Abstract
Key Clinical Message
Klippel–Trénaunay syndrome can present with atypical manifestations such as a bleeding vulvar hemangioma. This case report, the first documented in Uganda, highlights the need for awareness of such presentations and underscores the importance of continuous follow‐up in female patients to manage potential complications throughout adolescence and pregnancy.
Abstract
Klippel–Trénaunay syndrome (KTS) is a rare congenital disorder marked by bone and soft tissue hypertrophy, port‐wine stains, and varicosities. Cases involving genital hemangiomas are rare. This report highlights a 5‐year‐old girl in Uganda with typical KTS features, including hypertrophy and port‐wine stains, along with a bleeding vulvar hemangioma, emphasizing its uncommon presentation and potential complications. Treatment involved compression bandaging and timolol 0.2% solution. This case underscores the importance of awareness of atypical manifestations of hemangiomas with KTS and continuous follow‐up for female patients, especially through adolescence and pregnancy, due to potential complications such as prepubertal per vaginal bleeding, menorrhagia, and post‐partum bleeding.
Keywords: bleeding vulvar hemangioma, Klippel–Trénaunay syndrome, Mbarara University of Science and Technology, port‐wine stain, Uganda
1. INTRODUCTION
Klippel‐Trénaunay syndrome (KTS) is an uncommon congenital disorder that presents with a triad of bone and soft tissue hypertrophy, port‐wine stains (PWS), and varicosities. 1 The presence of two of these three features is sufficient to make the diagnosis. 2 Other findings in KTS include lipomatosis, lymphedema, and vascular lesions of the gastrointestinal and genitourinary tracts. 3 The incidence ranges from 2 to 5 per 100,000 individuals, with an equal distribution observed across both genders. Only one case of KTS has been reported in Uganda and this case had a PWS and unilateral lower limb hypertrophy but with no vascular tumors. 4 Even though many instances of KTS with PWS and varicosities have been reported, fewer cases with hemangioma have been described. Genital tract involvement with an infantile hemangioma is uncommon and may cause bleeding. 5 In KTS, hypertrophy of the affected limb is characteristic and manifests as increased girth and length and this is attributed to overgrowth of soft tissue, fat, and bone. A nevus flammeus is the most frequent and primary manifestation of KTS localized to the affected extremity. The PWS is commonly well‐circumscribed terminating abruptly at the midline but may occasionally spread bilaterally. 6 Patients may exhibit digital abnormalities such as macrodactyly, syndactyly, and camptodactyly. 7 Hemangiomas are classified as infantile or congenital. Infantile hemangiomas (IHs) are GLUT1 positive, appear within 2 months of birth, proliferate for the first year, then slowly regress. Congenital hemangiomas are present at birth. 8 Most hemangiomas regress spontaneously and are managed with periodic evaluation and conservative therapy. However, 10%–20% of large hemangiomas affecting aesthetics or function require specific treatment. 9 Few cases of hemangiomas have been reported to present in patients with KTS including, a diffuse cavernous hemangioma in the colon with KTS, 10 intraneural hemangioma in KTS, 11 mediastinal cavernous hemangioma in KTS, 12 renal hemangioma with KTS 13 and splenic hemangiomas with KTS. 14 This is the first reported instance of a vulvar hemangioma occurring in a patient with KTS in Uganda.
We present a case of a 5‐year‐old girl referred to our dermatology department with Klippel–Trénaunay syndrome and a bleeding focal vulvar hemangioma.
2. CASE HISTORY AND EXAMINATION
A 5‐year‐old girl presented to the skin department with her grandfather who reported that the child was born with red patches over her left buttock, left thigh, and left lower back where small red swellings had developed over the last 4 months. At birth, the lower limbs were equal but over the years, the left leg, foot, fourth and fifth toes became bigger. In the first few weeks of life, the child also developed a red swelling on her left vulva that gradually increased in size over the years turning violaceous in color with occasional bleeding.
On examination, the child had left leg and foot hypertrophy (Figure 1) with left fourth and fifth toe macrodactyly (Figure 2). There was a slight leg length difference with a longer left lower limb. Presence of erythematous patches on the left lower lumbosacral area, left intergluteal cleft and left thigh, solitary erythematous papules over the left lumbosacral patch (Figure 3), nodular soft fluctuant violet swellings over the left vulva (Figure 4) with positive diascopy and hyperpigmented patches on the dorsum of the left foot were observed.
FIGURE 1.
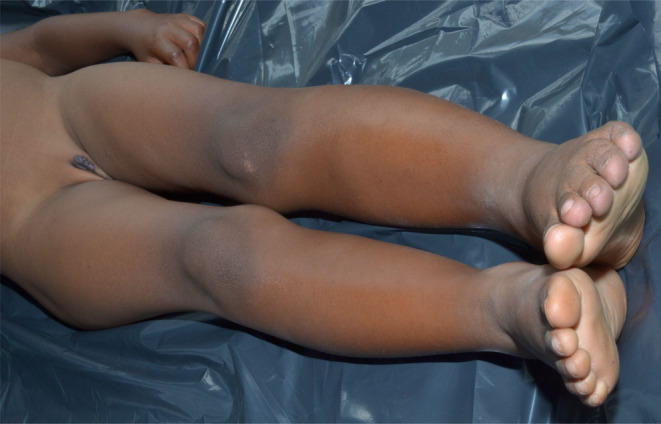
Left leg and foot hypertrophy.
FIGURE 2.

Left fourth and fifth toe macrodactyly.
FIGURE 3.
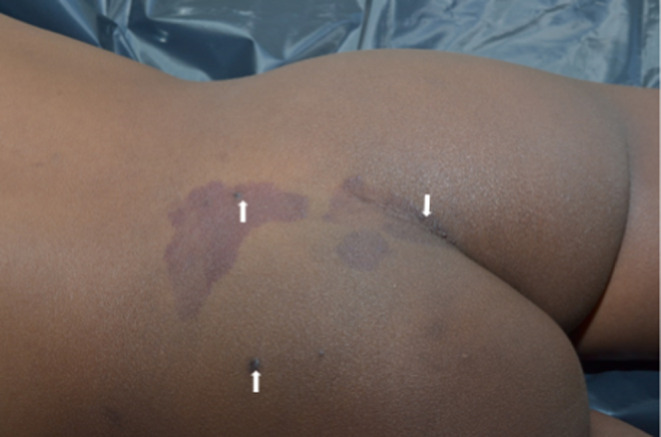
Lumbosacral port wine stain with overlying erythematous papules.
FIGURE 4.

Genital hemangioma.
3. DIAGNOSIS
A 5‐year‐old girl with Klippel–Trénaunay syndrome presented with a bleeding vulvar hemangioma.
4. MANAGEMENT AND FOLLOW‐UP
This child was managed with compression bandaging, timolol 0.2% solution application on hemangioma three times daily, and regular follow‐up.
5. DISCUSSION
KTS may appear clinically insignificant but can lead to functional and aesthetic concerns and is also associated with severe complications. Vascular anomalies in the female genital tract are rarely reported and if present, they may mimic other genital tumors. 15 According to the International Society for the Study of Vascular Anomalies (ISSVA), vascular anomalies are classified into tumors and malformations. Tumors include infantile hemangioma, kaposiform hemangioendothelioma (KHE), tufted angioma, and pyogenic granuloma (PG). Vascular malformations, such as PWS, are abnormal blood vessels present at birth that persist without endothelial proliferation or cellular turnover. 7 PWS, or nevus flammeus, are lifelong congenital capillary malformations while salmon patches, or nevus simplex, are venous malformations that typically appear on the upper eyelids, forehead (angel's kiss), and neck (stork bite marks) that disappear within 1–2 years. 16 Infantile hemangioma (IH) is the most common benign soft‐tissue tumor in children, occurring in 2%–12% of infants. It is three times more common in female infants and has a higher incidence in premature neonates. IHs are less common in those of African descent. 17 IHs can be classified as superficial, deep, or mixed based on their depth, with superficial ones appearing as bright red, dome‐shaped papules or plaques, and deep ones as subcutaneous nodules with a blue hue. Combined IHs have both superficial and deep components. They can also be categorized by their anatomic configuration into focal lesions, which are discrete and round, and segmental lesions, which cover a larger area. Some IHs are congenital and present at birth. They can be fully formed in‐utero (non‐involuting congenital hemangioma [NICH]) or can be rapidly involuting congenital hemangiomas (RICH) that do not undergo postnatal involution. Typically, IHs go through phases of growth, stability, and spontaneous regression, with complete involution occurring at a rate of about 10% per year, leading to over 90% involution by 9 years of age. 18 IHs typically present in the head and neck regions but can occasionally appear in the genitalia. 7 When a vulvar hemangioma is left untreated can cause functional and emotional disability 19 and because it may look like other tumors as the patients grow, the condition may be misdiagnosed and therefore the patient may be subjected to various unnecessary treatments. Cutaneous hemangiomas may be complicated by occasional bleeding which may result in acute or chronic anemia. Children may experience fewer complications of genital hemangiomas, but as they grow old, bleeding from the lower genital tract may become noticeable such as heavy menstrual bleeding and complications of pregnancy.
Mutations involving the angiogenic factor VG5Q have been described in the etiology of KTS, 6 this may result in local hyperemia and increased arterial blood flow. 7 Risk factors for IH include multiple gestation pregnancy, advanced maternal age, placenta previa, and preeclampsia. There is also evidence of autosomal dominant inheritance within families. 17 Our patient had no family history. The presence of erythrocyte‐type glucose transporter (GLUT)‐1 protein in lesions suggests that IHs may arise from angioblasts that have differentiated into a placental cell type. 7
Clinical complications with KTS include varicose veins which develop as the child grows older, hematuria following extensive lympho‐venous distortion to the bladder, 20 and gastrointestinal bleeding due to cavernous hemangiomas of the distal colon and rectum. 21 KTS can complicate pregnancy and its outcome by causing venous thromboembolism and postpartum hemorrhage. 22 Chronic pain is also a common challenge in patients with KTS and can arise from thrombosis, cellulitis, calcifications, arthritis, and neuropathic origins. About 95% of patients have unilateral lower limb involvement and the presence of arteriovenous (AV) fistula is indicative of Klippel–Trénaunay–Weber (KTW) syndrome or Parkes‐Weber syndrome. KTS can also present with intermittent claudication, venous ulcers, hyperpyrexia, alopecia, hypertrichosis, lymphedema, or altered tear and sweat production. Bone overgrowth results in compensatory scoliosis and hip dislocation. Cases of recurrent pulmonary embolism related to deep venous thrombosis, neurological manifestations such as multiple giant intracranial aneurysms, intracranial AV malformation, cerebral, and spinal cavernomas, intradural spinal cord AV malformations, epidural hemangioma, and epidural angiomyolipoma occurring at the same segmental level as cutaneous lesions of KTS have been documented. 6 KTS has also been reported to cause clitoromegaly. Complications of IH include disfigurement, psychosocial stress, bleeding, ulceration, infection, pain, and scarring. 3
Clinical assessment of KTS includes color duplex ultrasonography, MRI, and conventional radiography of both extremities. 6 Differential diagnoses for KTS include Proteus syndrome, Parkes‐Weber syndrome, and CLOVES syndrome. 2 Diagnosis of IHs is made clinically in 90% of cases 23 as was done in this child with a positive diascopy. Further tests like ultrasonography, CT, MRI, angiography, and histopathological exams can verify the uncertain cases.
Differential diagnoses for hemangiomas include KHE, tufted angioma, mucocele, and PG. 3 KTS has been associated with spindle cell hemangioendothelioma (SCHE), a low‐grade vascular tumor that histologically presents with cavernous blood spaces containing phleboliths, which are separated by spindle‐shaped fibroblast cells. These characteristics are reminiscent of both cavernous hemangioma and Kaposi's sarcoma. 24
A conservative approach is used for the symptomatic treatment of KTS by the use of elastic or non‐elastic compression stockings, 1 psychological support, and physiotherapy. 6 Laser therapy can also be used in these patients for the varicosities. 7 The prognosis of KTS is generally good but it can worsen based on associated conditions. 2 Conservative management for IH involves careful monitoring and regular follow‐ups, as well as pharmacotherapy options such as propranolol and corticosteroids. Cryotherapy, laser therapy, injection of sclerosing agents, or excision are other treatment modalities. 25 Our patient received a timolol 0.2% solution to apply three times daily.
The prognosis for IHs is typically excellent, as they seldom recur or transform into malignant tumors after proper treatment. 26
6. CONCLUSION
We present a case of KTS with a genital hemangioma that had started to bleed, an association and a complication that is rarely reported. Earlier reports indicate lower female genital tract hemangiomas that come with more complications as children grow thus, we advocate for regular follow‐up of girls through adolescence, before and during pregnancy.
6.1. Recommendations
Early diagnosis of KTS and its rare associations, like genital hemangiomas, is crucial. Continuous follow‐up, especially for female patients, is essential to monitor complications. Multidisciplinary care and patient education are key to managing KTS effectively and improving quality of life.
AUTHOR CONTRIBUTIONS
Mundeli Simon Peter: Conceptualization; methodology; writing – original draft. Sibali Gidimali Gibu: Conceptualization; writing – original draft. Mirembe Stephen Kizito: Conceptualization; methodology; supervision; writing – original draft.
FUNDING INFORMATION
This publication did not receive any external funding.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
We acknowledge the consent from the grandfather to have this case published.
Peter MS, Gibu SG, Kizito MS. A rare case of a 5‐year‐old girl with Klippel–Trénaunay syndrome and a bleeding focal vulvar hemangioma in Uganda. Clin Case Rep. 2024;12:e9501. doi: 10.1002/ccr3.9501
DATA AVAILABILITY STATEMENT
Data sharing not applicable–no new data generated: Data sharing does not apply to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Asghar F, Aqeel R, Farooque U, Haq A, Taimur MJC. Presentation and Management of Klippel–Trenaunay syndrome: a review of available data. Cureus. 2020;12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pavone P, Marino L, Cacciaguerra G, et al. Klippel–Trenaunay syndrome, segmental/focal overgrowth malformations: a review. Children. 2023;10(8):1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology e‐Book. Elsevier Health Sciences; 2012. [Google Scholar]
- 4. Sikakulya FK, Egesa WI, Kiyaka SM. A neonate with Klippel–Trénaunay syndrome: a case report. J Med Case Rep. 2021;15:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Essabar L, Amenzouy S, Oudghiri FZ, Kerboubi L. Klippel‐Trenaunay syndrome: a rare cause of vaginal bleeding in the prepubescent girl. J Biosci Med. 2016;4(11):1‐7. [Google Scholar]
- 6. James WD, Elston D, Berger T. Andrew's Diseases of the Skin E‐Book: Clinical Dermatology. Elsevier Health Sciences; 2011. [Google Scholar]
- 7. Hurwitz S, Paller AS, Mancini AJJ. Bullous disorders of childhood. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. Elsevier publishing company; 2016. [Google Scholar]
- 8. Hasan S, Khan A, Banerjee A, Ramalingam KJC. Infantile hemangioma of the upper lip: report of a rare case with a brief review of literature. Cureus. 2023;15(7):e42556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koltsidopoulos P, Skoulakis CJC. Intramuscular hemangioma with Turkey wattle sign. CMAJ. 2015;187(4):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghahremani GG, Kangarloo H, Volberg F, Meyers MAJR. Diffuse cavernous hemangioma of the colon in the Klippel–Trenaunay syndrome. Radiology. 1976;118(3):673‐678. [DOI] [PubMed] [Google Scholar]
- 11. Deka JB, Deka NK, Shah MV, Bhatnagar N, Nanni AL. Intraneural hemangioma in Klippel–Trenaunay syndrome: role of musculo‐skeletal ultrasound in diagnosis–case report and review of the literature. J Ultrasound. 2020;23(3):435‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuo P‐H, Chang Y‐C, Liou J. Mediastinal cavernous haemangioma in a patient with Klippel–Trenaunay syndrome. Thorax. 2003;58(2):183‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schofield D, Zaatari GS. Klippel‐Trenaunay and Sturge‐Weber syndromes with renal hemangioma and double inferior vena cava. J Urol. 1986;136(2):442‐445. [DOI] [PubMed] [Google Scholar]
- 14. Misawa T, Shiba H, Fujiwara Y, et al. Massive splenomegaly caused by cavernous hemangiomas associated with Klippel–Trenaunay syndrome: report of a case. Surg Today. 2014;44:197‐200. [DOI] [PubMed] [Google Scholar]
- 15. Wang S, Lang JH, Zhou HM. Venous malformations of the female lower genital tract. Eur J Obstet Gynecol Reprod Biol. 2009;145(2):205‐208. [DOI] [PubMed] [Google Scholar]
- 16. Leung AK, Barankin B, Hon KL. Persistent salmon patch on the forehead and glabellum in a Chinese adult. Case Rep Med. 2014;2014(1):139174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haggstrom AN, Drolet BA, Baselga E, et al. Prospective study of infantile hemangiomas: demographic, prenatal, and perinatal characteristics. J Pediatr. 2007;150(3):291‐294. [DOI] [PubMed] [Google Scholar]
- 18. Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122(2):360‐367. [DOI] [PubMed] [Google Scholar]
- 19. Silva JM, Calife ER, Cabral JVS, Andrade HPF. Vulvar hemangioma: case report. Rev Bras Ginecol Obstet. 2018;40:369‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rubenwolf P, Roosen A, Gerharz EW, Kirchhoff‐Moradpour A, Darge K, et al. Life‐threatening gross hematuria due to genitourinary manifestation of Klippel–Trenaunay syndrome. Int Urol Nephrol. 2006;38:137‐140. [DOI] [PubMed] [Google Scholar]
- 21. Yoshinaga T, Yagi K, Morishita T, Abe H, Nonaka M, Inoue TJAN. Cerebral and spinal cavernomas associated with Klippel‐Trenaunay syndrome: case report and literature review. Acta Neurochir. 2018;160:287‐290. [DOI] [PubMed] [Google Scholar]
- 22. Horbach S, Lokhorst M, Oduber C, Middeldorp S, der Post V, et al. Complications of pregnancy and labour in women with Klippel–Trénaunay syndrome: a nationwide cross‐sectional study. BJOG. 2017;124(11):1780‐1788. [DOI] [PubMed] [Google Scholar]
- 23. Hoornweg MJ, Theunissen CI, Hage JJ. Malignant differential diagnosis in children referred for infantile hemangioma. Ann Plast Surg. 2015;74(1):43‐46. [DOI] [PubMed] [Google Scholar]
- 24. Saikrishna D, Mahesh K, Hiriyanna NMJOMSC. Spindle cell haemangioma in head and neck: report of an uncommon vascular lesion and review of treatment modalities till present. Oral Maxillofac Surg Cases. 2020;6:100149. [Google Scholar]
- 25. Ţarcă E, Cojocaru E, Roşu ST, Butnariu LI, Plămădeală P, Moisă ŞM. Differential diagnosis difficulties related to infantile hemangioma–case report and literature review. Romanian J Morphol Embryol. 2019;60:1375‐1379. [PubMed] [Google Scholar]
- 26. Błochowiak KJ, Kamiński B, Witmanowski H, JJAiD S. Selected presentations of lip enlargement: clinical manifestation and differentiation. Postepy Dermatol Alergo. 2018;35(1):18‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable–no new data generated: Data sharing does not apply to this article as no new data were created or analyzed in this study.


