Abstract
Loss to follow-up (LTFU) in tuberculosis (TB) management increases morbidity and mortality, challenging effective control strategies. This study aims to develop and evaluate machine learning models to predict loss to follow-up in TB patients, improving treatment adherence and outcomes. Retrospective data encompassing tuberculosis patients who underwent treatment or registration at the National Center for Clinical Medical Research on Infectious Diseases from January 2017 to December 2021 were compiled. Employing machine learning techniques, namely SVM, RF, XGBoost, and logistic regression, the study aimed to prognosticate LTFU. A comprehensive cohort of 24,265 tuberculosis patients underwent scrutiny, revealing a LTFU prevalence of 12.51% (n = 3036). Education level, history of hospitalization, alcohol consumption, outpatient admission, and prior tuberculosis history emerged as precursors for pre-treatment LTFU. Employment status, outpatient admission, presence of chronic hepatitis/cirrhosis, drug adverse reactions, alternative contact availability, and health insurance coverage exerted substantial influence on treatment-phase LTFU. XGBoost consistently surpassed alternative models, boasting superior discriminative ability with an average AUC of 0.921 for pre-treatment LTFU and 0.825 for in-treatment LTFU. Our study demonstrates that the XGBoost model provides superior predictive performance in identifying LTFU risk among tuberculosis patients. The identification of key risk factors highlights the importance of targeted interventions, which could lead to significant improvements in treatment adherence and patient outcomes.
Keywords: Machine learning, Tuberculosis, Loss to follow-up, Anti-TB treatment, Predictive models, Artificial intelligence
Subject terms: Health care, Risk factors, Mathematics and computing
Introduction
Tuberculosis remains a pressing global health challenge, demanding sustained follow-up and treatment adherence for successful outcomes1. Robust treatment adherence is crucial for the effectiveness of TB treatment1. Loss to follow-up (LTFU) is a significant barrier to effective TB management, leading to treatment failure, drug resistance, and increased mortality, which negatively impacts families and society2,3.
LTFU is a global issue, with the World Health Organization reporting an average global rate of 6%, and some countries showing rates from 4 to 38% before treatment2,4. While studies have reported treatment attrition rates in France (15%)5, India (19%)6, and Japan (7.8%)7, comprehensive data from China remain limited. Notably, diverse factors contribute to the LTFU, encompassing cultural nuances, social contexts, and geographic disparities. Moreover, the triggers for visit loss at distinct stages can exhibit considerable variability8.
Machine learning has become increasingly important in detecting, diagnosing, and predicting TB outcomes. Techniques like Support Vector Machines (SVM), Deep Neural Networks, and Random Forests have demonstrated utility in aiding physicians to identify tuberculosis-related lesions in medical images, thereby enhancing diagnostic precision9. Convolutional neural network (CNN) models have successfully analyzed chest X-ray images to detect tuberculosis lesions with greater accuracy compared to conventional physician diagnosis10. Neural network models have integrated patients’ clinical attributes and biomarkers to prognosticate the likelihood of drug resistance in TB patients11. XGBoost is particularly powerful for handling complex data and enhancing predictive accuracy, especially with large, high-dimensional datasets12. Logistic regression, known for its simplicity and interpretability, is often used as a benchmark to compare more complex models like XGBoost, which has shown superior predictive power in our study.
This study uses machine learning to develop predictive models for LTFU in TB patients before and during treatment. Leveraging patient data from the National Center for Clinical Research in Infectious Diseases and follow-up data from the CDC, our objective is to craft robust and efficient models that empower healthcare practitioners to make informed decisions and mitigate the challenges posed by LTFU.
Method
Study design and participants
This study retrospectively collected data on tuberculosis patients treated or registered at the National Center for Clinical Medical Research on Infectious Diseases between January 1, 2017, and December 31, 2021, to create the training and validation datasets for the algorithm models. Follow-up data from the National Center for Disease Control (CDC) were used to verify and develop algorithm models, including SVM, Random Forest (RF), Extreme Gradient Boosting (XGBoost), and logistic regression, to predict LTFU before and during treatment. This retrospective study used anonymized data, ensuring privacy. Ethical approval and a waiver for obtaining informed consent from the study participants were granted by the Ethics Review Committee of the Third People’s Hospital of Shenzhen (Approval Number: [2022-027]). As the study did not involve direct patient participation, informed consent was not required. All research methods adhered to relevant ethical guidelines and legal regulations.
Definition of LTFU
LTFU, as defined by the WHO tuberculosis reporting framework13, includes two groups: (1) patients who did not start treatment after diagnosis; and (2) patients who started treatment but had interruptions of two or more consecutive months. Employing this definition, patients who hadno more than one treatment record were labeled as LTFU before the treatment. Meanwhile, individuals exhibiting intervals surpassing two months between consecutive medical visits subsequent to treatment initiation were categorized as LTFU during the treatment trajectory.
Include and exclude
The following two groups were included in this study: (1) Bacteriologically confirmed TB cases: patients with positive results confirmed by smear microscopy, culture, or WHO-approved rapid diagnostic methods (e.g. GeneXpert MTB/RIF). (2) Clinically diagnosed TB cases: patients with TB who show negative results on bacteriologic tests but are diagnosed with active TB by a clinician and start a full course of treatment regimen. The exclusion criteria were (1) Cases that were regularly seen as of December 30, 2021 and have not yet finished treatment; (2) Cases registered as dead and treatment failure; (3) Cases with incomplete patient identifiers, such as no inpatient and outpatient numbers or errors.
Data collection
Clinical data were obtained from the Hospital Information System (HIS), Laboratory Information System (LIS), Picture Archiving and Communication System (PACS), and Shenzhen Chronic Disease Follow-up Management System (SCDFMS) of the Shenzhen Third People’s Hospital. The Hospital Information System and the Chronic Disease Follow-up Management System are two separate information systems that operate independently. Therefore, in order to integrate all the medical and follow-up management data of patients, this study used the hospitalization number and outpatient number for patient information matching, and the processing process used Python V3.9.7 to call packages such as pandas and numpy for the initial integration of patient data, and two-person review of the matched data and saving the data as files in .csv formats (Fig. 1).
Fig. 1.
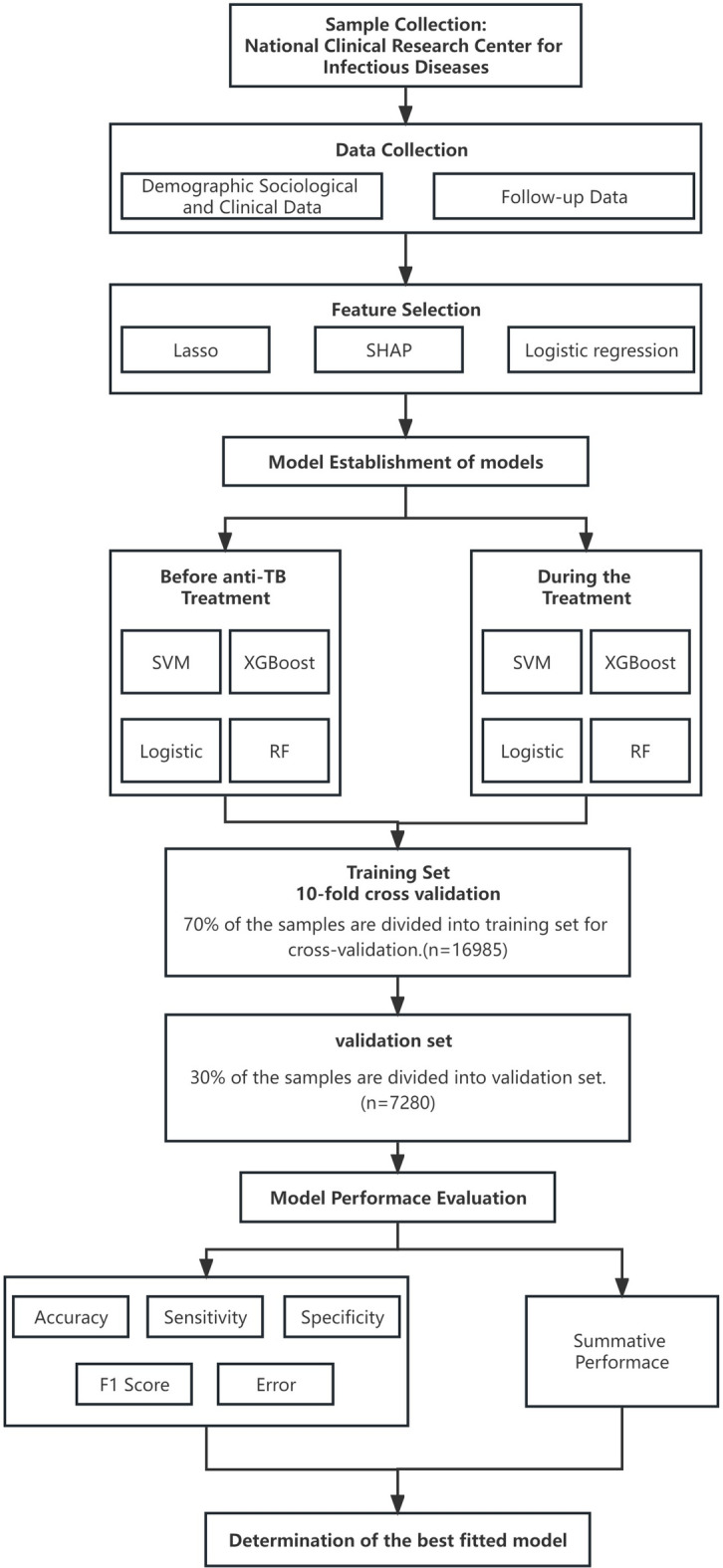
Study flowchart.
Feature selection variables
We drop the null value variables of the raw data to eliminate useless information, after which 35 variables were left three categories: (1) general patient information: name, age, gender, ethnicity, marital status, education, occupation, weight, BMI, household registration, and health insurance status, etc.; and (2) clinical medical care information: route of admission, mode of admission, year of diagnosis, clinical diagnosis, whether or not hospitalization was performed, bacteriological diagnosis, month of treatment, whether or not the patient had comorbid conditions such as hypertension, diabetes mellitus, chronic hepatitis or liver cirrhosis, or cancer, adverse reactions to anti-tuberculosis medication, history of smoking, history of drinking, psychological status, and self-care ability score; (3) Patient follow-up information: whether death occurred, time of death, patient-reporting hospital, whether treatment failed, time of treatment failure, and admission status.
Statistical analysis
Mean values were used for normally distributed continuous variables, while categorical variables were described using values and proportions. Chi-square tests, t-tests, and logistic regression were used for between-group comparisons. We selected variables with non-zero coefficients in the least absolute shrinkage and selection operator (LASSO) analysis for further analysis. Variables with P < 0.05 in univariate logistic regression analyses were included in multivariate logistic regression analyses to identify independent risk factors. Variables with high significance were screened by SHapley Additive exPlanations (SHAP) values, and the results of the LASSO and regression analyses were combined to determine the final variables to be included in the model. Multivariate logistic algorithms such as RF, SVM, XGB and logistic regression were used to build the model. XGBoost is a gradient boosting algorithm that integrates multiple decision trees, improving on traditional gradient tree boosting techniques. Describe the results of tenfold cross-validation with a line graph. The area under the receiver operating characteristic curve (ROC) (AUC) is calculated, which is the most commonly used parameter to measure the summed performance of the model. Sensitivity, specificity and accuracy were also calculated to evaluate and compare the performance of all predictive models. All machine learning models were constructed and validated in R (version 4.2.1).
Results
Basic characteristics
This study analyzed a cohort of 24,265 tuberculosis patients, with 3036 (12.51%) experiencing LTFU. LTFU occurred in 2.79% (n = 678) of patients before treatment and 9.72% (n = 2358) during treatment. Among those LTFU before treatment, 66.5% were male, with an average age of 40.9 ± 17.0 years, and 60.2% had only primary education or less. During treatment, 64.4% of the 2358 LTFU patients were male, 68.2% were married, and 20.0% had primary education or less. Employment status showed slight variations, with 54.8% of those LTFU before treatment being employed compared to 52.5% during treatment. Smoking and drinking habits were more prevalent among those LTFU before treatment, with 15.5% and 28.0% respectively, compared to 11.2% and 17.5% during treatment. Additionally, health insurance coverage was slightly lower in the LTFU group both before (67.1%) and during treatment (66.5%) compared to those who did not experience LTFU (Table 1).
Table 1.
Characters of TB patients with LTFU label before the treatment.
| Variables | Category | Before treatment initiation | P-value | After treatment initiation | P-value | ||
|---|---|---|---|---|---|---|---|
| Non-LTFU n = 23,587 (%) |
LTFU n = 678 (%) |
Non-LTFU n = 21,219 (%) |
LTFU n = 2368 (%) |
||||
| Sex | Male | 15,444 (65.5) | 445 (66.5) | 0.568 | 13,913(65.6) | 1529(64.4) | 0.234 |
| Female | 8152(34.5) | 224(33.5) | 7305(34.4) | 847(35.6) | |||
| Ethnicity | Han | 23,160(98.2) | 604(90.3) | < 0.001 | 20,840(98.2) | 2320(97.6) | 0.052 |
| Others | 436(1.8) | 65(9.7) | 380(1.8) | 56(2.4) | |||
| Education | Primary School or under | 5028(21.3) | 403(60.2) | < 0.001 | 4552(21.5) | 476(20.0) | 0.206 |
| Junior | 5434(23.0) | 57(8.5) | 4896(23.1) | 538(22.6) | |||
| Senior | 7991(33.5) | 160(23.9) | 7072(33.3) | 839(35.3) | |||
| Associate Degree | 2838(12.0) | 30(4.5) | 2542(12.0) | 296(12.5) | |||
| Bachelor’s Degree and above | 2385(10.1) | 19(2.9) | 2158(10.2) | 227(9.6) | |||
| Marital status | Married | 16,217 (68.7) | 452 (67.6) | 0.522 | 14,595 (68.8) | 1622 (68.2) | 0.609 |
| Single/Divorced /Widowed | 7397(31.3) | 217(32.4) | 6625(31.2) | 754(31.7) | |||
| Occupation | Employee | 10,079 (50.0) | 319 (54.8) | 0.022 | 9018 (49.7) | 1061 (52.5) | 0.018 |
| Unemployed | 9943(42.1) | 259(39.5) | 9265(42.2) | 937(40.4) | |||
| Students | 1490(6.3) | 39(5.9) | 1384(6.3) | 145(6.2) | |||
| Civil | 1061(4.4) | 24(3.6) | 969(4.4) | 116(5.0) | |||
| Others | 836(3.5) | 20(3.0) | 769(3.5) | 87(3.7) | |||
| Smoking | Yes | 2619(11.1) | 104(15.5) | < 0.001 | 2354(11.1) | 265(11.2) | 0.930 |
| No | 20,977(88.9) | 565(84.5) | 18,866(88.9) | 2111(88.8) | |||
| Drinking | Yes | 3474(14.7) | 187(28.0) | < 0.001 | 3058(14.4) | 416(17.5) | < 0.001 |
| No | 20,122(85.3) | 482(72.0) | 18,162(85.6) | 1960(82.5) | |||
| Health insurance | Yes | 16,836(71.4) | 449 (67.1) | 0.017 | 15,257(71.9) | 1579 (66.5) | < 0.001 |
| No | 6760(28.6) | 220(32.9) | 5963(28.1) | 797(33.5) | |||
| Residential status | Permanent | 14,416(61.1) | 412 (61.6) | 0.798 | 12,999(61.3) | 1417 (59.6) | 0.124 |
| Temporary | 9180(38.9) | 257(38.4) | 8221(38.7) | 959(40.4) | |||
| Residence registration | Urban | 17,592(97.3) | 492(2.7) | 0.553 | 15,827(74.6) | 1765(74.3) | 0.750 |
| Rural | 6004(25.4) | 177(26.5) | 5393(25.4) | 611(25.7) | |||
| Hypertension | 921(3.9) | 28(4.2) | 0.710 | 846(4.0) | 75(3.2) | ||
| Extrapulmonary TB | Yes | 3198 (13.6) | 98 (14.6) | 0.415 | 2856 (13.5) | 342 (14.4) | 0.207 |
| Underlying disease | Diabetes | 1731 (7.3) | 61 (9.1) | 0.082 | 1540 (7.3) | 191 (8.0) | 0.166 |
| Chronic Hepatitis/Cirrhosis | 1350 (5.7) | 45 (6.7) | 0.271 | 1185 (5.6) | 165 (6.9) | 0.007 | |
| Cancer | 302(1.3) | 6(0.9) | 0.383 | 269(1.3) | 33(1.4) | ||
| No | 20,398(86.4) | 571(85.4) | 18,364(86.5) | 2034(85.6) | |||
| RR/DR-TB | Yes | 709 (3.0) | 20 (3.0) | 0.982 | 646 (3.0) | 63 (2.7) | 0.288 |
| No | 22,887(97.0) | 649(97.0) | 20,574(97.0) | 2313(97.3) | |||
| Negative results of AFB smear/GeneXpert/Culture | Yes | 10,907(46.2) | 428 (64.0) | < 0.001 | 9326 (43.9) | 1581 (66.5) | < 0.001 |
| No | 12,689(53.8) | 241(36.0) | 11,894(56.1) | 795(33.5) | |||
| Previous history of TB | Yes | 1163 (4.9) | 46 (6.9) | 0.022 | 1042 (4.9) | 121 (5.1) | 0.697 |
| No | 22,433(95.1) | 623(93.1) | 20,178(95.1) | 2255(94.9) | |||
| Had been hospitalized | Yes | 15,284(64.8) | 131 (13.1) | < 0.001 | 14,809(69.8) | 475 (20.0) | < 0.001 |
| No | 20,398(86.4) | 571(85.4) | 18,364(86.5) | 2034(85.6) | |||
| RR/DR-TB | Yes | 709 (3.0) | 20 (3.0) | 0.982 | 646 (3.0) | 63 (2.7) | 0.288 |
| No | 22,887(97.0) | 649(97.0) | 20,574(97.0) | 2313(97.3) | |||
| Negative results of AFB smear/GeneXpert/Culture | Yes | 10,907(46.2) | 428 (64.0) | < 0.001 | 9326 (43.9) | 1581 (66.5) | < 0.001 |
| No | 12,689(53.8) | 241(36.0) | 11,894(56.1) | 795(33.5) | |||
| Previous history of TB | Yes | 1163 (4.9) | 46 (6.9) | 0.022 | 1042 (4.9) | 121 (5.1) | 0.697 |
| No | 22,433(95.1) | 623(93.1) | 20,178(95.1) | 2255(94.9) | |||
| Had been hospitalized | Yes | 15,284(64.8) | 131 (13.1) | < 0.001 | 14,809(69.8) | 475 (20.0) | < 0.001 |
| No | 8312(35.2) | 538(80.4) | 6411(30.2) | 1901(80.0) | |||
| Provision of an alternative contact | Yes | 19,525(82.7) | 546 (81.6) | 0.445 | 17,611(83.0) | 1914(80.6) | 0.003 |
| No | 4071(17.3) | 123(18.4) | 3609(17.0) | 462(19.4) | |||
| Admission method | Emergency | 8687 (36.8) | 511 (76.4) | < 0.001 | 6936 (32.7) | 1751(73.7) | < 0.001 |
| Outpatient | 14,909(63.2) | 158(23.6) | 14,284(67.3) | 625(26.3) | |||
| Occurrence of Adverse drug reaction | Yes | – | – | 1222 (5.8) | 183 (7.7) | < 0.001 | |
| No | – | – | 19,998(94.2) | 2193(93.2) | |||
| Age (Median [IQR]) | – | 41.3 ± 17.0 | 40.9 ± 17.0 | 0.502 | 41.3 ± 17.0 | 40.1 ± 16.9 | 0.248 |
| Weight, kg | – | 56.1 ± 7.2 | 56.8 ± 5.4 | 0.006 | 56.04 ± 7.2 | 56.16 ± 7.6 | 0.454 |
| BMI, kg/m2 | – | 20.5 ± 2.2 | 20.5 ± 1.6 | 0.973 | 20.5 ± 2.2 | 20.5 ± 2.3 | 0.500 |
| ADL score | – | 90.0 ± 13.5 | 89.8 ± 10.7 | 0.620 | 90.0 ± 13.5 | 90.2 ± 13.4 | 0.647 |
A preliminary univariate analysis, detailed in Table 1, was conducted to select relevant risk factors. This analysis revealed associations between LTFU before treatment and factors including education, ethnicity, smoking, drinking, occupation, health insurance, weight, history of hospitalization, and admission method. Comparing the non-LTFU and LTFU groups during treatment, notable intergroup disparities surfaced in ethnicity, drinking habits, occupation, health insurance status, underlying medical conditions, negative outcomes in smear/GeneXpert/culture tests, history of hospitalization, provision of alternative contacts, admission methods, and occurrences of adverse drug reactions (Table 1).
Predictor variable selection of LTFU
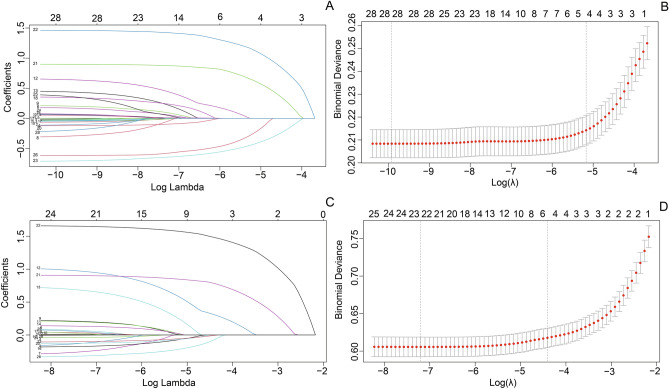
Univariate analyses identified predictive variables associated with LTFU before and during treatment. For the period preceding treatment initiation, protective factors included various educational levels, including junior high (aOR 0.12, 95% CI 0.09–0.17), senior high school (aOR 0.27, 95% CI 0.22–0.34), associate degree (aOR 0.15, 95% CI 0.10–0.22), and undergraduate (aOR 0.10, 95% CI 0.06–0.17), alongside a history of hospitalization (aOR 0.22, 95% CI 0.17–0.28). Conversely, being employed (aOR 1.21, 95% CI 1.02–1.43), engaging in alcohol consumption (aOR 2.18, 95% CI 1.79–2.65), outpatient admission (aOR 2.18, 95% CI 1.79–2.65), and having a prior history of tuberculosis (aOR 1.64, 95% CI 1.18–2.29) emerged as independent risk factors for LTFU before treatment. During the treatment phase, influential factors encompassed employment status (aOR 1.15, 95% CI 1.04–1.27), history of hospitalization (aOR 0.19, 95% CI 0.17–0.22), outpatient admission (aOR 2.53, 95% CI 2.25–2.85), presence of chronic hepatitis/cirrhosis (aOR 1.26, 95% CI 1.04–1.53), experiencing drug adverse reactions (aOR 1.24, 95% CI 1.02–1.50), availability of an alternative contact (aOR 0.82, 95% CI 0.72–0.94), and health insurance coverage (aOR 0.76, 95% CI 0.69–0.85). The Lasso regression and SHAP algorithm identified that BMI and smoking served as predictive factors for LTFU prior to treatment, while drinking proved significant in constructing the risk model for LTFU during treatment (Fig. 2 and Supplementary Fig. S1 and Table S1).
Fig. 2.
Variable selection using LASSO method. (A and C) A coefficient profile plot. evertical axis represents the coefficients, and the horizontal axis represents log (lambda). (B and D) A binomial deviance curve.
Prediction by SVM, Logistic, XGBoost and RF
For the prediction models assessing the risk of LTFU before the treatment initiation, the outcomes of tenfold cross-validation demonstrated consistent superiority of XGBoost over the other three models. XGBoost achieved the highest average AUC (0.921), outperforming Random Forest (0.828), Logistic Regression (0.736), and SVM (0.677), demonstrating its superior predictive capability for LTFU before treatment (Fig. 3).
Fig. 3.
Ten-foldcross-validation of 4 ML algorithms for predicting LTFU before and after the initiation.
In the validation set, XGBoost showed the highest sensitivity (0.81), F1 score (0.85), and AUC (0.921). The descending sequence of performance for the other models was Logistic (0.811), RF (0.755), and SVM (0.712). Remarkably, both XGBoost and RF models yielded the same F1 score of 0.75 among the models addressing LTFU during the treatment. Notably, XGBoost demonstrated the highest AUC value (0.825), surpassing Logistic (0.818), RF (0.692), and SVM (0.688) (Table 2).
Table 2.
Validation outcomes of the models.
| AUC | Accuracy | Sensitivity | Specificity | F1 score | |
|---|---|---|---|---|---|
| Logistic(A) | 0.811 | 0.79 | 0.64 | 0.88 | 0.77 |
| RF(A) | 0.755 | 0.85 | 0.76 | 0.90 | 0.83 |
| XGBoost(A) | 0.921 | 0.87 | 0.81 | 0.91 | 0.85 |
| SVM(A) | 0.712 | 0.79 | 0.72 | 0.82 | 0.73 |
| Logistic(B) | 0.818 | 0.73 | 0.59 | 0.82 | 0.69 |
| RF(B) | 0.692 | 0.76 | 0.55 | 0.88 | 0.75 |
| XGBoost(B) | 0.825 | 0.76 | 0.53 | 0.89 | 0.75 |
| SVM(B) | 0.688 | 0.76 | 0.74 | 0.77 | 0.62 |
*(A) The model for LTFU before treatment in tuberculosis patients, (B) The model for LTFU during the treatment period.
Discussion
Tuberculosis (TB) remains a global health challenge, with fatalities rising from 1.4 million in 2019 to 1.6 million in 20211. A significant contributor to TB mortality is the loss of follow-up (LTFU), highlighting the need for effective disease management2. However, there has been limited exploration of this issue among Chinese patients Thus, This study aims to address this gap by using retrospective data to develop a risk prediction model. Our goal is to identify key determinants and provide insights to improve TB management, enhance treatment adherence, and elevate patient outcomes in China.
This study uses advanced machine learning, particularly XGBoost, to predict treatment interruption in TB patients. This approach is essential in the big data era, where traditional methods may fall short. Compared to previous studies, our XGBoost model demonstrated significantly higher predictive accuracy. Rodrigo et al. used logistic regression for predicting lost visits in TB patients, achieving an AUC of 0.6714. whereas our model achieved an AUC of 0.921 for pre-treatment LTFU and 0.825 for in-treatment LTFU. Similarly, Hokino Yamaguti et al. developed a classification tree model with an accuracy of 0.76 and an F1 score of 0.7715, both of which were outperformed by our XGBoost model. These comparisons underscore the relevance of our study, as it demonstrates the superiority of machine learning techniques like XGBoost in handling complex variable relationships and improving prediction accuracy in the context of TB treatment interruption. XGBoost’s robustness comes from its ability to handle complex nonlinear relationships, fast training, and efficient memory usage, making it ideal for large healthcare datasets. Additionally, its gradient boosting framework ensures high accuracy even with small sample sizes, a crucial advantage in healthcare16.
Machine learning, particularly XGBoost, has proven highly effective in capturing complex data patterns, as demonstrated by our study. This approach significantly enhances prediction accuracy in TB treatment outcomes, offering a robust tool for addressing the challenges of treatment interruption. The superior performance of the XGBoost model in predicting treatment interruption risk for TB patients aligns with previous research showcasing its remarkable capabilities in various prediction tasks17. Our study further validates the superiority of the XGBoost model over SVM, RF, and logistic regression models in predicting treatment interruption risk, highlighting its prowess in handling complex variable relationships18. Our study contributes to the growing evidence supporting XGBoost as a potent tool in healthcare prediction tasks. Its versatility in medical scenarios makes it an invaluable asset for healthcare professionals seeking accurate risk assessments. Future research should assess the generalizability of our findings across diverse populations, enhancing the applicability of XGBoost. High-performance models like XGBoost offer a proactive strategy to reduce treatment interruption and improve TB treatment success rates. XGBoost accurately identifies high-risk patients, enabling efficient resource allocation, reducing workload, and improving patient care. This personalized approach significantly lowers treatment interruption rates, optimizing TB management. As a result, our study offers insightful strategies for data-driven approaches in TB treatment and underscores the potential of XGBoost as a valuable tool in predictive healthcare analytics.
We identified key risk factors for TB patient dropout before treatment, with outpatient clinic patients showing higher LTFU rates. Consequently, tailoring educational efforts towards patients who have experienced relapses and are attending outpatient clinics assumes paramount importance in augmenting treatment adherence. A notable revelation from our study was the independent association of alcohol consumption with TB patient dropout the trend that aligns with findings from a prospective study in Ethiopia19, this underscores the urgency of providing meticulous guidance to patients who consume alcohol, encouraging strict adherence to medication regimens throughout the TB treatment process. Our study’s insights underscore the significance of addressing specific risk factors like a history of TB and alcohol consumption, providing avenues for curbing treatment dropout in TB patients. Targeted interventions and enhanced patient education could improve treatment adherence, leading to better outcomes and reduced disease burden.
The inclusion of hospitalized TB patients in our study demonstrated a noteworthy decrease in the risk of LTFU. Hospitalization of TB patients exhibited a notable reduction in the risk of treatment interruption. Hospitalization provided advantageous conditions for treatment adherence by facilitating interaction with healthcare professionals, delivering health education, and ensuring continuous medical care until discharge criteria were met. This vigilant supervision and support during hospitalization contributed to enhanced adherence rates20, while the gradual recovery process during hospital stay instilled patient confidence in maintaining their treatment regimen21. These findings underscore the pivotal role of hospitalization, particularly in urgent cases, in fortifying treatment adherence for TB patients. Moreover, educational attainment emerged as a significant factor in reducing the likelihood of treatment interruption among TB patients. Patients with higher educational backgrounds often possess a more profound understanding of health concepts, heightened health awareness, and comprehensive knowledge of their health status and treatment options22. This heightened awareness drives them to prioritize TB management and treatment, recognizing the value of prompt medical attention and strict adherence to prescribed treatment protocols. Thus, targeted educational initiatives tailored to patients with lower educational levels can effectively enhance TB awareness, underscoring the significance of both treatment adherence and timely medical intervention.
Interestingly, chronic hepatitis or cirrhosis emerged as a unique risk factor for treatment interruption during antituberculosis treatment, yet intriguingly, it did not exert the same influence on treatment interruption before treatment initiation. This divergence can be attributed to the heightened vulnerability of patients with chronic liver disease to hepatotoxicity during antituberculosis treatment, prompting preemptive treatment discontinuation to prevent further liver damage23. Additionally, our study highlights that patients who experience adverse effects are more susceptible to treatment interruption after initiating therapy. Severe drug side effects compel patients to discontinue treatment, and frequent changes in the treatment regimen due to adverse reactions can test patients’ resilience, ultimately leading to treatment discontinuation and interruptions in follow-up24. Hence, enhancing the training of medical personnel in effectively monitoring and managing adverse drug reactions becomes crucial. Establishing a comprehensive framework for reporting and managing adverse drug reactions can assist patients in overcoming treatment challenges and reinforcing treatment adherence. Moreover, independent employment status emerged as a risk factor for treatment interruption, a finding contrasting with prior research associating unemployment with such interruptions25. Our hypothesis suggests that a substantial proportion of employed or self-employed individuals might experience social and occupational pressures that contribute to a negative impact and elevate the risk of LTFU.
The registration of backup contact information plays a crucial role in mitigating the risk of treatment interruption during antituberculosis therapy. Typically, these backup contacts, often family members, provide essential support to healthcare providers by facilitating communication, scheduling follow-up appointments, and ensuring adherence to treatment plans. Furthermore, meticulous documentation of patient information throughout treatment holds significant value, aiding in patient management, treatment decision-making, and providing invaluable data to support local TB elimination strategies26. Additionally, the presence of health insurance emerges as a protective factor against treatment interruption. Our study underscores previous research by providing compelling evidence that patients lacking health insurance face a higher risk of poor treatment outcomes27. The absence of health insurance leads to increased medical expenses. In alignment with Article 21 of the Measures of the People’s Republic of China on the Management of Tuberculosis, TB patients are entitled to health insurance coverage for examinations and medication during treatment, with both national and local governments offering subsidies and exemptions for medical expenses related to tuberculosis treatment28. Considering the association between TB and poverty, effective management interventions should prioritize alleviating the economic burden faced by TB patients.
While this study offers valuable insights, some limitations should be noted. We analyzed LTFU separately before and during treatment, which provides stage-specific insights but may not capture the overall impact of LTFU. Additionally, we did not compare binary outcomes of LTFU versus non-LTFU across the entire cohort, nor did we conduct a ternary analysis of non-LTFU, LTFU before treatment, and LTFU during treatment, which could highlight different epidemiological factors. The duration and reasons for hospitalization were also not explored in depth, which may influence treatment outcomes. Despite these limitations, our findings contribute significantly to understanding the factors influencing LTFU in TB patients and provide a solid foundation for future research to build upon.
Conclusion
In conclusion, our study highlights the superior predictive power of the XGBoost model in forecasting LTFU among TB patients, outperforming traditional machine learning methods. The identification of key risk factors, such as a history of previous tuberculosis, alcohol consumption, and lower educational attainment, offers valuable guidance for designing targeted interventions to enhance treatment adherence. Additionally, hospitalization was identified as a crucial factor in reducing the risk of LTFU, further emphasizing the importance of continuous care. By employing advanced machine learning techniques like XGBoost, we can more effectively address LTFU, thereby improving TB treatment success rates and patient outcomes. Our research advances the development of data-driven strategies in TB management and supports the implementation of personalized interventions to mitigate the impact of LTFU and optimize patient care.
Supplementary Information
Author contributions
J.F.C. and Y.L.J. conceived and wrote the manuscript. Z.H.L., M.S.Z., L.L.L., and A.L. participated in the experimental design. H.Z.L. conceived the presented idea and supervised the study. All authors approved the final paper and accepted accountability for all aspects of the work.
Funding
Our study were supported by Science, Technology and Innovation Commission of Shenzhen Municipality (JCYJ20220530163403008) and Shenzhen High-level Hospital Construction Fund (G2022006).
Data availability
The datasets used and/or analyzed during the current study are not publicly available due to privacy concerns and institutional regulations regarding the handling of patient data. However, the datasets are available from the corresponding author on reasonable request. Requests for access to the data should include a clear justification, and access will be granted in accordance with relevant ethical guidelines and institutional policies.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was designed and conducted in accordance with the tenets of the Declaration of Helsinki. Previous ethical approval was obtained from the Ethics Review Board of the Third people’s Hospital of Shenzhen(2022–027).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jingfang Chen and Youli Jiang.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-74942-z.
References
- 1.Chakaya, J. et al. Global Tuberculosis Report 2020—Reflections on the global TB burden, treatment and prevention efforts. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Diseases113(Suppl 1), S7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global tuberculosis report 2021. (2021).
- 3.Lei, X. et al. Are tuberculosis patients adherent to prescribed treatments in China? Results of a prospective cohort study. Infect. Dis. Poverty5, 38 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zawedde-Muyanja, S. et al. Estimating the effect of pretreatment loss to follow up on TB associated mortality at public health facilities in Uganda. PLoS ONE15, e0241611 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tetart, M. et al. Factors of loss to follow-up during tuberculosis treatment in a low-incidence region. Med. Mal. Infect.50(1), 28–35 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Zhou, T. J. et al. Predictors of loss to follow-up among men with tuberculosis in Puducherry and Tamil Nadu, India. Am. J. Trop. Med. Hyg.103(3), 1050–1056 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawatsu, L. et al. A combination of quantitative and qualitative methods in investigating risk factors for lost to follow-up for tuberculosis treatment in Japan—Are physicians and nurses at a particular risk?. PLoS ONE13(6), e0198075 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu, Q. et al. Reminder systems to improve patient adherence to tuberculosis clinic appointments for diagnosis and treatment. Cochrane Database Syst. Rev.2014(11), CD006594 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang, Y. et al. Factors associated with loss to follow-up before and after treatment initiation among patients with tuberculosis: A 5-year observation in China. Front. Med.10, 1136094 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latif, J. et al. Medical imaging using machine learning and deep learning algorithms: A review. (IEEE, 2019).
- 11.Evora, L., Seixas, J. M. & Kritski, A. L. Neural network models for supporting drug and multidrug resistant tuberculosis screening diagnosis. Neurocomputing265, 116–126 (2017). [Google Scholar]
- 12.Kanesamoorthy, K. & Dissanayake, M. B. Prediction of treatment failure of tuberculosis using support vector machine with genetic algorithm. Int. J. Mycobacteriol.10(3), 279–284 (2021). [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Definitions and reporting framework for tuberculosis–2013 revision: updated December 2014 and January 2020. (World Health Organization, 2020).
- 14.Linh, N. N. et al. World Health Organization treatment outcome definitions for tuberculosis: 2021 update. Eur. Respir. J.58(2), 2100804 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Hokino Yamaguti, V. et al. Development of CART model for prediction of tuberculosis treatment loss to follow up in the state of São Paulo, Brazil: A case-control study. Int. J. Med. Inform.141, 104198 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Chen, T. & Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining; August 13, 2016; San Francisco, California, USA 785–794 (2016).
- 17.Wang, C. et al. Comparison of machine learning algorithms for the identification of acute exacerbations in chronic obstructive pulmonary disease. Comput. Methods Programs Biomed.188, 105267 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Chen, T. & Guestrin, C. Xgboost: Reliable large-scale tree boosting system. (2015).
- 19.Soboka, M. et al. Substance use disorders and adherence to antituberculosis medications in Southwest Ethiopia: A prospective cohort study. BMJ Open11(7), e043050 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volmink, J. & Garner, P. WITHDRAWN: Interventions for promoting adherence to tuberculosis management. Cochrane Database Syst. Rev.4, CD000010 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Alipanah, N. et al. Adherence interventions and outcomes of tuberculosis treatment: A systematic review and meta-analysis of trials and observational studies. PLoS Med.15(7), e1002595 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sreeramareddy, C. T. et al. Delays in diagnosis and treatment of pulmonary tuberculosis in India: A systematic review. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Against Tuberc. Lung Dis.18(3), 255–266 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tweed, C. D. et al. Liver toxicity associated with tuberculosis chemotherapy in the REMoxTB study. BMC Med.16(1), 46 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Padilla, E. et al. Reasons for defaulting from drug-resistant tuberculosis treatment in Armenia: A quantitative and qualitative study. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Against Tuberc. Lung Dis.18(2), 160–167 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Choi, H. et al. The impact of social conditions on patient adherence to pulmonary tuberculosis treatment. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Against Tuberc. Lung Dis.20(7), 948–954 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ausi, Y. et al. Between curing and torturing: Burden of adverse reaction in drug-resistant tuberculosis therapy. Patient Prefer. Adherence15, 2597–2607 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paniagua-Saldarriaga, L. A., Pelissari, D. M. & Rueda, Z. V. Factors associated with unsuccessful outcomes of tuberculosis treatment in 125 municipalities in Colombia 2014 to 2016. Am. J. Trop. Med. Hyg.105(5), 1326–1334 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ministry of Health of the People’s Republic of China. Measures of the People’s Republic of China on the Management of Tuberculosis, in 029 (Beijing, Ministry of Health, 2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are not publicly available due to privacy concerns and institutional regulations regarding the handling of patient data. However, the datasets are available from the corresponding author on reasonable request. Requests for access to the data should include a clear justification, and access will be granted in accordance with relevant ethical guidelines and institutional policies.