Abstract
Twice each year, millions of night-migratory songbirds migrate thousands of kilometers. To find their way, they must process and integrate spatiotemporal information from a variety of cues including the Earth's magnetic field and the night-time starry sky. By using sensory-driven gene expression, we discovered that night-migratory songbirds possess a tight cluster of brain regions highly active only during night vision. This cluster, here named “cluster N,” is located at the dorsal surface of the brain and is adjacent to a known visual pathway. In contrast, neuronal activation of cluster N was not increased in nonmigratory birds during the night, and it disappeared in migrants when both eyes were covered. We suggest that in night-migratory songbirds cluster N is involved in enhanced night vision, and that it could be integrating vision-mediated magnetic and/or star compass information for night-time navigation. Our findings thus represent an anatomical and functional demonstration of a specific night-vision brain area.
Keywords: behavioral molecular mapping; bird orientation; cognition; magnetic sense; ZENK (zif268, Egr-1, NGF-1A, and Krox-24)
Night-migratory songbirds use both a geomagnetic and a star compass to orient during migration (1-6) but the underlying brain circuits are unknown. Star-compass orientation requires vision in dim light for processing constellations of the night-time starry sky. Surprisingly, magnetic-compass orientation also seems to require night vision: magnetic-compass orientation is dependent on the wavelength of the dim ambient light (4, 7), birds with their right eye covered seem unable to perform magnetic compass orientation (8), and birds can still perform magnetic compass orientation with their pineal gland removed (9). Current evidence suggests that visual sensing of the magnetic field occurs through the eyes by means of a light-activated, radical-pair based magnetodetector (4, 7, 10-13). Another type of magnetodetector based on magnetite seems to be predominantly involved in detecting changes in magnetic intensity and/or inclination (4, 14-16). Together, these findings predict that processing of both magnetic- and star-compass information during night-time migration requires specialized night-time visual processing in night-migratory songbirds. We tested this hypothesis by using sensory-driven gene expression (17) to identify brain regions involved in night- and day-time vision in migratory and nonmigratory songbirds. Our results suggest that night-migratory songbirds do indeed possess a brain area specialized for night vision.
Methods
Test Subjects. We examined two distantly related species of wild-caught night-migratory songbirds [garden warblers (GWs), Sylvia borin; and European robins (ERs), Erithacus rubecula] and two distantly related species of nonmigratory songbirds [zebra finches (ZFs), Taeniopygia guttata; and canaries (CNs), Serinus canaria]. The GWs, ERs, and ZFs were kept inside a wooden building under the local photoperiod for at least 5 days before the experiment. Behavioral tests were performed during the GW and ER migratory seasons from August 22 to October 18, 2002 and 2003 and April 15 to May 25, 2003, except for three GWs tested July 17 to August 3, 2003. The canaries were taken from the Jarvis laboratory collection of birds when awake at night and day to confirm consistency between nonmigrants. All animal procedures were approved by the Animal Care and Use Committees of Bezirksregierung Weser-Ems (Oldenburg, Germany) and/or Duke University Medical Center (Durham, NC).
Test Procedures. On testing day, our night-group birds (n = 12 GWs, 4 ERs, and 5 ZFs) were individually put into a custom-designed cylindrical, transparent Plexiglas cage fitted with a circular perch placed 8.5 cm above the ground in the center of the cage (18) (the two CNs were placed in sound isolation boxes). The birds were allowed to get used to the cage or soundbox for 3-12 h. At dusk (local photoperiod), the room lights were turned off except for four small, diffused light bulbs simulating a natural moonlit night (0.04 lux). This light intensity is typically used in behavioral orientation tests with night-migrants (2, 6, 7, 10). Our day-group birds (n = 5 GWs, 5 ZFs, and 5 CNs) were tested in full room light (≈275 lux). Each bird's behavior was continuously observed in real time by two infrared (840 nm) video cameras (top-view and side-view) connected to a split-screen surveillance monitor and in parallel recorded to videotape (25 frames per sec). No night-bird was collected earlier than 100 min after the lights went off, thereby ensuring that any possible brain activity induced by the day/night transition had decreased to its baseline level by the time the bird was collected. We also took care that no external acoustic signals reached the bird. To minimize brain activity because of movement and other factors, we collected birds only after they had been sitting relatively still but awake (eyes open) for at least 45 min but mostly for 60 min or more, while a minimum of other behaviors occurred (0-16 flights [most performed 0 flights] and occasional walking on the perch or floor; exception: ZFs tested during the day did not sit still). After the birds were killed, their brains were rapidly dissected, embedded in Tissue-Tek O.C.T. (Sakura Finetek, Zoeterwoude, The Netherlands), and quick-frozen in a dry ice/ethanol bath at -80°C.
Eye Capping. The ERs (n = 4) night-time group with eyes open was compared with another group of ERs (n = 4) fitted with light-tight eye caps to prevent any visual stimulus from reaching the eyes. The eye caps were built of small black plastic-velour cylinders (diameter = 10 mm) enlarged with Leucoplast tape (Beiersdorf AG, Hamburg, Germany) and light-tight black tape. Three to 5 h before onset of darkness, the eye caps were glued onto the bird's head to cover the eyes completely without any direct contact to the eyes themselves. The bird was put into the cage, observed continuously, and collected as described.
Gene Expression Analyses. We measured expression of ZENK [acronym for zif268, Egr-1, NGF-1A, and Krox-24 (19)] and c-fos immediate early genes in the brain. Expression of ZENK and c-fos mRNA in the brain is driven by neuronal activity and can be detected in neurons ≈5 min after onset of neural firing with peak expression after 30-45 min (17, 20). Therefore, increased cumulative mRNA expression marks brain regions that were active during the last 45-60 min of sensory stimulation or behavior. However, ZENK and c-fos are not activated by neural activity in all brain cell types. These exceptions in birds are some thalamic neurons, telencephalic neurons receiving primary sensory input from the thalamus, and globus pallidus neurons (21). It has been suggested that these neurons do not express the specific neurotransmitter receptors necessary to induce ZENK and c-fos expression in response to neuronal activity (20). In all other neuron types, which constitute roughly two-thirds of the avian brain, expression of ZENK and/or c-fos follows neuronal firing.
For one bird per group, 12-μm frozen sections were cut throughout the entire brain (left hemisphere in the sagittal plane and the right hemisphere in the coronal plane). We observed no differences in gene expression between left and right hemispheres and therefore continued cutting only sagittal sections of the left hemisphere for all remaining birds. We chose sagittal sections because cluster N was best seen in this plane. Corresponding sections of all birds were fixed in 4% paraformaldehyde and processed by in situ hybridization with antisense 5′-[α-[35S]thio]UTP-labeled riboprobes of a ZF ZENK (K.W. and E.D.J. clone), c-fos (K.W. and E.D.J., unpublished data), or glutamate receptor 1 subunit (GluR1) of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor cDNAs (22) by following described procedures (22). We used GluR1 as an anatomical marker in combination with Nissl staining to identify anatomical boundaries of cerebral subdivisions. The hybridized sections were first exposed to x-ray films (Biomax MR, Kodak) for 1-4 days, dipped into an autoradiographic emulsion (NTB2, Kodak), incubated for 3-4 weeks at 4°C, processed with Kodak developer (D-19) and fixer, and Nissl-stained with cresyl violet acetate solution (Sigma). X-ray film brain images were digitally scanned from a dissecting microscope connected to a SPOT III charge-coupled device camera with spotadvanced imaging software. For quantification, a person naïve to the experimental conditions used photoshop 6.0 (Adobe Systems, San Jose, CA) to measure the mean pixel intensities in the brain regions of interest on a 256-level gray scale pixel range. We measured ZENK expression by calculating the difference in mean pixel density between the relevant brain region and a comparably sized region anterior to it in the same brain subdivision(s) [e.g., cluster N - (anterior hyperpallium + anterior dorsal mesopallium)]. We use the term “region” to refer to a specific part of a brain subdivision and the term “area” to refer to a cluster of regions. To test for significant differences between individual groups, the sigmastat software package was used to perform one-way ANOVA followed by a Student-Newman-Keuls multicomparison.
Results
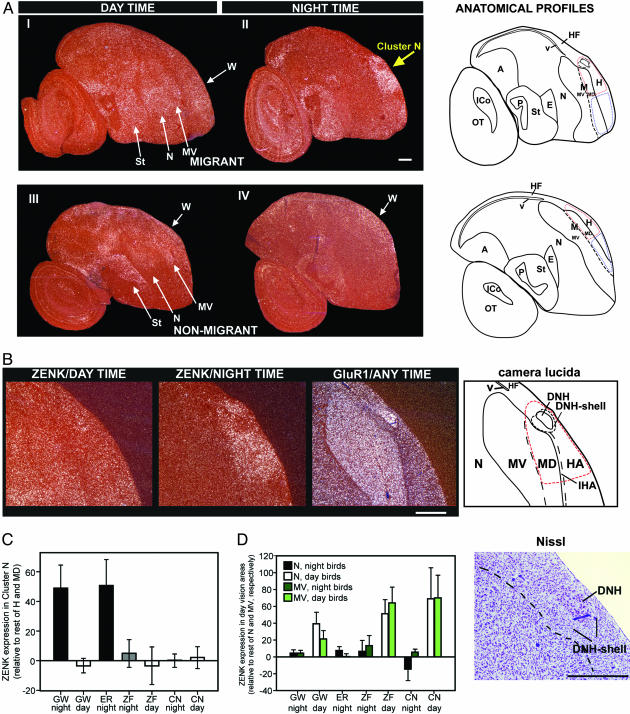
Both species of night-migratory songbirds when awake at night showed a striking pattern of high ZENK expression confined to the dorsal part of the cerebrum (Fig. 1A II, yellow arrow). ZENK expression in this area was not affected by movement behavior (unpublished work) and only medium to low levels of ZENK expression were found throughout the rest of the brain. To anatomically define this cerebral area showing very high expression, in addition to examining Nissl stains, we hybridized adjacent brain sections to GluR1, which distinguishes the boundaries between the avian cerebral brain subdivisions (22, 23). The GluR1- and Nissl-stained pattern revealed that this area consists of five brain regions (Fig. 1B): (i) a portion of the hyperpallium apicale, (ii) a portion of the interstitial region of the hyperpallium apicale, (iii) a portion of the dorsal mesopallium (iv) a nucleus embedded within this portion of the hyperpallium apicale that we named dorsal nucleus of the hyperpallium (DNH), and (v) a shell of cells around DNH that we named the DNH-shell. We designated these regions according to the recent anatomical nomenclature of the avian brain (23). The DNH had higher GluR1 and lower ZENK expression relative to the surrounding hyperpallium, indicating that it behaves differently from the surrounding hyperpallium. The DNH-shell had slightly lower ZENK expression and was characteristically different from the other parts of the hyperpallium (Fig. 1B; and Dominik Heyers, personal communication). In contrast to the strong night-time activation, this cluster of regions showed no significant ZENK induction during the day (Fig. 1 AI and C). c-fos, another activity-dependent gene, like ZENK, also showed strikingly high expression in this cluster of regions at night (Fig. 2). As expected, GluR1 did not show a difference between day and night (data not shown). We therefore named this group of five regions “cluster N” (N for night-activation). Cluster N is fairly large, taking up ≈40% of the hyperpallium and dorsal mesopallium in the sagittal slice where cluster N activation is most prominent. It extends ≈1 mm rostrocaudal, ≈1.5 mm mediolateral, and ≈1.5 mm dorsoventral.
Fig. 1.
ZENK brain activation patterns. (A) Day- and night-time visual activation in night-migratory GWs (I and II) and nonmigratory ZFs (III and IV). Parasagittal brain sections are shown. (Dorsal is up; anterior is right; white-silver grains, ZENK expression; red, Nissl stain; white arrows in I and III, regions activated during day-time vision; yellow arrow in II, regions activated in migrants during night-vision.) Arrows are drawn at different angles for each species to reflect that the relative orientation of the GW cerebrum is naturally angled more forward than the ZF cerebrum. The red and blue highlighted regions in the anatomical drawing indicate the regions quantified from for C.(B) Anatomical characterization of cluster N in GWs. The red-dashed line highlights the cluster N area of ZENK expression. (C) Differences in cluster N ZENK expression among groups were highly significant {cluster N, i.e., [posterior hyperpallium (H) + dorsal mesopallium (MD)] - [anterior H + MD]; by one-way ANOVA, df = 6; dfresidual = 29; F = 22.4; P < 0.001}. Relative cluster N ZENK expression in GWs and ERs at night was significantly higher compared with all other groups (Student-Newman-Keuls multicomparison test: P < 0.001 for all individual comparisons). Relative cluster N ZENK expression in GWs during the day and in the comparable region in nonmigrants (ZFs; CNs) during the day or night was not significantly different among each other (Student-Newman-Keuls multicomparison test: P > 0.73 for all comparisons) nor from anterior H + MD (all 95% confidence intervals include 0, which indicates identical expression in cluster N and the rest of H + MD.) The y axis shows relative pixel density on a 256 gray scale. (D) ZENK induction in known day-vision regions was significantly higher during the day (one-way ANOVA: P < 0.001 for all within-species comparisons between day and night birds except the mesopallium ventrale (MV) comparison for GWs where P = 0.06). Error bars = standard deviations. A, arcopallium; P, pallidum; E, entopallium; St, striatum; N, nidopallium; M, mesopallium; H, hyperpallium; ICo, intercollicular complex; v, ventricle; OT, optic tectum; HF, hippocampal formation; IHA, interstitial region of the hyperpallium apicale (HA); DNH, dorsal nucleus of the hyperpallium; W, visual Wulst). (Scale bars, 0.5 mm.) A high-resolution PDF version of Fig. 1 is available in the supporting information on the PNAS web site.
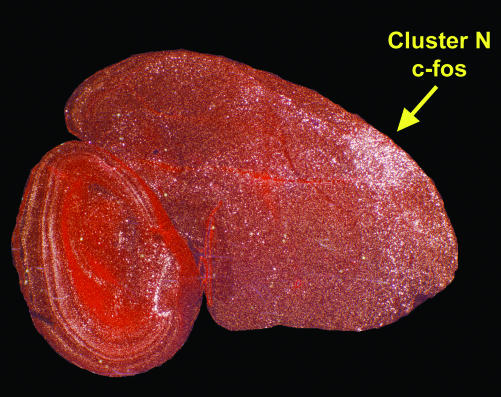
Fig. 2.
Example of cluster N c-fos induction at night in night-migrants (GW). The sagittal section is more lateral than that in Fig. 1, where the DNH nucleus is no longer present. A high-resolution PDF version of Fig. 2 is available in the supporting information on the PNAS web site.
The strong night-time activation in cluster N observed in the two migratory songbird species was not found in the two nonmigratory species (Fig. 1 A and C). Although, in nonmigrants, expression in the entire hyperpallium and MD (posterior to anterior) in the equivalent sagittal plane was slightly higher than in other brain regions at night, there was a decreasing tendency in absolute ZENK expression levels at night [mean intensity index: nonmigrants day = 95 ± 13 (SD), nonmigrants night = 76 ± 20; t test P = 0.15].
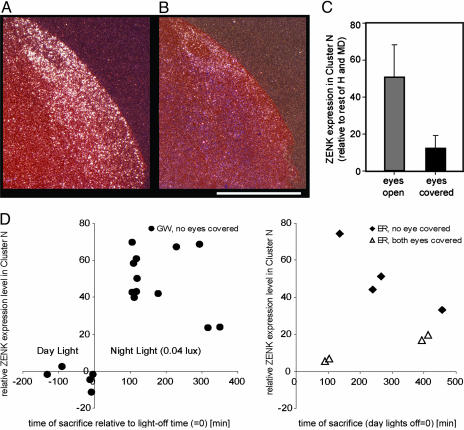
The hyperpallium is also known as the “Wulst” (meaning bulge) with its central-dorsal part functioning as the “visual Wulst” (24). Because no defined anatomical boundaries are known of the visual Wulst, we cannot prove whether cluster N is a part of the visual Wulst (being either a specialization or having evolved out of it) or whether it is located adjacent to it. However, a relationship to the visual Wulst is very likely because the anterior part of cluster N is localized in the mediodorsal part of the hyperpallium, which corresponds to the described posterior extensions of the visual Wulst (24). Based on this knowledge and on the predicted visual nature of magnetic- and star-compass input to the brain in combination with the fact that cluster N activation seems specific to night-migrants, we suspected that cluster N could be processing visual information at night. To test this hypothesis, we performed ZENK in situ hybridization on brains from night-migrants that had both eyes covered by light-tight eye caps during the dim-light night. After becoming used to the eye caps, these birds sat relatively still for extended periods of time, as did the birds without eye cover. Blocking visual input resulted in strong reduction of dim-light, night-time-induced cluster N ZENK expression (Fig. 3 A-C). The DNH-shell did not show reduction of ZENK expression, and thus these cells are still active in complete darkness (Fig. 3B). We conclude that the majority of cluster N activation requires dim-light, visual input.
Fig. 3.
Cluster N ZENK induction at night in night-migrants is visually driven. (A and B) ZENK expression in cluster N in a night-migrant, ER, with eyes open (A) or with both eyes covered by light-tight eye-caps (B). (Scale bar, 1 mm.) (C) Covering the birds' eyes led to a significant reduction in cluster N ZENK expression (t test, t = 4.080; df = 6; P < 0.01; n = 4 per group). The expression in the covered-eye birds is still above zero because the cluster N area quantified included the shell of high expression still present around the DNH nucleus (refer to B). (D) Cluster N ZENK expression as a function of time for all migratory birds used in this study. (Left) GWs with both eyes open. Along the x axis, time point 0 is when the day-lights were turned off and the dim night-time lights were turned on. The amount of time the lights were off did not affect increased cluster N ZENK expression (linear regression of night-time values, F = 1.55, P = 0.24). (Right) Birds with covered eyes show low ZENK expression at times when other birds sitting in dim-light show high ZENK expression throughout cluster N. A high-resolution PDF version of Fig. 3 is available in the supporting information on the PNAS web site.
To check for possible circadian effects, we compared cluster N expression levels in birds at multiple time points after the onset of night-time, dim-light levels (within a 6-h range) and found that the expression levels in cluster N in birds with both eyes open or closed did not correlate with the time of brain collection relative to the onset of dim-light levels (Fig. 3D). Furthermore, birds with covered eyes showed low ZENK expression at times when other birds sitting in dim-light show high ZENK expression in cluster N (Fig. 3D). Thus, cluster N activation cannot simply be because of circadian rhythms.
To test for a possible seasonal variable, we examined three GWs during their nonmigratory breeding season in July and early August, when they did not show evidence of migratory activity behavior at night. These animals showed increased ZENK expression in cluster N during dim-light, night-time conditions and at expression levels that did not differ from those seen in birds tested during the migration season (t test, P = 0.37). Thus, cluster N night-time activation is not purely due to the increase of night-time activity during the migratory season in the migrants but appears to be specific to night-migratory species at all times of the year.
In contrast to the night-time activation in cluster N, during day-light hours consistent increases in expression occurred in a set of regions surrounding the entopallium in both night-migratory and nonmigratory birds (Fig. 1 AI, 1 AIII, and 1D). The entopallium receives thalamic visual input, and like other primary sensory telencephalic neurons it does not show prominent ZENK expression (21). The day-time activated regions around the entopallium included a portion of the nidopallium and a portion of the ventral mesopallium, forming a ventral to dorsal column of brain activation. We suggest that these regions are involved in day-time vision. Support for this suggestion comes from visual pathway connectivity and vision-activated electrophysiological neural firing in these brain regions in ZFs and other bird species (25, 26).
Discussion
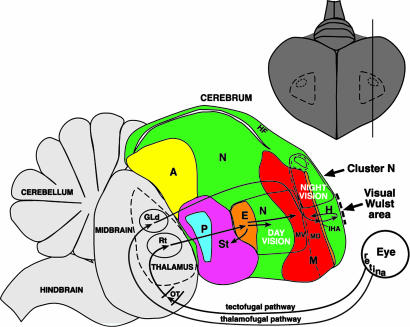
Our results identify a brain area, cluster N, active specifically in night-vision. We suggest that cluster N is evolutionarily related to the visual Wulst. It appears to be localized either posterior to or as a specialized part of the visual Wulst, a visual area comparable to the mammalian striate visual cortex V1 (27). The visual Wulst is part of the thalamofugal pathway (striate visual pathway in mammals) that transfers information directly from the retina to the thalamus, then to the interstitial region of the hyperpallium apicale, and finally to hyperpallium apicale (Fig. 4). The thalamofugal pathway is separate from another visual system called the tectofugal pathway (extrastriate visual pathway in mammals). The avian tectofugal pathway transfers information from the retina to the tectum of the midbrain and, from there, via the thalamus to the entopallium, surrounding nidopallium, and ventral mesopallium (Fig. 4) (25). In nonmigrants, we found that both cerebral visual pathways showed day-time activation. At night, only the visual Wulst area of the thalamofugal pathway still showed some activation, albeit decreased in relation to the day-time level. However, in migrants cluster N showed very high activation at night. These findings cannot prove whether nonmigrants actually do not possess an area comparable to cluster N or whether they do have such an area that does not show enhanced activation during the night. However, because the visual part of the Wulst is typically more centrally located (posterior-anterior) in the hyperpallium, and cluster N is located at the posterior end of hyperpallium, the results suggest that in night-migratory birds, cluster N evolved out of the preexisting thalamofugal visual pathway as a specialized posterior part of or an attachment to the visual Wulst.
Fig. 4.
Schematic drawing of a brain showing the relative locations of the day- and night-vision (cluster N) activated brain regions in night-migrant songbirds. The thamalofugal and tectofugal visual pathways have been determined in other bird species. Upper right, the extent of cluster N seen from the dorsal surface of the brain, determined from serial parasagittal and coronal sections hybridized to ZENK. GLd, lateral geniculate nucleus, dorsal part; Rt, nucleus rotundus; additional abbreviations are as in legend of Fig. 1.
The pattern of night-time and day-time activation suggests another view of the functional organization of the avian cerebrum (Fig. 4). The dorsal mesopallium and the hyperpallium above it are activated as a columnar unit (i.e., cluster N), whereas the ventral mesopallium and the nidopallium ventral to it are activated as another columnar unit (day-time visual activation). This pattern of activation suggests that the hyperpallium is functionally associated with the dorsal mesopallium, whereas the nidopallium is functionally associated with the ventral mesopallium, and that perhaps cluster N with its five subregions represents a functional cerebral system.
The increased ZENK and c-fos expression in cluster N and the fact that this increased expression disappears when the birds' eyes were covered suggest that cluster N increased neural firing during night vision. The consequence of induction of ZENK and other immediate early genes in cluster N of night-migratory songbirds is presumably the same as that proposed for other brain areas that express ZENK (17, 20) (i.e., it regulates the transcription of other genes to either replace proteins that get metabolized when the involved neurons become highly active or to stabilize circuits involved in new information processing).
Why would night-migrants need to evolve a distinct night-vision system, when all songbird species are able to see at night? We suggest that night-migrants may require specialized development of a cerebral system such as cluster N for seeing better at night and/or for visual night-time navigation. The navigation signals sensed and the information processed could be star-light constellations and/or the Earth's magnetic field. The latter possibility would be in line with theoretical (11), molecular (13), and behavioral (4, 7, 8, 10, 12, 18) evidence suggesting that the Earth's magnetic field modulates the light sensitivity of specialized receptor molecules differently in various parts of the retina, leading to perception of the magnetic field as visual patterns (11, 13). As a consequence, the brain regions ultimately extracting the reference direction provided by the geomagnetic field should process and compare purely visual input from the retina. This prediction is in line with ZENK and c-fos expression patterns in the eyes of migratory GWs. Retinal ganglion cells showed high neuronal activity levels at night during magnetic orientation, and these active cells contained high concentrations of cryptochromes, a class of photoreceptor molecules suggested to be involved in magnetodetection (13). In contrast, in nonmigratory ZFs retinal ganglion cells showed lower ZENK expression at night when very little cryptochrome was found in the eyes (13).
The discovery of a distinct night-vision brain area in night-migratory songbirds will allow investigators to probe a specific site in the central nervous system of vertebrates for biological mechanisms of night vision and navigation. Verifying these suggestions will require future studies with electrophysiological recordings and lesions of cluster N.
Supplementary Material
Acknowledgments
We thank Andreas Sommer (University of Oldenburg) for building the observation cages; Drs. Toru Shimizu, Scott Husband, and two anonymous referees for comments on the manuscript; the electronics work-shop at the University of Oldenburg for other assistance; the Institut für Vogelforschung, (Wilhelmshaven and Helgoland, Germany); and the Biological Station Rybachy (Russia) for help catching and keeping birds. This research was supported by the Volkswagen Stiftung Nachwuchsgruppe grant and University of Oldenburg (to H.M.) and the National Science Foundation's 2002 Waterman Award (to E.D.J.).
Author contributions: H.M. and E.D.J. designed research; H.M., G.F., M.L., and K.W. performed research; H.M., K.W., and E.D.J. contributed new reagents/analytic tools; H.M., G.F., M.L., and E.D.J. analyzed data; and H.M., G.F., and E.D.J. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GW, garden warbler (Sylvia borin); ER, European robin (Erithacus rubecula); ZF, zebra finch (Taeniopygia guttata); CN, canary (Serinus canaria); ZENK, zif268, Egr-1, NGF-1A, and Krox-24; MD, dorsal mesopallium; DNH, dorsal nucleus of the hyperpallium; GluR1, glutamate receptor 1 subunit.
References
- 1.Emlen, S. T. (1967) Auk 84, 309-342. [Google Scholar]
- 2.Wiltschko, W. & Wiltschko, R. (1972) Science 176, 62-64. [DOI] [PubMed] [Google Scholar]
- 3.Mouritsen, H. (1998) Anim. Behav. 55, 1311-1324. [DOI] [PubMed] [Google Scholar]
- 4.Wiltschko, W. & Wiltschko, R. (2002) Naturwissenschaften 89, 445-452. [DOI] [PubMed] [Google Scholar]
- 5.Cochran, W. W., Mouritsen, H. & Wikelski, M. (2004) Science 304, 405-408. [DOI] [PubMed] [Google Scholar]
- 6.Mouritsen, H. & Larsen, O. N. (2001) J. Exp. Biol. 204, 3855-3865. [DOI] [PubMed] [Google Scholar]
- 7.Wiltschko, W., Munro, U., Ford, H. & Wiltschko, R. (1993) Nature 364, 525-527. [Google Scholar]
- 8.Wiltschko, W., Traudt, J., Gunturkun, O., Prior, H. & Wiltschko, R. (2002) Nature 419, 467-470. [DOI] [PubMed] [Google Scholar]
- 9.Schneider, T., Thalau, H. P., Semm, P. & Wiltschko, W. (1994) J. Exp. Biol. 194, 255-262. [DOI] [PubMed] [Google Scholar]
- 10.Muheim, R., Backman, J. & Akesson, S. (2002) J. Exp. Biol. 205, 3845-3856. [DOI] [PubMed] [Google Scholar]
- 11.Ritz, T., Adem, S. & Schulten, K. (2000) Biophys. J. 78, 707-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritz, T., Thalau, P., Phillips, J. B., Wiltschko, R. & Wiltschko, W. (2004) Nature 429, 177-180. [DOI] [PubMed] [Google Scholar]
- 13.Mouritsen, H., Janssen-Bienhold, U., Liedvogel, M., Feenders, G., Stalleicken, J., Dirks, P. & Weiler, R. (2004) Proc. Natl. Acad. Sci. USA 101, 14294-14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirschvink, J. L., Walker, M. M. & Diebel, C. E. (2001) Curr. Opin. Neurobiol. 11, 462-467. [DOI] [PubMed] [Google Scholar]
- 15.Fleissner, G., Holtkamp-Rotzler, E., Hanzlik, M., Winklhofer, M., Petersen, N. & Wiltschko, W. (2003) J. Comp. Neurol. 458, 350-360. [DOI] [PubMed] [Google Scholar]
- 16.Mora, C. V., Davison, M., Wild, J. M. & Walker, M. M. (2004) Nature 432, 508-511. [DOI] [PubMed] [Google Scholar]
- 17.Jarvis, E. D. & Nottebohm, F. (1997) Proc. Natl. Acad. Sci. USA 94, 4097-5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mouritsen, H., Feenders, G., Liedvogel, M. & Kropp, W. (2004) Curr. Biol. 14, 1946-1949. [DOI] [PubMed] [Google Scholar]
- 19.Mello, C. V., Vicario, D. S. & Clayton, D. F. (1992) Proc. Natl. Acad. Sci. USA 89, 6818-6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarvis, E. D. (2004) in Nature's Music: The Science of BirdSong, eds. Marler, P. & Slabbekoorn, H. (Elsevier, New York), pp. 226-271.
- 21.Mello, C. V. & Clayton, D. F. (1995) J. Neurobiol. 26, 145-161. [DOI] [PubMed] [Google Scholar]
- 22.Wada, K., Sakaguchi, H., Jarvis, E. D. & Hagiwara, M. (2004) J. Comp. Neurol. 476, 44-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiner, A., Perkel, D. J., Bruce, L. L., Butler, A. B., Csillag, A., Kuenzel, W., Medina, L., Paxinos, G., Shimizu, T., Striedter, G., et al. (2004) J. Comp. Neurol. 473, 377-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medina, L. & Reiner, A. (2000) Trends Neurosci. 23, 1-12. [DOI] [PubMed] [Google Scholar]
- 25.Krutzfeldt, N. O. & Wild, J. M. (2004) J. Comp. Neurol. 468, 452-465. [DOI] [PubMed] [Google Scholar]
- 26.Colombo, M., Frost, N. & Steedman, W. (2001) Brain Res. 917, 55-66. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu, T. & Bowers, A. N. (1999) Behav. Brain Res. 98, 183-191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.