Abstract
Herpes simplex virus type 1 immediate early protein ICP0 influences virus infection by inducing the degradation of specific cellular proteins via a mechanism requiring its RING finger and the ubiquitin-proteasome pathway. Many RING finger proteins, by virtue of their RING finger domain, interact with E2 ubiquitin-conjugating enzymes and act as a component of an E3 ubiquitin ligase. We have recently shown that ICP0 induces the accumulation of colocalizing, conjugated ubiquitin, suggesting that ICP0 can act as or contribute to an E3 ubiquitin ligase. In this report we demonstrate that the ICP0-related RING finger proteins encoded by other alphaherpesviruses also induce colocalizing, conjugated ubiquitin, thereby suggesting that they act by similar biochemical mechanisms.
The mechanisms governing the switch between latent and lytic herpes simplex virus type 1 (HSV-1) infection have been a subject of continued interest. HSV-1 immediate-early protein ICP0 stimulates both entry into the lytic cycle and reactivation from latency, so it has been proposed that ICP0 may play a specific role in the control of the balance between the latent and lytic states (reviewed in reference 11).
ICP0 has profound effects on the cell, associating with and subsequently disrupting centromeres and other nuclear structures known as ND10 domains, PML nuclear bodies, or promyelocytic oncogenic domains (6, 9, 19, 20). The disruption occurs because ICP0 induces the proteasome-dependent degradation of components of both structures and also other characterized and uncharacterized proteins (7, 9, 17, 21, 22). Since the roles of ICP0 during infection and its effects on the host cell both require its zinc-binding RING finger domain and an active ubiquitin-proteasome pathway, it appears likely that a major part of ICP0's function in viral biology is achieved through its induction of proteasome-dependent degradation of one or more of its known target proteins. In support of this hypothesis is the increasing evidence that many RING finger proteins participate in the proteasome-dependent protein degradation pathway by interacting with E2 ubiquitin-conjugating enzymes in E3 ubiquitin ligase complexes, thereby controlling the stability of specific target proteins (reviewed in references 1, 12, 15, 16, and 26). Given the experimental background, it seems likely that ICP0 acts as or forms part of an E3 ligase. Consistent with this suggestion, we have recently shown that in both transfected and infected cells ICP0 causes the accumulation of colocalizing, conjugated ubiquitin in a RING finger-dependent manner (10).
HSV-1 is an alphaherpesvirus, and ICP0-related proteins are encoded by other family members: BICP0 in bovine herpesvirus 1 (27); the product of gene 63, Eg63, in equine herpesvirus 1 (25); the product of gene 61, Vg61, in varicella zoster virus (3); and EP0 in pseudorabies virus (2). We have recently demonstrated by immunofluorescence assays that these ICP0-related proteins also affect ND10 and in some cases centromeres (although to differing extents) and that in the case of Eg63 this involves proteasome-dependent degradation (22). These functional similarities among the ICP0 family members are consistent with the presence of a RING finger domain near their N termini, the only obvious similarity common to all the proteins. This region in ICP0 is essential for its functions in regulating gene expression, stimulating lytic infection and reactivation from quiescence, disruption of ND10 and centromeres, induced proteasome-dependent degradation of cellular proteins, interaction with cyclin D3, and induction of colocalizing, conjugated ubiquitin (4, 5, 10). The aim of this study was to determine if the other members of the ICP0 family were also able to induce the appearance of colocalizing, conjugated ubiquitin using the immunofluorescence assay used for ICP0 (10).
Cells and viruses.
Human epithelial HEp-2 cells were grown in Dulbecco's modified Eagle's medium with 10% fetal calf serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml.
Plasmids.
Plasmids expressing the ICP0 family of proteins tagged with an epitope from the C-terminal peptide of HSV-1 UL30, which is recognized by rabbit polyclonal serum r113 (18), were produced. First, intermediate plasmid pET-rtag-ICP0, containing a 5′- and 3′-modified ICP0 open reading frame (ORF) between the NdeI and HindIII sites of pET24a, was created. The 5′ end of the ICP0 ORF had been modified by an oligonucleotide containing a 5′ NdeI site, a start codon, an in-frame UL30 epitope tag, and a 3′ NcoI site, and the 3′ end of the ICP0 ORF had been modified by an oligonucleotide containing a 5′ SalI site, the last eight codons of ICP0, the termination codon, and a 3′ HindIII site. The UL30 epitope-tagged ICP0 cassette was then cloned into the mammalian expression vector pCIneo, forming pCI-rtag-ICP0. To create the corresponding plasmids expressing the other family members, the NcoI-HindIII ICP0 fragment was removed from pET-rtag-ICP0 for BICP0, Eg63, and Vg61, but for EP0, the NcoI-SalI fragment was removed and fragments containing the other ORFs were inserted. These fragments were as previously described (22), except for that of BHV-1, which was created by removing an NcoI (partial)-PvuII fragment from pp65-BICP0 (22). Cassettes containing the UL30 epitope-tagged ORFs were removed from the pET intermediates and cloned into pCIneo to form the pCI-rtag series of plasmids (Fig. 1A). Other plasmids used were pCIneo (Promega), pCIFXE (8), and pCIQEUb52 (10).
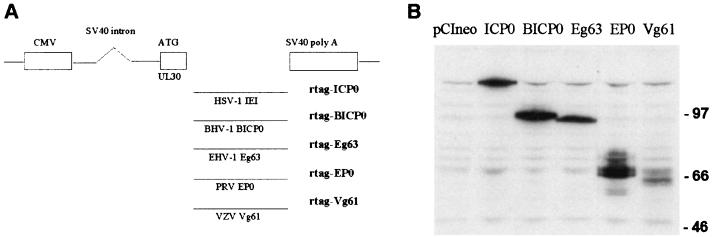
FIG. 1.
(A) Schematic diagram of the pCI-rtag series of plasmids. CMV, cytomegalovirus; SV40, simian virus 40; BHV-1, bovine herpesvirus 1; EHV-1, equine herpesvirus 1; PRV, pseudorabies virus; VZV, varicella zoster virus. (B) Expression of UL30-tagged ICP0 homologues. HEp-2 cells were transfected with pCIneo or plasmids expressing UL30-ICP0 or the UL30 homologues, as indicated. Samples were harvested into SDS-gel loading buffer at 24 h posttransfection and analyzed by Western blotting using polyclonal anti-UL30 r113 at a dilution of 1/5,000. The positions of the molecular size markers (in kilodaltons) are indicated.
Antibodies.
Polyclonal rabbit r113 and r190 have been previously described (18, 22), monoclonal antibody (MAb) FK2 was obtained from International Bioscience, Inc., and MAb MRGS, which recognizes the MRGS–6-His tag, was from Qiagen. Anti-PML antibody MAb 5E10 (24), polyclonal anti-CENP-C rabbit serum r554 (23), and polyclonal anti-Sp100 rat serum Sp26 (1/2,000) (14) have been described previously.
Transfections.
HEp-2 cells (105), on coverslips in 24-well plates, were transfected with 0.4 μg of DNA using Lipofectamine Plus (Gibco) according to the manufacturer's instructions as previously described (22). Cells were processed for microscopy or washed in phosphate-buffered saline and then harvested into sodium dodecyl sulfate (SDS)-gel loading buffer for immunoblot analysis at 24 h posttransfection.
Western blot (immunoblot) analysis.
Proteins in cell extracts were analyzed by separation on SDS–7.5% polyacrylamide gels, transferred to nitrocellulose membranes, and probed with antibody as previously described (22).
Confocal microscopy.
Cells seeded onto glass coverslips were prepared for immunofluorescence and examined by confocal microscopy as previously described (9, 21). Secondary antibodies used were fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin G (IgG) (Sigma; used at 1/100) and Cy3-conjugated goat anti-mouse or goat anti-rat IgG (Amersham; used at 1/1,000 and 1/500, respectively). Stained cells were examined using a Zeiss LSM 510 confocal microscope system, with two lasers giving excitation lines at 488 nm (FITC) and 543 nm (Cy3).
Characterization of rabbit epitope-tagged ICP0 family members.
The aim of the present study was to determine if, like ICP0 (10), other members of the ICP0 family of proteins are also able to induce the formation of colocalizing, conjugated ubiquitin. This assay depends on the use of MAb FK2, which recognizes conjugated but not free ubiquitin (13). In a previous study we used plasmids expressing ICP0 family members tagged with a human cytomegalovirus pp65 epitope recognized by a MAb (22), but because the conjugated ubiquitin is also recognized by a MAb, these plasmids were not suitable for the present study. Therefore, pCI-based plasmids expressing proteins tagged with an epitope from the C-terminal peptide of HSV-1 UL30, which is recognized by rabbit polyclonal serum r113 (18), were produced (Fig. 1A).
To determine the expression of the UL30-tagged proteins, HEp-2 cells were transfected with the plasmids and samples were analyzed by immunoblotting at 24 h posttransfection. All the UL30-tagged proteins were of the expected size and were efficiently and stably expressed (Fig. 1B). Although Vg61 was not as highly expressed as the other family members, it was nonetheless better expressed than the pp65 epitope-tagged version (22). Before assessing the effect of the UL30-tagged proteins on conjugated ubiquitin, it was important to determine whether their distribution in transfected cells and their effects on cellular structures and proteins were as previously characterized (22). The fluorescence intensity of transfected cells expressing the UL30-tagged proteins was greater than in the previous study using the pp65-tagged proteins, which could be due to the better performance of the r113 serum in immunofluorescence or to increased translational efficiency because of sequence changes around the initiating ATG codon, or both. This has allowed analysis in greater detail and with greater clarity than hitherto, and therefore we present here selected images obtained using the more recent plasmids which confirm and extend our previous conclusions. Overall, the staining patterns of the UL30-tagged proteins were very similar to those of the pp65-tagged proteins (a nuclear diffuse background with inset nuclear dots and accumulations) (22), except that the nuclear dots or accumulations were more evident, especially in cells expressing large amounts of the proteins (Fig. 2 [green staining] and 3). This was especially the case with rtag-Vg61-expressing cells, which appeared to be both clearer and more numerous than in the results with the pp65-tagged version. In a large proportion of transfected cells, Vg61 was mostly present in numerous small nuclear dots with little or no nuclear diffuse staining (Fig. 2I and 3R). Occasionally, however, transfected cells contained many irregular nuclear accumulations of rtag-Vg61 (Fig. 2J). As in the previous study, BICP0 frequently formed nuclear arcs and circles within a nuclear diffuse background (Fig. 2A to D and Fig. 3F and H). Cells expressing large amounts of EP0 in the nucleus also had diffuse cytoplasmic EP0 staining (Fig. 2H and 3P), and the nuclear staining of Eg63 frequently had an unusual mottled appearance (Fig. 2E and 3L). Thus, the change in epitope tag did not alter the essential characteristics of the ICP0 family of proteins but did make it easier to observe them in more detail.
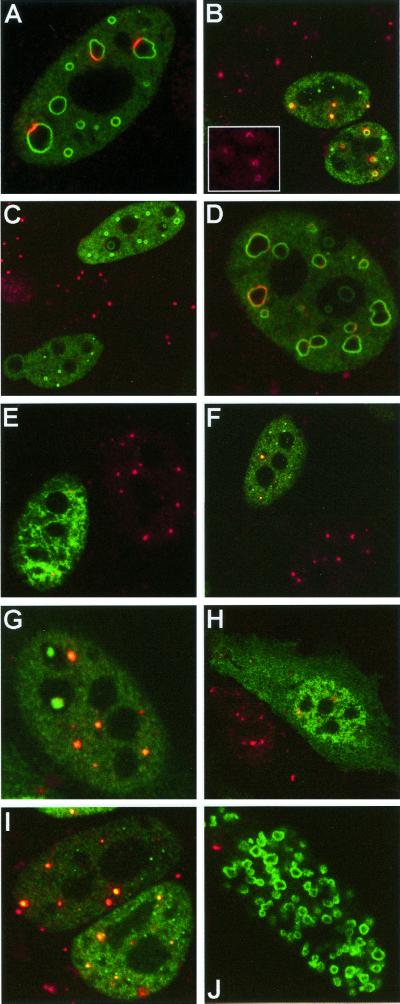
FIG. 2.
Effect of tagged ICP0 homologues on cellular proteins. HEp-2 cells were transfected with UL30-BICP0 (A to D), UL30-Eg63 (E and F), UL30-EP0 (G and H), or UL30-Vg61 (I and J). At 24 h posttransfection, cells were processed for confocal microscopy and costained with polyclonal anti-UL30 r113 at a dilution of 1/3,500 (A to J [green staining]) and either MAb anti-PML 5E10 at a dilution of 1/15 (A, B, and E to J [red staining]) or polyclonal rat anti-Sp100 Sp26 at a dilution of 1/2,000 (C and D [red staining]). Secondary antibodies used were FITC-conjugated goat anti-rabbit IgG (Sigma) at 1/100 and Cy3-conjugated goat anti-mouse IgG (Amersham) at 1/1,000 or Cy3-conjugated goat anti-rat IgG (Amersham) at 1/500. The inset (B) shows PML remaining in the lower right cell
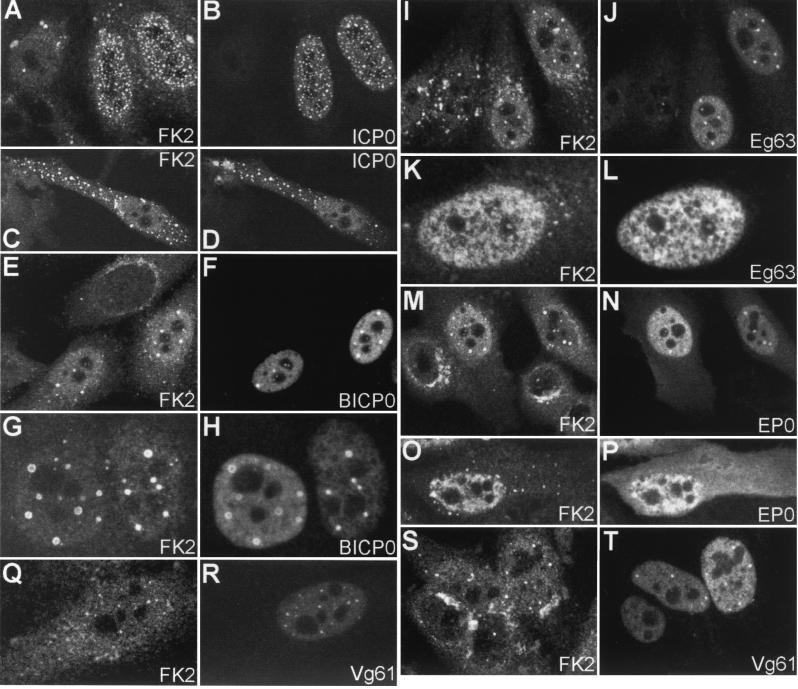
FIG. 3.
Effect of tagged ICP0 and homologues on ubiquitin conjugation seen by confocal microscopy. HEp-2 cells were transfected with UL30-ICP0 (A to D), UL30-BICP0 (E to H), UL30-Eg63 (I to L), UL30-EP0 (M to P), or UL30-Vg61 (Q to T). At 24 h posttransfection, cells were processed for confocal microscopy and costained with MAb FK2 at a dilution of 1/10,000 (A, C, E, G, I, K, M, O, Q, and S) and polyclonal anti-UL30 r113 at a dilution of 1/3,500 (B, D, F, H, J, L, N, P, R, and T). Secondary antibodies used were FITC-conjugated goat anti-rabbit IgG (Sigma) at 1/100 and Cy3-conjugated goat anti-mouse IgG (Amersham) at 1/1,000. The original colored images of this multichannel figure may be viewed at http://www.vir.gla.ac.uk./staff/parkinsonj/ICP0family_Ub.shtml.
Effect of UL30-tagged ICP0 homologues on cellular proteins.
The UL30-tagged proteins affected the immunofluorescence staining patterns of the ND10 proteins PML and Sp100 in an almost identical manner to the pp65-tagged proteins (22). The improved reagents allowed a clearer analysis of these effects, and we present here a selection of images to confirm and extend our previous findings. For BICP0 it was clear that any PML remaining in the transfected cells was localized to the outside of BICP0 ring-like structures (Fig. 2A and B; the inset in panel B shows the PML remaining in the lower right cell), and in the few BICP0-transfected cells where a minor amount of Sp100 remained, this also localized to the BICP0 ring structures with a nonuniform intensity (Fig. 2D). The UL30-tagged version of Eg63 (Fig. 2E and F) also disrupted ND10 in most transfected cells, while UL30-tagged EP0 (Fig. 2G and H) disrupted ND10 in some cells but not in others. For both Eg63 and EP0, in those cells with remaining PML foci, this clearly colocalized with a subset of the foci of the viral proteins. UL30-tagged Vg61 had a greater effect on the decrease in immunofluorescence staining of the cellular proteins than pp65-tagged Vg61, probably attributable to its greater level of expression and stability as noted above, but many cells retained some PML staining, which again colocalized with viral protein accumulations (Fig. 2I and J).
Because the only available reagent for detecting CENP-C is also a rabbit serum, we could not confirm directly that the UL30-tagged proteins also affected centromeres in the same manner as the previously studied pp65-tagged proteins. However, cells expressing the ICP0 family proteins could be identified indirectly by their effects on MAb FK2-detected endogenous ubiquitin (Fig. 3). This allowed confirmation that the effects of the UL30-tagged ICP0 family members on centromeres were exactly as described for the pp65-tagged versions (22).
All ICP0 family members induce colocalization of conjugated ubiquitin.
To assess the effect of the ICP0 family members on cellular conjugated ubiquitin, transfected HEp-2 cells were costained with r113 and MAb FK2. All ICP0 family members were able to induce concentrations of colocalizing, conjugated ubiquitin, although to different extents (Fig. 3), which were consistent with the degree of their effects on cellular structures previously seen (22). As expected, rtag-ICP0 had the same effect on the appearance of cellular ubiquitin conjugation as untagged ICP0 (10) (Fig. 3A to D). The general level of conjugated ubiquitin staining increased, and accumulations of punctate conjugated ubiquitin colocalized with rtag-ICP0 foci both within the nucleus and also in the occasional cells with cytoplasmic ICP0 accumulations. In rtag-BICP0-transfected cells, conjugated ubiquitin colocalized with BICP0 dots and circles (Fig. 3E to H). The mottled nuclear signal of rtag-Eg63 evident in transfected cells was exactly mirrored by an increase in the nuclear signal of conjugated ubiquitin, and accumulations which colocalized with rtag-Eg63 dots were also seen (Fig. 3I to L). In contrast to rtag-ICP0, although rtag-EP0 was present in both the nuclei and cytoplasms of transfected cells expressing large amounts of the protein, an increased conjugated ubiquitin signal was seen only in the nucleus, either as an increase in the nuclear diffuse signal or as colocalizing dots (Fig. 3M to P). Finally, rtag-Vg61 had the least obvious effect on conjugated ubiquitin and did not generally lead to an increase in the diffuse nuclear conjugated ubiquitin staining, but dots of conjugated ubiquitin were frequently seen colocalizing with nuclear dots of rtag-Vg61 (Fig. 3Q to T). In the occasional transfected cells which contained many irregular nuclear accumulations of rtag-Vg61 (Fig. 2J), corresponding accumulations of conjugated ubiquitin were evident (data not shown).
Using a variety of approaches, it had previously been shown that the results using MAb FK2 in ICP0-transfected cells were not due to fluorescence overlap or antibody cross-reactivity (10). In a further confirmation, we found that cell monolayers transfected with the rtag plasmids and then singly stained with FK2 contained an equivalent proportion of cells with a conjugated ubiquitin signal characteristic of transfected cells identified by staining directly for the UL30 tag. With respect to possible antibody specificity artifacts, the present results with the other ICP0 family members strongly suggest a lack of cross-reactivity, since even in their related RING finger domains, there is considerable primary sequence variation between the ICP0-related proteins. Additionally, plasmid-expressed untagged ICP0 and BICP0 had the same effect on ubiquitin as the UL30-tagged versions, which eliminates the possibility that the above results were due to recognition of the UL30 epitope tag by FK2.
Induced concentration of colocalizing epitope-tagged exogenous ubiquitin.
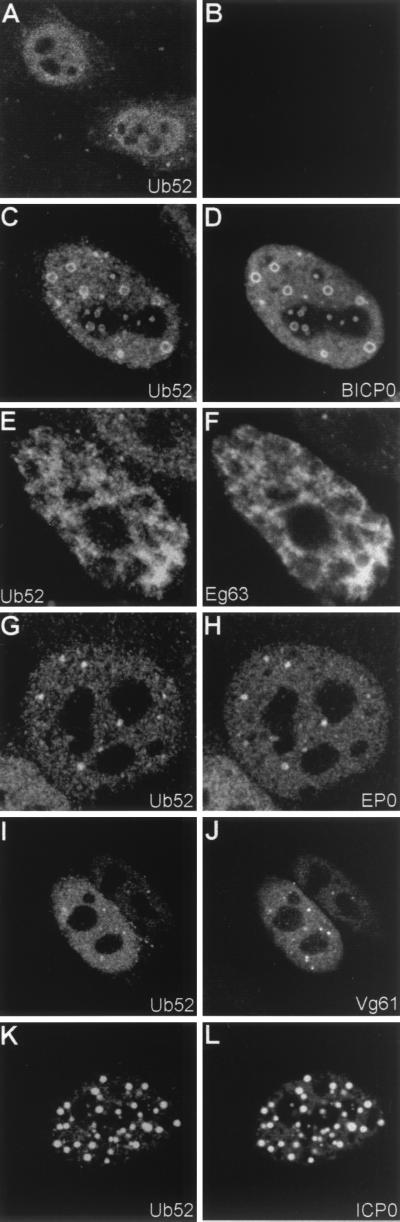
As an alternative approach, the effect of the ICP0 family members on exogenous ubiquitin was determined in HEp-2 cells cotransfected with the rtag-ICP0 plasmids and plasmid pCIQEUb52, which expresses an epitope-tagged ubiquitin precursor (10). As previously noted for ICP0 (10), all the ICP0 family members induced redistribution of the normally diffuse exogenous ubiquitin signal into colocalizing foci, exactly as for endogenous ubiquitin (Fig. 4), although their activity in sequestering ubiquitin was saturated in cells expressing high levels of exogenous ubiquitin, so that free ubiquitin was also diffuse throughout the cell. These results indicate that, like ICP0 itself, the related family members affect the distribution and accumulation of both exogenous ubiquitin and endogenous conjugated ubiquitin.
FIG. 4.
Effect of tagged ICP0 and homologues on exogenously expressed ubiquitin precursor. HEp-2 cells were cotransfected with pCIQEUb52 (expressing the tagged ubiquitin precursor Ub52) and either vector alone (A and B) or the UL30-tagged ICP0 homologues as indicated (C through L). At 24 h posttransfection, cells were processed for confocal microscopy and costained with MAb MRGS at a dilution of 1/1,000 (A, C, E, G, I, and K) and polyclonal anti-UL30 r113 at a dilution of 1/3,500 (D, F, H, J, and L). Secondary antibodies used were FITC-conjugated goat anti-rabbit IgG (Sigma) at 1/100 and Cy3-conjugated goat anti-mouse IgG (Amersham) at 1/1,000.
This report demonstrates that all the ICP0 family members affect the accumulation of intracellular conjugated ubiquitin in a related manner, thereby strengthening the hypothesis that, like several other RING finger proteins, they act via a related biochemical mechanism involving E3 ubiquitin ligase activity. However, our data in this and a previous paper (22) indicate that some members of the family are more potent in these assays than others. These intrinsic differences could be a reflection of the primary sequence divergence between the individual proteins (even within their RING finger domains), which could result in differences in their interactions with the cellular components of the active complex and the protein targets. These differences could be a reflection of the individual interactions between the viruses and their hosts.
Acknowledgments
We thank Duncan McGeoch for constructive criticism.
This research was supported by the Medical Research Council.
REFERENCES
- 1.Borden K L B. RING domains: master builders of molecular scaffolds? J Mol Biol. 2000;295:1103–1112. doi: 10.1006/jmbi.1999.3429. [DOI] [PubMed] [Google Scholar]
- 2.Cheung A K. DNA nucleotide sequence analysis of the immediate-early gene of pseudorabies virus. Nucleic Acids Res. 1989;17:4637–4646. doi: 10.1093/nar/17.12.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davison A J, Scott J E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 4.Everett R D. Analysis of the functional domains of herpes simplex virus type 1 immediate-early polypeptide Vmw110. J Mol Biol. 1988;202:87–96. doi: 10.1016/0022-2836(88)90521-9. [DOI] [PubMed] [Google Scholar]
- 5.Everett R D. Construction and characterisation of herpes simplex virus type 1 mutants with defined lesions in immediate-early gene 1. J Gen Virol. 1989;70:1185–1202. doi: 10.1099/0022-1317-70-5-1185. [DOI] [PubMed] [Google Scholar]
- 6.Everett R D, Maul G G. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J. 1994;13:5062–5069. doi: 10.1002/j.1460-2075.1994.tb06835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everett R D, Freemont P, Saitoh H, Dasso M, Orr A, Kathoria M, Parkinson J. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J Virol. 1998;72:6581–6591. doi: 10.1128/jvi.72.8.6581-6591.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Everett R D, Meredith M, Orr A. The ability of herpes simplex virus type 1 immediate-early protein Vmw110 to bind to a ubiquitin-specific protease contributes to its role in the activation of gene expression and stimulation of virus replication. J Virol. 1999;73:417–426. doi: 10.1128/jvi.73.1.417-426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everett R D, Earnshaw C E, Findley J, Lomonte P. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J. 1999;18:1526–1538. doi: 10.1093/emboj/18.6.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everett R D. ICP0 induces the accumulation of conjugated ubiquitin. J Virol. 2000;74:9994–10005. doi: 10.1128/jvi.74.21.9994-10005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett R D. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioassays. 2000;22:761–770. doi: 10.1002/1521-1878(200008)22:8<761::AID-BIES10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 12.Freemont P S. Ubiquitination: RING for destruction? Curr Biol. 2000;10:R84–R87. doi: 10.1016/s0960-9822(00)00287-6. [DOI] [PubMed] [Google Scholar]
- 13.Fujimuro M, Sawada H, Yokoswa H. Production and characterisation of monoclonal antibodies specific to multi-ubiquitin chains of polyubiquitinated proteins. FEBS Lett. 1994;349:173–180. doi: 10.1016/0014-5793(94)00647-4. [DOI] [PubMed] [Google Scholar]
- 14.Grotzinger T, Sterndorf T, Jenson K, Will H. Interferon-modulated expression of genes encoding the nuclear-dot-associated proteins Sp100 and promyelocytic leukemia protein (PML) Eur J Biochem. 1996;238:554–560. doi: 10.1111/j.1432-1033.1996.0554z.x. [DOI] [PubMed] [Google Scholar]
- 15.Jackson P K, Eldridge A G, Freed E, Furstenthal L, Hsu J Y, Kaiser B K, Reimann J D R. The lore of the RINGs: substrate recognition and catalysis by ubiquitin ligases. Trends Cell Biol. 2000;10:429–439. doi: 10.1016/s0962-8924(00)01834-1. [DOI] [PubMed] [Google Scholar]
- 16.Joazeiro C A, Weissman A M. RING finger proteins: mediators of ubiquitin ligase activity. Cell. 2000;102:549–552. doi: 10.1016/s0092-8674(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 17.Lees-Miller S P, Long M C, Kilvert M A, Lam V, Rice S A, Spencer C A. Attenuation of DNA-dependent protein kinase activity and its catalytic subunit by the herpes simplex virus type 1 transactivator ICP0. J Virol. 1996;70:7471–7477. doi: 10.1128/jvi.70.11.7471-7477.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsden H S, Murphy M, McVey G L, MacEachran K A, Owsianka A M, Stow N D. Role of the carboxy terminus of herpes simplex virus type 1 DNA polymerase in its interaction with UL42. J Gen Virol. 1994;75:3127–3135. doi: 10.1099/0022-1317-75-11-3127. [DOI] [PubMed] [Google Scholar]
- 19.Maul G G, Guldner H H, Spivack J G. Modification of discrete nuclear domains induced by herpes simplex virus type 1 immediate-early gene 1 product ICP0. J Gen Virol. 1993;74:2679–2690. doi: 10.1099/0022-1317-74-12-2679. [DOI] [PubMed] [Google Scholar]
- 20.Maul G G, Everett R D. The nuclear location of PML, a cellular member of the C3HC4 zinc binding domain protein family, is rearranged during herpes simplex virus infection by the C3HC4 viral protein ICP0. J Gen Virol. 1994;75:1223–1233. doi: 10.1099/0022-1317-75-6-1223. [DOI] [PubMed] [Google Scholar]
- 21.Parkinson J, Lees-Miller S P, Everett R D. Herpes simplex virus type 1 immediate-early protein Vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA-dependent protein kinase. J Virol. 1999;73:650–657. doi: 10.1128/jvi.73.1.650-657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parkinson J, Everett R D. Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 affect cellular structures and proteins. J Virol. 2000;74:10006–10017. doi: 10.1128/jvi.74.21.10006-10017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saitoh H, Tomkiel J, Cooke C A, Ratrie H, Maurer M, Rothfield N F, Earnshaw W C. CENP-C, an autoantigen in scleroderma, is a component of the human inner kinetochore plate. Cell. 1992;70:115–125. doi: 10.1016/0092-8674(92)90538-n. [DOI] [PubMed] [Google Scholar]
- 24.Stuurman N, DeGraaf A, Josso A, Humbel B, DeYong L, van Driel R. A monoclonal antibody recognising nuclear matrix associated nuclear bodies. J Cell Sci. 1992;101:773–784. doi: 10.1242/jcs.101.4.773. [DOI] [PubMed] [Google Scholar]
- 25.Telford E A R, Watson M S, McBride K, Davison A J. The DNA sequence of equine herpesvirus 1. Virology. 1992;189:304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- 26.Tyers M, Willems A R. One RING to rule a superfamily of E3 ubiquitin ligases. Science. 1999;284:601–604. doi: 10.1126/science.284.5414.601. [DOI] [PubMed] [Google Scholar]
- 27.Wirth U V, Fraefel C, Vogt B, Vlcek C, Paces V, Schwyzer M. Immediate-early RNA 2.9 and early RNA 2.6 of bovine herpesvirus 1 are 3′ coterminal and encode a putative zinc finger transactivator protein. J Virol. 1992;66:2763–2772. doi: 10.1128/jvi.66.5.2763-2772.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]