guidelines published by Filippi and colleagues in 20195. All patients with fulfillment of the latter were excluded. Laboratory tests included peripheral blood workup, microbiology tests, antibody tests (presence of Aquaporin-4 and Myelin-Oligodendrocyte-Glycoprotein [MOG] antibodies), CSF parameters such as cell count, microbiological tests, protein, albumin, lactate, cytology and oligoclonal bands (OCB). OCB were interpreted as positive when at least two bands were present in the CSF sample but not in the corresponding serum sample6. Aquaporin-4 and MOG antibodies were retrospectively tested in 22 biobanked CSF samples, while from 2013 onwards, 20 patients had both serum and CSF tested as part of routine clinical work-up. These tests were conducted using a live cell-based assay at a reference center (the lab of Prof. Höftberger). A retrospective review of all data was performed to examine potential underlying causes of isolated ATM, including systemic autoimmune diseases, infectious or malignant origins. The diagnosis of MS was made retrospectively according to the revised 2017 McDonald criteria in respective cases7.
Electrophysiological measurements were retrieved and data on visual evoked potentials (VEP) were extracted. Therefore, P100 component of VEP response, potential configuration and amplitude were analyzed. Pathological findings were determined on the basis of internal standard operating procedures (SOP).
Finally, all 80 patients were evaluated for fulfillment of the proposed diagnostic criteria for isolated ATM in accordance with the TMCWG1.
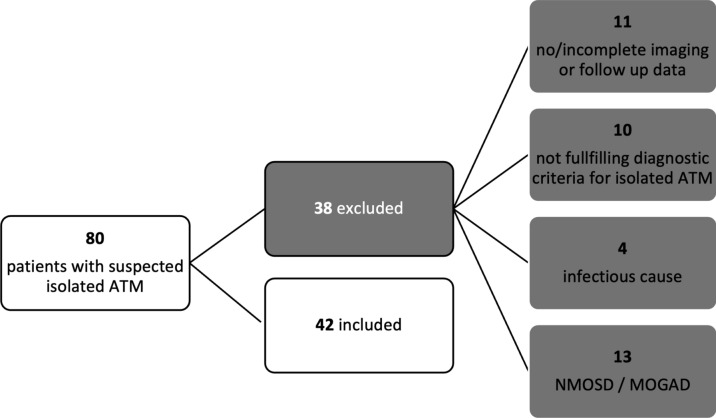
The detailed inclusion process is shown in Fig. 1.
Fig. 1.
Inclusion flow chart. ATM acute transverse myelitis, MOGAD myelin-oligodendrocyte-glycoprotein associated disease; NMOSD neuromyelitis-optica-spectrum disorder.
Ethics
The ethics committee of the Medical University Vienna (MUV) approved the study (ethical approval number: 1455/2021) and therefore all methods were performed in accordance with the relevant guidelines and regulations. As datasets were exported pseudonymously from local databases including data obtained in routine practice, the need for written informed consent from study participants was waived by the ethics committee of the MUV. This study adheres to the reporting guidelines outlined within the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.
Statistics
All statistical analyses and graphical representations were performed in R (Version 4.2.1). Univariate group comparisons were done by Chi-square test, Mann–Whitney U test, or independent t-test (with Welch’s correction in case of unequal standard deviations in the groups) as appropriate.
For the multivariate binary logistic regression model: initially, all explanatory variables were tested using a univariate model, and significant p-value less than 0.05) variables were included in the final multivariate binary logistic regression model.
Sex, time to last follow up and age were included in the multivariate model regardless of their significance in the univariate model due to their a priori potential explanatory power. The significance of individual variables was assessed using the Wald Chi-Squared Test.
We checked for linearity assumption (martingale residuals and deviance residuals) and proportional hazard's assumption and found them acceptable. We tested all variables for normal distribution using the Lilliefors-test and for collinearity using variance inflation factor (VIF). Any variables with a VIF greater than 2.0 were excluded from the multivariate regression analysis. McFadden's R squared was used to evaluate the goodness of fit for the logistic regression models. We considered a two-sided p-value less than 0.05 as statistically significant.
Missing values were reported and for subgroup analysis only complete cases were analysed.
Results
A total of 80 patients were identified, of whom 38 were excluded due to lack of imaging data, lack of follow-up, and/or retrospectively known underlying cause of ATM (Fig. 1).
Demographics and characteristics of the groups (ATM-non-MS; ATM-MS) are shown in Table 1. Of the total 42 patients included in the analysis, 12 (29%) were diagnosed with MS at the last follow-up.
Table 1.
1n / N (%); Median (IQR). 2Fisher's exact test, Pearson's Chi-squared test, Wilcoxon rank sum exact test, Wilcoxon rank sum test.
| Characteristics | ATM-non-MS, N = 301 | ATM-MS, N = 121 | p-value2 |
|---|---|---|---|
| Clinical | |||
| Sex | 0.45 | ||
| Male | 10/30 (33%) | 2/12 (17%) | |
| Female | 20/30 (67%) | 10/12 (83%) | |
| Age of onset | 42 (31, 50) | 32 (25, 39) | 0.032 |
| Time to last FU (y) | 7.7 (2.9, 9.4) | 7.7 (3.4, 8.8) | 0.9 |
| Onset to nadir (d) | 3 (1, 10) | 6 (4, 17) | 0.16 |
| Motor symptoms | 11/30 (37%) | 5/12 (42%) | > 0.99 |
| Sensory symptoms | 30/30 (100%) | 12/12 (100%) | > 0.99 |
| Sensory level | 0.3 | ||
| Cervical | 7/30 (23%) | 6/12 (50%) | |
| Thoracal | 17/30 (57%) | 5/12 (42%) | |
| Lumbal | 6/30 (20%) | 1/12 (8.3%) | |
| EDSS at onset | 1.00 (1.00, 2.88) | 1.50 (1.00, 2.13) | > 0.99 |
| Time to LP | 5.0 (3.0,6.0) | 5.5 (3.8, 7.0) | 0.47 |
| Time to MRI | 5.0 (3.0, 9.8) | 8.5 (3.8, 10.3) | 0.41 |
| Steroids | 20/30 (67%) | 9/11 (82%) | 0.46 |
| n.a | 0 | 1 | |
| PLEX/IA | 3/30 (10%) | 0/11 (0%) | 0.55 |
| n.a | 0 | 1 | |
| Antiviral | 5/30 (17%) | 0/11 (0%) | 0.3 |
| n.a | 0 | 1 | |
| MRI | |||
| Segments involved on MRI | 1 (, 2) | 2 (1, 2) | 0.29 |
| Level MRI | 0.81 | ||
| Cervical | 16/30 (53%) | 8/12 (67%) | |
| Thoracic | 13/30 (43%) | 4/12 (33%) | |
| Lumbar | 1/30 (3.3%) | 0/12 (0%) | |
| CEL | 22/27 (81%) | 10/11 (91%) | 0.65 |
| n.a | 3 | 1 | |
| Multiple spinal MRI lesions present | 5/30 (17%) | 5/12 (42%) | 0.12 |
| LETM | 0.3 | ||
| No | 25/30 (83%) | 12/12 (100%) | |
| Yes | 5/30 (17%) | 0/12 (0%) | |
| CSF | |||
| CSF cell count (cell/mm3) | 4 (1, 13) | 7 (3, 11) | 0.55 |
| CSF total protein (mg/dL) | 37 (27, 52) | 29 (24, 35) | 0.037 |
| CSF AlbuminQ | 5.5 (3.4, 8.5) | 4.5 (2.8, 4.8) | 0.023 |
| Intrathecal IgG | 0.00 (0.00, 0.00) | 0.80 (0.00, 2.85) | 0.014 |
| Intrathecal IgM | 0 (0, 0) | 0 (0, 0) | 0.39 |
| Intrathecal IgA | 0/30 (0%) | 0/12 (0%) | |
| OCB | 0.002 | ||
| Neg | 21/30 (70%) | 2/12 (17%) | |
| Pos | 9/30 (30%) | 10/12 (83%) | |
| Electrophysiological | |||
| VEP | 0.38 | ||
| Normal | 17/21 (81%) | 6/10 (60%) | |
| Pathological | 4/21 (19%) | 4/10 (40%) | |
| n.a | 9 | 2 | |
Significant values are in bold.
CEL contrast-enhancing lesion, d days, EDSS expanded disability status scale at initial presentation, FU follow-up, IA immunoadsorption, ATM-non-MS acute transverse myelitis not subsequently diagnosed with MS, ATM-MS acute transverse myelitis subsequently diagnosed with MS, LETM longitudinal extensive transverse myelitis, n.a. not available, OCB oligoclonal bands, PLEX plasma exchange, VEP visual evoked potential, y years.
Patients who were later diagnosed with definite MS were younger at the age of the ATM (32 years [25–39] vs. 42 years [31–50], p = 0.032), had lower total protein and a lower CSF/serum albumin ratio (29 [24–35] vs. 37 [27–52], p = 0.037; 4.5 [2.8–4.8] vs 5.5 [3.4–8.5], p = 0.023, respectively), as well as more often positive CSF-specific OCB (10/12 (83%) vs. 9/30 (30%), p = 0.002). The prevalence of motor or sensory symptoms, sensory levels, and electrophysiological measurements did not show any significant differences (Table 1). Of note, in the retrospectively reviewing of the medical records, we were only able to retrieve the final report of the investigation categorizing it as either normal or pathological, without detailed information about latency and/or amplitude, though it is taken into account for the final report according to the internal SOPs.
In the next step, univariate regression analysis was used to identify variables associated with a later diagnosis of MS (Table 2).
Table 2.
CEL contrast-enhancing lesion, d days, EDSS expanded disability status scale, FU follow-up, LETM Longitudinal extensive transverse myelitis, OCB oligoclonal bands, OR odds ratio, CI confidence interval, VEP visual evoked potential, y years.
| Variables | N | Diagnosis of MS | OR | 95% CI | p-value |
|---|---|---|---|---|---|
| Sex | 42 | 12 | 0.26 | ||
| Male | – | – | |||
| Female | 2.5 | 0.53–18.3 | |||
| Age of onset | 42 | 12 | 0.93 | 0.86–0.99 | 0.022 |
| Time to last FU (y) | 42 | 12 | 1.05 | 0.89–1.23 | 0.58 |
| Onset to nadir (d) | 42 | 12 | 1.06 | 0.97–1.16 | 0.2 |
| Motor symptoms | 42 | 12 | 0.76 | ||
| No | – | – | |||
| Yes | 1.23 | 0.30–4.85 | |||
| Sensory level | 42 | 12 | 0.22 | ||
| Cervical | 2.91 | 0.67–13.5 | |||
| Thoracal | – | – | |||
| Lumbal | 0.57 | 0.03–4.57 | |||
| EDSS at onset | 42 | 12 | 0.98 | 0.60–1.47 | 0.91 |
| Steroids | 41 | 11 | 0.33 | ||
| No | – | – | |||
| Yes | 2.25 | 0.46–16.6 | |||
| Segments involved on MRI | 42 | 12 | 1.04 | 0.58–1.74 | 0.88 |
| Level MRI | 42 | 12 | 0.56 | ||
| Cervical | 1.63 | 0.41–7.23 | |||
| Thoracic | – | – | |||
| Lumbar | 0 | ||||
| CEL | 38 | 11 | 0.45 | ||
| No | – | – | |||
| Yes | 2.27 | 0.31–46.6 | |||
| Multiple spinal MRI lesions | 42 | 12 | 0.1 | ||
| No | – | – | |||
| Yes | 3.57 | 0.79–16.7 | |||
| LETM | 42 | 12 | 0.057 | ||
| No | – | – | |||
| Yes | < 0.1 | ||||
| CSF cell count (cell/mm3) | 42 | 12 | 0.99 | 0.94–1.00 | 0.22 |
| CSF total protein (mg/dL) | 42 | 12 | 0.93 | 0.85–0.99 | 0.013 |
| CSF AlbuminQ | 42 | 12 | 0.7 | 0.45–0.94 | 0.011 |
| OCB | 42 | 12 | 0.001 | ||
| Neg | – | – | |||
| Pos | 11.7 | 2.47–86.8 | |||
| VEP | 31 | 10 | 0.22 | ||
| Normal | – | – | |||
| Pathological | 2.83 | 0.52–15.9 |
Significant values are in bold.
In the univariate regression analysis, age (OR: 0.93; CI 0.86 to 0.99, p = 0.022), CSF total protein (OR: 0.93, CI 0.85 to 0.99, p = 0.013), CSF/serum albumin ratio (OR: 0.70, CI 0.45 to 0.94, p = 0.011) and CSF-specific OCB (OR: 11.7, CI 2.47 to 86.8, p = 0.001) were significantly associated with the diagnosis of MS after ATM presentation. Neither clinical parameters nor imaging parameters or electrophysiological measurements were associated with the risk of MS diagnosis.
In the multivariate model, the only factor significantly associated with a risk for subsequent conversion to MS were positive CSF-specific OCB (OR: 14.42, CI 1.39–149.48, p = 0.03) (Fig. 2, Table 3).
Fig. 2.
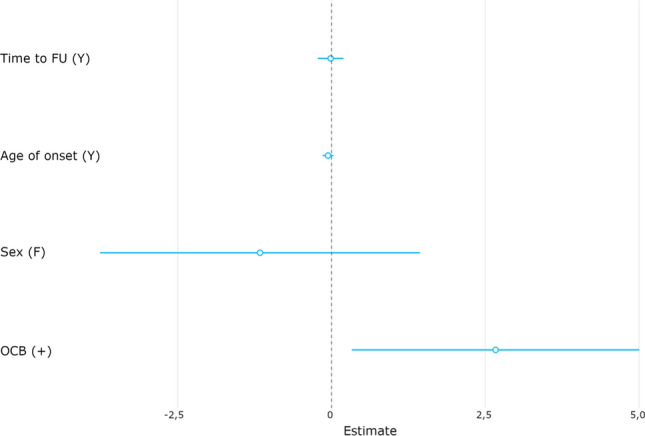
f female, FU Follow-up, OCB oligoclonal bands; visualization of the results from the adapted multivariate regression model with OCB being the only significant factor associated with conversion from ATM to MS. Values represent non transformed coefficients.
Table 3.
FU: follow up, y: years, OCB: Oligoclonal bands, OR: Odds Ratio, CI: confidence interval, p: p-value
| Risk of MS diagnosis | OR | 95% CI | p | |
|---|---|---|---|---|
| 2.5% | 97.5% | |||
| Multivariate model | ||||
| Intercept | 1.77 | 0.01 | 230.33 | 0.82 |
| Age of onset | 0.95 | 0,86 | 1.03 | 0.22 |
| Sex(male) | 0.31 | 0.02 | 4.22 | 0.38 |
| Time to last FU (y) | 0.99 | 0.80 | 1.22 | 0.90 |
| OCB (positive) | 14.42 | 1.39 | 149.48 | 0.03 |
| Pseudo-R2 (McFadden) = 0.25 | ||||
Significant values are in bold.
Discussion
In this retrospective study, we present findings from a well-characterized collective of 42 patients fulfilling the proposed diagnostic criteria for isolated ATM (termed idiopathic ATM in the criteria), followed for a median time of 7.7 years. Of this cohort, 29% were diagnosed with MS at the last follow-up, and OCB emerged as the sole significant but highly robust predictive factor for later conversion to MS.
This early available predictive biomarker holds crucial implications for clinicians, to inform risk stratification and monitoring process for patients presenting with isolated ATM.
Our findings are in line with the literature reporting OCB as highly predictive biomarker for the later diagnosis of MS in the context of isolated ATM8–11. This can be explained by the fact that OCB is an indicator of chronic intrathecal immunoglobulin synthesis and is thus considered a pathophysiological predictor of chronic CNS inflammation. At the same time, however, the limited specificity and the resulting necessary differential diagnostics must be taken into account6. Likewise in line with the literature, CSF parameters such as protein levels were higher in the non-MS group, albeit not significantly associated in the multivariate models8,9.
We did not find any MRI specific characteristics to be more prominent in MS-converters or non-converters. Recently, LETM was proposed as an important variable in stratifying risk of MS in the context of isolated ATM, though this was not the case in other studies8,9. While LETM is rare in the MS population and data support clinical and paraclinical differences in these populations in comparison to short-segment myelitis, LETM can occur in the MS population. One explanation is the misinterpretation of LETM due to several short myelitis lesions with edema12,13. In our cohort, none of the patients with LETM were diagnosed with MS at the last follow-up.
Visual Evoked Potentials (VEPs) did not show a significant predictive value in stratifying the risk for subsequent conversion to MS. This lack of significance may be attributed to various factors, including technical limitations, language barriers, the limited specificity of pathological Evoked Potentials (EP), and the relatively small sample size of the study14. Of note, in this study, VEPs were not repeated at intervals15. In this context, it should be mentioned that absence of normal VEP is not an exclusion criterion for diagnosis of isolated ATM according to the diagnostic criteria published in 20021.
Future studies will show if VEPs may have a value in patients with ATM or other modalities to assess retinal integrity such as OCT measures may also predict risk of conversion to MS in the context of ATM16.
Limitations
Similar to previous studies regarding this topic, the small sample size and the retrospective study design represent limitations, although the rarity of isolated ATM with an annual incidence of 6.2 per million should be kept in mind in this context3,8,17. More precisely, the small sample size is clearly a possible reason for the lack of statistical significance of other possible predictors. Particularly with regard to the MRI and electrophysiological measurements, this could be a potential reason for the missing of statistical significance in the different number of subjects with multiple spinal cord lesions in the ATM-MS (n = 5/12, 42%) versus the ATM-non-MS (n = 5/30, 17%) group. Beyond that and in contrast to this work, other studies have also considered additional factors such as somatosensory evoked potentials or family history of MS and thus it would be desirable for future studies to investigate further factors such as these or, for example, imaging factors such as lesion pattern or pattern of contrast enhancement17. Further, the diagnostic criteria for isolated ATM were published in 2002 and therefore do not consider relevant advancements in the field of MRI as well as highly specific serological marker like Aquaporin-4 or MOG antibodies for NMSOD and MOGAD, respectively18–20. (see Fig. 1).
Although we carefully reviewed clinical records and excluded patients with features suggestive of alternative diagnoses, the lack of comprehensive antibody testing for all patients in serum and CSF (20 patients underwent both serum and CSF testing, while 22 patients underwent only CSF testing) represents a potential limitation.
Despite the study's extensive follow-up period, there remains a chance that some patients initially classified in the ATM-non-MS group might meet the diagnostic criteria for multiple sclerosis (MS) in the future, introducing a potential source of bias.
Conclusion
In this retrospective real-world study with an extensive follow-up duration, the presence of OCB emerges as a highly influential predictive factor for conversion to MS in patients with an initial manifestation of isolated ATM. This emphasizes the significance of conducting timely CSF analysis in such patients and underscores the need for tailored monitoring and follow-up treatment strategies in this specific group.
Author contributions
Conception and design of the work: TM, FL, TZ. Data analysis and interpretation: TM, MP, GB, NK, GZ, PR, BK, TB, FL, TZ. Drafting the article: TM, TZ. Critical revision of the article: TM, MP, GB, NK, GZ, PR, BK, TB, FL, TZ. Final review and approval of the version to be published TM, MP, GB, NK, GZ, PR, BK, TB, FL, TZ.
Data availability
De-identified data can be made available from the corresponding author upon reasonable request by a qualified researcher and upon approval by the data-clearing committee of the Medical University of Vienna.
Competing interests
Tobias Monschein: has participated in meetings sponsored by or received travel funding from Biogen, Celgene, Merck, Novartis, Roche, Sanofi-Genzyme and Teva. Markus Ponleitner: has participated in meetings sponsored by or received travel funding from Amicus, Merck, Novartis and Sanofi-Genzyme. Gabriel Bsteh: has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Celgene/BMS, Janssen-Cilag, Lilly, Merck, Novartis, Roche, Sanofi-Genzyme and Teva, and received honoraria for consulting Biogen, Celgene/BMS, Merck, Novartis, Roche, Sanofi-Genzyme and Teva. He has received financial support in the past 12 months by unrestricted research grants (Celgene/BMS, Novartis). Nik Krajnc: has participated in meetings sponsored by, received speaker honoraria or travel funding from Alexion, BMS/Celgene, Janssen-Cilag, Merck, Novartis, Roche and Sanofi-Genzyme and held a grant for a Multiple Sclerosis Clinical Training Fellowship Programme from the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). Gudrun Zulehner: has participated in meetings sponsored by or received travel funding from Biogen, Merck, Novartis, Roche, Sanofi-Genzyme and Teva. Paulus Rommer: has received honoraria for consultancy/speaking from AbbVie, Allmiral, Alexion, Biogen, Merck, Novartis, Roche, Sandoz, Sanofi Genzyme, has received research grants from Amicus, Biogen, Merck, Roche. Barbara Kornek: has received honoraria for speaking and for consulting from Biogen, BMS-Celgene, Johnson&Johnson, Merck, Novartis, Roche, Teva and Sanofi-Genzyme outside of the submitted work. No conflict of interest with respect to the present study. Thomas Berger: has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS: Allergan, Bayer, Biogen, Bionorica, BMS/Celgene, Genesis, GSK, GW/Jazz Pharma, Horizon, Janssen-Cilag, MedDay, Merck, Novartis, Octapharma, Roche, Sandoz, Sanofi-Genzyme, Teva and UCB. His institution has received financial support in the past 12 months by unrestricted research grants (Biogen, Bayer, BMS/Celgene, Merck, Novartis, Roche, Sanofi-Genzyme, Teva and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Octapharma, Roche, Sanofi-Genzyme, Teva. Fritz Leutmezer: has participated in meetings sponsored by, received speaker honoraria or travel funding from Actelion, Almirall, Biogen, Celgene, Johnson & Johnson, MedDay, Merck, Novartis, Roche, Sanofi-Genzyme and Teva, and received honoraria for consulting Biogen, Celgene, Merck, Novartis, Roche, Sanofi-Genzyme and Teva. Tobias Zrzavy: has participated in meetings sponsored by or received travel funding from Biogen, Merck, Novartis, Roche, Sanofi-Genzyme and Teva.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Román, G. C. & Kerr, D. Proposed diagnostic criteria and nosology of acute transverse myelitis [6] (multiple letters). Neurology60, 730–731. 10.1212/WNL.60.4.730 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Zalewski, N. L., Flanagan, E. P. & Keegan, B. M. Evaluation of idiopathic transverse myelitis revealing specific myelopathy diagnoses. Neurology90, e96-102. 10.1212/WNL.0000000000004796 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Young, J. et al. Clinically isolated acute transverse myelitis: Prognostic features and incidence. Mult. Scler.15, 1295–1302. 10.1177/1352458509345906 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Bsteh, G. et al. Quantifying the risk of disease reactivation after interferon and glatiramer acetate discontinuation in multiple sclerosis: The VIAADISC score. Eur. J. Neurol.28, 1609–1616. 10.1111/ene.14705 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filippi, M. et al. Assessment of lesions on magnetic resonance imaging in multiple sclerosis: Practical guidelines. Brain142, 1858–1875. 10.1093/brain/awz144 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deisenhammer, F. et al. The cerebrospinal fluid in multiple sclerosis. Front. Immunol.10.3389/fimmu.2019.00726 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson, A. J. et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol.17, 162–173 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Murphy, O. C. et al. Early factors associated with later conversion to multiple sclerosis in patients presenting with isolated myelitis. J. Neurol. Neurosurg. Psychiatry92, 831–838. 10.1136/jnnp-2020-325274 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Cobo Calvo, Á. et al. Idiopathic acute transverse myelitis: Outcome and conversion to multiple sclerosis in a large series. BMC Neurol.13, 135. 10.1186/1471-2377-13-135 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gastaldi, M. et al. Predictors of outcome in a large retrospective cohort of patients with transverse myelitis. Mult. Scler. J.24, 1743–1752. 10.1177/1352458517731911 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Bruna Escuer, J. et al. Idiopathic acute transverse myelitis: A clinical study and prognostic markers in 45 cases. Mult. Scler.12, 169–173. 10.1191/135248506ms1260oa (2006). [DOI] [PubMed] [Google Scholar]
- 12.Das, S. et al. Radiological illusion of longitudinally extensive myelitis in multiple sclerosis. Neuroimmunol. Rep.2, 100088. 10.1016/j.nerep.2022.100088 (2022). [Google Scholar]
- 13.Asnafi, S. et al. The frequency of longitudinally extensive transverse myelitis in MS: A population-based study. Mult. Scler. Relat. Disord.37, 101487. 10.1016/j.msard.2019.101487 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Leocani, L., Guerrieri, S. & Comi, G. Visual evoked potentials as a biomarker in multiple sclerosis and associated optic neuritis. J. Neuro-Ophthalmol.38, 350–357. 10.1097/WNO.0000000000000704 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Calugaru, L., Calugaru, G. T. & Calugaru, O. M. Evoked potentials in multiple sclerosis diagnosis and management. Curr. Health Sci. J.42, 385–389. 10.12865/CHSJ.42.04.08 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bsteh, G. et al. Diagnostic performance of adding the optic nerve region assessed by optical coherence tomography to the diagnostic criteria for MS. Neurology10.1212/WNL.0000000000207507.10.1212/WNL.0000000000207507 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sellner, J. et al. Acute partial transverse myelitis: Risk factors for conversion to multiple sclerosis. Eur. J. Neurol.15, 398–405. 10.1111/j.1468-1331.2008.02088.x (2008). [DOI] [PubMed] [Google Scholar]
- 18.Wattjes, M. P. et al. 2021 MAGNIMS–CMSC–NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol.20, 653–670. 10.1016/S1474-4422(21)00095-8 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Wingerchuk, D. M. et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology85, 177–189 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banwell, B. et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol.22, 268–282 (2023). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified data can be made available from the corresponding author upon reasonable request by a qualified researcher and upon approval by the data-clearing committee of the Medical University of Vienna.