Abstract
Continuous use of antibiotics in poultry feed as growth promoters poses a grave threat to humanity through the emergence of antibiotic resistance, necessitating the exploration of novel and sustainable alternatives. The present study was carried out to evaluate the performance of postbiotics derived from Lactobacillus acidophilus in broiler birds. The postbiotics were harvested by culturing probiotic bacteria from the stock cultures at the required temperature and duration under laboratory conditions and supplemented to broilers via feed. For experimentation, 480-day-old CARI-Bro Dhanraja (slow-growing broiler) straight-run chicks were randomly split up into six groups. Treatment groups diets are as follows: T1- Basal diet (BD)+0.2%(v/w) MRS Broth/ uninoculated media; T2 – BD + Antibiotic (CTC); T3- BD + Probiotic; T4, T5 & T6 – BD + postbiotics supplementation of 0.2%, 0.4% and 0.6% (v/w) respectively. The chicks were raised under an intensive, deep litter system with standard protocol for 6 weeks. Results showed that 0.2% of postbiotics (T4) had significantly (P < 0.001) higher body weight (1677.52 g) with better FCR (1.75) and immune response. Postbiotic supplementation altered various serum attributes positively, in this study. Significant (P < 0.001) reductions in total plate counts (TPC), coliform counts, and maximum Lactobacillus counts were recorded in all postbiotic-supplemented groups. The villus height (1379.25 μm), width (216.06 μm) and crept depth (179.25 μm) showed significant (P < 0.001) improvement among the treatment groups on the 21st and 42nd day of the experimental trial, with the highest value in the T4 group (0.2% postbiotic supplementation). Jejunal antioxidant values also noted significantly (P < 0.001) higher values in T4 group. The study concludes that 0.2% postbiotic supplementation can act as a substitute to antibiotic growth promoters and also combat the disfavour activity of probiotics in broilers.
Keywords: Antibiotic resistance, Postbiotics, Broilers, Gut health
Subject terms: Nutrition, Antimicrobials, Animal physiology
Introduction
Food derived from animals such as meat, egg, fish considered as major source of protein and fats to mankind for which lots of animals reared in different rearing systems across the globe. Each animal husbandry system has its own advantage and disadvantage but beyond that one major issue which is disputably threat to universe is Anti-microbial resistance (AMR). The reason beyond this alarm were many, one among them is that continuous use of antibiotics at sub therapeutic dosage level in animal’s feed as Antibiotic Growth Promotors1. Poultry meat consumption holds highest compared to other species, where birds often raised intensively and subjected to significant antimicrobial usage for disease prevention, treatment, and growth enhancement2. Antibiotics at sub therapeutic dosage level supports in reducing or preventing the pathogenic organisms in the intestine, boost poultry wellbeing, improve FCR and alleviate carbon emission3. The continuous use of antibiotics develops certain resistance called as antimicrobial-resistant where pathogens acclimatised to the antibiotics and becomes unresponsive, leading to treatment failures. This ignites the emanation of drug (Antibiotic) resistant among bacterial populations4, and subsequently, causes detrimental effect on the well-being of poultry and humans5. At this juncture, economic transformations also drawing attention to sound health; hence consumers are very keen in knowing the safety, quality, judicious use of inputs such as antibiotics and adoption of best practices are calls forth. This worrisome problem spurred numerous countries to enact bans on the use of antibiotics as growth promoters (EU regulations:1831/2003; India – INFAAR 2012–2017). Owing to the rise of antimicrobial resistance (AMR), the hunt for innovative and economical feed additives as alternatives to the exploitation of antibiotics in poultry as a growth promoter in need of hour.
Live probiotics or direct fed microbials containing several LAB strains are the most popular feed additives in chicken feed. Direct fed microbials are boon to the poultry production, because the expansionism of these microbiota will be more in the gut thereby reducing the pathogenic organism6. Despite their proven benefits, concerns persist regarding the biosafety considerations associated with live probiotics, efficiency of microbial production, handling and storage; route of probiotic administration; stability; and survivability within the host, and tolerance to bile7. The antimicrobial substances that produced by probiotic bacteria called as Postbiotics, which can be better choice than probiotics. Postbiotics are the substances or metabolites of probiotic organisms, produced during their growth but also when they die which tends to have great impact on host health. Postbiotics refers to bioactive substances created by food-grade bacteria during the fermentation process, and is the latest addition of the biotics heritage8. Postbiotics include microbial cells, cell constituents and metabolites. Furthermore, postbiotics avoid the technological challenges of colonisation efficacy and maintaining viable and stable microorganisms in the product at large doses9. The advantages of postbiotics over parent bacterial live cells include: stable structures, no tendency to pass drug resistance, no biogenic amine (BA) production, specified chemical composition and protection, ease of use and storage, stability in an extensive range of pH and temperature, and broad-spectrum antimicrobial activity10.
When postbiotics incremented in an animal’s feed, it has a vast array of favourable probiotic effects on animal growth and, in particular to gastrointestinal health. Furthermore, to meet increasing global demands by sustainable poultry production we require the immediate development of generally acceptable innovative feed additive(s) like Postbiotics that will favourably impact overall poultry performance and health. With this background, the present experimental study was put forwarded to understand the postbiotics and its performance in broilers at the tropical regions.
Materials and methods
Ethics declarations
The study conducted in accordance with the regulatory framework outlined by the “Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) 2012,” as laid down under the “Prevention of Cruelty to Animals Act 1960” of the Indian Penal Code. The experimental protocols employed in this investigation received explicit approval from the Institutional Animal Ethics Committee (IAEC) of ICAR-Central Avian Research Institute, Bareilly, 243,122, India. The approval number is 452/GO/S/03/CPCSEA-25/07/2021 (Project code– P-1/2021/1-IAV/L34/3900/6100, Duration: 01-04-2021 to 31.03.2024). Reporting of in Vivo Experiments (ARRIVE) guidelines, ensuring the ethical conduct and reporting standards in the realm of animal experimentation.
Procedure followed for postbiotics collection
A procedure11 was followed to isolate and collect postbiotic metabolites from probiotic bacteria (Lactobacillus acidophilus). The stock culture of probiotic (Lactobacillus acidophilus) procured from NCDC − 15 (National Collection of Dairy Cultures) at National Dairy Research Institute, India. Stock culture was revived twice by using de-Mann Rogosa Sharpe (MRS) broth (Hi Media – M369, granulated) with incubation at 30 °C for 24 h, followed by spread plate inoculation of culture and incubation again at 30 °C for 48 h. Each time, the incubation conditions were static. Then, a single colony was picked up and put into 10 ml MRS broth and incubated at 30 °C for 24 h. This will be followed by sub-culturing in 10 ml MRS broth and incubation at 30 °C for 24 h. From this culture, 1% (v/v) inoculum was added to the appropriate reconstitution media and incubated at 30 °C for 24 h. The bacterial cells were separated by centrifugation at 10,000 x g for 15 min, and the cell-free supernatant (CFS) was then collected by filtration through a syringe filter (Millex® Syringe Filters, 0.22 μm). The postbiotics (Supernatants) were collected and kept at 4 °C until the feeding trial was conducted. The postbiotics produced were examined in vitro study to evaluate characteristic property of the postbiotic metabolites harvested from GRAS probiotic Lactobacillus sps. i.e., Lactobacillus acidophilus12. The Results exhibited significantly reduced pH (4.22), increased osmolarity and higher calcium (10.71 mg/dl), sodium(12.83 mg/dl) and chloride(124.16mmol/l) concentration than the un-inoculated media12. Further, the postbiotics showed improved antioxidant, anti-inflammatory activity (55.80) and clear zone of inhibition (56.48) against E. Coli and Salmonella12. Then the liquid Postbiotics was daily mixed in mash feed manually only once during early morning hours by premix method. The premixing of Postbiotics and feed was done every day based upon the feed intake of the birds and mixed well before feeding to the respective treatments.
Management, diets, and experimental design of birds
The present experimental study was conducted in experimental broiler farm at ICAR- Central Avian Research Institute, Bareilly, U.P. India. A total of 480 Day old CARI-Bro Dhanraja (slow growing-coloured broilers) chicks (straight run) were obtained from experimental hatchery of the institute and were randomly split up into six groups (completely randomized design). Each group was comprised of four replicates, and each replicates contained twenty chicks. Six different dietary treatments groups were, T1- Basal diet (BD) + 0.2%(v/w) MRS Broth/ uninoculated media; T2 - BD + Antibiotic (CTC@335 mg/kg) Antibiotic (CTC- Chlorotetracycline) was procured from Altron Biotech; T3 BD + Probiotic (Lactobacillus acidophilus − 1 × 106 cfu/g); T4 - BD + Postbiotics @ 0.2% (v/w); T5 - BD + Postbiotics @ 0.4% (v/w) and T6 - BD + Postbiotics @ 0.6% (v/w). The total duration of the experiment was 42 days. Birds were fed with three different phases of diet such as pre-starter (0-14d), starter (15-28d) and finisher (29-42d) according to ICAR (2013) recommendations. The detailed outline of the feed ingredients employed in the study is given in the Table 1. The chicks were raised under an intensive, deep litter system. The birds were provided with ad libitum amounts of their respective feed and water. The birds have also administered with pertinent vaccinations in accordance with the standard vaccination schedule followed at the institute’s farm. The housing systems made as per the standard protocol, at initial for a week, birds were provided with 24 h of light, after that light duration has been decreased one hour each day until they had 18 h of light, which they continued to receive until the end of trial.
Table 1.
Ingredient and chemical composition of basal diet (ICAR-2013).
| Ingredients (%) | Pre-starter | Starter | Finisher |
|---|---|---|---|
| Maize | 54.60 | 54.20 | 57.62 |
| Soybean meal (CP, 44%) | 39.58 | 37.80 | 32.58 |
| Rice bran oil | 02.12 | 04.24 | 05.86 |
| Calcite | 01.54 | 01.52 | 01.73 |
| Di-calcium phosphate | 0.90 | 0.95 | 1.10 |
| Salt | 0.18 | 0.18 | 0.18 |
| L – Lysine | 0.30 | 0.15 | 0.17 |
| DL – Methionine | 0.30 | 0.28 | 0.27 |
| Phytase | 0.015 | 0.015 | 0.015 |
| Vitamin AD3EKa | 0.014 | 0.014 | 0.014 |
| B-complex vitaminsb | 0.015 | 0.015 | 0.015 |
| Trace Mineralsc | 0.010 | 0.010 | 0.010 |
| Coccidiostat | 0.01 | 0.01 | 0.01 |
| Toxin binder | 0.05 | 0.05 | 0.05 |
| Total | 100 | 100 | 100 |
| Nutrient composition (%-)Calculated | |||
| Crude protein | 22.65 | 21.65 | 19.70 |
| Metabolizable energy (kcal/kg) | 3000 | 3125 | 3250 |
| Calcium | 0.96 | 0.95 | 0.90 |
| Available Phosphorus | 0.45 | 0.46 | 0.46 |
| Lysine | 1.42 | 1.25 | 1.14 |
| Methionine | 0.62 | 0.59 | 0.55 |
*Calculated values.
aOne gram of Vitamin AD3 EK supplement contained 82,500 IU of Vitamin-A, 12,000 IU of Vitamin-D3, 80 mg of Vitamin-E, and 10 mg of Vitamin-K.
bOne gram of B-complex supplement contained 8 mg of Vitamin-B1, 16 mg of Vitamin-B6, 80 mcg of Vitamin-B12, 120 mg of Niacin, 8 mg of Folic acid, 80 mg of Calcium -D-pantothenate and 86 mg of Calcium.
cOne gram of Trace mineral mixture contained 54 mg of Manganese, 52 mg of Zinc, 20 mg of Iron, 2 mg of Iodine and 1 mg of Cobalt.
Zootechnical performance
Weekly and cumulative production parameters including body weight, weight gain, feed intake, and feed conversion ratio (FCR) were methodically recorded for each treatment group. Each morning, precise quantities of feed were dispensed to the birds according to their respective dietary regimens, and the remaining feed was weighed the following day to determine total consumption. FCR was calculated on a weekly and cumulative basis using data from feed intake (FI) and body weight gain (BWG). Mortality among the experimental birds was monitored daily through meticulous observation and individual record-keeping.
Immune response
The immune status of the birds was evaluated in terms of humoral13 and cell mediated14 immune response, using standard methodologies15. The weight of the immune organs was also recorded in this study.
Humoral immunity
Humoral immunity was evaluated by determining the antibody titre values against Newcastle disease (ND) in vaccinated birds. To facilitate this assessment, a booster dose of the La-Sota strain vaccine was administered to the birds via drinking water during their 3rd week of age. Antibody titre values were quantified using the haem-agglutination (HA) test procedure, conducted in U-bottom micro-titre plates.
Blood samples were collected from the jugular vein of healthy sheep using Alsever’s solution as an anticoagulant. The collected blood was centrifuged at 2500 rpm for approximately 15 min after which the red blood cells were completely washed three times with phosphate buffer solution (PBS − 7.6) and the supernatant was discarded. Then, 1% v/v SRBC suspension was prepared in PBS and stored at 4 °C in refrigerator. 1.0 ml suspension of 1% v/v SRBC was injected intravenously to eight-birds/treatment on 28th day post hatch and the immunized birds were marked properly for easy identification. Blood (2 ml) from jugular veins of immunized birds were aseptically collected 5 days post immunization, i.e. on 34th day post hatch. The serum was separated and kept under frozen condition (−20 °C), following which antibody titres to SRBC were analysed. A total of 98 U-bottom microtiter plates were utilized for the experiment. Each well received 50 µl of PBS as the initial solution. Subsequently, 50 µl of serum sample was added to the first well, followed by consecutive two-fold serial dilutions up to the 11th well. The 12th row served as the control. Following this, 50 µl of a 1% v/v suspension of sheep red blood cells (SRBC) in PBS was dispensed into each well. The microtiter plates were then placed in an incubator at 37 °C for 1 h to facilitate incubation. After the incubation period, the plates were examined under bright light. The titration results were expressed as the log2 of the maximum dilution, which displayed irregular haemagglutination.
In-vivo cell mediated immune response (CMIR)
In this study, the cell-mediated immune response in birds was investigated by assessing their reaction to phytohemagglutinin type P (PHA-P), a protein variant of phytohemagglutinin-P (PHA-P) sourced from Hi-Media (TC226). On the 27th day post-hatch, each bird received an intradermal injection of 0.1 mL of PHA-P solution (1 mg/mL in PBS) between the 3rd and 4th interdigital spaces of the right foot. Concurrently, the left foot was injected with 0.1 mL of sterile phosphate-buffered saline (PBS) to serve as a control. Skin thickness measurements of both the right and left foot webs were taken using a micro-meter at both the time of injection (0 h) and 24 h post-injection of the mitogen. The in vivo response to PHA-P was quantified using the Foot Pad Index (FPI), computed as the difference between the swelling in the right foot and the left foot before and after the 24-hour injection period, and was reported in milli-meters.
Relative weight of lymphoid organs
Weights of lymphoid organs such as bursa of Fabricius, spleen and thymus (g) were recorded at 42 days of age and expressed as percentage of pre-slaughter live weight.
Serum assays
Biochemical serum profiling encompassing of protein, lipid, liver, kidney and mineral profiles was conducted utilizing commercial kits from Coral Clinical Systems (Tulip Diagnostics, Pvt. Ltd. Goa) along with spectrophotometer sourced from Bio-Rad Laboratories. At the age of 42 days, blood samples were randomly collected from 8 birds per treatment, into red-capped serum vials devoid of anticoagulants. After blood collection, the plasma samples were separated by centrifugation at 3000 rpm for 10 min and plasma was decanted into sterilized plastic vials, and then stored at -20 °C for further use. The study employed various methods to determine serum biomarkers. Total protein estimation was performed using the Biuret method16, while the Bromocresol Green method was employed for albumin assessment17. Globulin estimation was derived using the formula: Globulin (g/dl) = Total Proteins (g/dl) – Albumin (g/dl), whereas the Albumin/Globulin Ratio (A/G Ratio) was calculated as Albumin (g/dl) divided by Globulin (g/dl). For the evaluation of plasma lipid profile, the enzymatic CHOD-PAP method was utilized for cholesterol determination, as per the methodology outlined by18; GPO/PAP method for triglycerides19. For plasma enzymes such as SGOT & SGPT the Reitman and Frankel method20, Kidney Profile, the Uricase method for uric acid21 and Modified Jaffe’s Kinetic Method for creatine22. For mineral profile, Calcium (OCPC method)23 - Phosphorus (Molybdate U.V. method)24, Calorimetric method for Sodium25, Potassium26 & Chloride (thiocyanate method) were employed.
Gut health
Caecal flora quantification
On the 21st and 42nd day, samples of intestinal content (specifically caecal content), weighing approximately one gram, were meticulously collected from six birds per treatment and transferred into sterile containers to undergo microbiological analysis. Each gram of the sample was then diluted with 9 ml of 0.85% sterile saline solution, followed by homogenization and subsequent tenfold serial dilution. The diluted samples were plated in duplicate according to the standard procedure27, onto selective media suitable for enumerating coliform, lactic acid bacteria, and total plate count. For the same MacConkey agar for coliform, MRS media for Lactic acid Bacteria and Hi-Veg Plate count agar for total plate count (Hi media) was utilized, and all media containing digesta samples (Spread plate method) were uniformly incubated at 37 °C for 24 h. Following incubation, colonies were selected and counted using a colony counter. The average count for each treatment was then calculated by multiplying the count obtained with the dilution factor and was expressed as log10 colony-forming units per gram (cfu/g) of the sample.
Morphometric analysis of jejunal tissue
For the histo-morphological assessment, samples of the jejunum were collected from six birds per treatment on days 21 and 42 of age. The jejunum samples were fixed in neutral buffered formalin and then processed for histology. Tissues were dehydrated using an ascending graded series of ethanol and embedded in paraffin wax28. Using a microtome serial section of 5 μm thickness were cut from the jejunum samples. Four cross-sections of the jejunum per bird were then stained with Mayer’s Haematoxylin and Eosin (H&E) from Hi-Media Manufactures Ltd. Subsequently, morphometric and morphological analyses were conducted using light microscopy. The histological sections were examined under low magnification (10x), and parameters such as villus height, villus width, Villus height: Villus width ratio, crypt width and cryptal depth were measured using Zeiss Primo Star Software. In each cross-section, measurements were taken for ten villi and ten crypts per bird.
Jejunal tissue antioxidant activity
To ascertain the total antioxidant activity of the jejunal tissues, samples were procured from six birds per treatment on days 21 and 42 of the broilers’ age. The assessment was conducted utilizing the Cayman Chemical Antioxidant Assay kit (709001) in strict accordance with the manufacturer’s guidelines. The results were determined employing the specified formula. Antioxidant (mM) = [(Sample average absorbance) – (y-intercept) / Slope] x Dilution.
Carcass characteristics
For carcass characteristics, the birds were slaughtered at the end of the trial, i.e., 42nd day, four birds from each replicate of the treatment group (20 birds/dietary treatment, n = 120) were randomly selected. The selected birds were fastened for about 12 h, while having unrestricted access to drinking water. Afterwards, birds were euthanized to assess carcass traits and cut-up parts in percentage.
Statistical analysis
The statistical analysis was executed using Statistical Package for Social Sciences (SPSS) version 20.0, software. The data obtained from the conducted experiments underwent rigorous statistical analysis employing a one-way ANOVA framework, expressed as: Xij = µi + εij; Xij: The observed value for the dependent variable for the jth observation in the ith group; µi: Mean value for the ith group; εij: Represents the random error associated with the jth observation in the ith group. The group means (represented by µi) were compared using Tukey’s multiple range test at a significance level of P ≤ 0.05 to determine if the differences between them were statistically significant.
Results
Zootechnical performance
Table 2 depicts the zootechnical performance findings of the broilers supplemented with different dietary regimen, accounting weekly and overall (0–6 weeks). Statistical analysis revealed that significantly better (P < 0.001) results obtained on body weight, weight gain trend, feed intake and FCR of the birds on weekly and also on cumulative basis. The body weight was significantly (P < 0.01) varied in the study specifically, the group receiving 0.2% postbiotic supplementation (T4) exhibited the highest body weight, followed by the antibiotic-treated group (T2) and the probiotic supplemented group (T3). Similarly, the pattern of cumulative weight gain was also significantly (P < 0.001) highest in 0.2% postbiotic group (1636.72 g) followed by antibiotic group (1590.4 g). Regarding feed intake, significant differences (P < 0.01) among treatment groups were noted at the first and sixth week of age. The T4 group demonstrated the highest feed intake at both time points, followed by T2. Moreover, significant improvement (P < 0.001) in FCR were observed between treatment groups, with T4 exhibiting a notably superior over all FCR (1.75) compared to the other groups. There were no notable variances in mortality between the treatment groups in the study, and the rates remained within the expected range.
Table 2.
Effect of postbiotic on zootechnical performance in broiler chickens (0–6 weeks).
| Zootechnical Performance | T1 | T2 | T3 | T4 | T5 | T6 | P-Value |
|---|---|---|---|---|---|---|---|
| Body weight (g) | |||||||
| Hatch | 39.80 ± 0.30 | 40.63 ± 0.26 | 40.15 ± 0.32 | 40.80 ± 0.26 | 40.13 ± 0.22 | 40.33 ± 0.28 | 0.126 |
| 1st week | 89.00d ± 0.82 | 111.20b ± 1.27 | 105.52bc ± 1.41 | 122.95a ± 1.06 | 104.07bc ± 1.34 | 94.32c ± 1.00 | 0.000 |
| 2nd week | 216.73d ± 1.81 | 241.30b ± 1.79 | 234.00c ± 2.04 | 253.52a ± 1.68 | 232.52c ± 1.72 | 224.15d ± 1.61 | 0.000 |
| 3rd week | 399.78c ± 3.14 | 428.23b ± 2.83 | 418.67b ± 3.30 | 441.13a ± 2.78 | 417.08b ± 2.55 | 409.68c ± 2.87 | 0.000 |
| 4th week | 694.30c ± 5.37 | 728.90bc ± 4.64 | 715.80bc ± 5.52 | 743.12a ± 4.66 | 714.12b ± 4.06 | 708.15c ± 4.96 | 0.000 |
| 5th week | 1012.73d ± 7.80 | 1094.60ab ± 6.93 | 1077.15bc ± 8.30 | 1110.32a ± 6.98 | 1075.32bc ± 6.00 | 1071.15c ± 7.51 | 0.000 |
| 6th week | 1394.78e ± 10.71 | 1631.03b ± 10.34 | 1478.65cd ± 11.43 | 1677.52a ± 10.60 | 1512.78c ± 8.41 | 1474.48cd ± 10.35 | 0.000 |
| Body weight gain (g) | |||||||
| 1st week | 50.67e ± 0.64 | 70.57b ± 1.18 | 65.37c ± 1.31 | 82.15a ± 0.92 | 63.93c ± 1.27 | 53.99d ± 0.82 | 0.000 |
| 2nd week | 127.73 ± 0.97 | 130.10 ± 0.83 | 128.48 ± 1.00 | 130.57 ± 0.82 | 128.45 ± 0.69 | 129.83 ± 0.91 | 0.121 |
| 3rd week | 183.78 ± 1.40 | 186.93 ± 1.21 | 184.67 ± 1.45 | 187.62 ± 1.19 | 184.57 ± 1.03 | 185.53 ± 1.31 | 0.126 |
| 4th week | 294.55 ± 2.26 | 300.67 ± 1.92 | 297.13 ± 2.33 | 301.98 ± 1.93 | 297.03 ± 1.64 | 298.47 ± 2.11 | 0.126 |
| 5th week | 318.40b ± 2.44 | 365.70a ± 2.34 | 361.35a ± 2.84 | 367.20 a±2.34 | 361.20a ± 2.00 | 363.00a ± 2.56 | 0.000 |
| 6th week | 382.05e ± 2.93 | 536.43b ± 3.43 | 401.50d ± 3.15 | 567.20a ± 3.64 | 437.47c ± 2.45 | 403.33d ± 2.85 | 0.000 |
| 0-6th week | 1390.18e ± 2.93 | 1590.4 ab±4.23 | 1438.5 d±3.43 | 1636.72 a±2.15 | 1472.65 bc±2.65 | 1434.15cd ± 2.74 | 0.000 |
| Feed Intake (g) | |||||||
| 1st week | 60.30 ab±1.64 | 78.33 ab±2.55 | 73.87 a±6.61 | 80.51 a±0.50 | 71.60 ab±8.11 | 62.33 b±1.75 | 0.007 |
| 2nd week | 185.21 ± 1.71 | 174.33 ± 2.55 | 176.02 ± 2.03 | 171.05 ± 2.12 | 170.84 ± 2.38 | 185.66 ± 3.99 | 0.076 |
| 3rd week | 308.75 ± 8.97 | 300.96 ± 7.92 | 301.01 ± 8.98 | 298.32 ± 6.80 | 302.69 ± 6.91 | 307.98 ± 5.32 | 0.827 |
| 4th week | 553.75 ± 10.24 | 526.17 ± 12.38 | 528.89 ± 12.67 | 525.45 ± 10.35 | 531.68 ± 10.35 | 537.25 ± 10.24 | 0.544 |
| 5th week | 627.25 ± 13.81 | 691.17 ± 30.11 | 693.79 ± 13.59 | 690.34 ± 5.92 | 682.67 ± 8.79 | 704.22 ± 13.81 | 0.996 |
| 6th week | 900.66 a±18.06 | 1051.40 c±2.55 | 815.05 b±2.34 | 1100.37 c±0.50 | 870.57 b±11.29 | 794.83ab ± 1.76 | 0.000 |
| 0–6th week | 2635.92 a±27.47 | 2822.14 bc±30.73 | 2588.64 abc±17.65 | 2866.36 c±8.93 | 2630.15 ab±14.06 | 2590.25 a±22.54 | 0.001 |
| Feed Conversion Ratio | |||||||
| 1st week | 1.19 ± 0.06 | 1.11 ± 0.05 | 1.13 ± 0.04 | 0.98 ± 0.00 | 1.12 ± 0.16 | 1.13 ± 0.04 | 0.379 |
| 2nd week | 1.45a ± 0.03 | 1.34bc ± 0.01 | 1.37 bc±0.03 | 1.31b ± 0.01 | 1.33b ± 0.04 | 1.43a ± 0.03 | 0.005 |
| 3rd week | 1.68 ± 0.04 | 1.61 ± 0.02 | 1.63 ± 0.03 | 1.59 ± 0.02 | 1.64 ± 0.01 | 1.66 ± 0.04 | 0.566 |
| 4th week | 1.88a ± 0.02 | 1.75c ± 0.01 | 1.78b ± 0.02 | 1.74c ± 0.02 | 1.79b ± 0.02 | 1.80b ± 0.03 | 0.000 |
| 5th week | 1.97 ± 0.03 | 1.89 ± 0.03 | 1.92 ± 0.01 | 1.88 ± 0.01 | 1.89 ± 0.01 | 1.94 ± 0.04 | 0.159 |
| 6th week | 2.17a ± 0.03 | 1.96ab ± 0.02 | 2.03ab ± 0.02 | 1.94b ± 0.01 | 1.99ab ± 0.02 | 1.97ab ± 0.04 | 0.000 |
| 0-6th week | 1.90a ± 0.01 | 1.77b ± 0.03 | 1.80ab ± 0.05 | 1.75b ± 0.04 | 1.79ab ± 0.02 | 1.81a ± 0.04 | 0.000 |
Means bearing different superscript (a, b, c, d) in rows differ significantly (p < 0.001). T1 = BD + 0.2%(v/w) MRS Broth/ uninoculated media, T2 = BD + CTC@335 mg/kg (w/v), T3 = BD + Probiotic; T4 = BD + Postbiotics @ 0.2% (v/w); T5 = BD + Postbiotics @ 0.4% (v/w); T6 = BD + Postbiotics @ 0.6% (v/w). MRS- de-Mann Rogosa Sharpe (MRS) broth; CTC- Chlortetracycline.
Immune response
From results its evident that dietary supplementation of postbiotics had significantly improved the immune status of the birds (Table 3). Treatment T4 had the highest (P ≤ 0.01) index of humoral and cell-mediated immunity. The results of the study showed that postbiotic inclusion not had a significant impact on the weight of immune related organs. But, T6 manifested quantitatively higher thymus (0.58 g), bursa (0.43 g) & spleen (0.18 g) weight.
Table 3.
Effect of postbiotic on immune response & immune organs weight (%) in broiler chickens.
| T1 | T2 | T3 | T4 | T5 | T6 | P-Value | |
|---|---|---|---|---|---|---|---|
| HI (log2) | 2.71b ± 0.11 | 3.01b ± 0.13 | 2.76b ± 0.20 | 3.36b ± 0.21 | 3.11b ± 0.15 | 4.01a ± 0.23 | 0.001 |
| CMI (mm) | 0.63c ± 0.05 | 0.78 ab±0.0.04 | 0.72b ± 0.08 | 0.80a ± 0.03 | 0.73 b ±0.12 | 0.66c ± 0.04 | 0.001 |
| Spleen Weight | 0.13 ± 0.01 | 0.17 ± 0.02 | 0.17 ± 0.03 | 0.15 ± 0.02 | 0.13 ± 0.01 | 0.18 ± 0.02 | 0.446 |
| Bursa weight | 0.39 ± 0.02 | 0.29 ± 0.04 | 0.32 ± 0.05 | 0.41 ± 0.02 | 0.34 ± 0.02 | 0.38 ± 0.01 | 0.521 |
| Thymus Weight | 0.49 ± 0.04 | 0.42 ± 0.09 | 0.29 ± 0.05 | 0.33 ± 0.04 | 0.49 ± 0.09 | 0.55 ± 0.05 | 0.048 |
Means bearing different superscript (a, b, c, d) in rows differ significantly (p < 0.001); T1 = BD + 0.2%(v/w) MRS Broth/ uninoculated media, T2 = BD + CTC@335 mg/kg (w/v), T3 = BD + Probiotic; T4 = BD + Postbiotics @ 0.2% (v/w); T5 = BD + Postbiotics @ 0.4% (v/w); T6 = BD + Postbiotics @ 0.6% (v/w). MRS- de-Mann Rogosa Sharpe (MRS) broth; CTC- Chlortetracycline.
Serum assays
The sequels of various dietary treatment on biochemical serum profiling in broiler chicken on 42nd day of study were portrayed in the Table 4. A significantly noteworthy variation (P < 0.001) was observed in the protein profile attributes, encompassing total protein, albumin, and globulin (g/dl) values, across various treatment groups. Interestingly, the results of the A/G estimate remained consistent among all treatment groups. In terms of plasma lipid profile (mg/dl), significantly (P < 0.001) lowered cholesterol values were recorded in the T4 group, while plasma triglycerides showed no significant (P > 0.05) alterations among the treatment groups. The kidney profile (mg/dl) parameters such as uric acid and creatine did not exhibit any significant (P > 0.05) differences among the treatment groups in this investigation. The impact of postbiotics on the plasma mineral profile of broilers, specifically calcium (mg/dl), phosphorus (mg/dl), sodium (mmol/l), potassium (mmol/l), and chloride (mmol/l), was examined and found significant (P < 0.001) disparity only in plasma potassium, phosphorus and calcium but chloride & sodium concentration of broilers among different experimental groups were comparable.
Table 4.
Effect of postbiotic on serum attributes in broiler chickens.
| Serum parameters | T1 (BD + MRS) | T2 (CTC) | T3 Probiotic | T4 (0.2% Postbiotic) | T5 (0.4% Postbiotic) | T6 (0.6% Postbiotic) | P Value |
|---|---|---|---|---|---|---|---|
| Serum protein profile | |||||||
| Albumin (g/dl) | 0.87c ± 0.06 | 1.75bc ± 0.17 | 2.24ab ± 0.16 | 2.9a ± 0.21 | 2.23ab ± 0.35 | 2.29ab ± 0.43 | 0.000 |
| Total Protein (g/dl) | 4.58ab ± 0.11 | 4.28b ± 0.20 | 4.44ab ± 0.10 | 5.26a ± 0.11 | 5.31a ± 0.42 | 4.61ab ± 0.20 | 0.005 |
| Globulin (g/dl) | 1.67b ± 0.22 | 2.53ab ± 0.34 | 2.33ab ± 0.46 | 3.03a ± 0.20 | 3.08a ± 0.40 | 3.57a ± 0.13 | 0.002 |
| A/G ratio | 2.20 ± 0.40 | 0.90 ± 0.16 | 0.25 ± 0.02 | 0.80 ± 0.10 | 1.38 ± 0.74 | 6.69 ± 3.79 | 0.063 |
| Serum lipid profile | |||||||
| Cholesterol (mg/dl) | 182.74a ± 1.52 | 151.92bc ± 4.13 | 174.90ab ± 2.49 | 122.82d ± 3.89 | 124.39d ± 2.99 | 128.37cd ± 5.22 | 0.000 |
| Triglycerides (mg/dl) | 42.30 ± 2.78 | 34.83 ± 3.93 | 39.98 ± 3.73 | 52.88 ± 1.32 | 38.77 ± 2.29 | 79.86 ± 2.31 | 0.855 |
| Serum kidney profile | |||||||
| Uric acid (mg/dl) | 7.29 ± 0.34 | 7.47 ± 0.34 | 6.93 ± 0.33 | 6.91 ± 0.33 | 7.88 ± 0.41 | 7.57 ± 0.45 | 0.050 |
| Creatine (mg/dl) | 0.27 ± 0.01 | 0.26 ± 0.01 | 0.26 ± 0.01 | 0.27 ± 0.01 | 0.26 ± 0.01 | 0.25 ± 0.01 | 0.403 |
| Serum liver enzymes profile | |||||||
| SGOT (mg/dl) | 115.51 ± 1.06 | 101.85 ± 1.72 | 95.85 ± 2.62 | 91.43 ± 3.10 | 93.69 ± 2.63 | 99.23 ± 1.95 | 0.754 |
| SGPT (mg/dl) | 6.49 ± 0.51 | 6.36 ± 0.22 | 5.98 ± 0.70 | 5.88 ± 0.41 | 6.74 ± 0.60 | 5.36 ± 0.45 | 0.352 |
| Serum Mineral profile | |||||||
| Phosphorus (mg/dl) | 4.17c ± 0.25 | 5.71a ± 0.55 | 5.64a ± 0.42 | 5.50a ± 2.40 | 4.95b ± 0.18 | 4.59b ± 1.75 | 0.006 |
| Chloride (mg/dl) | 101.62 ± 15.25 | 111.74 ± 7.81 | 112.09 ± 8.57 | 116.10 ± 17.58 | 121.15 ± 5.65 | 114.24 ± 13.72 | 0.921 |
| Potassium (mg/dl) | 5.95abc ± 0.88 | 4.06bc ± 1.05 | 3.80c ± 0.24 | 7.06a ± 0.29 | 7.35a ± 0.59 | 6.83ab ± 0.54 | 0.001 |
| Sodium (mg/dl) | 144.80 ± 10.12 | 171.19 ± 13.10 | 155.20 ± 23.29 | 153.53 ± 23.86 | 163.26 ± 7.81 | 173.42 ± 11.24 | 0.799 |
| Calcium (mg/dl) | 8.79b ± 0.30 | 10.91a ± 0.41 | 8.85b ± 0.54 | 10.60a ± 1.04 | 8.49b ± 0.28 | 9.28ab ± 1.51 | 0.004 |
Means bearing different superscript (a, b, c, d) in rows differ significantly (p < 0.001). T1 = BD + 0.2%(v/w) MRS Broth/ uninoculated media, T2 = BD + CTC@335 mg/kg (w/v), T3 = BD + Probiotic; T4 = BD + Postbiotics @ 0.2% (v/w); T5 = BD + Postbiotics @ 0.4% (v/w); T6 = BD + Postbiotics @ 0.6% (v/w). MRS- de-Mann Rogosa Sharpe (MRS) broth; CTC- Chlortetracycline.
Enumeration of caecal microbes
In connection with gut health, caecal microbial count was performed to understand better about interaction of postbiotics in proliferation of gut friendly bacteria, the results of the study were précised in the Table 5. Investigation of microbes such as coliform counts, Lactic Acid Bacteria and total plate count, (log10 cfu/g) in the caecal contents of birds carried out at 21st and 42nd day of age. Concerning with coliform counts, significantly lower coliform numbers were noticed with postbiotic supplementation on 21st day whereas the results were comparable between the treatment groups on 42nd day of age. The study also found that, Lactobacillus counts were significantly higher in T4, T5 & T6 groups not in control and antibiotic groups on both 21st and 42nd days of age. Similarly, total plate count results showed that postbiotic supplementation at all levels significantly (P < 0.001) subsided the bacterial growth when compared to control and other treatment groups on 21st day, despite no significance (P > 0.001) was observed on 42nd day of age.
Table 5.
Effect of postbiotic on gut health and gut integrity in broiler chickens.
| Gut health attributes | T1 (BD + MRS) | T2 (CTC) | T3 Probiotic | T4 (0.2% Postbiotic) | T5 (0.4% Postbiotic) | T6 (0.6% Postbiotic) | P Value |
|---|---|---|---|---|---|---|---|
| Microbial Count (21d) | |||||||
| Coliform Count (× 107cfu/g) | 9.43a ± 0.02 | 8.16b ± 0.30 | 8.64b ± 0.11 | 7.57bc ± 0.18 | 7.13c ± 0.14 | 7.40c ± 0.14 | 0.001 |
| Lactobacillus Count (× 107cfu/g) | 10.54b ± 0.12 | 11.33a ± 0.34 | 10.25bc ± 0.19 | 9.50c ± 0.39 | 9.39c ± 0.12 | 9.69c ± 0.03 | 0.001 |
| Total Plate count (× 107cfu/g) | 7.94b ± 0.09 | 8.64b ± 0.18 | 8.61b ± 0.42 | 10.03a ± 0.05 | 9.95a ± 0.11 | 9.76a ± 0.07 | 0.012 |
| Microbial Count (42 d) | |||||||
| Coliform Count (× 107cfu/g) | 8.57 ± 0.26 | 8.14 ± 0.15 | 8.04 ± 0.22 | 8.18 ± 0.28 | 7.81 ± 0.30 | 7.83 ± 0.38 | 0.726 |
| Lactobacillus Count (× 107cfu/g) | 10.33 ± 0.32 | 9.93 ± 0.27 | 10.05 ± 0.18 | 9.27 ± 0.32 | 8.64 ± 0.16 | 9.58 ± 0.31 | 0.078 |
| Total Plate count (× 107cfu/g) | 8.38ab ± 0.10 | 8.33ab ± 0.26 | 9.11ab ± 0.50 | 9.25a ± 0.48 | 9.36a ± 0.24 | 9.52a ± 0.15 | 0.014 |
| Jejunal Histomorphometry – 21 days of age | |||||||
| Villus height (µm) | 915.03b ± 14.59 | 1060.51a ± 14.65 | 867.72b ± 31.19 | 1149.38a ± 10.08 | 1076.90a ± 42.28 | 921.51b ± 10.56 | 0.000 |
| Villus width (µm) | 102.36c ± 4.70 | 150.65ab ± 15.25 | 117.39bc ± 16.59 | 180.05a ± 9.32 | 152.29ab ± 9.29 | 115.72bc ± 4.55 | 0.000 |
| Crypt depth (µm) | 86.47bc ± 7.14 | 116.50ab ± 5.06 | 73.01c ± 14.83 | 149.37a ± 7.65 | 100.37bc ± 4.59 | 77.67c ± 1.72 | 0.000 |
| Crypt width (µm) | 29.07b ± 1.85 | 47.41a ± 5.67 | 30.49c ± 1.68 | 37.82ab ± 2.24 | 42.70ab ± 6.77 | 33.54ab ± 2.33 | 0.015 |
| VH: CD | 8.98ab ± 0.32 | 8.61ab ± 0.19 | 8.73ab ± 0.24 | 7.69b ± 0.36 | 9.91a ± 0.30 | 9.89a ± 0.78 | 0.005 |
| Jejunal Histomorphometry – 42 days of age | |||||||
| Villus height (µm) | 1098.04b ± 1.51 | 1272.61a ± 3.25 | 1041.26b ± 3.43 | 1379.25a ± 2.09 | 1313.82a ± 1.58 | 1124.25b ± 1.89 | 0.000 |
| Villus width (µm) | 122.84c ± 1.64 | 180.77ab ± 1.30 | 140.87bc ± 4.91 | 216.06a ± 1.18 | 185.79ab ± 1.34 | 141.17bc ± 2.56 | 0.000 |
| Crypt depth (µm) | 103.77bc ± 2.57 | 139.80ab ± 1.08 | 87.61c ± 1.79 | 179.25a ± 3.18 | 122.45bc ± 2.60 | 94.75c ± 2.10 | 0.000 |
| Crypt width (µm) | 34.88b ± 2.22 | 56.89a ± 1.81 | 36.58ab ± 2.02 | 45.38ab ± 2.69 | 52.09ab ± 3.26 | 40.92ab ± 2.85 | 0.015 |
| VH: CD | 9.13ab ± 0.33 | 8.76ab ± 0.20 | 8.88ab ± 0.25 | 7.82b ± 0.37 | 10.24a ± 0.31 | 10.23a ± 0.81 | 0.002 |
| Jejunal Antioxidant activity (21d) | 0.87c ± 0.03 | 0.97ab ± 0.03 | 0.91bc ± 0.01 | 1.00a ± 0.00 | 0.91bc 0.03 | 0.96ab ± 0.00 | 0.000 |
| Jejunal Antioxidant activity (42 d) | 0.89c ± 0.02 | 1.02b ± 0.01 | 1.04a ± 0.00 | 1.05a ± 0.00 | 1.05a ± 0.00 | 1.03ab ± 0.00 | 0.000 |
Means bearing different superscript (a, b, c, d) in rows differ significantly (p < 0.001); cfu- Colony Forming Units; T1 = BD + 0.2%(v/w) MRS Broth/ uninoculated media, T2 = BD + CTC@335 mg/kg (w/v), T3 = BD + Probiotic; T4 = BD + Postbiotics @ 0.2% (v/w); T5 = BD + Postbiotics @ 0.4% (v/w); T6 = BD + Postbiotics @ 0.6% (v/w). MRS- de-Mann Rogosa Sharpe (MRS) broth; CTC- Chlortetracycline.
Jejunum histo-morphometry
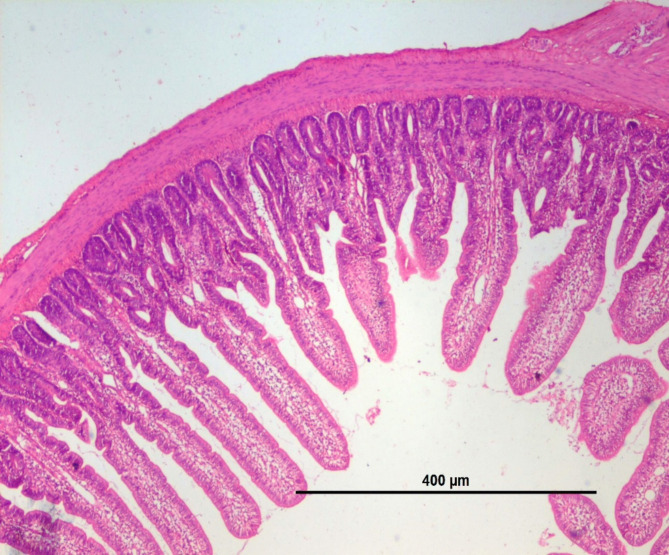
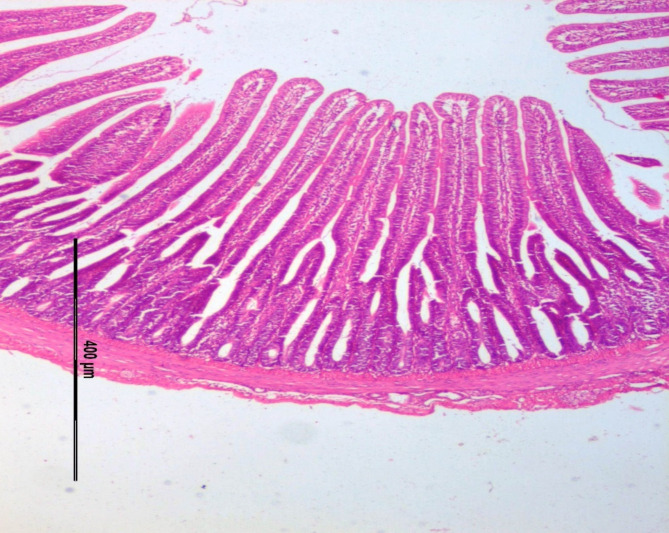
The effect of different dietary supplementation on the jejunal histomorphology attributes such as jejunum villi length, width (µm) and crypt depth, width (µm) in broilers was measured at different periods (21st & 42nd days of age) is presented in the Table 5. The villi height, width & crept depth showed significant (P < 0.001) improvement among the treatment groups on day 21st as well as 42nd of the experimental trial with highest value in T4 group with 0.2% postbiotic supplementation. Crept depth also exhibited significantly (P < 0.05) higher among the treatment groups on day 21st of the experimental trial. The villi height and crypt depth ratio showed significant (P < 0.05) improvement among the treatment groups on day 21st as well as 42nd the experimental trial with highest value in T5 group with 0.4% postbiotic supplementation. The representative histological sections of jejunum at 21 and 42 days of age in broiler chicken were depicted in Figs. 1, 2, 3 and 4 respectively.
Fig. 1.
BASAL DIET + MRS (21 day).
Fig. 2.
BASAL DIET + Postbiotics (21 day).
Fig. 3.
BASAL DIET + MRS (42 day).
Fig. 4.
BASAL DIET + Postbiotics (42 day).
Jejunal antioxidant
The effect of postbiotic supplementation on jejunal antioxidant concentration (Trolox, mM) in broiler chicken on 21st and 42nd days of age is presented in Table 5. The total antioxidant concentration (mM) significantly varied (P < 0.001) among the treatment diets at 21st and 42nd days of the experimental period. The postbiotic supplemented groups at day 21st and 42nd shown significantly highest values then other groups.
Carcass characteristics
The postbiotic supplements had no effect (p > 0.05) on the slaughter weights (Table 6). No trend of significance (p > 0.05) was observed in the slaughter weights and cut off parts between the treatment groups in the study.
Table 6.
Effect of postbiotics on carcass characteristics (%) in broiler chickens.
| Carcass characteristics | T1 (BD + MRS) | T2 (CTC) | T3 Probiotic | T4 (0.2% Postbiotic) | T5 (0.4% Postbiotic) | T6 (0.6% Postbiotic) | P Value |
|---|---|---|---|---|---|---|---|
| Post Slaughter Weight | 98.33 ± 0.97 | 97.32 ± 0.77 | 97.91 ± 0.53 | 98.69 ± 0.78 | 97.23 ± 0.48 | 96.05 ± 1.45 | 0.209 |
| Defeathered Weight | 80.33 ± 1.39 | 79.21 ± 1.47 | 79.17 ± 1.10 | 80.08 ± 0.48 | 79.64 ± 1.11 | 81.82 ± 0.70 | 0.555 |
| Dressed weight | 71.86 ± 1.75 | 73.60 ± 1.34 | 72.13 ± 1.26 | 74.01 ± 1.38 | 73.51 ± 1.63 | 72.56 ± 1.32 | 0.297 |
| Breast weight | 15.75 ± 0.24 | 14.71 ± 0.45 | 14.81 ± 0.44 | 15.04 ± 0.44 | 15.10 ± 0.48 | 15.56 ± 0.43 | 0.370 |
| Thigh weight | 9.99 ± 0.20 | 10.16 ± 0.31 | 10.08 ± 0.21 | 9.47 ± 0.15 | 10.30 ± 0.39 | 10.70 ± 0.27 | 0.224 |
| Drumstick weight | 9.96 ± 0.10 | 9.58 ± 0.22 | 9.25 ± 0.21 | 9.29 ± 0.16 | 9.81 ± 0.16 | 9.78 ± 0.15 | 0.050 |
| Back weight | 13.29 ± 0.25 | 14.27 ± 0.57 | 14.66 ± 0.52 | 14.59 ± 0.65 | 14.59 ± 0.28 | 15.19 ± 0.45 | 0.082 |
| Wing weight | 7.02 ± 0.14 | 6.69 ± 0.12 | 6.59 ± 0.14 | 6.50 ± 0.17 | 6.98 ± 0.16 | 7.12 ± 0.17 | 0.019 |
| Neck weight | 2.87 ± 0.14 | 3.26 ± 0.19 | 2.90 ± 0.21 | 3.04 ± 0.12 | 2.48 ± 0.14 | 2.73 ± 0.18 | 0.105 |
Means bearing different superscript (a, b, c, d) in rows differ significantly (p < 0.001). T1 = BD + 0.2%(v/w) MRS Broth/ uninoculated media, T2 = BD + CTC@335 mg/kg (w/v), T3 = BD + Probiotic; T4 = BD + Postbiotics @ 0.2% (v/w); T5 = BD + Postbiotics @ 0.4% (v/w); T6 = BD + Postbiotics @ 0.6% (v/w). MRS- de-Mann Rogosa Sharpe (MRS) broth; CTC- Chlortetracycline.
Discussion
Zootechnical performances
From the results of the study its evident that performance of the postbiotic supplementation treatments was comparable to the antibiotic-fed chickens and, in some cases, even better. This could be due to myriad reasons: Antibacterial (bacteriostatic and bactericidal) properties of postbiotics which combat pathogenic bacteria and inhibit toxin production in the gut12. Consequently, postbiotics mimic antibiotics by reducing subclinical infections, promoting nutrient absorption through intestinal wall thinning thereby enhancing growth performance of birds. Moreover, postbiotics have metabolites such as short-chain fatty acids, microbial cell fragments, extracellular polysaccharides, cell lysates, teichoic acid, vitamins, etc. facilitating improved nutrient absorption by the villi of jejunum in the gut. The pH range of Postbiotics of Lactobacillus acidophilus is in acidic range (4 to 5), observed in the in vitro study12, which range is required for adequate protein digestion and general reduction in bacteria community in the different segments of the gut. Acidic pH used to reduce digesta viscosity in the duodenum and jejunum which is an essential part of the gut where digestion and most absorption of nutrients take place. The combination of acidic pH and decreased pathogens in the gut, facilities nutrient uptake much greater29. Improvement in the absorption and digestion of the nutrients results in increased weight gain which eventually leads to a better feed conversion ratio30.
Remarkably, postbiotics offer a safer alternative to probiotics, owing to their composition rich in functional fermentation compounds such as short-chain fatty acids, microbial fractions, functional proteins, secreted polysaccharides, extracellular polysaccharides, cell lysates, teichoic acid, and peptidoglycan-derived muropeptides31,32. Furthermore, their capacity to synergize with other compounds presents an opportunity to enhance animal health status33. However, no significant differences among treatment groups regarding final body weight (FBW), cumulative weight gain (CWG), and feed conversion ratio (FCR)34. This finding contrasts with previous research35 indicating higher FCR associated with postbiotic RI11. The disparity in results could potentially be attributed to the controlled environment of the closed-house system32 which differs from the open-house system33. Further the supplementation of postbiotics improves villi height & as we know villi as well as crypts microarchitecture are linked with the gut function. Improvement gut mucosal villi is directly proportional to expand the absorptions activity of the nutrients in the GIT leading to the animal growth15. All above deliberation supports our study, that the postbiotics obtained from Lactobacillus acidophilus at 0.2% were effective at promoting the growth performance of broilers compared with the antibiotic group.
Immune traits
The immune response of the birds in relation with the dietary treatments were computed in terms of humoral, cell mediated response and immune organ weight. Significant (p < 0.001) results observed in immune response attributes in the present study. Our results were in harmony with findings of36,who evinced that postbiotic supplementation in broilers under normal condition, improves growth performance and health by promoting the immune status. Metabolic compounds produced by probiotic bacteria demonstrated significant efficacy in ameliorating disease symptoms, enhancing growth performance, bolstering immune responses, improving bursa to body weight ratio, and reducing coliform counts in the intestines of challenged chickens37. He also defined that, symbiotic relationship between the immune system and the microbiota is vital for the proper development and functioning of immunity. This could be due to bioactive compounds, often of small molecular size, are derived from nutritional and environmental sources or are endogenously produced and regulated by the host and its microbiota38. explains that a complex interplay exists between the intestinal mucosal immune system and the microbiota, facilitated by the secretion and signalling of metabolites. This intricate cross-talk profoundly influences host immunity and physiology, impacting endocrine, metabolic, and nervous system functions in both health and disease states38. Disturbances in microbiome-associated metabolite levels and activity are implicated in the pathogenesis of an increasing array of illnesses. Our result was corroborated with other research finding39, where feeding a mixture of postbiotics and inulin had a positive effect on the humoral immune response in broiler chickens.
Serum assays
The impact of postbiotics inclusion in the broiler diet on various biochemical serum profiling such as protein, lipid, kidney, liver and mineral profiles were determined using Coral clinical kits. Significantly higher values observed in the protein profile shows that postbiotics from Lactobacillus acidophilus, whose pH is acidic12 could aid in protein digestion, which was reflected via increased serum protein levels. Cholesterol values in the present study were decreased significantly when compared to the other treatment groups and triglyceride values were remained unaffected. It was identified that feeding broilers with postbiotics procured from Lactobacillus plantarum resulted in lower (p < 0.05) total cholesterol as compared with broilers fed Negative Control, Positive Control or ascorbic acid added diets36. Postbiotics used in this study could increase the population of lactic acid bacteria, production of enzymes disintegrating bile salts and de-conjugating them in the gut, as well as reduction of the gut pH that can be efficient in decreasing the cholesterol of blood by reducing non-conjugate bile acids solvability at low pH, leading to less absorption from the intestine and more excretion in the faeces36.
Monitoring of liver enzymes such as SGOT and SGPT are essential for evaluating the function and viability of the liver. No significant (p > 0.05) differences were observed in the study which states that postbiotic supplementation influenced the liver profile of the broilers. Increase in values of SGOT and SGPT reflects the hepatic damage. SGOT is well distributed in several organs such as skeletal muscles, the heart, liver, whereas primary source of SGPT is mainly liver. Decreased levels of these enzymes may be expressed less liver and skeletal muscle damage40. The change in kidney profile due to postbiotic supplementation from Lactobacillus acidophilus was estimated by measuring the serum uric acid and creatine level in the current study. Results of the study stated that no significant (p > 0.05) differences were observed between the treatment groups in the kidney profile. Therefore, relatively low-level creatinine may be an indication of the renal protective effects of the probiotic’s bacteria.
Poultry interactions with the external environment may lead to disease burden but micronutrients ensure the preservation of homeostasis with a key role in the response process. Electrolytes can be defined as chemicals that break down into their ionic constituents, having as their main physiological function the maintenance of the body acid-base balance. Sodium (Na+), potassium (K+) and chloride (Cl-) are essential ions for the maintenance of osmotic pressure and acid-base balance of body fluids. The current study showed significant (P < 0.001) variations in plasma mineral levels where, T4 (0.2% postbiotics) birds exhibited higher values in potassium, phosphorus and calcium than other treatment birds. The optimum relationship between sodium (Na), potassium (K), and chloride (Cl) has been described by dietary electrolyte balance (DEB) as being essential for proper acid-base balance41. The elevated plasma potassium and phosphorus levels in postbiotic fed groups could be due to increased intestinal absorption of potassium and phosphorus levels caused by the metabolites like short-chain fatty acids, that cause reduction in the gut pH. Minerals estimation of postbiotics in the in vitro study results shows that when these mineral enriched postbiotics fed to the birds can influence the mineral status of the host directly or through gut microbiota which can increases the mineral bioavailability12. Postbiotics–Fermented products of probiotics/metabolites incorporation helps in natural assimilation of minerals by microbes’ biosynthesis, uptake, absorption, and bioavailability of micronutrients while the process is eco-friendly and cost-effective42.
Microbial count of caecal digesta
In the current study, gut health was analysed by estimating the caecal microbes such as coliform count, Lactobacillus count and total plant count. The outcome evinces that postbiotic supplementation at all the inclusion levels had a significant (p < 0.01) influence on the microbiota presence of broiler chickens on both 21st and 42nd days of age. Probiotic bacteria exerts certain mechanism in the intestine called competitive exclusion43. Competitive exclusion is the competition of attachment sites and nutrients of different species of bacteria in the same niche. Metabolites like organic acids, bacteriocins, hydrogen peroxide, and vitamins, mannans help diminish pathogen colonisation in an animal’s intestinal tract by adsorbing bacterial pathogens, such as Salmonella, E. coli, and Clostridia44. The metabolites/Postbiotics obtained from Lactobacillus acidophilus in our study not only expressed an inhibitory effect against intestinal pathogens but also reduced the gastrointestinal Enterobacteriaceae. These effects allowed LAB to increase its population in intestinal microflora via competitive exclusion.
Gut histomorphometry
Villi are important components involve in the absorption of nutrients in small intestine. The surface for absorption in the small intestine is enlarged enormously by folds and by villi45. The villi height, crypt depth and its ratios were measured in the current study on 21st and 42nd days of age (Figs. 1, 2, 3 and 4). The results presented that postbiotic inclusion at 0.2% Lactobacillus acidophilus significantly increased the villus height and crypt depth at different age of birds. This increased height of villi facilitates more nutrient absorption. This was in consistent with the findings of46 who observed that in absence of inflammatory reactions, the intestinal tissues undergo positive changes like increase in villus height and crypt depth, also lessens the muscularis thickness which will be beneficial in the absorption of nutrients.
Jejunal tissue antioxidant activity
The aim of measuring the jejunal antioxidant activity is to evaluate the potential of postbiotics to perform its antioxidant level. Oxidative stress leads to the production of varieties of ROS, including hydroxyl free radical and superoxide anions. Several studies showed that overflow of ROS could damage the biological macromolecules such as proteins and nucleic acids, and produce huge amounts of MDA causing tissue damage, consequently leading to the development of diseases46. The study results were significantly affected by postbiotics inclusion in the diet of broilers, 0.2% level portrayed higher antioxidant level at 21st day, whereas on 42nd day35. observed that postbiotics, apart from their ability to promote a healthy gut environment, the potential antioxidant capacity of postbiotics obtained from L. plantarum has been found to be particularly strong under heat-stress conditions. The high jejunal antioxidant level obtained in the metabolites added group suggests that postbiotics exhibited antioxidant effects due to an increase in the content of uronic acid which has an antioxidant effect as well as the chelating capability of ferrous ion which is involved in the formation of free radicals.
Carcass characteristics
In the present experiment, the percentages of carcass and viscera weights were assessed in relation to the live weight. Notably, the supplementation of postbiotics in the diet did not exert any discernible impact on the carcass yield at the 42nd day of age. This observation resonates with prior research findings35, who similarly reported no significant alteration in carcass yield in broiler chickens subjected to heat stress despite being fed diets supplemented with postbiotics. This outcome is consistent with the results elucidated by39, wherein the incorporation of a combination of postbiotics and inulin into the diet of broilers did not manifest any notable effect on carcass yield.
Conclusion
As the search for alternatives to antibiotic growth promoters intensifies, it is crucial to identify sustainable and practical options that prioritize environmental, avian, and human health within a holistic One Health framework. This study clearly demonstrates the potential of postbiotics as a powerful replacement for antibiotic growth promoters. Specifically, it highlights the effectiveness of 0.2% postbiotics derived from Lactobacillus acidophilus in improving production performance, enhancing immune response, and promoting superior gut morphology and health in broiler birds.
Acknowledgements
The authors would like to thank ICAR-CARI, Izatnagar, UP-243122 for providing the facilities for conducting the research.
Author contributions
(1) M.M. conducted the research as part of her Ph.D research work, diligently overseeing the trials, managing birds, and conducting laboratory tests, including microbiological and molecular work. She also authored the main manuscript text. (2) J.S.T. and J.J.R. were responsible for formulating the research hypothesis and designing the technical program for the trial. 4. A.B. contributed to ration formulation for the trial and methodology in feed additives supplementation. (3) N.S. provided guidance on bird management, assisted in data compilation, and conducted statistical analysis. 3. D.M. provided valuable inputs in laboratory analysis and also histomorphology reading (Figs. 1, 2, 3 and 4). 2. Sky assisted in conducting the trial and prepared Tables (1-6). 2. A.K.T. Conceptualization and financial assistance. All authors have reviewed the manuscript.
Data availability
The data provided in the manuscript is comprehensive and available upon request to the first author, Monika M .
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jagbir Singh Tyagi, Email: jbstyagi@gmail.com.
Jaydip Jaywant Rokade, Email: jaydeepvet@gmail.com.
References
- 1.Hudson, J. A. et al. The agri-food chain and antimicrobial resistance: A review. Trends Food Sci. Technol.69, 131–147. 10.1016/j.tifs.2017.09.007 (2017). [Google Scholar]
- 2.Saraiva, M. et al. Bastos Borges, K. Antimicrobial resistance in the globalized food chain: A one health perspective applied to the poultry industry. Braz J. Microbiol. 1–22. 10.1007/s42770-021-00635-8 (2022). [DOI] [PMC free article] [PubMed]
- 3.Lee, K. W. & Lillehoj, H. S. An update on direct-fed microbials in broiler chickens in post-antibiotic era. Anim. Prod. Sci.57(8), 1575–1581. 10.1071/an15666 (2016). [Google Scholar]
- 4.Founou, L. L., Founou, R. C. & Essack, S. Y. Antibiotic resistance in the food chain: A developing country-perspective. Front. Microbiol.7, 232834. 10.3389/fmicb.2016.01881 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodrigues, C. et al. High prevalence of Klebsiella pneumoniae in European food products: A multicentric study comparing culture and molecular detection methods. Microbiol. Spectr.10(1), 02376–02321. 10.1128/spectrum.02376-21 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng, Y. H. et al. Bacillus licheniformis-fermented products improve growth performance and intestinal gut morphology in broilers under Clostridium perfringens challenge. J. Poult. Sci.58, 30–39. 10.2141/jpsa.0200010 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khaneghah, A. M. et al. Interactions between probiotics and pathogenic microorganisms in hosts and foods: a review. Trends Food Sci. Technol.95, 205–218. 10.1016/j.tifs.2019.11.022 (2020). [Google Scholar]
- 8.Priyamvada, P., Debroy, R., Anbarasu, A. & Ramaiah, S. A comprehensive review on genomics, systems biology and structural biology approaches for combating antimicrobial resistance in ESKAPE pathogens: Computational tools and recent advancements. World J. Microbiol. Biotechnol.38(9), 153. 10.1007/s11274-022-03343-z (2022). [DOI] [PubMed] [Google Scholar]
- 9.Aguilar-Toalá, J. E. et al. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol.75, 105–114. 10.1016/j.tifs.2018.03.009 (2018). [Google Scholar]
- 10.Barros, C. P. et al. Paraprobiotics and postbiotics: concepts and potential applications in dairy products. Curr. Opin. Food Sci.32, 1–8. 10.1016/j.cofs.2019.12.003 (2017). [Google Scholar]
- 11.Kareem, K. Y., Loh, T. C., Foo, H. L., Asmara, S. A. & Akit, H. Influence of postbiotic RG14 and inulin combination on cecal microbiota, organic acid concentration, and cytokine expression in broiler chickens. Poult. Sci.96, 4: 966–975. 10.3382/ps/pew362 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Monika, M. et al. Characterization of lactic acid bacteria postbiotics & its in-vitro evaluation for antibacterial effect, anti-inflammatory and antioxidant properties. 57(3): 301–308 (2022). 10.5958/0974-8180.2022.00047.2
- 13.Siegal, F. P. et al. Opportunistic infections in acquired immune deficiency syndrome result from synergistic defects of both the natural and adaptive components of cellular immunity. J. Clin. Investig.78(1), 115–123 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corrier, D. E. & DeLoach, J. R. Evaluation of cell-mediated, cutaneous basophil hypersensitivity in young chickens by an interdigital skin test. Poult. Sci.69, 403–408. 10.3382/ps.0690403 (1990). [DOI] [PubMed] [Google Scholar]
- 15.Sikandar, A. et al. Growth performance, immune status and organ morphometry in broilers fed Bacillus subtilis-supplemented diet. S Afr. J. Anim.47(3), 378–388 (2017). https://hdl.handle.net/10520/EJC-7351aec91 [Google Scholar]
- 16.Mann, G. Chemistry of the Proteids. (Macmillan and Company Limited, 1906). 10.5962/bhl.title.32898
- 17.Doumas, B. T., Watson, W. A. & Biggs, H. G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta31, 87–96. 10.1016/0009-8981(71)90365-2 (1971). [DOI] [PubMed] [Google Scholar]
- 18.Allain, C. C., Poon, L. S., Chan, C. S. G., Richmond, W. & Fu, P. C. Enzymatic determination of total serum cholesterol. Clin. Chem.20, 470–475. 10.1093/clinchem/20.4.470 (1974). [PubMed] [Google Scholar]
- 19.Luley, C. et al. Point-of-care testing of triglycerides: evaluation of the accutrend triglycerides system. Clin. Chem.46(2), 287–291. 10.1093/clinchem/46.2.287 (2000). [PubMed] [Google Scholar]
- 20.Reitman, S. & Frankel, S. A. Colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol.28(1), 56–63. 10.1093/ajcp/28.1.56 (1957). [DOI] [PubMed] [Google Scholar]
- 21.Fossati, P., Prencipe, L. & Berti, G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin. Chem.26, 227–231. 10.1093/clinchem/26.2.227 (1980). [PubMed] [Google Scholar]
- 22.Junge, W. et al. Determination of reference intervals for serum creatinine, creatinine excretion and creatinine clearance with an enzymatic and a modified Jaffe method. Clin. Chim. Acta344, 137–148. 10.1016/j.cccn.2004.02.007 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Stern, J. & Lewis, W. H. The colorimetric estimation of calcium in serum with ocresolphthalein complexone. Clin. Chim. Acta2(6), 576. 10.1016/0009-8981(57)90063-3 (1957). 80. [DOI] [PubMed] [Google Scholar]
- 24.Amador, E. & Urban, J. Simplified serum phosphorus analyses by continuous-flow ultraviolet spectrophotometry. Clin. Chem.18, 601–604. 10.1093/clinchem/18.7.601 (1972). [PubMed] [Google Scholar]
- 25.Arnold, E. & Pray, A. Colorimetric method for determination of sodium. Ind. Eng. Chem. Anal. Ed.15, 294–296. 10.1021/i560116a029 (1943). [Google Scholar]
- 26.Hill, L. A. A colorimetric method for the determination of small quantities of potassium. J. Am. Chem. Soc.25, 990–992. 10.1021/ja02011a016 (1903). [Google Scholar]
- 27.Miles, A. A., Misra, S. S. & Irwin, J. O. The estimation of the bactericidal power of the blood. Epidemiol. Infect.38(6), 732–749. 10.1017/s002217240001158x (1938). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sikandar, A. et al. Effect of Bacillus subtilis on the microarchitectural development of the immune system in Salmonella-challenged broiler chickens. Vet. Med.-Czech. 67(1):28–37. 10.17221/231/2020-vetmed (2022). [DOI] [PMC free article] [PubMed]
- 29.Sikandar, A. Use of Bacillus subtilis probiotics as non-antibiotic gut modulator and growth promoter in broiler chickens. Adv. Stud. 21st Cent. Anim. Nutr.8, 59. 10.5772/intechopen.99400 (2021). [Google Scholar]
- 30.Żółkiewicz, J., Marzec, A., Ruszczyński, M. & Feleszko, W. Postbiotics—A step beyond pre-and probiotics. Nutrients12(8), 2189. 10.3390/nu12082189 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markowiak, P. & Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients9(9), 1021. 10.3390/nu9091021 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Grady, J., O’Connor, E. M. & Shanahan, F. Dietary fibre in the era of microbiome science. Aliment. Pharmacol. Ther.49(5), 506–515. 10.1111/apt.15129 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Wegh, C. A., Geerlings, S. Y., Knol, J., Roeselers, G. & Belzer, C. Postbiotics and their potential applications in early life nutrition and beyond. Int. J. Mol. Sci.20(19), 4673. 10.3390/ijms20194673 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danladi, Y., Loh, T. C., Foo, H. L., Sazili, A. Q. & Nasruddin, Y. Effects of postbiotics and paraprobiotics as replacements for antibiotics on growth performance, carcass characteristics, small intestine histomorphology, immune status and hepatic growth gene expression in broiler chickens. Animals12, 917. 10.3390/ani12070917 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humam, A. M. et al. Effects of feeding different postbiotics produced by Lactobacillus plantarum on growth performance, carcass yield, intestinal morphology, gut microbiota composition, immune status, and growth gene expression in broilers under heat stress. Animals9, 644. 10.3390/ani9090644 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Humam, A. M. et al. Supplementation of postbiotic RI11 improves antioxidant enzyme activity, upregulated gut barrier genes, and reduced cytokine, acute phase protein, and heat shock protein 70 gene expression levels in heat-stressed broilers. Poult. Sci.100, 100908. 10.1016/j.psj.2020.12.011 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abd El-Ghany, W. A. et al. Comparative efficacy of postbiotic, probiotic, and antibiotic against necrotic enteritis in broiler chickens. Poult. Sci.101, 101988. 10.1016/j.psj.2022.101988 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blacher, E., Levy, M., Tatirovsky, E. & Elinav, E. Microbiome-modulated metabolites at the interface of host immunity. J. Immunol.198, 572–580. 10.4049/jimmunol.1601247 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Kareem, K. Y., Loh, T. C., Foo, H. L., Akit, H. & Samsudin, A. A. Dietary supplementation of postbiotic and live lactic acid bacteria modulates the growth performance, serum lipid profile, immune response and caecal microflora in broilers. Benef. Microb. 7, 465–475. 10.3920/bm2015.0160 (2015). [Google Scholar]
- 40.Agbafor, K. N. & Ezeali, C. Effects of leaf and root extracts of Newbouldia Laevis on hepatic and renal systems in albino rats. J. Pharm. Chem. Biol. Sci.3, 367–372. 10.9734/ejmp/2015/17673 (2015). [Google Scholar]
- 41.Mongin, P. Recent advances in dietary anion-cation balance: Applications in poultry. Proc. Nutr. Soc.40(3), 285–294. 10.1079/pns19810045 (1981). [DOI] [PubMed] [Google Scholar]
- 42.Dinu, L. D., Avram, I., Pelinescu, D. R. & Vamanu, E. Mineral-Enriched Postbiotics: a New Perspective for Microbial Therapy to prevent and treat Gut Dysbiosis. Biomedicines10, 2392. 10.3390/biomedicines10102392 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuller, R. Probiotics in man and animals. J. Appl. Bacteriol.66, 365–378. 10.3390/biomedicines10102392 (1989). [PubMed] [Google Scholar]
- 44.Thorakkattu, P., Khanashyam, A. C., Shah, K., Babu, K. S., Mundanat, A. S., Deliephan, A., & Nirmal, N. P. Postbiotics: current trends in food and pharmaceutical industry. Foods11(19), 3094. 10.3390/foods11193094 (2022). [DOI] [PMC free article] [PubMed]
- 45.Fuller, M. F. (ed) The Encyclopedia of farm Animal Nutrition (CABI Publishing, 2004).
- 46.Salvi, S. P., Berde, C. V., Kajarekar, K. V., Joshi, S. A. & Berde, V. B. Insight into the animal models for microbiome studies (chap. 13). (Springer, 2021). 10.1007/978-981-16-3156-6_13
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data provided in the manuscript is comprehensive and available upon request to the first author, Monika M .