Abstract
Liver-expressed antimicrobial peptide 2 (LEAP2) is a peptide that counteracts the hunger hormone ghrelin-induced functions. Recently, we showed that vertical sleeve gastrectomy (VSG) did not alter the serum LEAP2 concentration in individuals with obesity. Here, we investigated the effects of VSG in both chow diet (CD)-fed and high-fat diet (HFD)-fed mice. In CD-fed mice, VSG increased plasma LEAP2 levels and hepatic Leap2 mRNA levels while decreasing body weight, blood glucose levels, and ghrelin levels. Intraperitoneal (ip) administration of ghrelin reversed these changes. These effects were found in both male and female mice. In contrast, VSG or weight loss in HFD-induced obese mice decreased LEAP2 levels. After fasting, the plasma LEAP2 concentration was in the following order: hepatic vein > abdominal aorta > portal vein. A high glucose concentration robustly increased the plasma LEAP2 concentration in the hepatic vein and abdominal aorta but not in the portal vein. In addition, corn oil or palmitate increased LEAP2 expression and secretion. The increase in LEAP2 levels after the meal tolerance test was delayed in the human subjects with diabetes. Our data suggest that various factors (metabolic, hormonal, and nutritional) regulate LEAP2, and the liver is the predominant site for the production and secretion of LEAP2. Furthermore, the interaction between ghrelin and LEAP2 is involved in the pathogenesis of obesity and diabetes.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-74048-6.
Keywords: LEAP2, Ghrelin, Hepatokine, Diabetes, Obesity, Vertical sleeve gastrectomy
Subject terms: Endocrine system and metabolic diseases, Gastrointestinal hormones
Introduction
Ghrelin is a 28-amino-acid peptide in which octanoyl fatty acid is added to serine 31. It increases food intake, weight gain, adiposity, blood glucose levels, and growth hormone (GH) secretion by activating its cognate receptor, which is a G protein-coupled receptor known as the growth hormone secretagogue receptor (GHSR)1,2. Ghrelin is primarily produced in stomach X/A-like cells localized in the oxyntic gland in the mucosa of the gastric fundus, and ghrelin-producing cells occupy 20–25% of the endocrine cell population in oxyntic glands3. Des-acyl ghrelin (DAG), which lacks fatty acid modification, is a major molecular form of fatty acid in the circulation that accounts for approximately 90% of the ghrelin moiety in the blood3.
Liver-expressed antimicrobial peptide 2 (LEAP2) is a 40-amino-acid peptide that was initially identified as an antimicrobial agent from human haemofiltration in 20034. LEAP2 is the second liver-expressed antimicrobial peptide, and LEAP1/hepcidin, a 25-amino-acid peptide, is the first4. Both LEAP1 and LEAP2 are cationic peptides predominantly expressed in the liver. LEAP1 possesses four disulfide bonds, and LEAP2 possesses two disulfide bonds4,5. In 2018, Ge et al.. examined metabolic regulators whose expression levels changed after vertical sleeve gastrectomy (VSG) in the stomach and the duodenum of mice with high-fat diet (HFD)-induced obesity6. They reported that LEAP2 is an endogenous GHSR antagonist6. LEAP2 suppressed food intake and decreased blood glucose levels in rodents and humans, indicating that it may be a potential target for obesity treatment6–9.
VSG is a bariatric surgery that reduces the size of the stomach, including ghrelin-producing cells. Hence, plasma ghrelin levels are decreased by VSG in humans and rodents10,11. Leap2 mRNA expression was upregulated in the stomach and downregulated in the duodenum by VSG in HFD-induced obese mice6. However, whether VSG affects plasma LEAP2 and hepatic Leap2 expression in chow diet (CD)-fed lean mice and HFD-induced obese mice is unknown.
In the present study, we investigated LEAP2 production and secretion induced by glucose, lipids, and fatty acids in mice. We examined the effect of nutrients on LEAP2 haemodynamics in humans with normal glucose tolerance and those with diabetes. We investigated the effect of VSG on LEAP2 production in CD-fed male and female mice and HFD-induced obese male mice. We studied the effect of ghrelin administration on LEAP2 production in VSG-treated mice. We also compared LEAP2 production between the VSG and pair-fed groups. We have shown that VSG increased plasma LEAP2 levels and hepatic Leap2 expression in CD-fed mice, while ghrelin administration to these mice reversed these changes. VSG surgery or ~ 10% weight loss resulting from caloric intake reduction in HFD-induced obese mice was associated with decreased plasma LEAP2 levels and Leap2 expression in the liver.
Results
Glucose enhanced LEAP2 production and secretion
To explore the impact of glucose on LEAP2 production and secretion, we administered glucose orally (2 g/kg body weight) or ip (2 g/kg body weight) to 24-h fasted mice (Fig. 1A). Glucose administration increased blood glucose levels, plasma LEAP2 levels and hepatic Leap2 mRNA levels but decreased plasma DAG levels and stomach Ghrelin (Ghrl) mRNA levels (Fig. 1B–F). We maintained primary mouse hepatocytes in Williams’ medium E supplemented with 190 mg/dl glucose. Glucose stimulation at 440 mg/dl for 6 h increased Leap2 mRNA levels in hepatocytes (Fig. 1G).
Fig. 1.
Alterations in plasma parameters and mRNA levels in male mice orally or intraperitoneally administered glucose. (A) Experimental design (glucose: 2 g/kg body weight). (B) Blood glucose, (C) plasma LEAP2, (D) hepatic Leap2 mRNA, (E) plasma des-acyl ghrelin (DAG), (F) stomach Ghrl mRNA, and (G) Leap2 mRNA levels in primary mouse hepatocytes. n = 6/group. The data were analysed by a two-tailed unpaired t test.
Corn oil and palmitate enhanced LEAP2 production and secretion
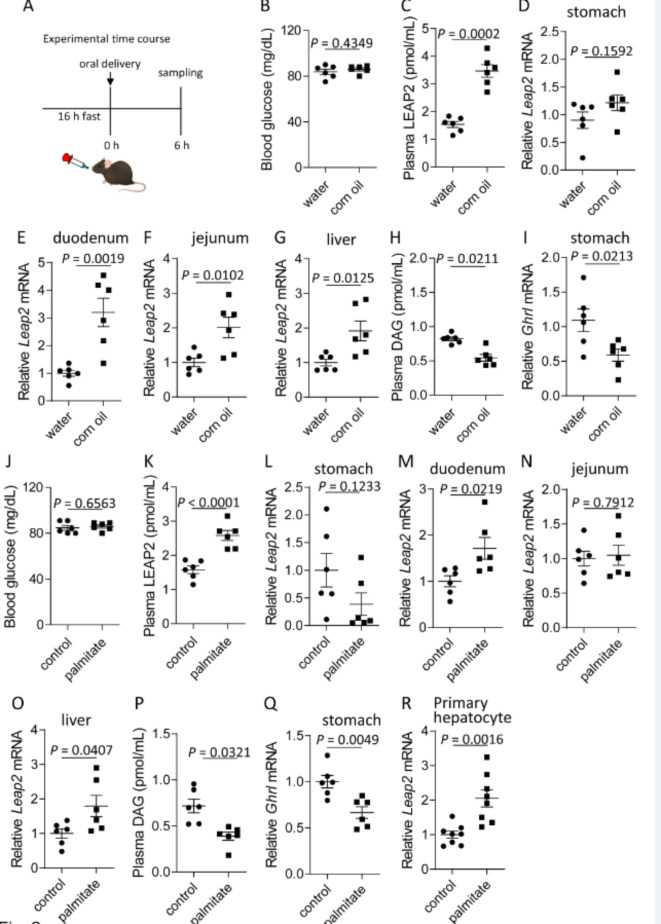
To explore the effects of fat on LEAP2 production and secretion, we administered corn oil (100 µl/mouse) orally to 16-h fasted mice (Fig. 2A). Corn oil did not affect blood glucose levels (Fig. 2B). Corn oil increased the plasma LEAP2 levels and Leap2 mRNA levels in the duodenum, jejunum, and liver but not in the stomach (Fig. 2C–G). Corn oil decreased plasma DAG levels and Ghrl mRNA levels in the stomach (Fig. 2H, I).
Fig. 2.
Acute responses to oral administration of corn oil or palmitate in lean male mice. (A) Experimental design (corn oil: 100 µl/mouse; palmitate: 3 µmol/mouse). (B) Blood glucose levels, (C) plasma LEAP2 levels, Leap2 mRNA levels in the (D) stomach, (E) duodenum, (F) jejunum and (G) liver, (H) plasma DAG levels, and (I) stomach Ghrl mRNA levels in mice that received oral administration of water or corn oil. (J) Blood glucose, (K) plasma LEAP2 levels, Leap2 mRNA levels in the (L) stomach, (M) duodenum, (N) jejunum, and (O) liver, (P) plasma DAG levels, and (Q) stomach Ghrl mRNA levels in mice that received oral administration of control solution (3.75% BSA in 0.01 M NaOH) or palmitate. (R) Primary mouse hepatocytes were stimulated with a control solution (3.75% BSA in 0.01 M NaOH) or palmitate. n = 6/group. The data were analysed by a two-tailed unpaired t test.
Next, palmitate (3 µmol/mouse) was orally administered to 16-h fasted mice (Fig. 2A). Palmitate did not affect blood glucose levels (Fig. 2J) but increased plasma LEAP2 levels and Leap2 mRNA levels in the duodenum and liver but not in the stomach or jejunum (Fig. 2K–O). Palmitate decreased plasma DAG levels and Ghrl mRNA levels in the stomach (Fig. 2P, Q). Palmitate upregulated Leap2 mRNA expression in primary mouse hepatocytes (Fig. 2R).
The liver is a major LEAP2-secretory organ
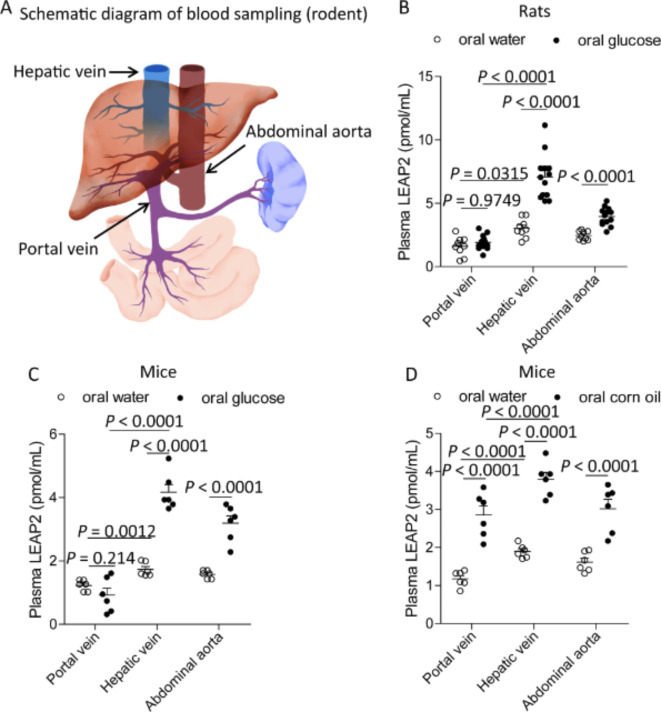
To identify the LEAP2-secreting organ, we examined plasma LEAP2 levels in three blood vessels in rodents, as shown in Fig. 3A. Oral glucose administration to rats increased blood glucose levels in these vessels via a concentration gradient in the portal vein, hepatic vein, and abdominal aorta (Supplementary Fig. S1A). During fasting, the highest plasma concentrations of LEAP2 and DAG were found in the hepatic and portal veins, respectively (Fig. 3B and Supplementary Fig. S1B). Oral glucose administration to rats increased the plasma LEAP2 concentration in the hepatic vein and the abdominal aorta but not in the portal vein (Fig. 3B). Oral glucose administration decreased plasma DAG levels in these vessels (Supplementary Fig. S1B). Oral glucose administration to mice also increased the plasma LEAP2 concentration in the hepatic vein and the abdominal aorta but not in the portal vein (Fig. 3C). Oral corn oil administration to mice increased the plasma LEAP2 concentration in all three vessels (Fig. 3D).
Fig. 3.
Plasma LEAP2 levels in rodents. (A) Schematic of the blood collection site. Plasma LEAP2 levels in the portal vein, hepatic vein, and abdominal aorta of rats (B) or mice (C) receiving oral glucose (2 g/kg body weight) or (D) mice receiving oral corn oil (100 µl/mouse). For rats, oral water (n = 9) or oral glucose (n = 13) was used. For mice, oral water (n = 6), oral glucose (n = 6), oral water (n = 6), or oral corn oil (n = 6) were used. The data were analysed by two-way ANOVA followed by Tukey’s HSD post hoc test (B–D).
Energy intake increased plasma LEAP2 levels in humans
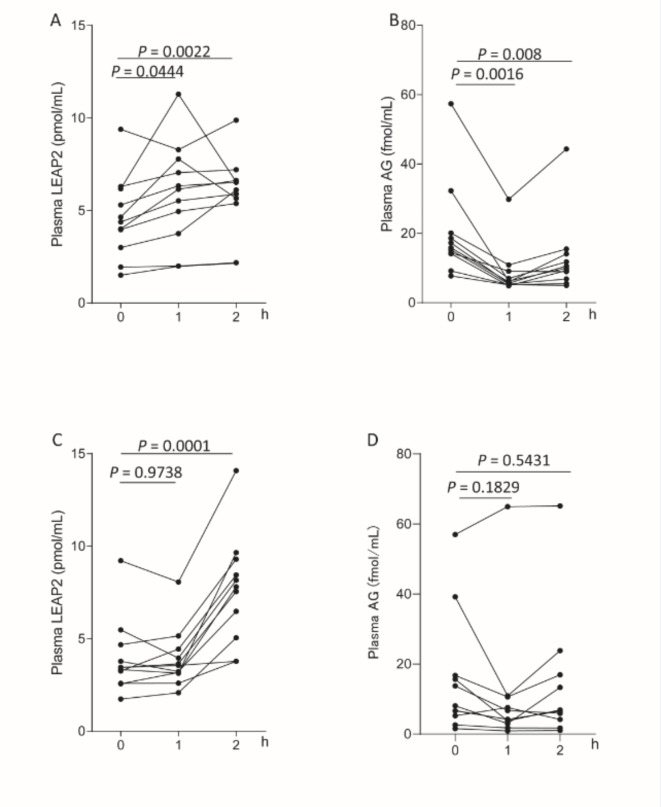
To investigate the effect of nutrients on LEAP2 haemodynamics, a meal tolerance test was performed on subjects with normal glucose tolerance or diabetes. Plasma LEAP2 levels in the subjects with normal glucose tolerance increased 1 h and 2 h after meal consumption (Fig. 4A), and plasma AG levels decreased 1 h after meal consumption (Fig. 4B). However, the increase in LEAP2 levels was delayed in the subjects with diabetes (Fig. 4C). Plasma AG concentrations did not decrease after meal consumption in the subjects with diabetes (Fig. 4D).
Fig. 4.
Plasma LEAP2 and AG levels in humans. (A) Plasma LEAP2 and (B) plasma acyl ghrelin (AG) levels in subjects with normal glucose tolerance (n = 11) and (C) plasma LEAP2 and (D) plasma AG levels in subjects with diabetes (n = 11) in response to 592 kcal/m2 meal intake. The data were analysed by one-way ANOVA followed by Dunnett’s multiple comparison test (A-D). Points represent average values, and upper and lower error bars represent maximum and minimum values, respectively.
Effects of VSG on mice and ghrelin administration after VSG
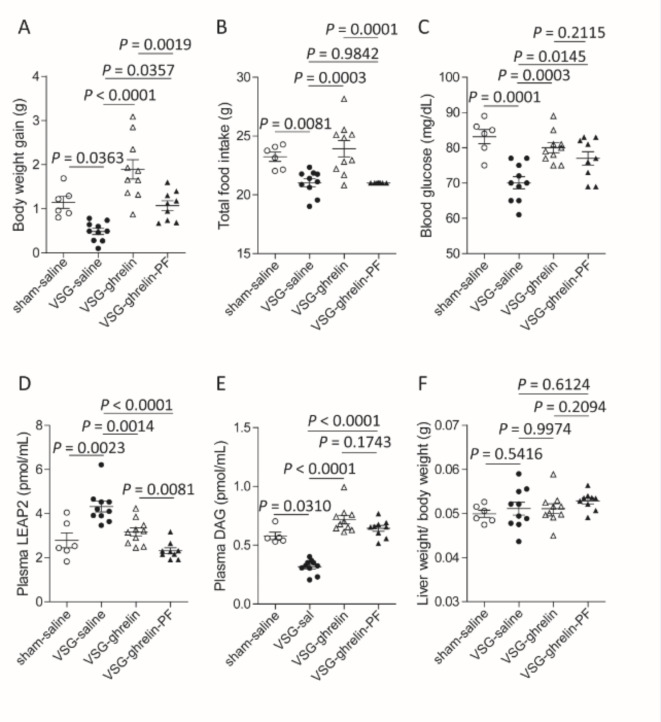
The level of LEAP2 in the stomach is increased by VSG in HFD-fed mice. To investigate the effect of VSG on LEAP2 secretion and expression, we examined VSG mice fed a CD or HFD. The experimental design for this section is shown in Fig. 5A. Sham-saline mice underwent sham surgery and then received saline for 7 days. VSG-saline mice and VSG-ghrelin mice underwent VSG and then received saline and ghrelin, respectively. The VSG-ghrelin-pair-fed (PF) mice consumed the same amount of food as the VSG-saline mice during ghrelin administration. Body weight gain, food intake, and blood glucose levels were lower in both male (Fig. 5B–D) and female (Fig. 6A–C) VSG-saline mice than in sham-saline mice. Compared with sham-saline mice, VSG-saline mice had higher plasma LEAP2 levels but lower plasma DAG levels. Ghrelin administration to VSG-saline mice restored these parameters to levels similar to those of sham-saline mice (Figs. 5B–F and 6A–E). Compared with those of the VSG-saline mice, the VSG-ghrelin-PF mice exhibited significantly greater body weight gain and blood glucose levels (Figs. 5B and D and 6A and C). The ratio of liver weight to body weight did not change among the 4 groups (Figs. 5G and 6F). Ghrelin administration to VSG-ghrelin-PF mice further decreased plasma LEAP2 levels compared to those in VSG-ghrelin mice (Figs. 5E and 6D). Compared with the VSG-saline mice, the VSG-ghrelin-PF mice had higher blood glucose levels but lower plasma LEAP2 levels (Figs. 5D and E and 6C and D). VSG upregulated Leap2 mRNA expression in the livers of male and female mice (Supplementary Fig. S2A, B). The food efficiency of the VSG-saline mice was significantly lower than that of the sham-saline mice. Ghrelin increased the food efficiency impaired by VSG (Supplementary Fig. S2C, D).
Fig. 5.
Restoration of metabolic parameters by i.p. administration of ghrelin in VSG-operated male mice. (A) Experimental design. (B) Body weight gain, (C) food intake for one week between 9 and 10 weeks, (D) blood glucose levels, (E) plasma LEAP2 levels, (F) plasma DAG levels, and (G) liver weight/body weight ratio. sham-saline mice, n = 6; VSG-saline mice, n = 13; VSG-ghrelin mice, n = 16; and VSG-ghrelin-pair fed (PF) mice, n = 10. The data were analysed by one-way ANOVA followed by Tukey’s HSD post hoc test (B–G).
Fig. 6.
Restoration of metabolic parameters by i.p. administration of ghrelin in VSG-operated female mice. (A) Body weight gain, (B) food intake for one week between 9 and 10 weeks, (C) blood glucose levels, (D) plasma LEAP2 levels, (E) plasma DAG levels, and (F) liver weight/body weight in sham or VSG-operated mice that received i.p. saline or ghrelin. Sham-saline mice, n = 6; VSG-saline mice, n = 10; VSG-ghrelin mice, n = 10; VSG-ghrelin-PF mice, n = 9. The data were analysed by one-way ANOVA followed by Tukey’s HSD post hoc test.
Metabolic improvements by VSG in HFD-fed mice
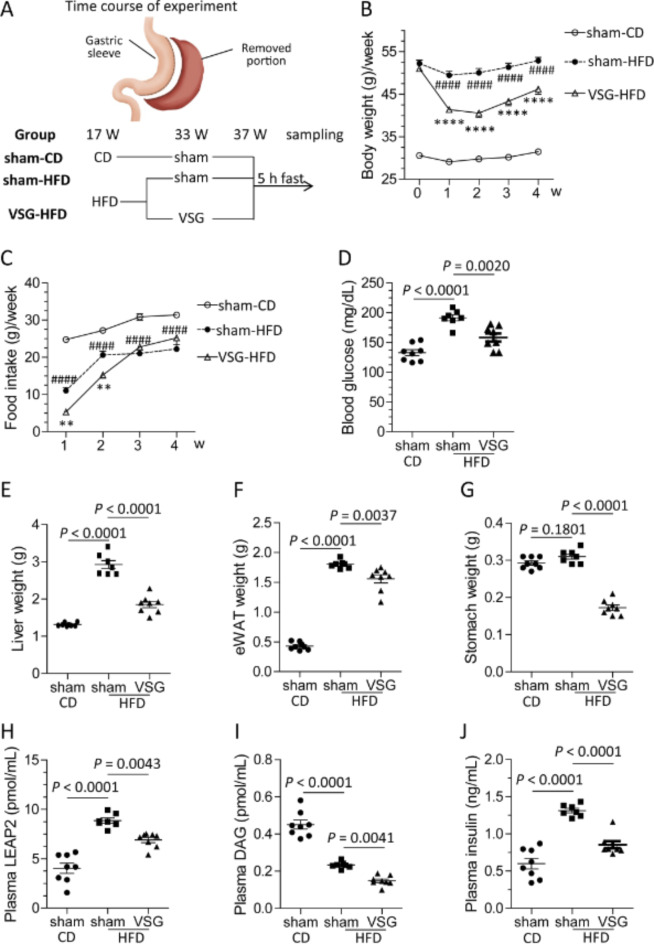
Next, we studied the effects of VSG on LEAP2 in mice fed a HFD for 20 weeks (Fig. 7A). Compared with HFD-fed mice that underwent a sham operation (sham-HFD), HFD-fed mice that underwent VSG (VSG-HFD) presented a reduced body weight from one week to four weeks after the operation (Fig. 7B). The VSG-HFD mice consumed less food than the sham-HFD mice did up to two weeks after the operation, and they consumed the same amount of food as the sham-HFD mice did three weeks after the operation (Fig. 7C). Compared with the sham-HFD mice, the VSG-HFD mice had lower blood glucose levels, liver weights, epididymal white adipose tissue (eWAT) weights, and stomach weights (Fig. 7D–G). A HFD elevated the plasma LEAP2 and insulin levels but decreased the plasma DAG level (Fig. 7H–J). Compared with the sham-HFD mice, the VSG-HFD mice presented reduced plasma LEAP2, insulin, and DAG levels (Fig. 7H–J). A HFD upregulated Leap2 mRNA expression in the duodenum, liver, and hypothalamus but not in the stomach (Supplementary Fig. S3A–D). A HFD downregulated Ghrl mRNA expression in the stomach but not in the duodenum (Supplementary Fig. S3E, F). A HFD also downregulated Ghsr mRNA expression in the hypothalamus (Supplementary Fig. S3G). VSG upregulated Leap2 mRNA expression in the stomach and hypothalamus but downregulated it in the liver and duodenum (Supplementary Fig. S3A–D). Moreover, VSG downregulated Ghrl mRNA expression in the stomach and duodenum (Supplementary Fig. S3E, F). VSG did not alter Ghsr mRNA levels in the hypothalamus (Supplementary Fig. S3G).
Fig. 7.
Improvement in metabolic parameters and plasma parameters by VSG in HFD-fed mice. (A) Experimental time course, (B) body weight, (C) food intake for one week, (D) blood glucose levels, (E) liver weight, (F) eWAT weight, (G) stomach weight, (H) plasma LEAP2 levels, (I) plasma DAG levels, and (J) plasma insulin levels. sham-CD, n = 8; sham-HFD, n = 7; VSG-HFD, n = 8. CD, chow diet; HFD, high-fat diet. The data were analysed by two-way ANOVA (B and C) or one-way ANOVA (D–J) followed by Tukey’s HSD post hoc test. #sham-CD vs. sham-HFD; *sham-HFD vs. VSG-HFD. **P < 0.01, ****P < 0.0001; ####P < 0.0001.
Weight loss downregulated LEAP2
As VSG induced weight loss (~ 10%) in our experiment, we aimed to obtain a similar degree of weight loss in HFD-fed mice by switching the diet from a HFD to a CD (Fig. 8A). Changes in the diet reduced body weight, blood glucose levels, liver weight, eWAT weight, and plasma LEAP2 levels but did not alter stomach weight or plasma DAG levels (Fig. 8B–G, M). Weight loss decreased Leap2 mRNA levels in the duodenum, jejunum, and liver but did not alter those in the stomach or hypothalamus (Fig. 8H–L). Weight loss did not alter stomach Ghrl mRNA levels or hypothalamic Ghsr mRNA levels (Fig. 8N, O).
Fig. 8.
Changes in plasma parameters and gene expression associated with weight loss in HFD-induced obese mice. (A) Experimental design. (B) Body weight, (C) blood glucose levels, (D) liver weight, (E) eWAT weight, and (F) stomach weight. (G) Plasma LEAP2 levels, and Leap2 mRNA levels in the (H) stomach, (I) duodenum, (J) jejunum, (K) liver, and (L) hypothalamus. (M) Plasma DAG levels and (N) stomach Ghrl mRNA levels. (O) Hypothalamic Ghsr mRNA levels. HFD, n = 8; HFD-CD, n = 12. CD, chow diet; HFD, high-fat diet. The data were analysed by two-tailed unpaired t tests.
Discussion
In this study, we investigated the nutritional factors regulating LEAP2 production. Oral glucose increased plasma LEAP2 levels, as previously reported10,17, and hepatic Leap2 mRNA levels. Oral corn oil or palmitate administration also increased plasma LEAP2 levels and hepatic Leap2 mRNA levels. Both glucose and palmitate increased Leap2 mRNA expression in primary mouse hepatocytes, indicating that they directly regulate Leap2 expression in hepatocytes. In intestinal organoids, oleic acid increased Leap2 mRNA expression, but linoleic acid did not18. Corn oil is rich in oleic acid (30% of total calories) and palmitate (10% of total calories), which may contribute to increased Leap2 expression in the liver.
Blood enters the liver through the portal vein and hepatic artery and exits the liver through the hepatic vein. The celiac artery branches from the abdominal aorta and then immediately branches into the hepatic artery. Thus, the LEAP2 concentrations in the hepatic artery and abdominal aorta are nearly identical, and we collected blood from the abdominal aorta instead of from the hepatic artery. The fasting LEAP2 concentration was greater in the hepatic vein than in the portal vein and abdominal aorta. In addition, oral glucose overload increased plasma LEAP2 concentrations in the hepatic vein and abdominal aorta but not in the portal vein. These findings suggest that the liver is the primary source of plasma LEAP2 and that glucose increases its secretion from the liver. In addition, the public RNA-seq data of mice (PRJNA66167)19 and humans (PRJEB4337)20 demonstrate predominant Leap2 expression in the liver. The oral corn oil load increased the plasma LEAP2 concentration in all three vessels, and the LEAP2 concentration in the hepatic vein was greater than that in the other vessels. Four-hour oral administration of olive oil to mice increased the plasma LEAP2 concentration in the portal vein18. These results suggest that the oral lipid load stimulated LEAP2 secretion from both the liver and intestine. Pro-LEAP2 is produced mainly in the liver and small intestine4,7, and enzymatic cleavage of furin, a member of the protein conversion enzyme family, results in the release of the mature 40-amino acid peptide LEAP2 from pro-LEAP24,21. Since furin is more abundant in the liver than in the gut4,21, the liver may be the primary organ of LEAP2 regulation through nutritional factors.
In humans with normal glucose tolerance, plasma ghrelin levels decrease, and plasma LEAP2 levels rapidly increase one hour after a meal. These results suggest that LEAP2 levels were increased by a postprandial decrease in the inhibitory effect of ghrelin as well as by nutrient stimulation. However, the decrease in ghrelin and increase in LEAP2 were delayed in humans with diabetes. The expression of GHSR is decreased in obese rodents22. These findings suggest that the regulation of LEAP2 expression by ghrelin is suppressed in subjects with diabetes and obesity. In the pathogenesis of diabetes and obesity, the secretion patterns of ghrelin and LEAP2 during feeding may be disrupted, resulting in abnormal feeding behaviour.
The plasma LEAP2 concentration is increased in individuals with obesity and is positively associated with body mass index (BMI)10. Despite their lower body weight, the VSG-CD mice had higher LEAP2 levels than the sham-CD mice. Like previous findings that ghrelin administration to wild-type mice decreased plasma LEAP2 levels and hepatic Leap2 mRNA levels7, ghrelin administration to VSG mice also decreased these levels. Ghrelin inhibited LEAP2 production via the GHSR in primary hepatocytes and Hepa 1–6 cells7. Blood LEAP2 levels were increased in the VSG-CD mice. The increase in LEAP2 production induced by reduced ghrelin levels could exceed the inhibition of LEAP2 production because of weight loss in these mice. The plasma LEAP2 concentration in the sham-HFD mice increased, as previously reported10, and the plasma LEAP2 concentration in the VSG-HFD mice decreased. GHSR expression is decreased in HFD-fed and obese mice, resulting in ghrelin resistance23. The inhibitory effect of ghrelin on LEAP2 production was reduced in HFD-fed mice due to ghrelin resistance, suggesting that the effect of weight loss on LEAP2 production was greater in HFD-fed mice.
Patients with gastric cancer, the fourth leading cause of cancer-related deaths worldwide, also undergo gastrectomy24. However, sarcopenia and cachexia may occur after surgery and can affect patient prognosis25. Anamorelin, a GHSR agonist, ameliorates gastric cancer-induced cachexia26. In this study, ghrelin was suggested to attenuate weight loss in VSG-ghrelin-PF mice compared with that in VSD-saline mice through a mechanism independent of increased food intake. Ghrelin might maintain body weight by reducing body temperature and energy expenditure through the suppression of sympathetic nerve and BAT activities27. In addition, GHSR, unlike the other GPCRs, has high basal activity28. The inhibition of GHSR activity by high LEAP2 levels after gastrectomy may contribute to anorexia and poor weight gain. HyperLEAP2emia is considered a therapeutic target for cachexia after gastrectomy.
LEAP2 is primarily known for its antimicrobial properties, functioning as part of the innate immune system. LEAP2 helps protect the body from infections by exerting direct antimicrobial effects against a range of pathogens4. LEAP2 may affect ghrelin`s activities under conditions of infection or inflammation in which energy balance is tightly regulated. Under normal physiological conditions, ghrelin promotes feeding, GH release, and energy storage. During infection or inflammation, increased levels of LEAP2 could suppress these ghrelin`s activities, which could be a protective response to conserve energy for immune function rather than growth or fat storage. Thus the interaction between LEAP2 and ghrelin may influence overall energy homeostasis.
In conclusion, the present study suggested that various factors (metabolic, hormonal, or nutritional) regulate the production and secretion of LEAP2 and that the liver is responsible for plasma LEAP2 levels (Fig. S4).
Materials and methods
Animal studies
Male and female C57BL/6J mice and male Wistar rats were purchased from Charles River Laboratories (Yokohama, Japan). Animals were group-housed (4 mice per cage and 3 rats per cage), fed a CD (CLEA Rodent Diet CE-2; CLEA Japan, Tokyo, Japan) and maintained under a constant 12-h light/12-h dark cycle (lights on at 08:00 and off at 20:00) at a constant temperature (23 ± 1 °C). Prior to the study, all the animals were housed singly. When necessary, the animals were anaesthetized by intraperitoneal (ip) injection of a mixture of three anaesthetics (dominator, 1 mg/ml; mazazole, 5 mg/ml; vetorphale, 5 mg/ml) in a volume of 100 µl/10 g body weight for and 100 µl/100 g body weight for rats. To investigate the nutritional regulation of LEAP2 and ghrelin, the following reagents were used: glucose (Nacalai Tesque, Inc. Kyoto, Japan), corn oil (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan), and palmitate sodium salt (Nacalai Tesque, Inc. Kyoto, Japan). All necessary steps were taken to minimize animal suffering. All animal experiments in this study complied with the ARRIVE guidelines 2.0 and were approved by the Animal Care and Use Committee of the University of Miyazaki and complied with the Guidelines for the Care and Use of Laboratory Animals at the University of Miyazaki.
Meal tolerance test in humans
Healthy adult individuals (8 men and 4 women) were enrolled at the University of Miyazaki. The mean age was 36.9 ± 2.6 years, and the mean body mass index (BMI) was 23.5 ± 0.9 kg/m2. The patients with type 2 diabetes (2 men and 9 women) had a mean age of 62.6 ± 2.3 years, a BMI of 28.3 ± 1.7 kg/m2 and a mean HbA1c level of 7.9 ± 0.2%. The details of the patients with type 2 diabetes are available in a previous publication12. The meal tolerance test (MTT) (592 kcal, 75 g carbohydrate, 8.0 g protein, 28.5 g fat; Saraya Corp., Osaka, Japan) was performed on both healthy individuals and T2DM patients after an overnight 12 h fast. Blood samples were taken at 0 (baseline), 1 h, and 2 h postprandial. The protocol for the human study was prepared in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the University of Miyazaki (approval date: February 2019; Approval No. O-462). Informed consent was obtained from all participants.
Oral glucose administration and blood sampling in rats
Nine-week-old male rats fasted for 24 h were administered glucose (2 g/kg body weight) or water orally by gavage under isoflurane anaesthesia. Rats were anaesthetized 1 h after oral gavage, and blood samples were collected simultaneously from the portal vein, hepatic vein, and abdominal aorta. Blood samples were kept in an ice-cold tube containing aprotinin and EDTA. The plasma samples were stored at − 80 °C until analysis.
Mouse model of diet-induced obesity and weight loss
At age 16 weeks, the mice were either continuously fed a CD (59.2% carbohydrate, 12.3% fat, 28.5% protein, 14.2 kJ/g; CLEA Rodent Diet CE-2, CLEA Japan, Tokyo, Japan) or a high-fat diet (HFD) (20% carbohydrate, 60% fat, 20% protein, 21.9 kJ/g; no. D12492; Research Diets, New Brunswick, NJ, USA) for 16 weeks to develop obesity. Mice then underwent sham or vertical sleeve gastrectomy (VSG) surgery. Blood sampling and tissue resection were performed 4 weeks after the surgery. In the second experiment, mice fed a HFD for 23 weeks were maintained on a HFD for the subsequent week or were switched to a CD for one week to induce weight loss at the same percentage as that of the VSG (Fig. 5A).
Vertical sleeve gastrectomy (VSG) surgery
Eight-week-old male and female mice (fed a CD) or thirty-three-week-old male mice (fed a HFD for 16 weeks) were anaesthetized via ip injection of anaesthesia. The abdominal area was wiped with 70% alcohol, and a transverse cut was made through the body wall. The stomach was gently elevated by blunt forceps, and all the attached tissues were carefully removed from the stomach by cotton buds. The lateral 40% (in the case of CD-fed mice) or 80% (in the case of HFD-fed mice) of the stomach was clipped with a sterilized titanium clip (LT400, Ethicon US LLC, Johnson & Johnson, Santa Ana, CA, USA) and a sterilized clip applier (LC307, Ethicon US LLC, Johnson & Johnson). The exterior part of the stomach was then surgically removed with sterilized scissors, and the remaining stomach was ligated along the clip using a 6 − 0 PDS suture. The abdominal muscle layer and skin were closed by suturing. The sham surgery involved opening the peritoneal cavity, and gentle pressure was applied to the stomach by blunt forceps. Mice received an intramuscular injection of an antianaesthetic agent (ANTISEDAN; Orion Pharma, Orion Corporation, Espoo, Finland) to wake them quickly. All the mice were returned to their home cages. For the CD-fed mice, a CZ-Hi liquid diet (Morinaga, Tokyo, Japan) was given for one postoperative day, followed by CD. Mice were allowed to recover for 7 days (Figs. 5 and 6). In the case of HFD-fed mice (Fig. 7), the CZ-Hi liquid diet was given for three postoperative days, followed by HFD feeding. CD-fed sham-operated mice (control group to HFD-sham-VSG mice) underwent the same experimental procedure but were given a CD instead of a HFD (Fig. 7).
Feeding experiment
Human ghrelin (Peptide Institute, Suita, Japan) or saline was ip injected into the VSG or sham-operated mice twice (10:00 and 18:00) a day for 7 consecutive days (Figs. 5 and 6). The dose of ghrelin was 10 nmol/mouse. Cumulative food intake and body weight were measured. Mice were divided into 4 groups (Figs. 5 and 6): group-1 (sham-operated mice received ip saline), group-2 (VSG-operated mice received ip saline), and group-3 (VSG-operated mice received ip ghrelin). In group-4, herein called VSG-ghrelin pair-fed (PF), VSG-operated mice received ip ghrelin and were immediately provided the average amount of food consumed by group-2 mice. After the final administration of ghrelin, the mice were fasted overnight (from 18:00 to the next day at 10:00), and blood sampling and tissue resection were performed.
Glucose administration to mice
Eight-week-old male mice fasted for 24 h were administered glucose (2 g/kg body weight) or water orally by gavage. The mice received another shot of glucose 1.5 h later. Blood sampling and tissue (liver, stomach) resection were performed 3 h after the first administration. The other groups of mice received ip injection of glucose (2 g/kg body weight) or water, and blood sampling and tissue (liver, stomach) resection were performed according to the same protocol as mentioned above.
In another experiment, eight-week-old male mice fasted for 24 h were administered glucose (2 g/kg body weight) or water orally by gavage under isoflurane anaesthesia. Mice were anaesthetized 1 h after oral gavage, and blood samples were collected simultaneously from the portal vein, hepatic vein, and abdominal aorta. Blood samples were kept in an ice-cold tube containing aprotinin and EDTA. The plasma samples were stored at − 80 °C until analysis.
Oral corn oil or palmitate administration to mice
Nine-week-old male mice fasted for 16 h were administered corn oil (100 µl/mouse) or water orally by gavage. Blood sampling and tissue (liver, duodenum, jejunum, and stomach) resection were performed 6 h after administration. The other groups of mice received palmitate (3 µmol (3.75% BSA in 0.01 M NaOH)/mouse) or control solution (3.75% BSA in 0.01 M NaOH), and blood sampling and tissue (liver, duodenum, jejunum, and stomach) resection were performed according to the same protocol as mentioned above.
In another experimental setting, nine-week-old male mice fasted for 16 h were administered corn oil (100 µl/mouse) or water orally by gavage under isoflurane anaesthesia. Mice were anaesthetized 1 h after oral gavage, and blood samples were collected simultaneously from the portal vein, hepatic vein, and abdominal aorta. Blood samples were kept in an ice-cold tube containing aprotinin and EDTA. The plasma samples were stored at − 80 °C until analysis.
Blood sampling and plasma hormone measurement
Blood glucose was measured in the nonanaesthetized mice via a tail prick using a glucometer (Terumo, Tokyo, Japan). The mice were then anaesthetized. Blood samples were taken via heart puncture and kept in an ice-cold tube containing aprotinin and EDTA. Plasma was collected and stored at − 80 °C until analysis. The plasma concentration of des-acyl ghrelin (DAG) was measured on an AIA-600II immunoassay analyser (Tosoh, Tokyo, Japan). Both intra- and interassay variations in DAG were < 3%8. Plasma samples were subjected to Sep-Pak cartridges for the measurement of LEAP2 as described elsewhere13, and the plasma LEAP2 concentration was measured using a commercial human/mouse LEAP2 EIA kit (Phoenix Pharmaceuticals, Burlingame, CA, USA; Catalogue # EK-075-40). The intra- and interassay variations in LEAP2 were < 3% and < 5%, respectively8,14. Plasma insulin concentrations were measured using a commercial assay kit (Morinaga Institute of Biological Science, Yokohama, Japan)8,15. The intra- and interassay variations in insulin EIA were < 10% and < 10%, respectively8.
Isolation of primary mouse hepatocytes
Primary hepatocytes were successfully isolated using a two-step perfusion method with some modifications, as described in a previous study16. The ingredients of perfusion buffer 1 (pH 7.4) were 33 mM KCl, 0.441 mM KH2PO4, 4.17 mM NaHCO3, 137.93 mM NaCl, 0.338 mM Na2HPO4, D-glucose (4.75 mg/mL), 26.6 µM phenol red, and 0.5 mM EGTA. The ingredients of perfusion buffer 2 (pH 7.4) were 1.26 mM CaCl2, 0.493 mM MgCl2, 0.407 mM MgSO4, 5.33 mM KCl, 0.441 mM KH2PO4, 4.17 mM NaHCO3, 137.93 mM NaCl, 0.338 mM Na2HPO4, D-glucose (4.75 mg/mL), 26.6 µM phenol red, and 0.72% BSA. Both perfusion buffers were passed through a 0.22 μm filter. The mice were anaesthetized, and the abdominal area was cleaned with 70% alcohol. The abdominal cavity was opened, and the portal vein was exposed. A catheter (26G needle) connected to prewarmed oxygenated perfusion buffer 1 was inserted into the portal vein, after which perfusion started. The inferior vena cava (IVC) was cut to drain the liquid. After an 80 mL infusion of perfusion buffer 1, the digestion step started by introducing perfusion buffer 2 containing collagenase type IV (15 mg/30 mL), and 30 to 40 mL of buffer was infused. Livers were minced in cold Williams’ medium E (W4128-50; Sigma‒Aldrich), and the homogenate was passed through a 70 μm cell strainer. Hepatocytes were isolated, and viable cells were counted by the trypan blue exclusion method. Culturing and experimentation were performed with Williams’ medium E (glucose concentration: 1.9 mg/mL) supplemented with 10% FBS, 10 nM dexamethasone, 1 nM insulin, streptomycin/penicillin, and 2 mM L-glutamine. Hepatocytes were stimulated with glucose or palmitate for 6 h. The whole experiment was performed within 24 h after the isolation of hepatocytes.
RNA extraction, cDNA synthesis, and quantitative RT‒PCR (qRT‒PCR) analysis
Tissue samples from the liver, stomach, duodenum, jejunum, and hypothalamus were resected, quickly snap-frozen in liquid nitrogen, and stored at −80°C until use. Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). A NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) was used to measure the concentration and determine the purity of the products. One microgram of RNA was reverse-transcribed to first-strand cDNA in a volume of 20 µl with the High Capacity RNA-to-cDNA Kit (Life Technologies, Tokyo, Japan). Fifty nanograms of cDNA from each sample was subjected to qRT‒PCR (listed in Supplementary Table 1) in a Thermal Cycler Dice Real-Time System II (Takara Bio, Kusatsu, Japan). The qRT‒PCR program was as follows: 95°C for 30 seconds; 40 cycles at 95°C for 30 seconds; and 60 seconds at 60°C. The mRNA levels were normalized to those of the control reference gene Gapdh and determined using the ∆∆Ct method. The reaction efficiency and optimal cDNA concentration were determined by generating standard curves. The reaction efficiency for all primers was between 95% and 110%.
Statistical analysis
All the data are presented as the means ± SEMs. Statistical analysis was performed by a two-tailed unpaired t test, one-way ANOVA, the Friedman test, Dunn’s multiple comparison test or two-way ANOVA followed by Tukey’s HSD post hoc test, as indicated in the figure legends, using GraphPad Prism (version 8.42 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com) or R software (version 4.2.2). P < 0.05 was considered to indicate statistical significance. P values are presented in each of the figures.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Mika Hara, Itsuki Morinaga, and Eiko Kurata (University of Miyazaki) for their technical support. The animal study of this work was performed at the Frontier Science Research Centre, University of Miyazaki. This study was also supported in part by grant from the Japan foundation for applied enzymology (Front Runner of Future Diabetes Research associated research grant, FFDR 2023 to Islam M. N.).
Author contributions
Authors Md.N.I. and H.N. contributed equally to this work. Md.I., H.N., T.N., A.N., H.S., W.Z., and M.N. designed the study; Md.I., H.N., H.U., T.N., and H.S. conducted the experiments and analyzed the data; Md.I., H.N., H.S., and M.N. wrote the manuscript. All authors commented on and approved the final version of the manuscript. M.N. is the guarantor of this work, has full access to all the data in the study, and takes responsibility for the integrity and accuracy of the data.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Authors Md Nurul Islam and Hiroki Nabekura contributed equally to this work.
References
- 1.Kojima, M. et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 402, 656–660 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Nakazato, M. et al. A role for ghrelin in the central regulation of feeding. Nature. 409, 194–198 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Yanagi, S., Sato, T., Kangawa, K. & Nakazato, M. The homeostatic force of ghrelin. Cell. Metab.27, 786–804 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Krause, A. et al. Isolation and biochemical characterization of LEAP-2, a novel blood peptide expressed in the liver. Protein Sci.12, 143–152 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krause, A. et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett.480, 147–150 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Ge, X. et al. LEAP2 is an endogenous antagonist of the ghrelin receptor. Cell. Metab.27, 461–469 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Islam, M. N. et al. Liver-expressed antimicrobial peptide 2 antagonizes the effect of ghrelin in rodents. J. Endocrinol.244, 13–23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Islam, M. N. et al. Liver-expressed antimicrobial peptide 2 functions independently of growth hormone secretagogue receptor in calorie-restricted mice. Peptides. 151, 170763 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Hagemann, C. A. et al. LEAP2 reduces postprandial glucose excursions and ad libitum food intake in healthy men. Cell. Rep. Med.3, 100582 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mani, B. K. et al. LEAP2 changes with body mass and food intake in humans and mice. J. Clin. Invest.129, 3909–3923 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings, B. P. et al. Vertical sleeve gastrectomy improves glucose and lipid metabolism and delays diabetes onset in UCD-T2DM rats. Endocrinology. 153, 3620–3632 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noda, T. et al. Concurrent use of teneligliptin and canagliflozin improves glycemic control with beneficial effects on plasma glucagon and glucagon-like peptide-1: a single-arm study. Diabetes Ther.10, 1835–1846 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakai, K. et al. Human liver-expressed antimicrobial peptide 2 elevation in the cerebrospinal fluid in bacterial meningitis. Brain Behav.11, e02111 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nabekura, H. et al. Liver-expressed antimicrobial peptide 2 is a hepatokine that predicts weight loss and complete remission of type 2 diabetes mellitus after vertical sleeve gastrectomy in Japanese individuals. Obes. Facts. 16, 392–400 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang, W. et al. Neuromedin U uses Gαi2 and Gαo to suppress glucose-stimulated Ca2+ signaling and insulin secretion in pancreatic β cells. PLoS One. 16, e0250232 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bate, T. S. R., Gadd, V. L., Forbes, S. J. & Callanan, A. Response differences of HepG2 and primary, mouse hepatocytes to morphological changes in Electrospun PCL scaffolds. Sci. Rep.11, 3059 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedersen, M. G. B. et al. Nutrient sensing: LEAP2 concentration in response to fasting, glucose, lactate, and β-hydroxybutyrate in healthy young males. Am. J. Clin. Nutr.118, 1091–1098 (2023). [DOI] [PubMed] [Google Scholar]
- 18.Gradel, A. K. J. et al. The dietary regulation of LEAP2 depends on meal composition in mice. FASEB J.37, e22923 (2023). [DOI] [PubMed] [Google Scholar]
- 19.Yue, F. et al. Mouse ENCODE consortium. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 20, 355–364 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fagerberg, L. et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom.13, 397–406 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roebroek, A. J. M. et al. Limited redundancy of the proprotein convertase furin in mouse liver. J. Biol. Chem.279, 53442–53450 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Naznin, F. et al. Restoration of metabolic inflammation-related ghrelin resistance by weight loss. J. Mol. Endocrinol.60, 109–118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briggs, D. I., Enriori, P. J., Lemus, M. B., Cowley, M. A. & Andrews, Z. B. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology. 151, 4745–4755 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Sekiguchi, M., Oda, I., Matsuda, T. & Saito, Y. Epidemiological trends and future perspectives of gastric cancer in eastern asia. Digestion. 103, 22–28 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Zhuang, C. L. et al. Cachexia versus Sarcopenia in clinical characteristics and prognostic value after radical gastrectomy for gastric cancer: A large-scale prospective study. Ann. Surg. Oncol.29, 2348–2358 (2022). [DOI] [PubMed] [Google Scholar]
- 26.Shiomi, Y. et al. Z-505, an oral ghrelin receptor agonist, attenuates anorexia after total gastrectomy in rats. J. Surg. Res.246, 527–534 (2020). [DOI] [PubMed] [Google Scholar]
- 27.St-Pierre, D. H. et al. Relationship between ghrelin and energy expenditure in healthy young women. J. Clin. Endocrinol. Metab.89, 5993–5997 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Müller, A., Berkmann, J. C., Scheerer, P., Biebermann, H. & Kleinau, G. Insights into basal signaling regulation, oligomerization, and structural organization of the human G-protein coupled receptor 83. PLoS One. 11, e0168260 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.