Abstract
Information on late complications in patients with acute leukemia who have undergone allogeneic hematopoietic cell transplantation (HCT) is limited. We performed a left‐truncated analysis of long‐term survival in patients with acute leukemia who were alive and disease‐free 2 years after HCT. We included 2701 patients with acute lymphoblastic leukemia (ALL) and 9027 patients with acute myeloid leukemia (AML) who underwent HCT between 2005 and 2012. The 10‐year overall survival (OS) rate was 81.3% for ALL and 76.2% for AML, with the main causes of late mortality being relapse (ALL‐33.9%, AML‐44.9%) and chronic graft‐versus‐host disease (ALL‐29%, AML‐18%). At 10 years, HCT‐related mortality was 16.8% and 20.4%, respectively. Older age and unrelated donor transplantation were associated with a worse prognosis for both types of leukemia. In addition, transplantation in the second or third complete remission and peripheral blood HSC for ALL are associated with worse outcomes. Similarly, adverse cytogenetics, female donor to male patient combination, and reduced intensity conditioning in AML contribute to poor prognosis. We conclude that 2‐year survival in remission after HCT for acute leukemia is encouraging, with OS of nearly 80% at 10 years. However, the long‐term mortality risk of HCT survivors remains significantly higher than that of the age‐matched general population. These findings underscore the importance of tailoring transplantation strategies to improve long‐term outcomes in patients with acute leukemia undergoing HCT.
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) is a curative therapy for patients with acute leukemia. Advances in transplantation techniques and supportive care have allowed a much larger population to be considered for transplantation and have resulted in significantly improved long‐term survival for HCT recipients. 1 , 2 In the early years of HCT, only younger patients were considered eligible due to the toxicity of conventional myeloablative conditioning (MAC) regimens. Reduced intensity conditioning (RIC) is now widely used to allow transplantation in the elderly and in patients with multiple comorbidities. Several studies have shown similar survival outcomes in both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) patients following HCT with RIC or MAC. 3 , 4 , 5 , 6 , 7 Over the past two decades, there has also been a significant increase in the number of human leukocyte antigen (HLA)‐matched unrelated donor (MUD), mismatched unrelated donor (MMUD), and haploidentical (haplo) transplants for patients without an HLA‐matched sibling donor (MSD). A number of retrospective studies have shown similar overall survival (OS) and leukemia‐free survival (LFS) between haplo‐HCT with high‐dose posttransplant cyclophosphamide and MUD‐HCT. 8 , 9 , 10 , 11 , 12 , 13 Most deaths after HCT occur in the first 2 years post transplantation due to disease relapse, infection, graft‐versus‐host disease (GVHD), or organ toxicity from conditioning regimens. 14 Within the first 2 years after transplantation, advances in GVHD prophylaxis have led to improvements in mortality. 15 , 16 , 17 , 18 , 19 , 20 , 21 However, long‐term survivors of HCT remain at risk of late complications resulting in higher morbidity and mortality compared to the general population. 22 , 23 The largest study of long‐term survivors reported by the Committee of the Center for International Blood and Marrow Transplant Research (CIBMTR) showed that the probability of survival at 10 years for patients who were alive and disease‐free 2 years after HCT was 84% in AML or ALL. 24 A major limitation was that the majority of patients transplanted for ALL were in the pediatric or adolescent and young adult (AYA) population, with only 1% of patients older than 50 years. In a more recent long‐term survivors study comparing RIC to MAC in MSD‐HCT for AML, the Acute Leukemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT) group showed that the 10‐year OS of patients alive and disease‐free at the 2‐year time point approached 75% in both groups. 25 The most common cause of late mortality was relapse, but chronic GVHD (cGVHD), infection, transplant‐related organ toxicity, and secondary malignancies were also important causes of late death. 26 , 27 , 28 Given the major advances in transplantation practices, there is likely to be a larger number of survivors with similar long‐term outcomes as reported in previous studies, despite the increased incidence of high‐risk transplantation. In addition, previous studies did not consider current cytogenetic risk categories and measurable residual disease (MRD) status, which are important prognostic factors for transplantation outcomes. 29 , 30 , 31 , 32 , 33 Long‐term survival after HCT needs to be reevaluated and updated. We thus conducted an analysis of long‐term survival and prognostic factors of late relapse in patients with acute leukemia who are alive and disease‐free at 2 years after HCT, reported to the ALWP of the EBMT.
MATERIALS AND METHODS
Study design and data collection
This is a retrospective, multicenter, registry‐based analysis. Data were provided by the EBMT registry, to which >600 transplant centers annually submit anonymized data on all their consecutive HCTs according to specific guidelines and audited quality measures, after informed consent of the patients and according to local regulations in place at the time of transplantation. The ALWP of the EBMT approved the study according to the tenets of the Declaration of Helsinki. Adult patients aged ≥18 years who received an HCT for AML or ALL, who were alive and relapse‐free 2 years after their first allogeneic HCT, and who were reported to the EBMT between 2005 and 2012, were included. Patients with ex vivo T cell depletion (TCD) or cord blood transplantation were excluded from the study.
Endpoints and statistical analysis
The primary objective of the study was to estimate 10‐year OS after HCT for patients with ALL or AML who were disease‐free at 2 years. OS was defined as the time from HCT to death, regardless of the cause. All surviving patients were censored at the time of last documented contact. Secondary endpoints included non‐relapse mortality (NRM), relapse incidence (RI), leukemia‐free survival (LFS), cGVHD and the composite of cGVHD‐free and relapse‐free survival (cGRFS). LFS was defined as survival without evidence of relapse or progression. RI was defined as leukemia recurrence at any site. NRM was defined as death without evidence of relapse or progression. cGRFS was defined as the time being alive with neither severe cGVHD nor disease relapse at any time point. Probabilities of OS, LFS, and cGRFS were calculated using the Kaplan–Meier method. Cumulative incidence was used to estimate the endpoints of RI and NRM, and cGVHD to accommodate for competing risks. When assessing the cumulative incidence of cGVHD, we considered relapse and death as competing events. The follow‐up time was calculated using the reverse Kaplan–Meier method.
Univariate analyses were performed using the Log‐rank test for OS, LFS, and cGRFS and Gray's test for cumulative incidence functions. Multivariate analysis was performed using the Cox proportional‐hazards model including variables known to potentially influence posttransplant outcomes. Covariates were not statistically selected. Patients with missing information were excluded from the Cox model, except missing cytogenetics which was included as a separate category. Results were expressed as the hazard ratio (HR) with a 95% confidence interval (95% CI). All tests were two‐sided with a type 1 error rate fixed at 0.05. HCT‐related crude mortality was estimated using common methods in relative survival 34 , 35 in which patients were matched by age, sex, and country in the year of HCT, to a cohort from the general population for whom survival information was available in the Human Mortality Database population tables (www.mortality.org). Overall mortality (1–OS) was split into the probability of dying from HCT or relapse and the probability of dying from other causes. 36 , 37 Statistical analyses were performed with SPSS 27.0 (SPSS Inc.) and R 4.1.1 (R Development Core Team, URL: https://www.R-project.org/).
RESULTS
A total of 29,327 acute leukemia patients underwent HCT between 2005 and 2012, 7229 with ALL and 22,098 with AML. Of these, 2701 ALL patients with a median age of 34 (range 18−73.5) years and 9027 AML patients with a median age of 48 (range 18−77.7) years met the study inclusion criteria (Data S1 and Figure S1). Most AML patients (85%) had de novo AML. The majority of patients (78.6% for ALL and 68.6% for AML) were in first complete remission (CR1) with undetectable MRD (68.3% for ALL and 78.9% for AML). There were similar numbers of MSD transplants (43.7% for ALL and 45.6% for AML) and unrelated donor (UD) transplants (53.2% for ALL and 49.5% for AML). Most patients received peripheral blood (PB) grafts (75.7% for ALL and 85.2% for AML) without antithymocyte globulin (ATG) in about half of the cases (55.7% for ALL and 46.8% for AML). Conditioning regimens also varied, with MAC used extensively in most ALL patients (86.5%) but only in half of AML cases (57.5%). Characteristics of patients in these two groups are shown in Table 1.
Table 1.
Population baseline characteristics of ALL and AML
| ALL patients, n = 2701 | AML patients, n = 9027 | ||||
|---|---|---|---|---|---|
| Follow‐up, months | Median [IQR] | 99.46 [97.85–101.82] | 98.18 [97.09–99.19] | ||
| Female | n (%) | 1087 (40.3%) | 4373 (48.5%) | ||
| Age at HCT, years | median (min‐max) [IQR] | 34 (18–73.5) [24.3–45.9] | 48.5 (18–77.7) [37.2–57.6] | ||
| KPS | ≥90 | 1592 (77.5%) | 5578 (78.9%) | ||
| Diagnosis | Phineg B ALL | 463 (17.1%) | AML de novo | 7632 (84.5%) | |
| Phipos B ALL | 635 (23.5%) | AML secondary | 1395 (15.5%) | ||
| T ALL | 663 (24.5%) | Cytogenetics favorable | 600 (6.6%) | ||
| Missing | 940 (34.8%) | Cytogenetics intermed | 3091 (34.2%) | ||
| Cytogenetics adverse | 682 (7.6%) | ||||
| Cytogenetics missing | 4654 (51.6%) | ||||
| FLT3 positive | 592 (24.7%) | ||||
| NPM1 positive | 476 (22.3%) | ||||
| Status at transplant | CR1 | 2124 (78.6%) | 6193 (68.6%) | ||
| ≥CR2 | 411 (15.2%) | 1567 (17.4%) | |||
| Advanced | 166 (6.1%) | 1267 (14%) | |||
| MRD pretransplant | Negative | 840 (68.3%) | 1726 (78.9%) | ||
| Positive | 389 (31.7%) | 461 (21.1%) | |||
| missing | 1306 | 5573 | |||
| Type of donor | MSD | 1180 (43.7%) | 4120 (45.6%) | ||
| UD | 1436 (53.2%) | 4472 (49.5%) | |||
| Other relative | 85 (3.1%) | 435 (4.8%) | |||
| Female to male | Yes | 516 (19.3%) | 1523 (17%) | ||
| Cell source | BM | 655 (24.3%) | 1339 (14.8%) | ||
| PB | 2046 (75.7%) | 7688 (85.2%) | |||
| Patient CMV | Positive | 1409 (58.4%) | 5346 (66.1%) | ||
| Donor CMV | Positive | 1167 (48.2%) | 4241 (52.3%) | ||
| ATG | Yes | 1091 (44.3%) | 4387 (53.2%) | ||
| Conditioning | MAC | 2320 (86.5%) | 5151 (57.5%) | ||
| RIC | 361 (13.5%) | 3806 (42.5%) | |||
| CT | 630 (23.5%) | 6309 (70.1%) | |||
| BuCy ± Other | 2013 (23.4%) | ||||
| BuFlu ± Other | 2171 (25.2%) | ||||
| Other CT | 1731 (20,1%) | ||||
| TBI | 2054 (76.5%) | 2686 (29.9%) | |||
| HCT‐CI | 0 | 538 (72.5%) | 1558 (62%) | ||
| 1 or 2 | 118 (15.9%) | 537 (21.4%) | |||
| ≥3 | 86 (11.6%) | 416 (16.6%) | |||
| Acute GVHD < 2 y | Yes | 837 (32.3%) | 2103 (24%) | ||
| Grade I | 577 | 1930 | |||
| Grade II | 616 | 1598 | |||
| Grade III | 183 | 424 | |||
| Grade IV | 38 | 81 | |||
| No aGvHD present | 1175 | 4727 | |||
| missing | 112 | 267 | |||
| Chronic GVHD < 2 y | Yes | 1335 (52.2%) | 4531 (52.5%) | ||
| GVHD prevention | CSA | 221 (9%) | 1200 (14.6%) | ||
| CSA + MTX | 1665 (67.8%) | 4015 (48.8%) | |||
| CSA + MMF | 288 (11.7%) | 1911 (23.2%) | |||
| Other | 282 (11,7%) | 1098 (13,4%) | |||
| PTCy | 126 (1.5%) | ||||
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATG, antithymocyte globulin; BM, bone marrow; BuCy, busulfan cyclophosphamide; BuFlu, busulfan fludarabine; CMV, Cytomegalovirus; CR, complete remission; CR1, 1st Complete Remission; CR2, 2nd Complete Remission; CR3, 3rd Complete Remission; CSA, cyclosporine A; CT, chemotherapy; Cytogenetics, Risk According to MRC Classification (Blood 2010;116:354–65); cGRFS, composite graft‐versus‐host disease‐free, relapse‐free survival; GVHD, graft‐versus‐host disease; HCT, hematopoietic cell transplantation; HCT‐CI, HCT‐Specific Comorbidity Index; HR, hazard ratio; IQR, interquartile range; Intermed, intermediate; KPS, Karnofsky Performance Status; LFS, leukemia‐free survival; MAC, myeloablative conditioning; min, minimum; max, maximum; MMF, mycophenolate mofetil; MSD, matched sibling donor; MTX, methotrexate; NA, not available; NRM, non‐relapse mortality; OS, overall survival; Phineg, Philadelphia chromosome negative; Phipos, Philadelphia chromosome positive; Ref, reference; RI, relapse incidence; TBI, total body irradiation; TCD, T cell depletion; UD, unrelated donor, y, year.
The 10‐year probabilities of OS and LFS were 81.3% and 78.2%, respectively, for ALL patients (Figure 1) and 76.2% and 72.3%, respectively, for AML patients (Figure 2). The cumulative RI and NRM at 10 years was 9.9% and 11.9%, respectively, for ALL patients (Figure 1) and 15.6% and 12.1%, respectively, for AML patients (Figure 2). The probability of cGRFS at 10 years was 73.3% for ALL patients and 68.1% for AML patients (Table 2).
Figure 1.
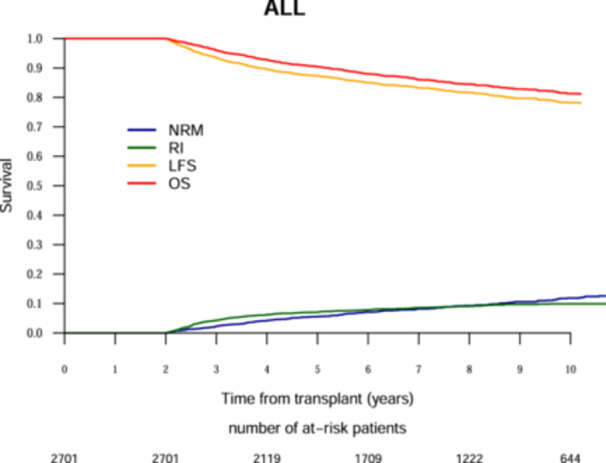
Ten‐year outcomes analysis after allogeneic hematopoietic cell transplantation for acute lymphoblastic leukemia. The red line represents overall survival (OS), the orange line represents leukemia‐free survival (LFS), the green line represents relapse incidence (RI), the blue line represents nonrelapse mortality (NRM).
Figure 2.
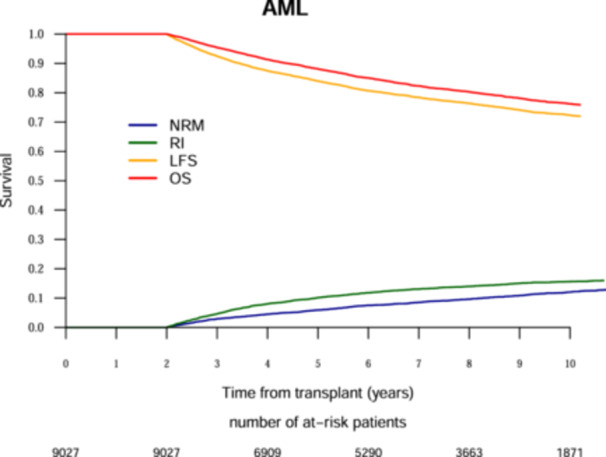
Ten‐year outcomes analysis after Allogeneic hematopoietic cell transplantation for acute lymphoblastic leukemia. The red line represents overall survival (OS), the orange line represents leukemia‐free survival (LFS), the green line represents relapse incidence (RI), the blue line represents nonrelapse mortality (NRM).
Table 2.
Main outcomes.
| ALL | AML | |||
|---|---|---|---|---|
| Outcomes post HCT % (95% CI) | 5 years | 10 years | 5 years | 10 years |
| RI | 7.1 (6.1−8.1) | 9.9 (8.7−11.2) | 10.1 (9.5−10.8) | 15.6 (14.7−16.5) |
| NRM | 5.6 (4.7−6.5) | 11.9 (10.4−13.4) | 5.9 (5.4−6.4) | 12.1 (11.3−12.9) |
| OS | 90.4 (89.2−91.5) | 81.3 (79.4−83) | 88.1 (87.4−88.8) | 76.2 (75.1−77.3) |
| LFS | 87.3 (86−88.6) | 78.2 (76.3−80) | 84 (83.1−84.7) | 72.3 (71.2−73.4) |
| CGRFS | 83.5 (81.9−84.9) | 73.3 (71.2−75.2) | 80.1 (79.2−81) | 68.1 (66.9−69.2) |
| Chronic GVHD after 2 y | 4.8 (4−5.7) | 6.3 (5.3−7.4) | 4.6 (4.2−5.1) | 5.9 (5.4−6.5) |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CGRFS, cGVHD‐free, relapse‐free survival; CI, confidence interval; GVHD, graft‐versus‐host disease; HCT, hematopoietic cell transplantation; LFS, leukemia‐free survival; NRM, nonrelapse mortality; OS, overall survival; RI, relapse incidence.
Multivariate analysis of long‐term HCT outcomes in ALL patients (Tables 3 and S2, 2 ) showed the following:
Table 3.
Multivariate analysis of long‐term HCT outcomes for ALL.
| Relapse | NRM | LFS | OS | CGRFS | Chronic GVHD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Donor MSC (ref) | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| UD | 0.77 (0.51–1.15) | 0.2 | 1.67 (1.17–2.39) | 0.005 | 1.19 (0.91–1.55) | 0.21 | 1.34 (1–1.8) | 0.047 | 1.15 (0.9–1.46) | 0.26 | 1.22 (0.75–1.97) | 0.42 |
| Other relative | 0.89 (0.38–2.09) | 0.79 | 0.68 (0.21–2.19) | 0.52 | 0.85 (0.43–1.68) | 0.64 | 0.84 (0.39–1.83) | 0.66 | 0.91 (0.5–1.64) | 0.75 | 0.93 (0.29–3.05) | 0.91 |
| CR2/3 | 2.32 (1.54–3.47) | <0.0001 | 1.56 (1.02–2.39) | 0.04 | 1.9 (1.42–2.54) | <0.0001 | 1.78 (1.29–2.46) | 0.0004 | 1.67 (1.29–2.18) | 0.0001 | 1.26 (0.72–2.2) | 0.41 |
| Active disease | 2.53 (1.48–4.32) | 0.0007 | 1.68 (0.97–2.88) | 6.20E−02 | 2.06 (1.41–3.01) | 0.0002 | 2.02 (1.35–3.05) | 0.0007 | 1.71 (1.2–2.43) | 0.003 | 1.19 (0.54–2.59) | 0.67 |
| Age (per 10 y) | 1.03 (0.9–1.19) | 0.64 | 1.36 (1.19–1.54) | <0.0001 | 1.19 (1.08–1.31) | 0.0003 | 1.2 (1.08–1.33) | 0.0005 | 1.19 (1.09–1.29) | <0.0001 | 1.16 (0.98–1.37) | 0.083 |
| Year of HSCT | 1 (0.92–1.09) | 0.96 | 0.98 (0.91−1.06) | 0.63 | 0.99 (0.93–1.05) | 0.73 | 1.01 (0.94–1.07) | 0.85 | 0.98 (0.94–1.04) | 0.55 | 0.96 (0.87–1.06) | 0.43 |
| Diagnosis (Ref = Phineg B ALL)a | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Phipos B ALL | 1.45 (0.9–2.33) | 0.12 | 1.16 (0.73−1.84) | 0.52 | 1.28 (0.92–1.77) | 0.15 | 0.99 (0.69–1.42) | 0.96 | 1.19 (0.89–1.59) | 0.24 | 1.2 (0.66–2.19) | 0.55 |
| T ALL | 0.72 (0.42–1.23) | 0.23 | 0.82 (0.5−1.36) | 0.44 | 0.77 (0.53–1.11) | 0.16 | 0.73 (0.49–1.07) | 0.11 | 0.74 (0.54–1.03) | 0.07 | 0.67 (0.34–1.32) | 0.24 |
| Missing | 0.76 (0.47–1.24) | 0.27 | 1.13 (0.73−1.76) | 0.59 | 0.94 (0.68–1.3) | 0.7 | 0.89 (0.63–1.26) | 0.52 | 0.96 (0.72–1.28) | 0.78 | 1.19 (0.67–2.11) | 0.56 |
| RIC vs. MAC | 1.05 (0.64–1.73) | 0.84 | 0.97 (0.61–1.55) | 0.91 | 1.04 (0.74–1.47) | 0.81 | 1.06 (0.73–1.54) | 0.77 | 0.96 (0.71–1.31) | 0.8 | 0.65 (0.32–1.3) | 0.22 |
| TBI | 0.77 (0.52–1.15) | 0.2 | 1.04 (0.7–1.54) | 0.84 | 0.91 (0.69–1.2) | 0.49 | 0.94 (0.69–1.27) | 0.68 | 0.91 (0.71–1.16) | 0.44 | 0.98 (0.59–1.64) | 0.95 |
| PB vs. BM | 1.42 (0.92–2.17) | 0.11 | 1.71 (1.12–2.61) | 0.012 | 1.57 (1.16–2.12) | 0.003 | 1.54 (1.11–2.14) | 0.009 | 1.64 (1.25–2.14) | 0.0003 | 1.99 (1.14–3.48) | 0.015 |
| Female to male donor | 0.85 (0.55–1.3) | 0.45 | 1.63 (1.14–2.32) | 0.007 | 1.21 (0.93–1.59) | 0.16 | 1.23 (0.91–1.66) | 0.18 | 1.21 (0.95–1.54) | 0.12 | 1.43 (0.9–2.26) | 0.13 |
| KPS > = 90 | 1.15 (0.78–1.7) | 0.48 | 0.81 (0.58–1.12) | 0.21 | 0.94 (0.74–1.21) | 0.65 | 0.83 (0.63–1.08) | 0.16 | 0.99 (0.79–1.23) | 0.9 | 1.11 (0.69–1.77) | 0.66 |
| In vivo TCD | 1.12 (0.74–1.69) | 0.59 | 0.48 (0.33–0.69) | <0.0001 | 0.7 (0.53–0.92) | 9.00E−03 | 0.62 (0.46–0.84) | 0.002 | 0.71 (0.56–0.9) | 0.005 | 0.71 (0.44–1.15) | 0.16 |
Abbreviations: ALL, acute lymphoblastic leukemia; BM, bone marrow; CGRFS, cGVHD‐free, relapse‐free survival; CI, confidence interval; GVHD, graft‐versus‐host disease; HCT, hematopoietic cell transplantation; HR, hazard ratio; LFS, leukemia‐free survival; MAC, myeloablative conditioning; NRM, nonrelapse mortality; OS, overall survival; PB, peripheral blood; RI, relapse incidence; RIC, reduced intensity conditioning; TCD, T cell depletion.
As a missing category was included in the model, HR is not reliable for a type of ALL.
Patient‐ and disease‐specific
OS and LFS decreased with increasing recipient age (HR [per 10‐year increment] 1.20, 95% CI: 1.08–1.33, p = 0.0005, and HR 1.19, 95% CI: 1.08–1.31, p = 0.0003, respectively and higher NRM [HR 1.36, 95% CI: 1.19–1.54, p < 0.0001]).
CR2/3 patients and those with advanced disease were associated with lower OS (HR: 1.78, 95% CI: 1.29–2.46, p = 0.0004 and HR: 2.02, 95% CI: 1.35–3.05, p = 0.0007, respectively) and worse LFS (HR: 1.90, 95% CI: 1.42–2.54, p < 0.0001 and HR: 2.06, 95% CI: 1.41–3.01, p = 0.0002, respectively) with higher rates of relapse (HR: 2.32, 95% CI: 1.54–3.47, p < 0.0001 and HR: 2.53, 95% CI: 1.48–4.32, p = 0.0007, respectively) and higher NRM for CR2/3 (HR: 1.56, 95% CI: 1.02–2.39, p = 0.04).
Transplant‐specific
Transplants from UD were associated with worse OS (HR: 1.34, 95% CI: 1–1.8, p = 0.047) and higher NRM (HR: 1.67, 95% CI: 1.17–2.39, p = 0.005) compared to transplants from MSD.
There were significant differences in long‐term outcomes based on graft source. PB grafts were associated with inferior OS (HR: 1.54, 95% CI: 1.11–2.14, p = 0.009), LFS (HR: 1.57, 95% CI: 1.16–2.12, p = 0.003), and cGVHD (HR: 1.99, 95% CI: 1.14–3.48, p = 0.015), as well as higher NRM (HR: 1.71, 95% CI: 1.12–2.61, p = 0.012) and cGRFS (HR: 1.64, 95% CI: 1.25–2.14, p = 0.0003) compared to bone marrow (BM) grafts.
ATG was associated with improved OS (HR: 0.62, 95% CI: 0.46–0.84, p = 0.002) and LFS (HR: 0.70, 95% CI: 0.53–0.92, p = 0.009] and lower NRM (HR: 0.48, 95% CI: 0.33–0.69, p < 0.0001).
-
Total body irradiation (TBI) was not significantly associated with differences in OS, LFS, or relapse.
In AML patients, multivariate analysis (Tables 4 and S2, 2 ) showed the following:
Table 4.
Multivariate analysis of long‐term HCT outcomes for AML.
| Relapse | NRM | LFS | OS | CGRFS | Chronic GVHD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Donor MSC (ref) | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| UD | 0.84 (0.72–0.98) | 0.026 | 1.28 (1.07–1.53) | 0.008 | 1.01 (0.9–1.14) | 0.88 | 1 (0.88–1.13) | 0.97 | 1.06 (0.95–1.18) | 0.31 | 1.13 (0.88–1.46) | 0.33 |
| Other relative | 0.59 (0.37–0.95) | 0.031 | 1.5 (0.99–2.26) | 0.054 | 0.93 (0.68–1.26) | 0.63 | 0.99 (0.71–1.38) | 0.98 | 0.93 (0.7–1.23) | 0.61 | 0.92 (0.48–1.76) | 0.8 |
| CR2/3 | 1.16 (0.97–1.4) | 0.11 | 1.12 (0.91–1.38) | 0.3 | 1.14 (0.99–1.31) | 0.063 | 1.15 (0.98–1.34) | 0.082 | 1.17 (1.03–1.33) | 0.018 | 1.25 (0.94–1.66) | 0.13 |
| Active disease | 1.7 (1.43–2.01) | <0.0001 | 1.48 (1.21–1.81) | <0.0001 | 1.59 (1.4–1.81) | <0.0001 | 1.65 (1.43–1.9) | <0.0001 | 1.49 (1.32–1.69) | <0.0001 | 1.25 (0.92–1.71) | 0.15 |
| Age (per 10 y) | 1.12 (1.06–1.19) | 0.0002 | 1.43 (1.32–1.54) | <0.0001 | 1.24 (1.18–1.3) | <0.0001 | 1.3 (1.23–1.37) | <0.0001 | 1.19 (1.14–1.25) | <0.0001 | 1.09 (0.99–1.19) | 0.092 |
| Year of HSCT | 1.04 (1.01–1.08) | 0.021 | 1 (0.96–1.04) | 0.86 | 1.02 (1–1.05) | 0.089 | 1.01 (0.98–1.04) | 0.47 | 1.03 (1.01–1.06) | 0.008 | 1.08 (1.01–1.14) | 0.014 |
| Cytogenetics (Ref = Favorable risk)a | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Intermediate | 1.57 (1.11–2.22) | 0.011 | 1.02 (0.72–1.45) | 0.91 | 1.29 (1.01–1.66) | 0.042 | 1.27 (0.96–1.68) | 0.088 | 1.32 (1.06–1.66) | 0.015 | 1.42 (0.86–2.36) | 0.18 |
| Adverse | 3 (2.06–4.36) | <0.0001 | 1.16 (0.76–1.79) | 0.49 | 2.06 (1.56–2.72) | <0.0001 | 2.27 (1.67–3.09) | <0.0001 | 2.09 (1.62–2.69) | <0.0001 | 1.96 (1.1–3.5) | 0.022 |
| Missing | 1.55 (1.1–2.18) | 0.011 | 1.05 (0.75–1.48) | 0.78 | 1.3 (1.02–1.66) | 0.031 | 1.32 (1.01–1.74) | 0.043 | 1.34 (1.07–1.67) | 0.009 | 1.42 (0.87–2.32) | 0.16 |
| RIC vs. MAC | 1.08 (0.92–1.25) | 0.35 | 1.21 (1.01–1.44) | 0.039 | 1.15 (1.02–1.29) | 0.021 | 1.21 (1.06–1.37) | 0.004 | 1.07 (0.97–1.2) | 0.19 | 0.75 (0.58–0.97) | 0.028 |
| TBI | 0.81 (0.69–0.95) | 0.009 | 0.99 (0.83–1.17) | 0.87 | 0.89 (0.79–1) | 0.054 | 0.91 (0.8–1.03) | 0.14 | 0.93 (0.84–1.04) | 0.22 | 1.12 (0.89–1.42) | 0.33 |
| PB vs. BM | 0.99 (0.81‐1.21) | 0.93 | 1.45 (1.11–1.89) | 0.006 | 1.16 (0.99–1.37) | 0.065 | 1.2 (1–1.43) | 0.048 | 1.13 (0.98–1.31) | 0.095 | 1.15 (0.84–1.57) | 0.39 |
| Female to male donor | 0.91 (0.76‐1.1) | 0.33 | 1.72 (1.44–2.06) | <0.0001 | 1.22 (1.08–1.39) | 0.002 | 1.32 (1.15–1.51) | <0.0001 | 1.16 (1.03–1.3) | 0.018 | 0.87 (0.64–1.17) | 0.35 |
| KPS > = 90 | 0.96 (0.82‐1.12) | 0.58 | 0.87 (0.73–1.03) | 0.11 | 0.92 (0.82–1.03) | 0.16 | 0.9 (0.79–1.02) | 0.097 | 0.95 (0.85–1.05) | 0.32 | 1.11 (0.84–1.46) | 0.46 |
| in vivo TCD | 1.14 (0.97‐1.34) | 0.11 | 0.48 (0.4–0.57) | <0.0001 | 0.78 (0.69–0.88) | <0.0001 | 0.67 (0.59–0.76) | <0.0001 | 0.74 (0.66–0.82) | <0.0001 | 0.6 (0.46–0.77) | <0.0001 |
Abbreviations: ALL, acute lymphoblastic leukemia; BM, bone marrow; CGRFS, cGVHD‐free, relapse‐free survival; CI, confidence interval; GVHD, graft‐versus‐host disease; HCT, hematopoietic cell transplantation; HR, hazard ratio; LFS, leukemia‐free survival; MAC, myeloablative conditioning; NRM, nonrelapse mortality; OS, overall survival; PB, peripheral blood; RI, relapse incidence; RIC, reduced intensity conditioning; TCD, T cell depletion.
As a missing category was included in the model, HR are not reliable for cytogenetics.
Patient‐ and disease‐specific
Increasing recipient age was associated with worse OS (HR [per 10‐year increment]: 1.30, 95% CI: 1.23–1.37, p < 0.0001) and LFS (HR: 1.24, 95% CI: 1.18–1.30, p < 0.0001), as well as higher rates of both relapse (HR: 1.12, 95% CI: 1.06–1.19, p = 0.0002) and NRM (HR: 1.43, 95% CI: 1.32–1.54, p < 0.0001).
Adverse cytogenetic profiles were associated with adverse OS (HR: 2.27, 95% CI: 1.67–3.09, p < 0.0001) and LFS (HR: 2.06, 95% CI: 1.56–2.72, p < 0.0001) outcomes.
Transplant‐specific
RIC versus MAC was associated with higher OS (HR: 1.21, 95% CI: 1.06–1.37, p = 0.004), LFS (HR: 1.15, 95% CI: 1.02–1.29, p = 0.021), and NRM (HR: 1.21, 95% CI: 1.01–1.44, p = 0.039), but no significant differences in relapse, cGRFS, or cGVHD.
PB grafts were associated with inferior OS (HR: 1.2, 95% CI: 1–1.43, p = 0.048) and higher NRM (HR: 1.45, 95% CI: 1.11–1.89, p = 0.006) compared to BM grafts.
Female donor to male patient combination was associated with inferior OS (HR: 1.32, 95% CI: 1.15–1.51, p < 0.0001) and LFS (HR: 1.22, 95% CI: 1.08–1.39, p = 0.002) with a higher risk of NRM (HR: 1.72, 95% CI: 1.44–2.06, p < 0.0001).
ATG was associated with improved OS (HR: 0.67, 95% CI: 0.59–0.76, p < 0.0001) and LFS (HR: 0.78, 95% CI: 0.69–0.88, p < 0.0001) and lower risk of NRM (HR: 0.48, 95% CI: 0.40–0.57, p < 0.0001) and cGVHD (HR: 0.60, 95% CI: 0.46–0.77, p < 0.0001).
TBI was associated with lower relapse rates (HR: 0.81, 95% CI: 0.69–0.95, p = 0.009) but did not significantly affect NRM, LFS, or OS.
For patients transplanted in the 26 countries with available mortality tables (Data S2) (92% of ALL patients and 91% of AML patients in our cohort), the probabilities of death from other causes were minimal (1.5% and 3.7% for ALL and AML, respectively), whereas the probabilities of death from HCT were 16.8% and 20.4%, respectively, 10 years after HCT. Survival probability stratified by time since BMT (≥2, ≥4, ≥6, and ≥8) among BMT survivors showed survival probabilities of 81.3% and 78.2% for 2‐year survivors, 89.3% and 85.6% for 4‐year survivors, 93.6% and 91.0% for 6‐year survivors, and 96.7% and 96.1% for 8‐year survivors for ALL and AML, respectively (Figure 3). Relapse and cGVHD were the most common causes of late mortality, accounting for 31.9% and 27.3% of ALL deaths and 42.7% and 17.0% of AML deaths, respectively, followed by infection, and secondary malignancy (Table S3). No patients reported to have died from infection had cGVHD. Out of 72 deaths by infection in ALL, 14 (19%) occurred after a relapse. Out of 221 deaths by infection in AML, 61 (28%) occurred after a relapse.
Figure 3.
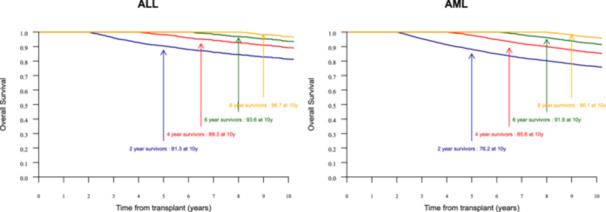
Ten‐year survival probability stratified by time survived since blood marrow transplantation (BMT) (≥2, ≥4, ≥6, and ≥8) among BMT survivors. Arrows indicate survival rates at specific time points: 2‐year survivors (blue), 4‐year survivors (red), 6‐year survivors (green), and 8‐year survivors (orange). BMT, blood marrow transplantation.
DISCUSSION
In the current landscape of advancing knowledge in acute leukemia, there is an urgent need for a better understanding of patient characteristics and hematologic conditions to sustain this positive trajectory. This is particularly important when considering candidate selection for allogeneic HCT, the only potentially curative strategy for high‐risk acute leukemia, which represents a significant portion of the patient population. In this analysis of two left‐truncated studies, we observed long‐term survival outcomes in patients who underwent HCT for acute leukemia. It is important to note that we include both ALL and AML but report the results separately because they differ between AML and ALL, as they are not the same disease. In patients with ALL who remained alive and relapse‐free at 2 years, the 10‐year OS was a remarkable 81.3%, while AML patients showed an encouraging 76.2% OS at 10 years. However, it is important to recognize the limitations of this study, including its retrospective nature, and significant gaps in data completeness, particularly with regard to cytogenetics, MRD, and cause of death.
In AML patients, older age and unfavorable cytogenetics are associated with a worse prognosis. Advanced age is associated with poorer performance status, lower white blood cell counts and a lower percentage of blasts in the BM. Patients aged over 75 years also have higher rates of chemotherapy resistance and less favorable cytogenetics. The effects of age on the patient and the disease are reflected in a higher incidence of early death after induction therapy, lower CR rates, and a decreased likelihood of long‐term survival as patients age. 38 , 39 , 40 , 41 In addition, NRM increased with age. A study conducted in Germany between 1998 and 2018 42 found similar results, with a higher NRM in patients over 70 years of age, a median relapse‐free survival shorter by more than 1 year, and therefore a higher relapse‐free mortality. This underscores the fact that despite major therapeutic advances over the past 20 years, age remains a limiting factor in the management of leukemia patients due to disease severity and unrelated comorbidities. Furthermore, despite the problem of missing data, unfavorable cytogenetics has shown a consistent association with unfavorable OS and LFS, underscoring the impact of disease biology on long‐term prognosis. 38 , 41 , 43 , 44 , 45 , 46 , 47
As previously described, pre‐transplant disease status is emerging as an important marker of long‐term survival. Indeed, several studies have already shown that achieving MRD‐negative status prior to allogeneic HCT improves relapse‐free survival and OS. Despite intensive induction/consolidation chemotherapy with complete hematologic remission rates of 80%–90%, approximately 30%–50% of adult patients with ALL develop MRD. However, many patients relapse while awaiting HCT, and detectable pre‐transplant MRD is associated with a higher relapse rate after HCT. 48 , 49 , 50 , 51 , 52 , 53
In ALL patients, the use of BM was associated with better outcomes. In an analysis spanning four decades, Bhatia et al. found a greater reduction in late mortality in people who received BM. 2
In AML patients, furthermore, the influence of donor sex on HCT outcomes remains controversial. 54 , 55 , 56 , 57 In our study, donor–recipient sex mismatch had a notable impact, particularly the association of lower OS and LFS with being male and receiving a female donor graft. Ali et al. 27 demonstrated that sex‐mismatched grafts were associated with increased GVHD, resulting in a decreased risk of relapse and improved OS. However, this observation contrasts with the findings of Bortin et al. 58 and Ramsay et al., 59 who did not consider gender mismatch as a significant parameter in the development of GVHD. Furthermore, RIC is associated with lower OS and LFS than MAC, fueling the ongoing debate about optimal conditioning intensity. The lower rates of GVHD observed with RIC contribute to the nuanced discussion regarding the trade‐off between antileukemic efficacy and transplant‐related morbidity. On the other hand, NRM seems to be higher with RIC than in MAC. Del Galy et al. 60 found a 10‐year incidence rate of relapse and relapse‐free mortality of 13.9% and 13.4%, respectively, after HCT with RIC. Late relapse was the most common cause of death, but a significant number of patients developed second malignancies, with a cumulative 10‐year incidence of 12.9%, which was significantly higher than in the general population. In addition, high rates of cardiovascular complications and venous thromboembolic events were observed at 10 years (15.1% and 11.7%, respectively).
In both AML and ALL, the use of ATG has shown significant benefits, including reduced NRM, improved OS and LFS, and a trend toward reduced GVHD. Currently, the optimal amount of T cells to introduce into the graft remains difficult to determine and is likely to vary between different donor–recipient pairs. 61 , 62 , 63 , 64 Donor T cells play a pivotal role in shaping the posttransplant immune response and significantly influence the incidence of GVHD. 65 The incidence of GVHD is dependent on graft characteristics, with unmanipulated grafts associated with a higher likelihood of acute GVHD. In contrast, T cell‐depleted grafts have demonstrated the ability to mitigate these risks and offer significant benefits in terms of patient quality of life. 66 , 67 Despite these advances, uncertainties remain regarding the optimal role of ATG in HCT. Critical issues such as the appropriate selection of eligible patients, optimal methods of marrow or stem cell purification, and the administration of adjunctive medications to manage the graft and prevent GVHD remain unresolved. 61 cGVHD is often a major factor in HCT‐related mortality. Severe complications of GVHD can lead to organ failure and other serious medical problems, contributing to posttransplant deaths. 65 , 68 , 69 , 70 , 71 , 72 , 73 Treatments to control GVHD, such as immunosuppression may also have adverse effects, including an increased risk of infection and other complications associated with a weakened immune system. 74 , 75 , 76 , 77
The 10‐year OS reported in this study, which includes patients transplanted between 2005 and 2012, shows some improvement but is not significantly better than previous studies by the CIBMTR 24 and the EBMT, 25 which included patients transplanted before 2004 and 2005, respectively. However, the most recent EBMT analysis highlights a significant improvement in survival for patients who underwent allogeneic HCT. This improvement is primarily attributed to a reduction in NRM. Several factors have likely contributed to this positive trend, including advances in supportive care, better management of GVHD, and improved infection control. 70 , 73 , 77 , 78 The introduction of novel immunosuppressive agents and improved GVHD prophylaxis protocols have significantly reduced the incidence and severity of acute and cGVHD, which were major contributors to NRM in earlier cohorts. 16 , 59 , 72 , 75 , 79 However, the current study did not provide detailed data on the severity of cGVHD or the need for systemic treatment. In addition, there were no reported deaths from infections in patients with cGVHD, which may indicate a gap in data collection and reporting. Improved infection control practices and the use of newer antiviral and antifungal agents have led to a significant reduction in infection‐related mortality. 80 , 81 These developments have led to a reduction in the complications that have historically contributed to NRM. Despite these advances, survival rates in the current cohort still reflect significant challenges.
A study conducted between 1974 and 2014 2 found that late mortality in allogeneic HCT has decreased over the past 40 years. The leading causes of all‐cause mortality included infections, subsequent malignancies, cardiovascular disease, and pulmonary disease. Relative mortality compared to the general population remained high even 30 years after transplantation, with a 20.8% reduction in life expectancy. Survival analyses show that each 2‐year relapse‐free period after transplantation significantly improves long‐term survival, with a particularly notable benefit at 6 years. This underscores the importance of rigorous, ongoing follow‐up, as each relapse‐free year significantly improves long‐term survival. 2 Similarly, another study of 4485 patients who underwent HCT for hematologic malignancies between 1976 and 2014 82 found that half of all deaths in the entire cohort were due to the primary disease, but this proportion was only 10% among survivors at 15 years. The main causes of late NRM were subsequent malignancies (26.1%) and cardiopulmonary disease (20.2%). In our study, one can speculate that the likelihood of death from other causes 10 years after HCT was minimal. Cardiac toxicity was not predominant, but there were 7.6% secondary cancers in ALL and 11.3% in AML. Therefore, it seems essential to develop new strategies to predict, monitor and treat these long‐term complications.
In conclusion, the landscape of HCT for acute leukemia has evolved significantly over the past two decades. For both leukemia types, the impact of donor type, graft source, and the use of ATG on transplant outcomes was evident. While ATG showed benefits in reducing NRM and cGVHD, differences in outcomes based on donor–recipient sex mismatch and graft source (PB vs. BM) require careful consideration in clinical decision‐making. Furthermore, late mortality was predominantly due to relapse and cGVHD, highlighting the critical need for targeted interventions and surveillance strategies in long‐term survivorship care. Importantly, infections emerged as a significant cause of mortality, particularly in the context of relapsed disease, highlighting gaps in infection prophylaxis and management strategies. Our study highlights the need to refine and optimize pretransplant strategies and integrate comprehensive survivorship programs to improve long‐term outcomes in acute leukemia patients undergoing HCT. Continued research efforts are essential to address remaining challenges and improve overall patient care in this evolving field.
AUTHOR CONTRIBUTIONS
Mohamad Mohty and Myriam Labopin conceived and designed the study; all authors collected the data. Mohamad Mohty, Marion Larue and Myriam Labopin analyzed the data. Mohamad Mohty, Marion Larue, and Myriam Labopin wrote the manuscript. All authors critically revised and approved the final manuscript. Mohamad Mohty is the guarantor of the study, has full access to all the data, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Data sharing is available through the ALWP office (myriam.labopin@upmc.fr).
FUNDING
None to declare.
ETHICS STATEMENT
The ALWP of the EBMT approved the study according to the tenets of the Declaration of Helsinki. Data were provided by the EBMT registry after informed consent of the patients and according to local regulations in place at the time of transplantation.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
We thank Khvedelidze Irma and the ALWP EBMT staff in the Paris office for data management. The study was accomplished thanks to the contributing centers of the EBMT registry which provided patient data. Following EBMT publication rules, co‐authorship was offered to centers contributing the highest number of patients.
DATA AVAILABILITY STATEMENT
REFERENCES
- 1. Kliman D, Nivison‐Smith I, Gottlieb D, et al. Hematopoietic stem cell transplant recipients surviving at least 2 years from transplant have survival rates approaching population levels in the modern era of transplantation. Biol Blood Marrow Transplant. 2020;26(9):1711‐1718. [DOI] [PubMed] [Google Scholar]
- 2. Bhatia S, Dai C, Landier W, et al. Trends in late mortality and life expectancy after allogeneic blood or marrow transplantation over 4 decades: a blood or marrow transplant survivor study report. JAMA Oncol. 2021;7(11):1626‐1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sengsayadeth S, Savani BN, Blaise D, Malard F, Nagler A, Mohty M. Reduced intensity conditioning allogeneic hematopoietic cell transplantation for adult acute myeloid leukemia in complete remission—a review from the Acute Leukemia Working Party of the EBMT. Haematologica. 2015;100(7):859‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu J, Du Y, Ahmad S, et al. Comparison of myeloablative versus reduced‐intensity conditioning regimens in allogeneic stem cell transplantation recipients with acute myelogenous leukemia with measurable residual disease‐negative disease at the time of transplantation: a retrospective cohort study. Transpl Cell Ther. 2021;27(8):663. [DOI] [PubMed] [Google Scholar]
- 5. Eom KS, Shin SH, Yoon JH, et al. Comparable long‐term outcomes after reduced‐intensity conditioning versus myeloablative conditioning allogeneic stem cell transplantation for adult high‐risk acute lymphoblastic leukemia in complete remission. Am J Hematol. 2013;88(8):634‐641. [DOI] [PubMed] [Google Scholar]
- 6. Mohty M, Labopin M, Volin L, et al. Reduced‐intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2010;116(22):4439‐4443. [DOI] [PubMed] [Google Scholar]
- 7. Bachanova V, Marks DI, Zhang MJ, et al. Ph+ ALL patients in first complete remission have similar survival after reduced intensity and myeloablative allogeneic transplantation: impact of tyrosine kinase inhibitor and minimal residual disease. Leukemia. 2014;28(3):658‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanz J, Galimard JE, Labopin M, et al. Post‐transplant cyclophosphamide after matched sibling, unrelated and haploidentical donor transplants in patients with acute myeloid leukemia: a comparative study of the ALWP EBMT. J Hematol Oncol. 2020;13(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luznik L, O'Donnell PV, Symons HJ, et al. HLA‐haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high‐dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Di Bartolomeo P, Santarone S, De Angelis G, et al. Haploidentical, unmanipulated, G‐CSF‐primed bone marrow transplantation for patients with high‐risk hematologic malignancies. Blood. 2013;121(5):849‐857. [DOI] [PubMed] [Google Scholar]
- 11. Peccatori J, Forcina A, Clerici D, et al. Sirolimus‐based graft‐versus‐host disease prophylaxis promotes the in vivo expansion of regulatory T cells and permits peripheral blood stem cell transplantation from haploidentical donors. Leukemia. 2015;29(2):396‐405. [DOI] [PubMed] [Google Scholar]
- 12. Webster JA, Luznik L, Tsai HL, et al. Allogeneic transplantation for Ph+ acute lymphoblastic leukemia with posttransplantation cyclophosphamide. Blood Adv. 2020;4(20):5078‐5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun Y, Beohou E, Labopin M, et al. Unmanipulated haploidentical versus matched unrelated donor allogeneic stem cell transplantation in adult patients with acute myelogenous leukemia in first remission: a retrospective pair‐matched comparative study of the Beijing approach with the EBMT database. Haematologica. 2016;101(8):352‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Socié G, Stone JV, Wingard JR, et al. Long‐term survival and late deaths after allogeneic bone marrow transplantation. N Engl J Med. 1999;341(1):14‐21. [DOI] [PubMed] [Google Scholar]
- 15. Keruakous AR, Holter‐Chakrabarty J, Schmidt SA, Khawandanah MO, Selby G, Yuen C. Azacitidine maintenance therapy post‐allogeneic stem cell transplantation in poor‐risk acute myeloid leukemia. Hematol Oncol Stem Cell Ther. 2023;16(1):52‐60. [DOI] [PubMed] [Google Scholar]
- 16. Gooptu M, Koreth J. Better acute graft‐versus‐host disease outcomes for allogeneic transplant recipients in the modern era: a tacrolimus effect? Haematologica. 2017;102(5):806‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khoury HJ, Wang T, Hemmer MT, et al. Improved survival after acute graft‐versus‐host disease diagnosis in the modern era. Haematologica. 2017;102(5):958‐966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brissot E, Labopin M, Beckers MM, et al. Tyrosine kinase inhibitors improve long‐term outcome of allogeneic hematopoietic stem cell transplantation for adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia. Haematologica. 2015;100(3):392‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y, Liu QF, Lin R, et al. Optimizing antithymocyte globulin dosing in haploidentical hematopoietic cell transplantation: long‐term follow‐up of a multicenter, randomized controlled trial. Sci Bull. 2021;66(24):2498‐2505. [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Liu DH, Liu KY, et al. Long‐term follow‐up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer. 2013;119(5):978‐985. [DOI] [PubMed] [Google Scholar]
- 21. Yan C, Wang Y, Sun Y, et al. Optimized therapeutic strategy for patients with refractory or relapsed acute myeloid leukemia: long‐term clinical outcomes and health‐related quality of life assessment. Cancer Commun. 2022;42(12):1387‐1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. 2010;116(17):3129‐3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mo XD, Xu LP, Liu DH, et al. Nonmalignant late effects in survivors of partially matched donor hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2013;19(5):777‐783. [DOI] [PubMed] [Google Scholar]
- 24. Wingard JR, Majhail NS, Brazauskas R, et al. Long‐term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230‐2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shimoni A, Labopin M, Savani B, et al. Long‐term survival and late events after allogeneic stem cell transplantation from HLA‐matched siblings for acute myeloid leukemia with myeloablative compared to reduced‐intensity conditioning: a report on behalf of the acute leukemia working party of European group for blood and marrow transplantation. J Hematol Oncol. 2016;9(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martin PJ, Counts GW, Appelbaum FR, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28(6):1011‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Armenian SH, Chen Y, Hageman L, et al. Burden of long‐term morbidity borne by survivors of acute myeloid leukemia treated with blood or marrow transplantation: the results of the BMT survivor study. J Clin Oncol. 2022;40(28):3278‐3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Socie G. Long‐term outcomes after transplantation for acute myelogenous leukemia. J Clin Oncol. 2022;40(28):3235‐3238. [DOI] [PubMed] [Google Scholar]
- 29. Daher‐Reyes G, Kim T, Novitzky‐Basso I, et al. Prognostic impact of the adverse molecular‐genetic profile on long‐term outcomes following allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia. Bone Marrow Transpl. 2021;56(8):1908‐1918. [DOI] [PubMed] [Google Scholar]
- 30. Anthias C, Dignan FL, Morilla R, et al. Pre‐transplant MRD predicts outcome following reduced‐intensity and myeloablative allogeneic hemopoietic SCT in AML. Bone Marrow Transpl. 2014;49(5):679‐683. [DOI] [PubMed] [Google Scholar]
- 31. Dillon R, Hills R, Freeman S, et al. Molecular MRD status and outcome after transplantation in NPM1‐mutated AML. Blood. 2020;135(9):680‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang YJ, Wang Y, Liu YR, et al. Haploidentical allograft is superior to matched sibling donor allograft in eradicating pre‐transplantation minimal residual disease of AML patients as determined by multiparameter flow cytometry: a retrospective and prospective analysis. J Hematol Oncol. 2017;10(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang YJ, Wang Y, Xu LP, et al. Haploidentical donor is preferred over matched sibling donor for pre‐transplantation MRD positive ALL: a phase 3 genetically randomized study. J Hematol Oncol. 2020;13(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pohar M, Stare J. Relative survival analysis in R. Comput Methods Programs Biomed. 2006;81(3):272‐278. [DOI] [PubMed] [Google Scholar]
- 35. Pohar Perme M, Estève J, Rachet B. Analysing population‐based cancer survival—settling the controversies. BMC Cancer. 2016;16(1):933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Greenfield DM, Salooja N, Peczynski C, et al. Metabolic syndrome and cardiovascular disease after haematopoietic cell transplantation (HCT) in adults: an EBMT cross‐sectional non‐interventional study. Bone Marrow Transpl. 2021;56(11):2820‐2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tichelli A, Passweg J, Wojcik D, et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93(8):1203‐1210. [DOI] [PubMed] [Google Scholar]
- 38. Bhansali RS, Pratz KW, Lai C. Recent advances in targeted therapies in acute myeloid leukemia. J Hematol Oncol. 2023;16:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481‐3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Juliusson G, Antunovic P, Derolf Å, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113(18):4179‐4187. [DOI] [PubMed] [Google Scholar]
- 41. Fröhling S, Schlenk RF, Kayser S, et al. Cytogenetics and age are major determinants of outcome in intensively treated acute myeloid leukemia patients older than 60 years: results from AMLSG trial AML HD98‐B. Blood. 2006;108(10):3280‐3288. [DOI] [PubMed] [Google Scholar]
- 42. Weller JF, Lengerke C, Finke J, et al. Allogeneic hematopoietic stem cell transplantation in patients aged 60‐79 years in Germany (1998‐2018): a registry study. Haematologica. 2024;109(2):431‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kumar CC. Genetic abnormalities and challenges in the treatment of acute myeloid leukemia. Genes Cancer. 2011;2(2):95‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lafage‐Pochitaloff M, Baranger L, Hunault M, et al. Impact of cytogenetic abnormalities in adults with Ph‐negative B‐cell precursor acute lymphoblastic leukemia. Blood. 2017;130(16):1832‐1844. [DOI] [PubMed] [Google Scholar]
- 45. Gupta M, Mahapatra M, Saxena R. Cytogenetics' impact on the prognosis of acute myeloid leukemia. J Lab Physicians. 2019;11(2):133‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boscaro E, Urbino I, Catania FM, et al. Modern risk stratification of acute myeloid leukemia in 2023: integrating established and emerging prognostic factors. Cancers. 2023;15(13):3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saygin C, Cannova J, Stock W, Muffly L. Measurable residual disease in acute lymphoblastic leukemia: methods and clinical context in adult patients. Haematologica. 2022;107(12):2783‐2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gale RP. Progress in transplants for acute lymphoblastic leukemia. Clin Cancer Res. 2022;28(5):813‐815. [DOI] [PubMed] [Google Scholar]
- 50. Loke J, Buka R, Craddock C. Allogeneic stem cell transplantation for acute myeloid leukemia: who, when, and how? Front Immunol. 2021;12:659595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maffini E, Labopin M, Beelen DW, et al. Measurable residual disease (MRD) status before allogeneic hematopoietic cell transplantation impact on secondary acute myeloid leukemia outcome. A Study from the Acute Leukemia Working Party (ALWP) of the European society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transpl. 2022;57(10):1556‐1563. [DOI] [PubMed] [Google Scholar]
- 52. Gökbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B‐cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522‐1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kreidieh F, Abou Dalle I, Moukalled N, et al. Relapse after allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia: an overview of prevention and treatment. Int J Hematol. 2022;116(3):330‐340. [DOI] [PubMed] [Google Scholar]
- 54. Shinohara A, Inamoto Y, Kurosawa S, et al. High non‐relapse mortality and low relapse incidence in gender‐mismatched allogeneic hematopoietic stem cell transplantation from a parous female donor with a male child. Leuk Lymphoma. 2017;58(3):578‐585. [DOI] [PubMed] [Google Scholar]
- 55. Ayuk F, Beelen DW, Bornhäuser M, et al. Relative impact of HLA matching and non‐HLA donor characteristics on outcomes of allogeneic stem cell transplantation for acute myeloid leukemia and myelodysplastic syndrome. Biol Blood Marrow Transpl. 2018;24(12):2558‐2567. [DOI] [PubMed] [Google Scholar]
- 56. Bross D, Tutschka P, Farmer E, et al. Predictive factors for acute graft‐versus‐host disease in patients transplanted with HLA‐identical bone marrow. Blood. 1984;63(6):1265‐1270. [PubMed] [Google Scholar]
- 57. Ali N, Ullah H, Shaikh MU, Adil SN. Outcome of donor and recipient sex match versus mismatch in stem cell transplant procedure. Int J Hematol Oncol. 2019;8(4):IJH21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bortin MM, Rimm AA. Treatment of 144 patients with severe aplastic anemia using immunosuppression and allogeneic marrow transplantation: a report from the international bone marrow transplant registry. Transplant Proc. 1981;13:227‐229. [PubMed] [Google Scholar]
- 59. Ramsay NKC, Kersey JH, Robison LL, et al. A randomized study of the prevention of acute graft‐versus‐host disease. N Engl J Med. 1982;306(7):392‐397. [DOI] [PubMed] [Google Scholar]
- 60. Del Galy AS, Rousseau A, Capes A, et al. Life expectancy and burden of late complications after reduced intensity conditioning allogeneic transplantation. Bone Marrow Transpl. 2022;57(9):1365‐1372. [DOI] [PubMed] [Google Scholar]
- 61. Ho VT, Soiffer RJ. The history and future of T‐cell depletion as graft‐versus‐host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. 2001;98(12):3192‐3204. [DOI] [PubMed] [Google Scholar]
- 62. Malard F, Labopin M, Cho C, et al. Ex vivo and in vivo T cell‐depleted allogeneic stem cell transplantation in patients with acute myeloid leukemia in first complete remission resulted in similar overall survival: on behalf of the ALWP of the EBMT and the MSKCC. J Hematol Oncol. 2018;11(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Forcade E, Chevret S, Finke J, et al. Impact of in vivo T‐cell depletion in patients with myelodysplastic syndromes undergoing allogeneic hematopoietic stem cell transplant: a registry study from the Chronic Malignancies Working Party of the EBMT. Bone Marrow Transpl. 2022;57(5):768‐774. [DOI] [PubMed] [Google Scholar]
- 64. Daniele N, Scerpa MC, Caniglia M, et al. Overview of T‐cell depletion in haploidentical stem cell transplantation. Blood Transf = Trasfusione del sangue. 2012;10(3):264‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ferrara JL, Levine JE, Reddy P, Holler E. Graft‐versus‐host disease. Lancet. 2009;373(9674):1550‐1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mackinnon S, Hows J, Goldman J. Induction of in vitro graft‐versus‐leukemia activity following bone marrow transplantation for chronic myeloid leukemia. Blood. 1990;76(10):2037‐2045. [PubMed] [Google Scholar]
- 67. Schaap N, Schattenberg A, Bär B, Preijers F, van de Wiel van Kemenade E, de Witte T. Induction of graft‐versus‐leukemia to prevent relapse after partially lymphocyte‐depleted allogeneic bone marrow transplantation by pre‐emptive donor leukocyte infusions. Leukemia. 2001;15(9):1339‐1346. [DOI] [PubMed] [Google Scholar]
- 68. Arai S, Arora M, Wang T, et al. Increasing incidence of chronic graft‐versus‐host disease in allogeneic transplantation: a report from the center for international blood and marrow transplant research. Biol Blood Marrow Transpl. 2015;21(2):266‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Styczyński J, Tridello G, Koster L, et al. Death after hematopoietic stem cell transplantation: changes over calendar year time, infections and associated factors. Bone Marrow Transpl. 2020;55(1):126‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Puerta‐Alcalde P, Chumbita M, Charry P, et al. Risk factors for mortality in hematopoietic stem cell transplantation recipients with bloodstream infection: points to be addressed by future guidelines. Transpl Cell Ther. 2021;27(6):501.e1‐501.e6. [DOI] [PubMed] [Google Scholar]
- 71. Atsuta Y, Hirakawa A, Nakasone H, et al. Late mortality and causes of death among long‐term survivors after allogeneic stem cell transplantation. Biol Blood Marrow Transpl. 2016;22(9):1702‐1709. [DOI] [PubMed] [Google Scholar]
- 72. Lee SJ. Classification systems for chronic graft‐versus‐host disease. Blood. 2017;129(1):30‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zeiser R, Polverelli N, Ram R, et al. Ruxolitinib for glucocorticoid‐refractory chronic graft‐versus‐host disease. N Engl J Med. 2021;385(3):228‐238. [DOI] [PubMed] [Google Scholar]
- 74. Zhang X, Wang J, Liu Y, et al. Long‐term survivors demonstrate superior quality of life after haploidentical stem cell transplantation to matched sibling donor transplantation. J Transl Med. 2022;20(1):596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gruber I, Koelbl O, Herr W, Holler E, Edinger M, Wolff D. Impact of chronic graft‐versus‐host disease on quality of life and cognitive function of long‐term transplant survivors after allogeneic hematopoietic stem cell transplantation with total body irradiation. Radiat Oncol. 2022;17(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bachier CR, Aggarwal SK, Hennegan K, et al. Epidemiology and treatment of chronic graft‐versus‐host disease post‐allogeneic hematopoietic cell transplantation: a US claims analysis. Transpl Cell Ther. 2021;27(6):504.e1‐504.e6. [DOI] [PubMed] [Google Scholar]
- 77. Malard F, Mohty M. Updates in chronic graft‐versus‐host disease management. Am J Hematol. 2023;98(10):1637‐1644. [DOI] [PubMed] [Google Scholar]
- 78. Jaime‐Pérez JC, Meléndez‐Flores JD, Ramos‐Dávila EM, et al. Infection‐related mortality after HLA‐identical and haploidentical hematopoietic cell transplantation using reduced‐intensity conditioning in an outpatient setting. Clin Transplant. 2023;37(6):e14972. [DOI] [PubMed] [Google Scholar]
- 79. Lee SJ, Vogelsang G, Flowers MED. Chronic graft‐versus‐host disease. Biol Blood Marrow Transpl. 2003;9(4):215‐233. [DOI] [PubMed] [Google Scholar]
- 80. Freyer CW, Carulli A, Gier S, et al. Letermovir vs. high‐dose valacyclovir for cytomegalovirus prophylaxis following haploidentical or mismatched unrelated donor allogeneic hematopoietic cell transplantation receiving post‐transplant cyclophosphamide. Leuk Lymphoma. 2022;63(8):1925‐1933. [DOI] [PubMed] [Google Scholar]
- 81. Ullmann AJ, Schmidt‐Hieber M, Bertz H, et al. Infectious diseases in allogeneic haematopoietic stem cell transplantation: prevention and prophylaxis strategy guidelines 2016. Ann Hematol. 2016;95(9):1435‐1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wong FL, Teh JB, Atencio L, et al. Conditional survival, cause‐specific mortality, and risk factors of late mortality after allogeneic hematopoietic cell transplantation. JNCI. 2020;112(11):1153‐1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


