Abstract
Heteroaryldihydropyrimidines (HAPs) are a new class of antivirals inhibiting production of hepatitis B virus (HBV) virions in tissue culture. Here, we examine the effect of a representative HAP molecule, methyl 4-(2-chloro-4-fluorophenyl)-6-methyl-2-(pyridin-2-yl)-1,4-dihydropyrimidine-5-carboxylate (HAP-1), on the in vitro assembly of HBV capsid protein (Cp). HAP-1 enhances the rate and extent of Cp assembly over a broad concentration range. Aberrant particles, dominated by hexagonal arrays of Cp, were observed from assembly reactions with high HAP-1 concentrations. HAP-1 also led to dissociation of metastable HBV capsids, overcoming a kinetic barrier to dissociation by scavenging Cp and redirecting its assembly into hexamer-rich structures. Thus, HAP drugs act as allosteric effectors that induce an assembly-active state and, at high concentration, preferentially stabilize noncapsid polymers of Cp. HAP compounds may have multiple effects in vivo stemming from inappropriate assembly of Cp. These results show that activating and deregulating virus assembly may be a powerful general approach for antiviral therapeutics.
Keywords: antiviral, virus assembly, protein polymerization
More than 350 million people worldwide are chronically infected with hepatitis B virus (HBV) (1). Approved treatments for chronic HBV are IFN-α and the polymerase inhibitors lamivudine and adefovir (2-4). IFN therapy has limited efficacy (5). Resistant viruses arise during treatment with polymerase inhibitors (6, 7), with rates for lamivudine resistance approaching 50% (2, 8). Clearly, there is a great need to diversify the therapeutic and prophylactic arsenal for HBV.
HBV persistence and transmission require HBV replication, which depends on the assembly of a core particle composed of capsid protein (Cp), polymerase, and pregenomic RNA. Reverse transcription to produce infectious DNA-containing particles occurs solely within the core residing in the cytoplasm (9, 10). Thus, core assembly is likely to be a high value target for therapeutics (11).
The capsid, the protein shell of the core, is built of 120 Cp dimers arranged with T = 4 symmetry (12, 13). The dimer interfaces are evident as spikes (14-16) that are the major epitope of the capsid (17). Cp in low ionic strength solution is dimeric (18). We have studied ionic strength-dependent capsid assembly extensively in vitro by using the Cp assembly domain (Cp149) (residues 1-149) lacking the 34-residue C-terminal RNA-binding domain (19-21). Assembly is nucleated by a trimer of Cp dimers and proceeds without accumulating observable populations of intermediates (22). Interactions between dimers are weak but sum to give a globally stable capsid (23). These capsids persist, even under conditions where they are not thermodynamically favored, because of hysteresis to dissociation (24). Some Cp mutations lead to faster assembly and greater stability (25), indicating that wild-type Cp is suboptimal for assembly and suggesting that assembly is regulated in vivo, possibly by conformational change. In support of this assertion, we found that Zn2+ alters the conformation of Cp dimers and enhances the rate of assembly, suggesting that capsid assembly is allosterically regulated (26).
Recently, it was discovered that heteroaryldihydropyrimidines (HAPs) (Fig. 1) affect the accumulation of HBV capsids (27, 28). HAP drugs decreased the yield of assembled core and HBV genomes from cells that constitutively produce HBV. Electron microscopy showed that Cp assembled in vitro in the presence of HAP drugs led to polymers that had abnormal morphology (29). Similarly, small molecules such as bis ANS {5,5-bis[8-(phenylamino)-1-naphthalenesulfonate]} alter Cp assembly in vitro (30). Recent reports suggest that other small molecules also inhibit normal HBV capsid assembly (31-33).
Fig. 1.
Structure and synthesis of HAP-1. See Materials and Methods for a description of the synthetic scheme. HAP-1 is an analog of BAY 41-4109, previously shown to inhibit HBV replication in tissue culture (27) and misdirect assembly (29).
Here, we describe the mechanism of a representative HAP compound, HAP-1 [methyl 4-(2-chloro-4-f luorophenyl)-6-methyl-2-(pyridin-2-yl)-1,4-dihydropyrimidine-5-carboxylate] (Fig. 1). In vitro, low concentrations of HAP-1 enhance both the rate and extent of assembly by favoring an assembly-active state; thus, HAP-1 acts like an allosteric effector. At higher concentrations, HAP-1 led to aberrant noncapsid polymers in vitro, even at the expense of preexisting capsids. We propose that both of these effects on assembly contribute to reducing HBV virion production.
Materials and Methods
Synthesis of HAP-1. Preparation of racemic HAP-1 (Fig. 1) was adapted from the patent literature (27, 28, 34). Condensation of a pyridylamidine with a substituted α-carboxymethyl enone gave a 30% yield of >97% pure HAP-1 after chromatographic purification. HAP-1 was characterized by 1H, 13C, and 19F NMR and mass spectrometry (see the supporting information, which is published on the PNAS web site). HAP-1 was quantitated by absorbance at either 280 nm (ε = 3,800 M-1·cm-1) or 332 nm (ε = 3,000 M-1·cm-1) in aqueous solution.
Protein Expression and Purification. Truncated HBV Cp (Cp149) was expressed and purified from Escherichia coli as Cp149 dimers, as described in refs. 21 and 30.
Light Scattering (LS). Assembly was monitored in real time by 90° LS as described in ref. 35, except that LS was measured at 400 nm, where HAP-1 absorbance was negligible. Assembly was initiated by mixing 2× Cp149 in 50 mM Hepes (pH 7.5) with 50 mM Hepes (pH 7.5)-buffered 2× NaCl. Protein and NaCl solutions were preequilibrated to 37°C, and assembly reactions were carried out at 37°C by using a jacketed cuvette holder.
Size-Exclusion Chromatography (SEC). Assembly reactions were examined by SEC on a Superose 6 10/30 column mounted on a HPLC system equipped with an auto injection module (Shimadzu). The column was equilibrated with 50 mM Hepes, pH 7.5/50 mM NaCl. For time-course experiments, assembly was initiated robotically and allowed to proceed for the indicated time before automatic loading. Recovered protein was assigned either to the void (6.5-7.0 ml), capsid (7.0-8.3 ml), dimer (15-16.5 ml), or intermediate (8.3-15 ml) elution. Without drug, ≈5% of the capsid is in the intermediate elution fraction, probably due to interaction with the column matrix (23). For capsid-binding studies, capsids were purified from assembly reactions (42 μM Cp149/500 mM NaCl/50 mM Hepes, pH 7.5/1 mM DTT, 21°C) by SEC using a Superose 6 column equilibrated in the same buffer without DTT and then stored at 21°C in 500 mM NaCl/50 mM Hepes, pH 7.5/1 mM DTT.
EM and Image Analysis. Assembly reactions were prepared for EM as described in ref. 30. Samples were visualized on an H-7600 transmission electron microscope (Hitachi, Tokyo), and images were collected by using an AMT 2K × 2K charge-coupled device camera (Advanced Microscopy Techniques, Danvers, MA). Fourier transforms of regions from selected micrographs were performed by using imagej software (http://rsb.info.nih.gov/ij).
Molecular Modeling. Coordinates for HBV dimers were obtained from the Protein Data Bank (PDB ID code 1QGT) (16). After placement in space groups P6 and P3, analogous to plane groups p6 and p3, structures were minimized by using cns (36). To optimize the fit of the dimer in the unit cell, the a = b unit cell lengths were systematically screened in 5-Å steps; c was set to 100 Å to eliminate interactions between layers. Minimization used 20 cycles of rigid body refinement, each with 30 steps, followed by 1,000 steps of 0.0005-ps constant-temperature Cartesian molecular dynamics using a dielectric constant of 80. Optimum unit cell dimensions were a = b = 90 Å for p6 and a = b = 95 Å for p3. Figures were prepared by using molscript (37) and rendered with raster3d (38).
Results
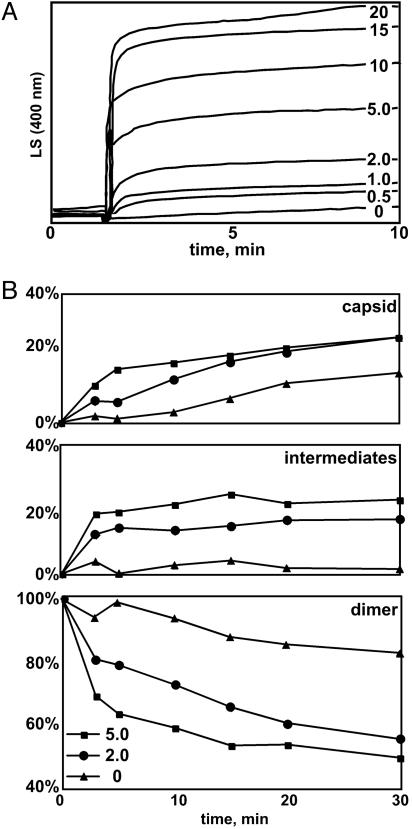
HAP-1 Induces Assembly. HBV Cp assembly was observed by LS and SEC using Cp149 (22). LS is proportional to the average molecular weight of solute. Without HAP-1, LS of assembly is dominated by capsid and shows sigmoidal kinetics (22). HAP-1 causes the initial LS to rapidly increase in a dose-dependent manner and then reach an asymptotic state (Fig. 2A). SEC showed that low HAP-1 concentration ([HAP-1]) resulted in faster formation of capsid (Fig. 2B): In the absence of HAP-1, only ≈0.3 μM capsid (as bound dimer) was formed in the first 10 min of the reaction (<10% of the final yield), whereas ≈2 μM capsid (≈60% of the final yield) was formed in the same time when 5 μM HAP-1 was present. In the presence of HAP-1, we also observed a continuous spectrum of intermediates eluting between capsid and dimer (Figs. 2B and 3A). The magnitude and rapid increase in LS suggests that the LS was probably dominated by the rapid appearance of a large pool of intermediates, which is not seen in the absence of HAP-1 (Figs. 2B and 3 and the supporting information). With HAP-1, intermediates reached steady-state concentrations in the first 5 min of assembly and persisted for at least 24 h (Fig. 2B). Thus, LS reflected enhanced assembly kinetics, even in the absence of aberrant structures. At high [HAP-1], more intermediates and their larger size are both likely to contribute to the greater increase in LS.
Fig. 2.
HAP-1 enhances the rate of production of both capsid and intermediate. (A) Kinetics of assembly were observed by 90° LS at 400 nm. Final concentrations of HAP-1 ranged from 0 to 20 μM, as indicated. (B) Assembly reactions performed in the presence of 0, 2.0, or 5.0 μM HAP-1 were subjected to SEC at the times indicated to determine the proportions of capsid, intermediate elution, and residual dimer (defined in Materials and Methods). The increased LS at higher [HAP-1] in A arises from increased capsid production and accumulation of intermediates. Assembly reactions contained 11 μM Cp149 dimer and were performed at 37°C in 150 mM NaCl at pH 7.5.
Fig. 3.
HAP-1 can increase the extent of assembly and alters assembly products. Assembly reactions performed as described in the legend of Fig. 2 were subjected to SEC after reaching equilibrium (≈24 h). (A) Representative chromatograms are shown. (B) Data from assembly reactions performed in triplicate are summarized. At higher HAP-1 concentrations, the amount of dimer progressively decreased, and the average size and amount of the assembly products increased (A Inset). At low [HAP], no change in free dimer concentration was observed. (C) Electron micrographs show an increase in the proportion of misshapen particles with increasing [HAP-1]. Assembly reactions for EM were performed at 21°C and contained 5 μM Cp149 and 0.5 M NaCl; each micrograph is labeled with the respective [HAP-1] (in micromolar).
At equilibrium, low concentrations of HAP-1 did not significantly alter the extent of assembly at 37°C with 150 mM NaCl (Fig. 3). At progressively higher [HAP-1], the first major peak broadened and eluted progressively earlier, in the void volume. Thus, the average size of Cp assembly products became larger than capsid with increasing [HAP-1] (Fig. 3). At higher [HAP-1], more free dimer was consumed, indicating a greater extent of assembly. Based on intrinsic protein fluorescence and circular dichroism, HAP-1 did not detectably change the structure of Cp: The α-helix-dominated CD spectrum and extremely blue-shifted fluorescence spectrum (24) remained unchanged (data not shown). Even at high [HAP-1], assembly products were recognized by a capsid-specific antibody, indicating that the material was not merely an aggregate of denatured protein. A reduction in the intensity of ELISA signal may indicate subtle differences in epitope conformation or antibody exclusion (see the supporting information).
Previous studies have shown that capsids assembled in vitro from Cp149 are indistinguishable from cores prepared from whole virus (12, 39). Low [HAP-1] did not detectably alter capsid morphology by EM. At higher [HAP-1], an increasing fraction of products was large and pleiomorphic (Fig. 3C and ref. 29). Thus, EM and SEC results are consistent.
HAP-1 Induces Dissociation of Capsids and Assembly of Dimers. Because high [HAP-1] led to decreased dimer and increased aberrant assembly (Fig. 3), HAP-1 must stabilize these products relative to free dimer. This working hypothesis led to two mutually exclusive predictions: (i) if HAP-1 preferentially bound capsids, it would stabilize preexisting capsids or (ii) if HAP-1 preferentially bound dimer, it would destabilize preexisting capsids and induce misdirected assembly.
To test whether the changes in assembly were to due to interaction of HAP-1 with capsid (27) or dimer (29), we examined the effect of the drug on capsid. HAP-1 was mixed with purified capsids under capsid-stabilizing conditions (500 mM NaCl at 21°C), metastable conditions (150 mM NaCl at 21°C), and capsid-destabilizing conditions (150 mM NaCl on ice). Capsids can normally be stored under conditions where they are metastable because of the hysteresis to disassembly (24). For example, in 150 mM NaCl at 21°C, at least 10 μM dimer is needed to support assembly (23); 4 μM dimer does not assemble, yet 4 μM capsid remains largely intact (Fig. 4B). In all cases, the presence of excess HAP-1 (10 μM) decreased the amount of free dimer and capsid observed by SEC, whereas the amount of material eluting in the void volume increased. In contrast to assembly reactions with HAP-1, where we observed a broad product peak (Fig. 2B), reactions where HAP-1 was incubated with capsid showed distinct “void” and “capsid” peaks (Fig. 4).
Fig. 4.
HAP-1 destabilizes metastable capsids and leads to aberrant structures. Purified capsid (4 μM Cp149 dimer) was incubated at either 21°C or on ice for 5 days at either 150 mM or 500 mM NaCl and with or without 10 μM HAP-1. As a control, 4 μM Cp dimer was incubated at 150 mM NaCl on ice with or without 10 μM HAP-1. (A) SEC showed that Cp segregates into distinct void and capsid peaks after incubation with HAP-1. Representative chromatograms are shown (offset vertically for clarity). (B) Summary of SEC data, as above (see the supporting information for data at 24 h). HAP-1 destabilizes the metastable capsids either by extracting Cp dimers during breathing or from the free dimer pool (24), resulting in polymers eluting in the void volume.
The loss of dimer and capsid and the formation of “void” material was particularly pronounced at lower [NaCl] or temperature, where capsid stability is reduced (23). Dimer was originally included in these experiments as a control; dimer does not assemble at 4°C and low ionic strength (Fig. 4B). When starting with dimer and high [HAP-1], the majority of the protein is assembled and eluted in the void peak after 24 and 120 h (Fig. 4 and the supporting information). Starting with capsid under the same conditions, void and capsid peaks were of almost equal height. In contrast, at higher temperature and [NaCl], conditions that enhance capsid stability, more material was retained in the capsid peak. These results lead us to generalize that HAP-1 enhances aberrant assembly. Furthermore, where capsids are less stable, they will be more sensitive to HAP-1.
HAP-1 Induces the Formation of Structures with Hexameric Repeats. HAP-1 had its most striking effect on free dimer on ice (Figs. 4 and 5). EM revealed large structures that were either sheets or tubes of protein (Fig. 5). These polymers were as long as 1,200 Å and up to 600-Å wide. Their crumpled appearance may be due to staining and drying during EM sample preparation.
Fig. 5.
HAP-1 favors the formation of hexameric structures. Electron micrograph of 4 μM Cp149 dimer with 10 μM HAP-1, incubated for 24 h on ice in 150 mM NaCl. No 30-nm HBV capsids were observed. The box shows the approximate area that was analyzed by Fourier transform (Inset); note the sixfold symmetry of the peaks spaced at 45-49 Å. These data are representative of the power spectrum obtained from multiple micrographs showing sheet- or tube-like structures. Model studies suggest that these peaks correspond to the 1,1 peaks from a planar p6 lattice with unit cell dimensions ≈ 90 Å, equivalent to the length of one Cp149 dimer (see the supporting information). (Scale bar, 100 nm.)
Striations in these polymers suggested regular structure. Fourier analysis of selected sheet or tube-like areas showed a hexagonal pattern of peaks at a spacing of 45-49 Å from the origin (Fig. 5 Inset). Thus, we concluded that the structures formed by dimer in the presence of HAP-1 must be composed mainly of hexamers. We were able to recapitulate this Fourier transform in a model study by using rectangular “dimers” arrayed in planar space group p6 (see the supporting information). Taken together, these results indicate that Cp149 dimers formed hexagonally ordered lattices. Similarly, the spacing between quasi-sixfold axes in the HBV capsid structure is ≈90 Å (16).
It was not possible to generate a sheet from hexamers of dimers extracted from the crystal structure of a T = 4 capsid (16), because the curvature of this structure results in clashes between subunits (see the supporting information). Using symmetry as the only constraint, we modeled flat hexameric sheets by placing either a monomer or dimer into the asymmetric unit of plane group p6 or p3, respectively. The residues involved in interactions around the sixfold (or quasi-sixfold in p3) maintained many features observed in the T = 4 quasi-sixfold axes (16). The rms deviation between α-carbons from a modeled dimer and the original AB dimer was small (1.3 Å), about the same rms deviation as between T = 4 quasi-equivalent dimers. The planar structures required modest movements of the C-terminal loop and extended structure (residues 128-142; Fig. 6A). We also observed a small systematic shift in relative positions of the four helices at the dimer interface, again within the variance between quasi-equivalent dimers.
Fig. 6.
Modeling HAP-1-induced assembly. A fragment from a hexagonal lattice modeled by minimizing an AB dimer from the crystal structure (16) in plane group p3 with unit cell dimensions of a = b = 95 Å. (A and B) Dimer is viewed down the spike (A) or from the side (B). Modest changes in the C terminus (residues 128-142) and at the dimer interface are demonstrated in the overlay of the starting (A, red; B, yellow) and minimized (A, indigo; B, cyan) dimers. Note that C termini in both A and B show similar changes, consistent with the quasi-sixfold nature of the p3 threefold. (C) A schematic of Cp assembly products without and with HAP. Small amounts of HAP-1 were tolerated in capsids with apparently normal morphology. Larger amounts of HAP-1 led to aberrant structures that contained larger fractions of hexameric capsomers. Dimers without HAP-1 are filled with gray and black; dimers with bound HAP-1 are white.
Discussion
HAP-1 Acts as an Allosteric Effector for Cp Assembly. HAP-1 enhanced the rate of assembly of HBV Cp and promoted assembly under otherwise nonpermissive conditions. We conclude that HAP-1 activates Cp dimers for assembly; thus, HAP-1 behaves like an allosteric activator. We proposed that the dimer must undergo a conformational change from an assembly-inactive to an assembly-active state based on thermodynamic considerations and the fact that unassembled dimer can be distinguished from capsids (and assembly intermediates) by antibodies recognizing the same amino acid sequence (23, 25, 26). We propose that HAP compounds promote a similar conformational change.
In many respects, low concentrations of HAP-1 and Zn2+ have similar effects (26). Low [Zn2+] led to accelerated assembly without changing the equilibrium, and high [Zn2+] led to persistence of intermediates due to kinetic trapping. Kinetic simulations suggested that the metal had its greatest effect on nucleation: high [Zn2+] induced too many nuclei, inducing kinetic traps. High [HAP-1] led to aberrant assembly, whereas lower HAP-1 increased the rate of capsid production and dimer consumption but also yielded a stable population of intermediates that rapidly accumulate and persist for at least 24 h. These intermediates are true intermediates, rather than “dead ends,” because the rate at which capsid appears increases with intermediate concentration. Species not involved in assembly would act as a “sink” for dimer and reduce the rate of capsid accumulation.
Deres et al. (27) reported that the IC50 and the Kd of BAY 39-5493, another HAP, for capsid were very similar (30 nM), suggesting that the biological effect is exerted at substoichiometric concentrations. Reduced secretion of mature virus in tissue culture was reported with treatment using low [BAY 39-5493] (less than the Kd of drug for capsid), whereas a significant reduction of total Cp levels required higher drug concentrations (27). At low HAP-1, apparently normal capsids were assembled more rapidly in vitro; large-scale misdirection of assembly required considerably higher drug concentrations. [The pure active isomer of BAY41-4109 shows similar activity to racemic HAP-1 in our in vitro assays, but at one-fifth the concentration (data not shown).] Thus, simply deregulating the rate of assembly may be sufficient to reduce the production of normal virus particles. Similarly, F97L, a common, naturally occurring mutant associated with replication defects and secretion of immature virus particles in some cell lines (42), increased rates and extents of assembly in vitro (25).
Enhanced assembly kinetics observed at lower drug concentrations may perturb assembly by inducing assembly inappropriately: e.g., by disrupting the coordination of capsid assembly and polymerase and/or pregenome recruitment or by altering maturation. In vitro, higher [HAP] leads to altered assembly products. Proteasomal destruction of Cp at higher [HAP] decreases total Cp (27) by destroying Cp oligomers or assembly-activated Cp, which we would expect to have exposed hydrophobic surfaces (23).
HAP-1 Can Act as a Morphological Switch. Small amounts of HAP-1 do not result in unusual morphology. At low concentrations, HAP-bound Cp may fit correctly or be tolerated as a minor defect in a globally stable lattice (11, 40, 43). Misdirected assembly of Cp was prevalent at higher [HAP-1], leading to a better understanding of the HAP mechanism. The presence of drug favors the formation of hexamers; sheets and tubes can be constructed exclusively of hexamers. Extra hexamers in a capsid would necessarily break icosahedral symmetry: Balloon-like particles observed at intermediate HAP-1 concentrations may have a higher fraction of HAP-1-bound protein and presumably include more hexamers, but by Euler's theorem, closed particles must still contain 12 pentamers (44). Increased assembly at high [HAP-1], where hexagonal geometry is dominant, indicates a stronger association energy. Surprisingly, HAP-1 has no effect on capsid stability at low concentration, although it enhances the assembly rate. If capsid stability is considered in terms of ΔGcapsid = 12ΔGfivefold + 30ΔGquasi-sixfold, then ΔGfivefold must be weaker in the presence of HAP-1. Thus, in addition to activating dimer for assembly, binding of HAP-1 favors assembly of Cp dimers into hexameric structures, resulting in the prevalence of spirals, tubes, sheets, and larger structures (Fig. 6B). This finding is also consistent with enhanced assembly kinetics at low [HAP-1] because increased intermediate stability increases the rate of capsid assembly (41).
Quasi-equivalence requires that a single protein conform to different environments. HBV subunits from different quasi-equivalent environments (16), and in a modeled hexagonal lattice (Fig. 6B), show only slight conformational differences. Systematically distorted subunits result in systematically distorted structures (11, 40, 43, 45). In models, a few distortions were sufficient to generate aberrant structures (46). However, we found that drug-bound dimers must be present in high proportions to yield an aberrant structure. Thus, consistent with our modeling and ELISA data, we conclude that HAP-1 binding has only a subtle effect on dimer conformation.
What Is the Target of HAP Compounds? No data unambiguously identify the site for HAP-1 binding. Deres et al. (27), using related HAP compounds, showed that HAP bound to capsid, the stoichiometry of binding was approximately one per dimer, and the binding site was near the cluster of histidine residues at the base of the intradimer interface. Hacker et al. (29) could not demonstrate binding to capsid but demonstrated that HAP did not disrupt dimer and led to aberrant capsids and so proposed that the binding site for drug was at the interdimer interface. We also consider a third possibility, that drug binds a small oligomer of dimers: for example, the trimer of dimers that nucleates capsid assembly (22). Each model leads to predictions.
If HAP binding is at the interdimer interface, it should be incorporated into capsids and should, in fact, stabilize them. Our studies with capsid suggest that HAP actually lowers the energy barrier to capsid dissociation (Fig. 4), which can best be explained if HAP-1 is able to extract dimers during capsid “breathing” or bind to the pool of free dimers (47, 48). This hypothesis is consistent with the mechanism proposed for HBV dissociation, where interdimer contacts are stochastically made and broken, resulting in metastable capsids due to hysteresis (24).
HAP binding to dimer and inducing a conformational change is much more consistent with our data, because it would predict the observed increase in the rate of assembly and drug-dependent assembly of dimer at low salt concentrations. Crosslinking studies suggest that the binding site involves histidine residues (27), which form a cluster at the base of the intradimer interface (the “spike”), which we also suggested was involved in the assembly-enhancing effects of Zn2+ that we observed in vitro (26). A conformational change at the intradimer interface at the base of the spike could propagate to the major core-specific antigen epitope at the tip of the spike, altering the local conformation enough to alter antibody binding, consistent with our reduced ELISA signal at higher [HAP-1]. It seems unlikely that binding at the interdimer contact would exert such a conformational effect.
Binding of drug to an oligomer of dimers, such as a preformed nucleus, would be expected to exert a strong kinetic effect on product accumulation. At higher drug concentrations, this effect could lead to kinetically trapped intermediates, consistent with our SEC data. Predictions for binding of drug to dimer or to an oligomer are not mutually exclusive, and the two scenarios would be almost indistinguishable experimentally.
Conclusions
Although an effective vaccine exists, worldwide, there are still many individuals with chronic HBV infections. We have shown that a new class of drugs acts by deregulating capsid assembly. In vitro, high HAP-1 concentration leads to the formation of aberrant structures, whereas substoichiometric [HAP-1] can induce assembly under conditions where assembly would not normally occur. In vivo, both mechanisms would be expected to deplete the pool of Cp available for virion assembly. Additionally, such drugs can destabilize preformed capsids and thus could be used to treat blood and blood products to reduce the threat of transmission of HBV.
Supplementary Material
Acknowledgments
We thank Dr. S. Goldmann (Bayer AG Pharmaceutical Research Centre, Wuppertal, Germany) for the kind gift of BAY41-4109, Pablo Ceres and Jennifer Johnson for useful discussions and critical reading of the manuscript, and the Oklahoma Medical Research Foundation Imaging Facility for use of the electron microscope. This work was supported by American Cancer Society Research Grant RSG 99-339-04-MBC (to A.Z.), David and Lucille Packard Foundation Interdisciplinary Science Program Grant 2001-1773, and National Institutes of Health Grant EB00432 (to M.G.F.).
Author contributions: S.J.S., M.G.F., and A.Z. designed research; S.J.S., C.R.B., S.P., and W.G.L. performed research; S.P., W.G.L., and M.G.F. contributed new reagents/analytic tools; S.J.S., C.R.B., M.G.F., and A.Z. analyzed data; and S.J.S. and A.Z. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Cp, capsid protein; Cp149, Cp assembly domain (amino acids 1-149); HAP, heteroaryldihydropyrimidine; HAP-1, methyl 4-(2-chloro-4-fluorophenyl)-6-methyl-2-(pyridin-2-yl)-1,4-dihydropyrimidine-5-carboxylate; HBV, hepatitis B virus; LS, light scattering; SEC, size-exclusion chromatography.
References
- 1.World Health Organization (2000) WHO Hepatitis B Fact Sheet (World Health Organization, Geneva).
- 2.Karayiannis, P. (2004) Expert Rev. Anti-Infect. Ther. 2, 745-760. [DOI] [PubMed] [Google Scholar]
- 3.Wands, J. (2004) N. Engl. J. Med. 351, 1567-1570. [DOI] [PubMed] [Google Scholar]
- 4.Hollinger, F. B. (1996) in Fields Virology, eds. Fields, B. N., Knipe, D. M., Howley, P. M., Chanock, R. M., Melnick, J. L., Monath, T. P., Roizman, B. & Straus, S. E. (Lippincott-Raven, Philadelphia), pp. 2738-2808.
- 5.Brunetto, M. R., Giarin, M., Saracco, G., Oliveri, F., Calvo, P., Capra, G., Randone, A., Abate, M. L., Manzini, P., Capalbo, M., et al. (1993) Gastroenterology 105, 845-850. [DOI] [PubMed] [Google Scholar]
- 6.Ling, R., Mutimer, D., Ahmed, M., Boxhall, E., Elias, E., Dusheiko, G. & Harrison, T. J. (1996) Hepatology 24, 711-713. [DOI] [PubMed] [Google Scholar]
- 7.Villeneuve, J.-P., Durantel, D., Durantel, S., Westland, C., Xiong, S., Brosgart, C., Gibbs, C., Parvaz, P., Werle, B., Trepo, C., et al. (2003) J. Hepatol. 39, 1085-1089. [DOI] [PubMed] [Google Scholar]
- 8.Liaw, Y. F., Sung, J., Chow, W. C., Farrell, G., Lee, C. Z., Yuen, H., Tanwandee, T., Tao, Q. M., Shue, K., Keene, O., et al. (2004) N. Engl. J. Med. 351, 1521-1531. [DOI] [PubMed] [Google Scholar]
- 9.Ganem, D. (1996) in Fields Virology, eds. Fields, B. N., Knipe, D. M., Howley, P. M., Chanock, R. M., Melnick, J. L., Monath, T. P., Roizman, B. & Straus, S. E. (Lippincott-Raven, Philadelphia), pp. 2703-2737.
- 10.Seeger, C. & Mason, W. S. (2000) Microbiol. Mol. Biol. Rev. 64, 51-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zlotnick, A. & Stray, S. (2003) Trends Biotechnol. 21, 536-542. [DOI] [PubMed] [Google Scholar]
- 12.Kenney, J. M., von Bonsdorff, C. H., Nassal, M. & Fuller, S. D. (1995) Structure (London) 3, 1009-1019. [DOI] [PubMed] [Google Scholar]
- 13.Crowther, R. A., Kiselev, N. A., Bottcher, B., Berriman, J. A., Borisova, G. P., Ose, V. & Pumpens, P. (1994) Cell 77, 943-950. [DOI] [PubMed] [Google Scholar]
- 14.Bottcher, B., Wynne, S. A. & Crowther, R. A. (1997) Nature 386, 88-91. [DOI] [PubMed] [Google Scholar]
- 15.Conway, J. F., Cheng, N., Zlotnick, A., Wingfield, P. T., Stahl, S. J. & Steven, A. C. (1997) Nature 386, 91-94. [DOI] [PubMed] [Google Scholar]
- 16.Wynne, S. A., Crowther, R. A. & Leslie, A. G. W. (1999) Mol. Cell 3, 771-780. [DOI] [PubMed] [Google Scholar]
- 17.Belnap, D. M., Watts, N. R., Conway, J. F., Cheng, N., Stahl, S. J., Wingfield, P. T. & Steven, A. C. (2003) Proc. Natl. Acad. Sci. USA 100, 10884-10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou, S. & Standring, D. N. (1992) Proc. Natl. Acad. Sci. USA 89, 10046-10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birnbaum, F. & Nassal, M. (1990) J. Virol. 64, 3319-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wingfield, P. T., Stahl, S. J., Williams, R. W. & Steven, A. C. (1995) Biochemistry 34, 4919-4932. [DOI] [PubMed] [Google Scholar]
- 21.Zlotnick, A., Cheng, N., Conway, J. F., Booy, F. P., Steven, A. C., Stahl, S. J. & Wingfield, P. T. (1996) Biochemistry 35, 7412-7421. [DOI] [PubMed] [Google Scholar]
- 22.Zlotnick, A., Johnson, J. M., Wingfield, P. W., Stahl, S. J. & Endres, D. (1999) Biochemistry 38, 14644-14652. [DOI] [PubMed] [Google Scholar]
- 23.Ceres, P. & Zlotnick, A. (2002) Biochemistry 41, 11525-11531. [DOI] [PubMed] [Google Scholar]
- 24.Singh, S. & Zlotnick, A. (2003) J. Biol. Chem. 278, 18249-18255. [DOI] [PubMed] [Google Scholar]
- 25.Ceres, P., Stray, S. & Zlotnick, A. (2004) J. Virol. 78, 9538-9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stray, S., Ceres, P. & Zlotnick, A. (2004) Biochemistry 43, 9989-9998. [DOI] [PubMed] [Google Scholar]
- 27.Deres, K., Schröder, C., Paessens, A., Goldmann, S., Hacker, H., Weber, O., Krämer, T., Niewöhner, U., Pleiss, U., Stoltefuss, J., et al. (2003) Science 299, 893-896. [DOI] [PubMed] [Google Scholar]
- 28.Weber, O., Schlemmer, K.-H., Hartmann, E., Hagelshuer, I., Paessens, A., Graef, E., Deres, K., Goldmann, S., Niewohner, U., Stoltefuss, J., et al. (2002) Antiviral Res. 54, 69-78. [DOI] [PubMed] [Google Scholar]
- 29.Hacker, H., Deres, K., Mildenberger, M. & Schroder, C. (2003) Biochem. Pharmacol. 66, 2273-2279. [DOI] [PubMed] [Google Scholar]
- 30.Zlotnick, A., Ceres, P., Singh, S. & Johnson, J. M. (2002) J. Virol. 76, 4848-4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu, X., Tran, T., Simsek, E. & Block, T. (2003) J. Virol. 77, 11933-11940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta, A., Carrouée, S., Conyers, B., Jordan, R., Butters, T., Dwek, R. A. & Block, T. M. (2001) Hepatology 33, 1488-1495. [DOI] [PubMed] [Google Scholar]
- 33.Mehta, A., Conyers, B., Tyrrell, D. L., Walters, K. A., Tipples, G. A., Dwek, R. A., Block, T. M. (2002) Antimicrob. Agents Chemother. 46, 4004-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoltefuss, J., Goldmann, S., Paessens, A., Graef, E. & Lottmann, S. (2002) U.S. Patent 6,436,943.
- 35.Zlotnick, A., Aldrich, R., Johnson, J. M., Ceres, P. & Young, M. J. (2000) Virology 277, 450-456. [DOI] [PubMed] [Google Scholar]
- 36.Brunger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J. S., Kuszewski, J., Nilges, M., Pannu, N. S., et al. (1998) Acta Crystallogr. D 54, 905-921. [DOI] [PubMed] [Google Scholar]
- 37.Kraulis, P. (1991) J. Appl. Crystallogr. 24, 946-950. [Google Scholar]
- 38.Merrit, E. & Bacon, D. (1977) Methods Enzymol. 277, 505-524. [DOI] [PubMed] [Google Scholar]
- 39.Zlotnick, A., Cheng, N., Stahl, S. J., Conway, J. F., Steven, A. C. & Wingfield, P. T. (1997) Proc. Natl. Acad. Sci. USA 94, 9556-9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zlotnick, A. (1994) J. Mol. Biol. 241, 59-67. [DOI] [PubMed] [Google Scholar]
- 41.Endres, D. & Zlotnick, A. (2002) Biophys. J. 83, 1217-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan, T. T., Lin, M. H., Qiu, S. M. & Shih, C. (1998) J. Virol. 72, 2168-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zandi, R., Reguera, D., Bruinsma, R. F., Gelbart, W. M. & Rudnick, J. (2004) Proc. Natl. Acad. Sci. USA 101, 15556-15560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ganser, B. K., Li, S., Klishko, V. Y., Finch, J. T. & Sundquist, W. I. (1999) Science 283, 80-83. [DOI] [PubMed] [Google Scholar]
- 45.Caspar, D. L. D. & Klug, A. (1962) Cold Spring Harbor Symp. Quant. Biol. 27, 1-24. [DOI] [PubMed] [Google Scholar]
- 46.Prevelige, P. E., Jr. (1998) Trends Biotechnol. 16, 61-65. [DOI] [PubMed] [Google Scholar]
- 47.Li, Q., Yafai, A. G., Lee, Y. M.-H., Hogle, J. & Chow, M. (1994) J. Virol. 68, 3965-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis, J. K., Bothner, B., Smith, T. J. & Siuzdak, G. (1998) Proc. Natl. Acad. Sci. USA 95, 6774-6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.