Abstract
Folates play a crucial role as cofactors in metabolic pathways, influencing biological methylation and nucleotide synthesis, which has a significant impact on overall health and disease susceptibility. Diabetic nephropathy (DN) is a prevalent and severe complication of diabetes mellitus (DM). The correlation between RBC folate and DN remains unclear currently. This study aims to assess whether RBC folate is associated with DN. Based on data from the NHANES (2011–2018), we conducted a cross-sectional study involving 3070 adults with type 2 DM (T2DM). Demographic factors, levels of folate and vitamin B12, dietary folate intakes, and relevant laboratory data were obtained from all participants. Logistic regression, fitting smooth curves, interaction effects were utilized to support the research objectives. Regression analyses demonstrated a positive relation between RBC folate and DN. (P < 0.001). A positive association between levels of RBC folate and the risk of DN was observed after full adjustment for all the confounding variables (odds ratio: 1.38, 95% confidence interval: 1.27–1.49, P < 0.001). Similar patterns of association were observed for subgroup analysis (all P values for interaction > 0.05). In addition, curve fitting after adjusting for all the confounding variables demonstrated that there was a linear relationship between RBC folate and DN (P for non-linearity = 0.147). Increased RBC folate levels were linked to a higher risk of DN in type 2 diabetes. RBC folate should be considered as a crucial indicator for folate status in DN.
Keywords: Diabetic nephropathy, Type2 diabetes mellitus, RBC folate, Serum folate, NHANES
Subject terms: Biomarkers, Diseases, Endocrinology
Introduction
The incidence of DM has indeed risen significantly in recent years, coinciding with improvements in living standards and alterations in lifestyle factors, such as dietary choices and physical activity. 1 In recent years, DM has become one of the most common chronic illnesses, leading to significant challenges for both patients and the healthcare system, especially for those suffering from diabetes-related complications like DN. 2–4 DN is a common and serious microvascular complication of DM. Prolonged exposure to high levels of glucose in the blood can indeed cause damage to the small blood vessels in the kidneys, leading to kidney disease, which is a significant cause of end-stage renal disease (ESRD) and can result in the need for dialysis or kidney transplantation. 5 DN can potentially reduce the lifespan of individuals with diabetes, and early identification of DN risks provides an opportunity to delay or stop disease onset. 6 Given the lack of effective pharmacological treatments, it is crucial to explore indicators for preventing the occurrence of DN. Because folate plays a central role in the one-carbon metabolism pathway, deficiencies and insufficiencies of it are associated with a wide range of negative health outcomes, from birth defects to cancer. 7 However, the evidence regarding the adverse effects of high folate level is inconsistent. Many reports suggested that elevated folate levels in the blood were primarily attributed to increased folate intake, without taking into account the interactions of homeostasis, which are mediated by use and excretion of the body. The kidneys are responsible for two crucial roles in folate metabolism. One is the excretion of metabolites, such as folates used as reducing agents and folic acid, as well as waste products, from the bloodstream into the urine. The other is the reabsorbing folates to save and return them to the blood for further use, ensuring that the body maintains an adequate level of folate. RBC folate is a blood biomarker that provides insight into human physiology and health status. 8 Measurement of RBC folate serves as a sensitive gauge of the body’s homeostatic control mechanisms, as it is a dynamic parameter that fluctuates in response to internal and external stimuli.9,10 Based on the current evidence, we postulate that changes in RBC folate concentrations in participants with T2DM are influenced by deteriorating kidney function, and higher concentrations of RBC folate may serve as a predictive factor for the early decline in kidney function in patients with T2DM, indicating a closer link between RBC folate levels and the development of diabetic nephropathy. However, there are limited and conflicting reports, and no substantial evidence from large population studies regarding the association between RBC folate and the risk of DN. In this study, we aim to explore the potential association between RBC folate and risk of DN in 3070 participants with T2DM using data from the National Health and Nutrition Examination Survey (NHANES) spanning from 2011 to 2018.
Materials and methods.
Participants enrollment.
The NHANES is a cross-sectional and nationwide study aimed at assessing the health and nutrition status of adults and children in the US. It has been periodically conducted since the 1960s. The study population is recruited through a complex, multistage, stratified sampling design, making it nationally representative. (Source: https://www.cdc.gov/nchs/nhanes/about_nhanes.Htm) The data we studied came from four NHANES cycles (2011–2012, 2013–2014, 2015–2016, and 2017–2018) because the data on RBC folate and DN in DM patients was available during these periods. Initially, the study included a total of 39,156 participants. Participants who aged younger than 20 were excluded (N = 22617). A total of 18,560 participants who were not diagnosed with diabetes were excluded (N = 4057). The patients who had no data about the urinary albumin to creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR) (N = 438), RBC folate (N = 516) and serum total folate (N = 27) were also excluded. 6 patients were excluded due to pregnancy. Finally, 3070 patients with T2DM were involved in the final analysis. The participants were considered to have DN (N = 1217) if their urinary albumin to creatinine ratio (UACR) was 30 mg/g or higher, or if their estimated glomerular filtration rate (eGFR) was less than 60 mL/min/1.73 m2. 11 (Fig. 1).
Fig. 1.
Flowchart of participant selection.
Data Collection.
Professionals collected basic population information and conducted experimental measurements in adherence to the technical standards published on the NHANES website. All data and experimental methods were available for download from the NHANES website. The laboratory work was performed at a facility in Minnesota. Demographics data (sex, age, race, family income, education level, etc.), examination Data (blood pressure, body mass index, etc.), health-related behaviors (smoking and alcohol using, etc.), laboratory data ( fasting blood glucose, glycated hemoglobin, albumin, total protein, alanine aminotransferase, gamma-glutamyltransferase, serum creatinine, cholesterol, high density lipoprotein cholesterol, RBC folate, serum total folate, vitamin B12, RBC count, etc.), and dietary data (dietary folate intake, dietary supplement folate intake)were selected 12,13. Subsequently, all measurements were quantified in accordance with international standard units.
Criteria for evaluation
Diagnosis of DN
Participants were considered to have diabetic nephropathy (DN) if their urinary albumin to creatinine ratio (UACR) was 30 mg/g or higher, or if their estimated glomerular filtration rate (eGFR) was less than 60 mL/min/1.73 m2. 11
Identification of DM
The diagnostic criteria for diabetes were developed based on international and previous research literature.14 The criteria include: the person who had ever been told that he or she had diabetes, or a fasting blood glucose level of ≥ 7.0 mmol/L, or a glycosylated hemoglobin level of ≥ 6.5 mmol/L.
Defining hypertension.
The diagnostic criteria for hypertension were identified as: individuals who had ever been told that they had hypertension, or individuals with a systolic blood pressure (SBP) of 140mmHg or higher, and/or a diastolic blood pressure (DBP) of 90mmHg or higher. 15.
Evaluation of BMI
The body mass index (BMI) is an indicator of whether a person is underweight, normal weight, overweight, or obese. The formula is: BMI = weight (kg) / (height (m))^2. According to the standards set by the World Health Organization, if a person’s BMI fell between 18.5 kg/m2 and 24.9 kg/m2, it was considered normal. A BMI of 25.0 to 29.9 kg/m2 was categorized as overweight, and a BMI of 30.0 kg/m2 or higher was classified as obese 16.
Assessment of of smoking and alcohol using.
Based on the analysis of data and previous research, individuals who have smoked more than 100 cigarettes in their lifetime were defined as smokers, while participants who have not smoked more than 100 cigarettes were considered non-smokers.A non-drinker was defined as a person who had consumed no more than 12 alcoholic drinks in a year, while those who exceeded this limit were categorized as drinkers. 17–20
Covariable Screening
The covariates were screened based on the following criteria:
Factors that have been identified as potential contributors to the development and advancement of DN in prior research. (11)
In consideration of our clinical experience.
-
(3)
Introduction of variability that may result in a change in the regression coefficient of the base model by over 10%
-
(4)
Avoidance of variables with collinearity.
The collected covariates included demographic data (sex, age, race, family income, education level, etc.), examination data (blood pressure, body mass index, etc.), health-related behaviors (smoking and alcohol use, etc.), laboratory data (albumin, total protein, alanine aminotransferase, gamma-glutamyltransferase, cholesterol, high-density lipoprotein cholesterol, vitamin B12, RBC count, etc.), and dietary data (dietary folate intake, dietary supplement folate intake). A dietary recall interview followed by an interview at the Mobile Examination Center (MEC) was conducted to gather participants’ 24-hour nutritional information, including dietary folate intake, and dietary supplement folate intake, which were determined by questions regarding nutritional consumption. Education level was categorized as less than 9 years, 9–12 years, and more than 12 years. Family income was classified as low (PIR ≤ 1.3), medium (PIR > 1.3–3.5), and high (PIR > 3.5) based on the poverty income ratio (PIR). 20
Statistical Analysis
The baseline characteristics of different RBC folate groups were analyzed using One-Way ANOVA (for normal distribution), Kruskal-Wallis H test (for skewed distribution), and chi-square test (for categorical variables). Histogram distribution, Q-Q plot, or Kolmogorov-Smirnov test was utilized to evaluate whether the variables followed a normal distribution. Continuous variables were presented as mean with standard deviation (SD) or medians with interquartile range (IQR), and categorical variables were presented as number and percentage. The participants were divided into two groups: those with DN and those without DN. Single-factor and multi-factor logistic regressions were used to explore the associations between RBC folate and DN. In the context of multi-factor analyses, RBC folate was treated as both continuous and categorical variables, in which RBC folate levels were transformed into their logarithmic base 2 values for analysis or categorized by quartiles with Q1 as the reference group, respectively. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to assess the relationship between RBC folate and the risk of DN.
Model 1 represented the basic model without accounting for any variables. Model 2 was controlled for age, gender, race/ethnicity, PIR, and education level. Model 3 was further adjusted from Model 2 to include BMI, smoking, alcohol use, and hypertension. Model 4 was adjusted as for Model 3, additionally adjusted for ALT, ALB, GGT, TP, cholesterol, HDL, dietary folate intake, dietary folate supplement intake, vitamin B12, and RBC count. A smooth curve fitting graph between RBC folate and DN using the restricted cubic spline approach was created and fine-tuned based on the covariates in model 4. Subgroup analysis was performed on all subgroups to assess the stability of the results. Interaction tests were conducted, followed by effect adjustment tests on subgroup measures, and likelihood ratio tests were carried out subsequently.
The analysis was performed using R 4.2.1 (http://www.Rproject.org; The R Foundation, Vienna, Austria) and the Free Statistics software (version 1.9.2; Beijing FreeClinical Medical Technology Co., Ltd, Beijing, China). A two-sided p-value less than 0.05 was deemed statistically significant.
Result
Basic information feature when collecting the data of participant.
This study involved 39,156 participants from the NHANES dataset over a duration of eight years across four cycles. Following the strict screening criteria outlined above, the final analysis included 3,070 participants with T2DM, among whom 1,217 had DN and 1,853 had no DN (refer to Fig. 1). Table 1 presented the baseline data for diabetic nephropathy and non-diabetic nephropathy. DN was detected in 1,217 participants (39.6%). Significant differences in age, race, serum folate, RBC folate, ALB, RBC count, hypertension, smoking, and ALT were observed between the DN and non-DN groups (all P-value < 0.05). The DN group showed higher levels of serum folate and RBC folate (P < 0.001) and lower levels of ALB, RBC count, and ALT (P < 0.001).
Table 1.
Comparison of baseline data of participants.
| Variables | Total (n = 3070) |
Without DN | DN | p |
|---|---|---|---|---|
| (n = 1853) | (n = 1217) | |||
| CHO, Mean ± SD | 4.8 ± 1.2 | 4.8 ± 1.2 | 4.7 ± 1.3 | 0.165 |
| Gender, n (%) | 0.550 | |||
| Male | 1589 (51.8) | 951 (51.3) | 638 (52.4) | |
| Female | 1481 (48.2) | 902 (48.7) | 579 (47.6) | |
| Age, (year) | 60.2 ± 13.5 | 57.1 ± 13.2 | 64.8 ± 12.7 | < 0.001 |
| Race, n (%) | < 0.001 | |||
| Mexican American | 539 (17.6) | 342 (18.5) | 197 (16.2) | |
| Other Hispanic | 357 (11.6) | 238 (12.8) | 119 (9.8) | |
| Non-Hispanic White | 935 (30.5) | 494 (26.7) | 441 (36.2) | |
| Non-Hispanic Black | 788 (25.7) | 482 (26) | 306 (25.1) | |
| Other Race | 451 (14.7) | 297 (16) | 154 (12.7) | |
| BMI, (kg/m2) | 32.5 ± 7.5 | 32.4 ± 7.6 | 32.5 ± 7.3 | 0.888 |
| Serum folate, (nmol/L) | 47.4 ± 41.3 | 45.3 ± 37.5 | 50.6 ± 46.3 | < 0.001 |
| RBC folate, nmol/L | 1324.6 ± 653.7 | 1243.9 ± 559.6 | 1447.5 ± 759.2 | < 0.001 |
| ALB, (g/l) | 41.3 ± 3.5 | 41.7 ± 3.3 | 40.7 ± 3.8 | < 0.001 |
| TP, (g/l) | 71.6 ± 5.0 | 71.6 ± 4.7 | 71.5 ± 5.4 | 0.501 |
| HDL, (mmol/l) | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.4 | 0.281 |
| RBC count, (million cells/uL) | 4.6 ± 0.5 | 4.7 ± 0.5 | 4.5 ± 0.6 | < 0.001 |
| Hypertension, n (%) | 2174 (72.2) | 1185 (65.2) | 989 (82.8) | < 0.001 |
| Education level, (n %) | 0.062 | |||
| < 9 years | 523 (17.1) | 306 (16.5) | 217 (17.9) | |
| 9–12 years | 1155 (37.7) | 676 (36.5) | 479 (39.5) | |
| > 12 years | 1389 (45.3) | 871 (47) | 518 (42.7) | |
| Alcohol using, n (%) | 1523 (64.3) | 916 (65) | 607 (63.4) | 0.424 |
| Smoking, n (%) | 1482 (48.3) | 862 (46.5) | 620 (50.9) | 0.016 |
| ALT, (U/L) | 22.0 (16.0, 30.0) | 23.0 (17.0, 32.0) | 20.0 (15.0, 27.0) | < 0.001 |
| GGT, (U/L) | 24.0 (17.0, 38.0) | 25.0 (17.0, 38.0) | 24.0 (17.0, 38.0) | 0.671 |
| DFI, (mcg) | 121.0 (59.0, 210.0) | 123.0 (59.2, 212.0) | 119.0(59.0, 205.0) | 0.673 |
| DFSI, (mcg) | 400.0(400.0, 400.0) | 400.0 (400.0, 400.0) | 400.0 (400.0, 400.0) | 0.485 |
| Vitamin B12, (pmol/L) | 393.4 (281.2, 575.6) | 381.9 (277.5, 568.3) | 415.5 (284.9, 577.9) | 0.163 |
Note: BMI, Body Mass Index; ALB, Albumin; TP, Total protein; Family income, Family poverty income ratio; Hypertension, High blood pressure; ALT, Alanine aminotransfease; GGT, Gamma-glutamyltransferase; Vitamin B12, Serum vitamin B12; HDL, High density lipoprotein cholesterol; Serum folate, Serum total folate. DFI, Dietary folate intake; DFSI, Dietary folate supplement intake.
The univariate logistic regression analysis showed that age, race, RBC folate, serum folate, ALT, ALB, RBC count, hypertension, smoking, and family income were related factors for DN. Non-Hispanic White individuals had an increased risk of DN compared to other races, and higher family income was associated with a decreased likelihood of developing DN. Serum folate, RBC folate, age, hypertension, and smoking were positively correlated with the occurrence of DN. Conversely, ALT, ALB, and RBC count were negatively correlated with the occurrence of DN (refer to Table 2).
Table 2.
Univariate analysis of association between correlated variables and DN.
| Variable | DN | |
|---|---|---|
| OR (95% CI) | P value | |
| Cholesterol, (mmol/l) | 0.96 (0.90 ~ 1.02) | 0.166 |
| Male | 1 | |
| Female | 0.96 (0.83 ~ 1.11) | 0.550 |
| Age, (years) | 1.05 (1.04 ~ 1.05) | < 0.001 |
| Mexican American | 1 | |
| Other Hispanic | 0.87 (0.66 ~ 1.15) | 0.324 |
| Non-Hispanic White | 1.55 (1.25 ~ 1.93) | < 0.001 |
| Non-Hispanic Black | 1.10 (0.88 ~ 1.38) | 0.400 |
| Other Race | 0.90 (0.69 ~ 1.17) | 0.431 |
| BMI, (kg/m2) | 1.00 (0.99 ~ 1.01) | 0.888 |
| Serum folate, (nmol/L) | 1.00 (1.00 ~ 1.01) | 0.001 |
| RBC folate, (nmol/L) | 1 .00 (1.00 ~ 1.00) | < 0.001 |
| ALT, (U/L) | 0.99 (0.98 ~ 0.99) | < 0.001 |
| ALB, (g/L) | 0.91 (0.90 ~ 0.93) | < 0.001 |
| GGT, (U/L) | 1.00 (1.00 ~ 1.00) | 0.440 |
| TP, (g/L) | 1.00 (0.98 ~ 1.01) | 0.501 |
| Dietary folate intake, (mcg) | 1.00 (1.00 ~ 1.00) | 0.812 |
| Dietary supplement folate intake, (mcg) | 1.00 (1 .00~ 1.00) | 0.695 |
| Vitamin B12, (pmol/L) | 1.00 (1.00 ~ 1.00) | 0.488 |
| HDL, (mmol/L) | 0.90 (0.74 ~ 1.09) | 0.281 |
| RBC count (million cells/uL) | 0.50 (0.43 ~ 0.57) | < 0.001 |
| Hypertension, (n %) | 2.56 (2.15 ~ 3.07) | < 0.001 |
| Education level, (n %) | ||
| < 9 years | 1 | |
| 9–12 years | 1.00 (0.81 ~ 1.23) | 0.994 |
| > 12 years | 0.84 (0.68 ~ 1.03) | 0.093 |
| Alcohol using | 0.93 (0.79 ~ 1.11) | 0.424 |
| Smoking | 1.19 (1.03 ~ 1.38) | 0.016 |
| Family income, (n %) | ||
| Low income | 1 | |
| Medium income | 0.91 (0.77 ~ 1.09) | 0.307 |
| High income | 0.62 (0.50 ~ 0.76) | < 0.001 |
Note: ALT, Alanine aminotransfease; GGT, Gamma-glutamyltransferase; HDL, High density lipoprotein cholesterol; BMI, Body Mass Index; ALB, Albumin; TP, Total protein; Hypertension, High blood pressure.
Univariate Analysis of Factors associated with DN.
Multivariate analysis of RBC folate and DN related factors
After multiple imputations were performed to account for missing covariates, we conducted a logistic multiple factor regression analysis to assess the relationship between RBC folate and DN. RBC folate was considered both as continuous and categorical variables. The RBC folate levels were transformed into their logarithmic base 2 values for analysis or categorized by quartiles, with Q1 serving as the reference group. We created four logistic regression models to explore the correlation between RBC folate and DN, and the results pointed to a positive correlation between RBC folate and DN. Table 3 provided a detailed analysis of the relationship between RBC folate and DN, with the effect value expressed as odds ratio (OR) and 95% confidence interval (CI). The effect value can be interpreted as the proportional increase in the risk of DN for each additional unit of RBC folate. For instance, in the unadjusted model 1, where RBC folate was transformed into logarithmic base 2 values, the effect size of 1.57 with a confidence interval of 1.47 to 1.68 suggested a 57% rise in the risk of DN for every additional 2 units of RBC folate. In the slightly adjusted model 2, the effect value of 1.38 (95% CI 1.28 ~ 1.48) implied a 38% increase in the risk of DN for every additional 2 units of RBC folate. In the further adjusted model 3, the effect size of 1.34 (95% CI 1.25 ~ 1.45) indicated a 34% increase in the risk of DN for every additional 2 units of RBC folate. In the fully adjusted model 4, the effect value was 1.38 (95% CI 1.27 ~ 1.49), suggesting that every additional 2 units of RBC folate increased the risk of DN by 38%. These results were statistically significant with a P value of less than 0.001. In the unadjusted model, participants in the highest quartile (Q4) group of RBC folate exhibited a higher risk of DN, with an effect value of 2.11 (95% CI 1.87 ~ 2.38) compared to the Q1 group. Moreover, as RBC folate levels increased, the risk of DN gradually increased. These associations remained statistically significant in all multivariate logistic regression models, even after adjusting for various potential influencing factors. In model 4, compared with Q1, participants in the Q4 group had a higher risk of DN (OR = 1.69, 95% CI 1.47–1.94). The Q2 and Q3 groups also showed significantly higher risk of DN (P for trend < 0.001). In all models, the rise in RBC folate was consistently associated with an increased risk of DN, as indicated by a consistent trend test, with P for trend < 0.001 (Table 3). To ensure the stability of the results, subgroup analyses were conducted. Additionally, smooth fitting curves of RBC folate and DN were plotted to verify the relationship between the two variables. After adjusting for potential confounding factors based on clinical consensus, if the influencing factors changed by more than 10%, RBC folate can be considered to have a strong positive correlation with the incidence of DN.
Table 3.
Multivariate analysis of association between RBC folate and DN in individuals of T2DM.
| Variable( nmol/L) | Model 1 | P value | Model 2 | P value | Model 3 | P value | Model 4 | P value |
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | |||||
| RBC folate logtrans | 1.57(1.47 ~ 1.68) | < 0.001 | 1.38(1.28 ~ 1.48) | < 0.001 | 1.34(1.25 ~ 1.45) | < 0.001 | 1.38(1.27 ~ 1.49) | < 0.001 |
| RBC folate group( nmol/L) | ||||||||
| Q1(219–894) | 1(Ref) | 1(Ref) | 1(Ref) | 1(Ref) | ||||
| Q2(894–1170) | 1.28 (1.13 ~ 1.45) | < 0.001 | 1.24 (1.09 ~ 1.42) | 0.001 | 1.25 (1.10 ~ 1.43) | 0.001 | 1.28 (1.12 ~ 1.46) | < 0.001 |
| Q3(1170–1580) | 1.33 (1.18 ~ 1.50) | < 0.001 | 1.25 (1.10 ~ 1.42) | 0.001 | 1.26 (1.11 ~ 1.44) | < 0.001 | 1.26 (1.11 ~ 1.44) | 0.001 |
| Q4(1580–6240) | 2.11 (1.87 ~ 2.38) | < 0.001 | 1.68 (1.47 ~ 1.91) | < 0.001 | 1.64 (1.44 ~ 1.87) | < 0.001 | 1.69 (1.47 ~ 1.94) | < 0.001 |
| P for trend | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
Model1:Non-adjusted.
Model2: Adjusted for age, sex, race. family poverty income ratio, and education level.
Model3: Adjusted for Model2 + BMI, hypertension, alcohol using, smoking.
Model4: Adjusted for Model3 + ALT, ALB, GGT, TP, Cholesterol, HDL, dietary folate intake, dietary folate supplement intake, VitaminB12, RBC count.
Subgroup Analysis and Curve Fitting
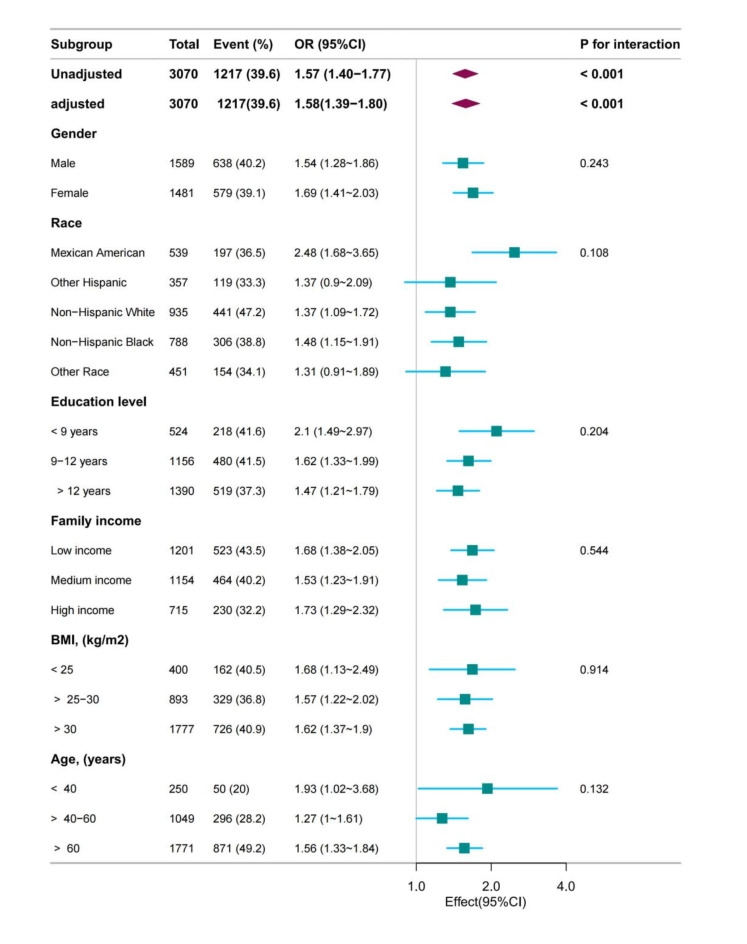
We investigated whether there were variations in age, BMI, race, gender, education level, and PIR in relation to RBC folate and DN. The findings revealed that the association between RBC folate and DN remained consistent in all subgroups (Fig. 2), and there was no significant interaction (P > 0.05). This suggests that the relationship between RBC folate and DN was not influenced by demographic or clinical characteristics, such as age, BMI, race, gender, education level, and PIR. These results support the idea that RBC folate may serve as a reliable biomarker for identifying individuals at risk of DN, regardless of these factors. Furthermore, to better understand the link between RBC folate and DN, fitting curves of RBC folate and DN were plotted after adjusting according to model 4 (Fig. 3). The results showed a linear relationship between RBC folate and DN (P for non-linearity = 0.147). This indicates that RBC folate was positively correlated with DN.
Fig. 2.
Forest plot of RBC folate and DN.
Fig. 3.
Fitting curves of RBC folate and DN after adjustment according to model 4.
Discussion
This extensive cross-sectional study of Type 2 Diabetes Mellitus utilized the NHANES 2011–2018 database to demonstrate a correlation between RBC folate concentrations and Diabetic Nephropathy (DN) in a nationally representative sample of the US population. The investigation aimed to explore the association between RBC folate levels and the occurrence of DN in a large, diverse population. The study findings suggested that RBC folate levels were positively correlated with the risk of DN, indicating that homeostatic changes in kidney function among patients with diabetes mellitus have a significant impact on RBC folate concentrations. Even after adjusting for demographic variables and other relevant factors, such as dietary folate intake, dietary supplement folate intake, levels of serum vitamin B12, and RBC count, which could potentially influence the results, the relationship between the variables remained robust and consistent. Furthermore, the relationship between RBC folate and DN was found to be stable across all subgroups (P for interaction > 0.05). These observations have significant implications for the current management strategies of Diabetic Nephropathy in Type 2 Diabetes Mellitus. The study suggested that there is a positive relationship between RBC folate and DN (P < 0.001), and that RBC folate concentrations in DN risk were independent of dietary folate intake, dietary supplement folate intake, levels of serum vitamin B12, and RBC count. The study also shed light on the potential oversight in studies that investigate the effects of elevated levels of RBC folates, which often assume that high folate concentrations indicate high folic acid intake, overlooking the potential impacts of other homeostatic and biological processes, including kidney function. These findings underscore the need to consider the complex interplay of factors influencing RBC folate levels and the risk of DN in individuals with Type 2 Diabetes Mellitus. This differs from the limited reports that have been conducted in the past. Wang A et al. reported that when examining the relationships between kidney function (measured CKD risk) and levels of RBC and serum folate, the levels of RBC and serum folate increased as kidney function declined without increased folic acid intake. 8 Additionally, elevated levels of RBC folate were independently linked to a higher risk of all-cause and cardiovascular disease (CVD) mortality in individuals with diabetes. Conversely, high levels of RBC folate appeared to have a mild protective effect in non-diabetic individuals.9 However, there are very few studies that have investigated the relation to RBC folate. 21–25 Folates are essential as cofactors in metabolic pathways, influencing biological methylation and nucleotide synthesis, significantly impacting overall health and susceptibility to diseases. 26–28 Serum total folate concentration is generally regarded as an indicator of recent folate intake, while RBC folate concentration is viewed as an indicator of long-term folate status. 29–32 There is a lack of extensive literature explaining the biological mechanism that associates high RBC folate with diabetic nephropathy (DN) risk among patients with type 2 diabetes.
The precise mechanism linking the effect of higher RBC folate and the risk of Diabetic Nephropathy (DN) is still unclear. One potential explanation is the adverse effects of unmetabolized folic acid (UMFA) in the bloodstream, which may disrupt cellular folate uptake and intracellular folate metabolism. UMFA in the blood has been associated with decreased natural killer (NK) cell cytotoxicity, which plays a role in inflammation and insulin resistance, and is hence involved in the development of diabetes mellitus (DM). Additionally, metabolic and homeostatic changes resulting from alterations in kidney function can lead to an increase in RBC folate concentration. 8,33–36 The studies mentioned above suggested a potential link between RBC folate and DN in Type 2 Diabetes Mellitus. Due to the current very limited reports, we emphasize the importance of conducting additional research to explore the potential link between kidney function, RBC folate concentrations, and the effects of unmetabolized folic acid on cellular processes. This could provide valuable insights into the underlying mechanisms and potential implications for conditions such as DM and DN.
It is important to note that previous research was limited by small sample sizes and a restricted number of covariates, which hindered the ability to make broad generalizations about the relationship between the variables under investigation. Future studies should aim to overcome these limitations by using larger and more diverse samples, as well as considering a wider range of potential confounding variables. The NHANES provided us with a unique opportunity to investigate the potential association between RBC folate and DN, as well as to comprehensively assess the level-risk connection between RBC folate and DN, fully adjusted for a large number of covariates and a range of stratified analyses.
The strength of the present study lies in its status as the first large-scale and ethnically diverse population-based investigation to uncover the link between RBC folate and DN in T2DM patients among US adults. Our findings have the potential to contribute to the existing limited literature on the association between RBC folate and DN.
However, our study was limited by its use of data from a cross-sectional survey, which prevented us from establishing a cause-and-effect relationship between RBC folate and DN. Therefore, additional longitudinal studies are necessary to verify and support our findings. Moreover, relying on self-reported data may have resulted in recall bias and potential misclassification of DN. Finally, the study’s narrow focus on a limited sample of US adults raises questions about the generalizability of the findings to other populations. Despite these limitations, our study offered valuable insights into the potential link between RBC folate and DN in individuals with T2DM. Future research should focus on addressing these limitations and delving deeper into this relationship.
Conclusions
In conclusion, our study suggested that a high level of RBC folate was independently associated with the presence of DN in diabetic patients, indicating RBC folate as a potential biomarker for early detection, prevention, and intervention planning for this serious complication. RBC folate might predict the possible existence of DN in patients with T2DM. Furthermore, when examining associations between disease outcomes and factors such as folic acid intake and folate biomarkers, it is crucial to take into account the potential mediating role of kidney function. It is essential for researchers to carefully assess and control for the potential influence of kidney function when investigating these relationships.
Author contributions
Peixia Yu contributed to the design of our study, and wrote the manuscript. Keyu Liu contributed to the design of our study, data analysis and interpretation. Yongjin Ji, and Hairu wang were responsible for the management and retrieval of data. All authors reviewed the manuscript and met the authorship of ICMJE criteria.
Data availability
All data and experimental methods were available for download from the NHANES website: https://www.cdc.gov/nchs/nhanes/index.htm.
Declarations
Competing interests
The authors declare no competing interests.
Informed consent
The NCHS Ethics Review Board has given approval for the NHANES research protocol, and all participants have given their written informed consent.
Study or trial registration: The study was registered in a registry with the registration number #2011-17, and data collection began in January 2011.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Demir S, Nawroth PP, Herzig S, et al. Emerging Targets in Type 2 Diabetes and Diabetic Complications. Adv Sci (Weinh). 2021;8(18):e2100275. [DOI] [PMC free article] [PubMed]
- 2.Dixon D, Edmonds M. Managing Diabetic Foot Ulcers: Pharmacotherapy for Wound Healing. Drugs. 2021;81(1):29–56. [DOI] [PubMed]
- 3.Vujosevic S, Aldington SJ, Silva P, et al. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. 2020;8(4):337 − 47 [DOI] [PubMed]
- 4.Umanath K, Lewis JB. Update on Diabetic Nephropathy: Core Curriculum 2018. Am J Kidney Dis. 2018;71(6):884 − 95. [DOI] [PubMed]
- 5.Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond). 2013;124(3):139 − 52. [DOI] [PubMed]
- 6.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12:2032–2045. [DOI] [PMC free article] [PubMed]
- 7.Wang W, Yang A, Zhang H, et al. Associations of RBC and Serum Folate Concentrations with Cervical Intraepithelial Neoplasia and High-Risk Human Papillomavirus Genotypes in Female Chinese Adults. J Nutr. 2022 ;8;152(2):466 − 74. [DOI] [PubMed]
- 8.Wang, A., Yeung, L. F., Ríos Burrows, N., et al. Reduced Kidney Function Is Associated with Increasing Red Blood Cell Folate Concentration and Changes in Folate Form Distributions (NHANES 2011–2018). Nutrients, 2022,14(5), 1054. [DOI] [PMC free article] [PubMed]
- 9.Xiong H, Li X, Cheng S, et al. Folate Status and Mortality in US Adults With Diabetes: A Nationally Representative Cohort Study. Front Cardiovasc Med. 2022;25;9:802247 [DOI] [PMC free article] [PubMed]
- 10.Wang J, Liu F, Kong R, et al. Association Between Globulin and Diabetic Nephropathy in Type2 Diabetes Mellitus Patients: A Cross-Sectional Study. Front Endocrinol (Lausanne). 2022;8;13:890273. [DOI] [PMC free article] [PubMed]
- 11.Yang H, Lin H, Liu X, Liu H, Chen T, Jin Z. Association between dietary fiber intake and diabetic nephropathy among adult diabetes mellitus in the United States: A cross-sectional study. Heliyon. 2024 Apr 23;10(9):e30036. [DOI] [PMC free article] [PubMed]
- 12.Yeung, L.F.; Cogswell, M.E.; Carriquiry, A.L, et al. Contributions of enriched cereal-grain products, ready-to-eat cereals, and supplements to folic acid and vitamin B-12 usual intake and folate and vitamin B-12 status in US children:National Health and Nutrition Examination Survey (NHANES), 2003–2006. Am. J. Clin. Nutr. 2011, 93, 172 − 85. [DOI] [PubMed]
- 13.Pfeiffer, C.M.; Sternberg, M.R.; Fazili, Z.; et al. Folate status and concentrations of serum folate forms in the US population: National Health and Nutrition Examination Survey 2011–2012. Br. J. Nutr. 2015, 113, 1965-77. [DOI] [PMC free article] [PubMed]
- 14.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011 Jan;34 Suppl 1(Suppl 1):S62-9. [DOI] [PMC free article] [PubMed]
- 15.Elliott WJ. Systemic Hypertension. Curr probl Cardiol (2007) 32(4):201 − 59. [DOI] [PubMed]
- 16.White GE, Mair C, Richardson GA, Courcoulas AP, King WC. Alcohol Use Among U.S. Adults by Weight Status and Weight Loss Attempt: NHANES, 2011–2016. Am J Prev Med. 2019;57(2):220 − 30. [DOI] [PubMed]
- 17.Ricci C, Schutte AE, Schutte R, et al. Trends in Alcohol Consumption in Relation to Cause-Specific and All-Cause Mortality in the United States: A Report From the NHANES Linked to the US Mortality Registry. Am J Clin Nutr (2020) 111(3):580–9. [DOI] [PubMed]
- 18.Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA (2016) 315(21):2284–91. [DOI] [PMC free article] [PubMed]
- 19.Mortada I. Hyperuricemia, Type 2 Diabetes Mellitus, and Hypertension: An Emerging Association. Curr hypertens Rep (2017) 19(9):69. [DOI] [PubMed]
- 20.Liu, H., Wang, L., Chen, C., et al. Association between Dietary Niacin Intake and Migraine among American Adults: National Health and Nutrition Examination Survey. Nutrients, 2022,14(15), 3052. [DOI] [PMC free article] [PubMed]
- 21.Shen M, Chaudhry SH, MacFarlane AJ, et al. Serum and red-blood-cell folate demonstrate differential associations with BMI in pregnant women. Public Health Nutr. 2016 Oct;19(14):2572-9. [DOI] [PMC free article] [PubMed]
- 22.Colapinto CK, O’Connor DL, Sampson M, et al. Systematic review of adverse health outcomes associated with high serum or red blood cell folate concentrations. J Public Health (Oxf). 2016;38(2):e84-97 [DOI] [PMC free article] [PubMed]
- 23.Bird JK, Ronnenberg AG, Choi SW, et al. Obesity is associated with increased red blood cell folate despite lower dietary intakes and serum concentrations. J Nutr. 2015 Jan;145(1):79–86. [DOI] [PMC free article] [PubMed]
- 24.Yetley EA, Pfeiffer CM, Phinney KW, et al. Biomarkers of folate status in NHANES: a roundtable summary. Am J Clin Nutr. 2011 Jul;94(1):303S-312S. [DOI] [PMC free article] [PubMed]
- 25.Liwinski, T., & Lang, U. E. Folate and Its Significance in Depressive Disorders and Suicidality: A Comprehensive Narrative Review. Nutrients, 2023;15(17), 3859. [DOI] [PMC free article] [PubMed]
- 26.Xie K, Xu P, Fu Z, et al. Association of maternal folate status in the second trimester of pregnancy with the risk of gestational diabetes mellitus. Food Sci Nutr. 2019 ;18;7(11):3759-65. [DOI] [PMC free article] [PubMed]
- 27.Hung J, Beilby JP, Knuiman MW, et al. Folate and vitamin B-12 and risk of fatal cardiovascular disease: cohort study from Busselton, Western Australia. BMJ. 2003;326:131. [DOI] [PMC free article] [PubMed]
- 28.Kyte B, Ifebi E, Shrestha S, et al. High red blood cell folate is associated with an increased risk of death among adults with diabetes, a 15-year follow-up of a national cohort. Nutr Metab Cardiovasc Dis. 2015;25:997–1006. [DOI] [PubMed]
- 29.Cho CE, Sánchez-Hernández D, Reza-López SA, et al. Anderson GH. High folate gestational and post-weaning diets alter hypothalamic feeding pathways by DNA methylation in Wistar rat offspring. Epigenetics. 2013;8(7):710-9. [DOI] [PMC free article] [PubMed]
- 30.Yajnik, C. S., Deshpande, S. S., Jackson, A. A., et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: The Pune maternal nutrition study. Diabetologia, 2008, 51(1), 29–38. [DOI] [PMC free article] [PubMed]
- 31.Marchetta CM, Hamner HC. Blood folate concentrations among women of childbearing age by race/ethnicity and acculturation, NHANES 2001–2010. Matern Child Nutr. 2016 Jan;12(1):39–50 [DOI] [PMC free article] [PubMed]
- 32.Mahabir S, Ettinger S, Johnson L, et al. Measures of adiposity and body fat distribution in relation to serum folate levels in postmenopausal women in a feeding study. Eur J Clin Nutr 2008;62:644–50 [DOI] [PMC free article] [PubMed]
- 33.Mojtabai R. Body mass index and serum folate in childbearing age women. Eur J Epidemiol 2004;19:1029–36. [DOI] [PubMed]
- 34.Twum F, Morte N, Wei Y, et al. Red blood cell folate and cardiovascular deaths among hypertensive adults, an 18-year follow-up of a national cohort. Hypertens Res. (2020) 43:938–12. [DOI] [PubMed]
- 35.Peng Y, Wang Z. Red blood cell folate concentrations and coronary heart disease prevalence: a cross-sectional study based on 1999–2012 National Health and Nutrition Examination Survey. Nutr Metab Cardiovasc Dis. 2017;27:1015–20. [DOI] [PubMed]
- 36.Chen MY, Rose CE, Qi YP, et al. Defining the plasma folate concentration associated with the red blood cell folate concentration threshold for optimal neural tube defects prevention: a population-based, randomized trial of folic acid supplementation. Am J Clin Nutr. 2019,1;109(5):1452-61. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and experimental methods were available for download from the NHANES website: https://www.cdc.gov/nchs/nhanes/index.htm.