Abstract
The olfactory bulb (OB) is the first relay station in the olfactory system and functions as a crucial hub. It can represent odor information precisely and accurately in an ever-changing environment. As the only output neurons in the OB, mitral/tufted cells encode information such as odor identity and concentration. Recently, the neural strategies and mechanisms underlying odor representation and encoding in the OB have been investigated extensively. Here we review the main progress on this topic. We first review the neurons and circuits involved in odor representation, including the different cell types in the OB and the neural circuits within and beyond the OB. We will then discuss how two different coding strategies—spatial coding and temporal coding—work in the rodent OB. Finally, we discuss potential future directions for this research topic. Overall, this review provides a comprehensive description of our current understanding of how odor information is represented and encoded by mitral/tufted cells in the OB.
Keywords: Olfactory bulb, Mitral/tufted cells, Odor identity, Neural representation, Information encoding
Abstract
嗅球是嗅觉系统中的第一个中继站,起着关键的枢纽作用,能在不断变化的环境中精确地表征气味信息。作为嗅球中唯一的输出神经元,僧帽细胞/簇状细胞编码诸如气味类型和浓度等信息。近年来,针对嗅球中气味表征和编码的潜在神经策略和机制已开展了广泛的研究。本文对该领域的主要进展进行了综述,首先回顾了涉及气味表征的神经元和相关环路,包括嗅球中的不同细胞类型以及嗅球内部和外部的神经回路;随后讨论了两种不同的编码策略(空间编码和时间编码)在啮齿动物嗅球中的工作原理;最后,对该研究领域的潜在未来方向展开讨论。综上,本文就目前僧帽细胞/簇状细胞在嗅球中如何表征和编码气味信息进行了全面总结。
Keywords: 嗅球, 僧帽/簇状细胞, 气味类型, 神经表征, 信息编码
1. Introduction
As one of the earliest sensory modes to emerge during evolution, the olfactory system plays a crucial role in individual survival and social functioning in animals. In humans, olfaction dramatically affects physiological, psychological, and emotional states. Strikingly, olfactory dysfunction is highly correlated with many diseases. For example, olfactory dysfunction is one of the earliest symptoms in both Alzheimer’s disease and Parkinson’s disease (Chen et al., 2021), two of the most common neurodegenerative diseases; abnormal olfaction has also been indicated in autism and epilepsy (Kuruppath et al., 2023; Wu J et al., 2023); and more recently, olfactory dysfunction is considered one of the most important manifestations of coronavirus disease 2019 (COVID-19) infection (Kay, 2022), evidenced not only by patients’ subjective reports but also by rigorous and objective psychophysical experimental tests that offer precise quantifications (Bhattacharjee et al., 2020; Menni et al., 2020; Moein et al., 2020). Given the prolonged impact of long COVID-19 on individuals’ health, developing new methods to measure olfactory function for predicting COVID-19 infection holds significant importance (Snitz et al., 2021; Bhowmik et al., 2023). As a result, olfaction has received intense scrutiny from neuroscientists, physiologists, and pathologists over the last several decades.
The major task of any sensory system is to precisely represent external and/or internal stimuli in the brain. For the olfactory system, this involves detecting chemical molecules called odorants using the receptors in the nose and transmitting the processed neural signals to the central olfactory regions of the brain (Ackels et al., 2021; Li et al., 2023; Verhagen et al., 2023). Thus, to study how the olfactory system works, we need to address two major issues: (1) how the receptors transduce different chemical stimuli to electrical neural signals; and (2) how the olfactory centers accurately encode these neural signals for olfactory perception. Pioneering work by Buck and Axel (1991), together with their subsequent work and that of others, identified the rules by which olfactory receptors in the olfactory sensory neurons (OSNs) interact with odorants and how the OSNs represent odor information (Fleischmann et al., 2008; Hanchate et al., 2015; Kim et al., 2022; Shayya et al., 2022; Billesbølle et al., 2023; Guo et al., 2023). Many studies have shed light on how odor information is represented in a number of olfaction-related areas, such as the olfactory bulb (OB), piriform cortex, anterior olfactory nucleus, lateral entorhinal cortex, and olfactory tubercle (Gadziola et al., 2015; Xiong and Wesson, 2016; Tsuji et al., 2019; Aqrabawi and Kim, 2020; Li et al., 2020; Wang et al., 2020; Wesson, 2020; Martiros et al., 2022; Brunert et al., 2023; Wu TT et al., 2023). The OB is the most intensively studied of these areas. Here, we review the strategies underpinning how the OB encodes odor information under different behavioral and brain states.
2. Neuronal and circuit bases of odor representation in the OB
The OB is the first relay station in the central olfactory system and receives direct input from the OSNs. Anatomically, the OB has a laminar structure and contains multiple neuronal types, including at least glutamatergic, γ-aminobutyric acid (GABA)ergic, and dopaminergic neurons. Mitral and tufted cells (M/Ts), located in the mitral cell layer and the external plexiform layer, respectively, are the main output neurons of the OB (Mori et al., 1981, 1983; Shipley and Ennis, 1996; Li et al., 2020; Lyons-Warren et al., 2023). Their dendrites form synapses with OSN axon terminals in structures called glomeruli, which form the major anatomical feature of the glomerular layer. The axons of the M/Ts project to many higher olfactory centers, including the anterior olfactory nucleus, piriform cortex, olfactory tubercle, and lateral entorhinal cortex, which are all considered part of the olfactory cortex. The major role of M/Ts is to transmit olfactory information from the OSNs to the olfactory cortex.
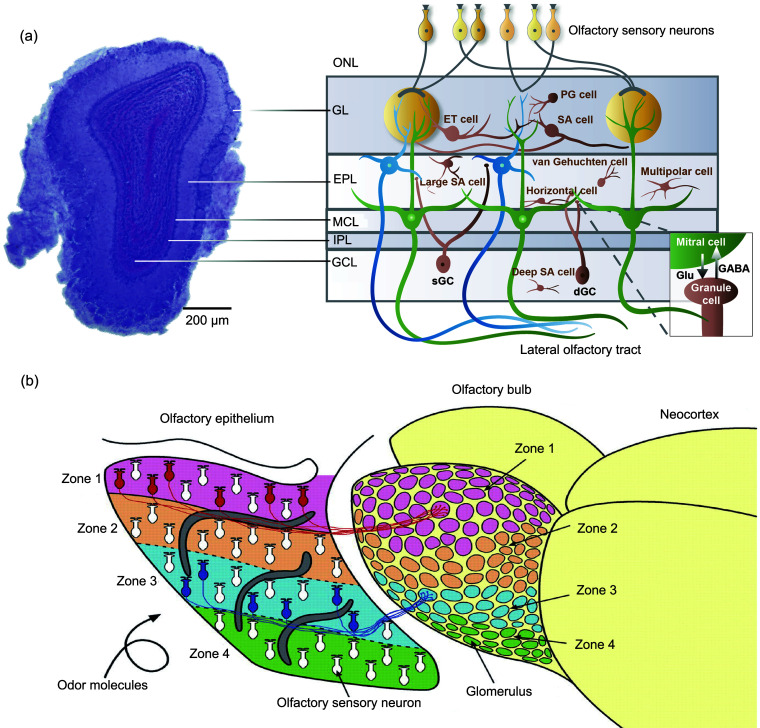
However, the M/Ts do more than passively convey this information: evidence suggests that they are also involved in the flexible processing of odor representations (Restrepo et al., 2009; Doucette et al., 2011; Kato et al., 2012; Chu et al., 2016; Jordan et al., 2018; Bhattacharjee et al., 2019; Wang et al., 2019; Ackels et al., 2021). The neural information transmitted by the M/Ts is dramatically modulated by neural circuits within the OB. At least two neural circuits mediate the neural representation of odor by M/Ts, one in the glomerular layer and the other in the external plexiform layer (Fig. 1a). In the glomerular layer, three types of neurons have been identified as follows: (1) External tufted cells are glutamatergic neurons that provide both feedforward and feedback excitation to the M/Ts to augment the otherwise weak signal from the OSNs to the M/Ts (Pressler and Strowbridge, 2022). (2) Periglomerular cells are GABAergic neurons. Some of them receive direct input from the OSNs and form presynaptic inhibitory synapses on the OSN terminals. Others receive indirect input from the external tufted cells and M/Ts and inhibit all of these. In general, periglomerular cells provide recurrent inhibition of odor-evoked activity in M/Ts (Najac et al., 2015; Burton, 2017; Li et al., 2020; Mori and Sakano, 2021). (3) Superficial short-axon cells can be GABAergic and dopaminergic, or co-release both GABA and dopamine (Lyons-Warren et al., 2023). Although the number of superficial short-axon cells is small, they have a strong mediating effect on odor-evoked neural activity in the M/Ts. Most of the superficial short-axon cells (approximately 70%) receive input from the OSNs indirectly via external tufted cells, with a minority (less than 30%) receiving direct OSN input (Kiyokage et al., 2010; Burton, 2017). The circuit role of superficial short-axon cells is to drive gain control and contrast enhancement of M/T activity via lateral inhibition (Li et al., 2020; Mori and Sakano, 2021).
Fig. 1. Anatomical basis for spatial coding in the olfactory bulb (OB). (a) Structure of the OB. Left: photomicrograph of a coronal section through the mouse OB; Right: diagram of the OB network. Reprinted from Li et al. (2020) by permisson of John Wiley & Sons. (b) A schematic diagram illustrating the projections from the olfactory epithelium to the OB. Reprinted with the permission from Mori et al. (2006). Copyright 2006 The American Physiological Society. ONL: olfactory nerve layer; GL: glomerular layer; EPL: external plexiform layer; MCL: mitral cell layer; IPL: internal plexiform layer; GCL: granule cell layer; PG cell: periglomerular cell; ET cell: external tufted cell; SA cell: short-axon cell; dGC: deep granule cell; sGC: superficial granule cell; Glu: glutamate; GABA: γ-aminobutyric acid.
In the external plexiform layer, the dendrites of the granule cells—whose cell bodies are located in the granule cell layer—form synapse with the dendrites of the M/Ts, yielding a unique type of dendrodendritic synapse. Through these connections, granule cells provide recurrent and lateral inhibition of M/Ts, and this circuit is critically involved in the encoding of odor identity and refinement information for complex odor discrimination by M/Ts (Abraham et al., 2010; Alonso et al., 2012; Markopoulos et al., 2012; Gschwend et al., 2015; Mori and Sakano, 2021). Interestingly, besides granule cells, there are other types of interneurons in the granule cell layer (López-Mascaraque et al., 1986). Although the anatomy and electrophysiological properties of these cells have been identified, their functions are still largely unknown (Schoppa, 2006). For example, Blanes cells, a class of short-axon interneurons in the granule cell layer, are distinguished by their dendritic arborizations. While the neural circuits involved in mediating the neural activity of mitral cells have been extensively investigated (Pressler and Strowbridge, 2006; Schoppa, 2006), how they contribute to the neural representation of odor information by mitral cells remains elusive. In addition, the external plexiform layer also contains a number of different interneurons. For example, parvalbumin (PV)-positive neurons inhibit the neural activity of M/Ts and are key regulators of gain control in the OB (Kato et al., 2013; Miyamichi et al., 2013). Meanwhile, vasoactive intestinal peptide (VIP)-positive interneurons form monosynaptic GABAergic inhibitory connections with M/Ts, playing critical roles in odor processing and olfactory behaviors (Wang et al., 2022). Additionally, somatostatin (SST)-positive interneurons not only contribute to the neural activity of M/Ts and olfactory behavioral performance, but are also critically involved in perceptual learning deficits in an early-life stress mouse model (Nocera et al., 2019; Pardasani et al., 2023). Thus, these neurons likely participate in odor representation by shaping the neural responses of M/Ts.
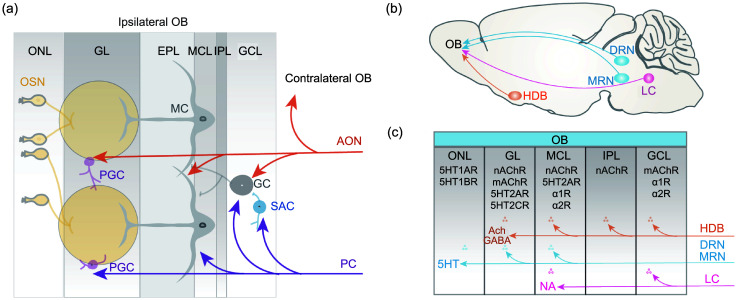
Besides direct input from the OSNs, the OB receives intensive inputs from other brain centers. In general, these inputs can be categorized as feedback projections from the olfactory cortex and modulatory centrifugal innervations, such as cholinergic inputs from the horizontal limb of the diagonal band of Broca (HDB), noradrenergic inputs from the locus coeruleus, and serotonergic inputs from the dorsal raphe nucleus (Fig. 2) (Fletcher and Chen, 2010; Linster and Cleland, 2016; Li et al., 2020; Brunert and Rothermel, 2021; Chae et al., 2022; Zhou et al., 2022). The most intensive feedback inputs to the OB are from piriform cortex and the anterior olfactory nucleus (Chae et al., 2022). While the projections to the OB from the piriform cortex are ipsilateral, the projections from the anterior olfactory nucleus are bilateral. Functionally, piriform-to-OB projections have a weak effect on the spontaneous activity of M/Ts but amplify the odor-evoked inhibition of M/Ts via complex microcircuits within the OB involving granule cells and short-axon cells (Boyd et al., 2012; Mazo et al., 2022). The anterior olfactory nucleus-to-OB projections strongly inhibit both spontaneous and odor-evoked activity in M/Ts (Markopoulos et al., 2012). Overall, the general effect of these feedback inputs to the OB is to decrease the amplitude of odor-evoked responses (Boyd et al., 2012; Markopoulos et al., 2012; Chae et al., 2022; Mazo et al., 2022). It has also been suggested that these inputs shape the encoding of odor information by M/Ts under different brain states, but more direct and detailed evidence for this is needed.
Fig. 2. Schema of olfactory bulb (OB) circuit modulation by cortical feedback and centrifugal neuromodulators. (a) The OB receives cortical feedback from the anterior olfactory nucleus (AON) and the piriform cortex (PC). The AON innervates both the ipsilateral and the contralateral OB. AON glutamatergic inputs can excite mitral cells (MCs) directly and inhibit MCs via granule cells (GCs) and periglomerular cells (PGCs). The PC projects widely to the OB through glutamatergic innervation of short-axon cells (SACs), GCs, MCs, and PGCs. (b) The OB is modulated by serotonergic projections from the dorsal raphe nuclei (DRN) and medial raphe nuclei (MRN), noradrenergic projections from the locus coeruleus (LC), and cholinergic projections from the nucleus of the horizontal limb of the diagonal band (HDB). (c) Schema of neuromodulator release and receptor distribution in different layers of the OB. OSN: olfactory sensory neuron; ONL: olfactory nerve layer; GL: glomerular layer; EPL: external plexiform layer; MCL: mitral cell layer; IPL: internal plexiform layer; GCL: granule cell layer; ACh: acetylcholine; GABA: γ-aminobutyric acid; 5HT: serotonin; NA: noradrenaline.
Projections from the HDB are the major source of centrifugal cholinergic inputs to the OB. Besides cholinergic neurons, a subset of GABAergic neurons in the HDB also project to the OB. These different projections have different modulatory effects on neural activity and odor representation in the OB M/Ts (Böhm et al., 2020). Interestingly, a subset of cholinergic neurons co-express markers for GABA, indicating co-transmission of acetylcholine and GABA from the same HDB neurons to the OB (Case et al., 2017; Zhou et al., 2022). The function of cholinergic inputs to the OB is complex and likely state-dependent. Specific optical activation of the cholinergic cell bodies in the HDB has a general inhibitory effect on the neural activity of M/Ts, but specific optical activation of the cholinergic axons that project to the OB has an excitatory effect on the M/Ts (Ma and Luo, 2012; Rothermel et al., 2014).
The noradrenergic inputs to the OB originate from the locus coeruleus. Although few studies have used specific manipulation of the noradrenergic neurons to investigate how they modulate M/T activity in the OB (Harley and Yuan, 2021), studies using electrical stimulation or pharmacological manipulation can provide some information (Manella et al., 2017). The effect of noradrenergic modulation on M/Ts is complex since both direct and indirect pathways are involved. The most obvious effect is a decrease in the spontaneous activity of M/Ts, but this enhances the signal-to-noise ratio of odor-evoked responses because of a reduction in the intrinsic noise of the M/Ts’ activity (Manella et al., 2017). Further direct evidence is required to support the role of noradrenergic inputs in odor representation and encoding by M/Ts under different brain states, especially in awake, behaving animals.
Serotonergic neurons from the raphe nuclei send direct projections to the OB. These serotonergic inputs dramatically modulate the activity of M/Ts. In contrast to the effects of cholinergic and noradrenergic modulation, the primary effect of serotonergic modulation is to increase the neural activity of the M/Ts (Brunert et al., 2016; Kapoor et al., 2016). It is still not clear whether and how these serotonergic inputs affect the neural representation of odors by M/Ts; however, since the serotonergic system is closely associated with reward, it is likely that the divergent responses of M/Ts to rewarded and unrewarded odors (Wang et al., 2019; Liu et al., 2020; Wang et al., 2020) are shaped by these serotonergic innervations.
Although some of the feedback and centrifugal modulation occur via direct innervation of M/Ts, most occur indirectly via innervation of interneurons within the OB, complicating the overall effects. Such complex modulation may be important for the precise representation of odors under different brain states, such as increased or decreased attention, with prior exposure to the situation or during learning. The centrifugal modulatory projections are particularly intricate because some individual neurons innervate other olfactory centers as well as the OB. These other centers include the piriform cortex and the anterior olfactory nucleus, which themselves send feedback to the OB, as discussed above. Many studies have focused on the separate roles of feedback and centrifugal inputs in the activity of M/Ts, but further studies are needed to investigate how these different modulatory systems cooperate. In particular, mathematical models that can predict the network effects of the various inputs should be established.
3. External factors that affect odor representation in the OB: odor plume and animal sniffing
In natural and open environments, the release of odorants into the air results in a plume that extends and widens downstream of the source. Turbulent plumes lead to rapid concentration fluctuations that contain rich information about the olfactory scenery (Szyszka et al., 2012; Ackels et al., 2021; Dasgupta et al., 2022). Therefore, to precisely perceive the odorants in everchanging natural environments, the olfactory system must have the ability to represent odor information using the temporal structure of the odor plumes. This ability has been confirmed in both insects and rodents (Szyszka et al., 2012; Ackels et al., 2021; Dasgupta et al., 2022). For example, honey bees can use a temporal difference of 6 ms in stimulus coherence for successful odor-object segregation (Szyszka et al., 2012, 2023). Mice have the ability to discriminate differences of 10 ms in duration (Li et al., 2014), as well as 10 ms input shifts in the sniff cycle (Smear et al., 2011). Interestingly, mice can also discriminate temporal correlations in rapidly fluctuating odors at frequencies of up to 40 Hz. Such correlation information can be readily extracted from the neural activity of M/Ts in the OB (Ackels et al., 2021).
Another important factor that affects the odor representation of M/Ts is respiration/sniffing (an active odor-sampling process with a respiration frequency higher than 4 Hz) (Wachowiak, 2011). In rodents, sniffing coordinates with other orofacial motor actions such as the movement of whiskers, chewing, licking, and lateral displacement of the nostrils (Moore et al., 2014). Exploratory sniffing is reliably evoked by novel odorant stimuli and is dominant during rapid odor‐source localization (Khan et al., 2012). An investigation by one rat towards the facial region of a conspecific often elicits a decrease in sniffing frequency in the conspecific, indicating that they use sniffing to communicate information (Wesson, 2013). Sniffing also plays a critical role in patients; for example, it has been reported that sniff responses signal consciousness in unresponsive patients with brain injuries, providing an accessible tool to determine whether the patient is unresponsive or perhaps minimally conscious (Arzi et al., 2020).
It is not surprising that respiration/sniffing plays a key role in the representation of odors in the olfactory system since the sampling of odorants—and the subsequent interaction between the odorants and OSN olfactory receptors—is not continuous but gated by the rhythm of the respiration/sniffing. Both the activity of OSNs and the transmission of neural signals from OSNs to the M/Ts are modulated by respiration/sniffing (Patterson et al., 2013; Jordan et al., 2018). Mice can discriminate between identical sensory stimuli based on their timing within the sniff cycle, distinguishing differences as small as 10 ms, suggesting that the mammalian olfactory system can interpret temporal patterns of neural activity, which may be critical for rapid and accurate odor recognition (Smear et al., 2011). Strikingly, the theta oscillations in the local field potential (LFP) in the OB are tightly coupled to the frequency of respiration/sniffing (Kay et al., 2009). As we will discuss below, the phase of respiration/sniffing provides a critical time frame for the firing of action potentials (spikes) in the M/Ts, and this, in turn, critically shapes the encoding of odor information by M/Ts.
4. Odor information encoded in olfactory maps in the OB
How is odor information, such as odor identity, represented by the olfactory output neurons, the M/Ts? Since this question is related to the organization of the OSNs and the projections from the OSNs to the OB, we first briefly review some important related properties of the OSNs. In the olfactory epithelium, olfactory receptors are expressed in the OSNs and interact with odorants that are sampled by respiration/sniffing. There are approximately 1000 different types of olfactory receptors in the mice and approximately 350 in humans; typically, only one type of receptor is expressed in each OSN (Fig. 1b) (Mori et al., 2006; Mori and Sakano, 2011), but for some non-G-protein-coupled receptors, more than one type of receptor can be expressed (Greer et al., 2016). OSNs that express the same olfactory receptor project their axons to two mirror-image glomeruli in the OB, one on the medial side and the other on the lateral side. This projection mode is unique among the sensory systems and is thought to play a role in odor representation. In each glomerulus, the axons from the OSNs express the same olfactory receptor synapse with the M/Ts and each M/T receives input from only one glomerulus. The glomeruli are functional units in the OB for odor representation (Xu et al., 2000). Studies using optogenetics to selectively activate individual glomerulus have shown that mice can perceive stimulation from a single glomerulus, even against a background of other odors (Smear et al., 2013). Additionally, variations in the amplitude and timing of glomerular stimulation affect odor perception. This suggests that individual glomerulus can convey unique signals that influence behavior based on the identity, intensity, and timing of the input (Smear et al., 2013). These findings indicate that glomeruli utilize a combinatorial code based on multiple signal modalities, enabling complex and nuanced odor detection and discrimination. However, it remains unclear whether, at the output level, the M/Ts also employ such a combinatorial encoding strategy to represent odor identity, intensity, and timing simultaneously.
The information flow from the OSNs to the M/Ts is highly convergent since the ratio of OSNs to M/Ts is approximately 5000:1 (Mori et al., 2006). Within a glomerulus, there are several thousand converging OSN axons, but dendrites from only 10–20 mitral cells and 50–70 tufted cells. The olfactory epithelium in rodents is organized into four distinct zones (Ressler et al., 1993; Mori et al., 1999). A given odorant receptor is expressed by OSNs located within one zone. These OSNs, which share a common receptor type, are broadly distributed throughout their respective zones in the epithelium. Their axons then converge onto several anatomically consistent glomeruli situated in the corresponding zone of the OB (Fig. 1b) (Mori et al., 2006). As a result, OSNs located in the ventral and lateral parts of the nasal turbinate project to the medial and lateral glomeruli of the OB, respectively; OSNs located in the central channel of the nose and more peripheral or ventral parts project to the dorsal and ventral glomeruli of the OB, respectively (Astic and Saucier, 1986; Schoenfeld et al., 1994; Johnson and Leon, 2007). Overall, it is clear from the projection properties that there is an odor map in the OB that reflects the activity of odorant-activated OSNs in the olfactory epithelium, and the existence of this odor map is highly conserved across species.
It has been hypothesized that information about odors is encoded in the OB via this odor map (Sharp et al., 1975; Johnson et al., 1998; Rubin and Katz, 1999; Xu et al., 2000; Mori et al., 2006; Johnson and Leon, 2007; Zhu et al., 2022). With a spatial coding strategy, information about odor identity and odor concentration can both be represented in the spatial pattern of activated glomeruli in the OB (Xu et al., 2000; Johnson and Leon, 2007). Although Adrian (1950) concluded that different odorants could activate different parts of the OB as early as 1950 using multi-site electrophysiological recordings, the first direct evidence for an OB odor map occurred in 1975 with the mapping of odor-evoked foci in the whole OB by 2-deoxyglucose (2-DG) uptake (Sharp et al., 1975). However, this mapping method lacks temporal information; that is, it does not include any information on how the odor map evolves during odor stimulation. Optical imaging methods, including both intrinsic optical responses and voltage- or calcium-sensitive dyes, have excellent temporal resolution but can only image odor responses in the dorsal and lateral parts of the OB (Spors et al., 2006; Johnson and Leon, 2007; Wachowiak et al., 2013; Leong and Storace, 2024). Functional magnetic resonance imaging (fMRI) has proven helpful for imaging odor maps in the OB: this method has good spatial and temporal resolution, can be used to image the entire OB, and can monitor activation maps during and after odor stimulation (Xu et al., 2003; Poplawsky et al., 2023).
Applying these and other mapping methods has revealed several characteristics of the odor map and related spatial coding (Xu et al., 2000; Mori et al., 2006; Johnson and Leon, 2007). First, the glomerulus is the functional unit of the odor maps. This property has been confirmed by 2-DG uptake and fMRI mapping (Johnson et al., 1998; Xu et al., 2003). Second, the activation map for a given odorant has constant topographic features across animals for the same experimental conditions. However, odor-evoked patterns can change under different experimental conditions, suggesting that the odor maps are dynamic and responsive to other functional properties such as experience, adaptation, and odor history (Schafer et al., 2005; Chu et al., 2016). Third, data indicate that different but overlapping foci are induced by different odorants. This observation has given rise to different odorants (Uchida and Mainen, 2003; Abraham et al., 2004; Bhattacharjee et al., 2019). Fourth, as well as odor identity, odor maps can contribute to the encoding of odor concentration. At weak concentrations, odorants often activate just one or a few glomeruli (Abraham et al., 2014; Wilson et al., 2017; Chong et al., 2020). However, recent studies suggest that as the concentration of an odor increases, leading to the activation of a greater number of olfactory glomeruli, the glomeruli activated at lower concentrations are activated earlier in time (Wilson et al., 2017; Chong et al., 2020). Consequently, these earliest activated glomeruli represent the type of odor and are capable of encoding the odor identity across varying concentrations (for details, see “primacy code” below). These changes in the odor map with different odor concentrations are correlated with perceptual changes. Overall, the spatial coding strategy has a solid structural and anatomical basis and is supported by a large body of experimental data, especially from mapping techniques.
5. Timing of neural activity provides abundant information for odor representation and encoding
Next, we discuss a different strategy used by M/Ts to encode odor information: temporal coding. First, we briefly introduce some properties of spontaneous and odor-evoked neural activities in the OB. M/Ts show strong spontaneous firing without odor stimulation, even when the animals are anesthetized (Li et al., 2011). As mentioned above, firing in these cells is highly coupled with the inhalation phase of respiration/sniffing. When odors are presented to anesthetized animals, increases in the firing rate of M/Ts are commonly observed; however, when odors are presented to awake, behaving animals, the responses are commonly weak and even inhibitory (Rinberg et al., 2006). Compared with mitral cells, tufted cells often show odor-evoked firing earlier in the sniffing cycle (Fukunaga et al., 2012).
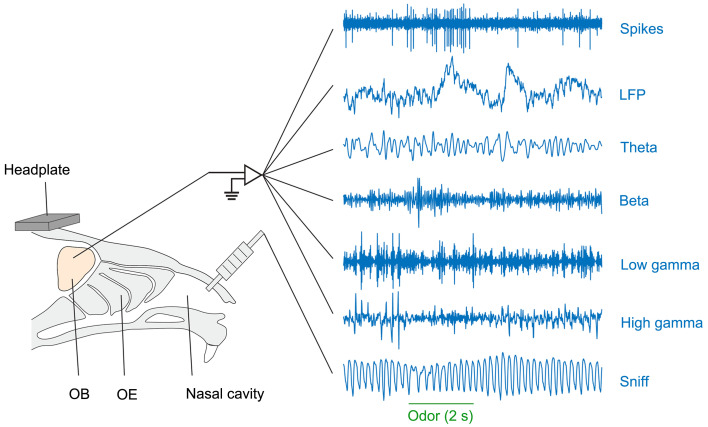
In addition to M/T spikes, electrodes placed in or near the mitral cell layer also pick up the LFP. Four bands of oscillations are usually filtered from the raw LFP (Fig. 3) (Li et al., 2011; Liu et al., 2020). Theta oscillations (approximately 2–12 Hz) tend to be highly correlated with respiration/sniffing and are considered to play an important role in sensorimotor performance (Sheriff et al., 2021). Beta oscillations (approximately 15–35 Hz) can be elicited by odors in both anesthetized and awake states (Li et al., 2011; Liu et al., 2020). In behaving rodents, beta oscillations are often induced by odorants in learning or odor-sensitization paradigms or in response to predator odorants such as 2,3,5-trimethyl-3-thiazoline (TMT) (Kay et al., 2009). Beta oscillations are likely generated by the neural circuits between the OB and the olfactory cortex since the beta oscillations disappear when these inputs to the OB are blocked (Osinski et al., 2018). Low gamma oscillations (35–65 Hz) can be elicited by odorants in anesthetized rodents but are rarely evoked in awake, behaving animals (Li et al., 2011; Liu et al., 2020). This oscillation band may be closely related to brain states since it can be an excellent indicator of anesthetized versus awake conditions and different sleep stages (Bagur et al., 2018). High gamma oscillations (65–90 Hz) are coupled to the theta oscillations and respiration/sniffing and often arise at the peak of the inhalation phase (Kay et al., 2009; Peace et al., 2024). High gamma oscillations are thought to be generated by neural circuits within the OB, specifically, the reciprocal connections between M/Ts and granule cells. Odor stimulation often evokes increased high gamma responses under anesthesia (Li et al., 2011) but decreased responses in the awake state (Liu et al., 2020). High gamma oscillations are critically involved in olfactory-related learning (Kay et al., 2009). Overall, the different oscillations in the OB all play important roles in odor detection, discrimination, and olfactory-related learning. The representation and encoding of odorants may also provide a critical time frame for spikes that is relevant for temporal coding (Li et al., 2015).
Fig. 3. Diagram showing the electrophysiological signals recorded in the olfactory bulb (OB) (spikes, raw local field potential (LFP), and four LFP frequency bands) and the respiration/sniffing signals recorded from the nasal cavity. Reprinted from Liu et al. (2020) with kind permission from Springer Nature. OE: olfactory epithelium.
One feature of neural activity that can be used for the temporal coding of odor information is the spiking latency (Uchida et al., 2014). As discussed above, odor-evoked spikes in M/Ts often appear at a certain phase in the respiration/sniffing cycle. The latency between sniffing onset (or the onset of the associated LFP oscillations) and the appearance of spiking may provide information on odor concentration and identity (Junek et al., 2010). While there is some indirect evidence to support this coding strategy, one study in locusts indicates that the spiking latency with respect to beta oscillations does not support the coding of odor concentration (Stopfer et al., 2003). More evidence will be needed to confirm that the information used is spiking latency rather than firing rate.
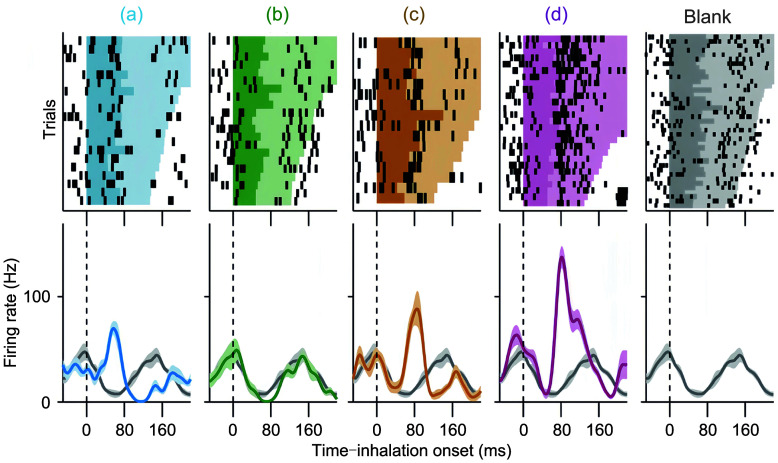
A second feature that can be used for temporal coding is the firing pattern. In awake rats, although odorants rarely evoke marked changes in the firing rate in M/Ts, they might evoke changes in firing patterns across a particular time window (Cury and Uchida, 2010). The respiration/sniffing cycle and the associated LFP oscillations are good candidates for such a time window. Indeed, the fine temporal structure within a respiration/sniffing cycle has been reported to carry information on odor identity (Cury and Uchida, 2010). Data from awake, behaving rats indicate that odor decoding performance is significantly improved if the sub-sniff temporal structure is considered (Fig. 4) (Cury and Uchida, 2010). This decoding performance is largely better than that obtained from the firing rate or from the latency to the first spike with respect to inhalation onset. Besides the sniffing cycle, other cycles, such as those in the beta or gamma oscillations, could form the temporal framework for fine firing patterns that convey odor information. For example, in mice performing a go/no-go task, gamma oscillation-coupled firing has been reported to carry information about both odor value and odor identity (Li et al., 2015).
Fig. 4. Firing of mitral/tufted cells with respect to the sniffing cycle for encoding odor information. Reprinted from Cury and Uchida (2010) . Copyright 2010, with permission from Elsevier. Top: raster plots of spikes from a representative mitral/tufted cell exposed to four different odorants (colors). Trials are aligned by the onset of the first odor inhalation; the colored shading indicates the first respiration cycle after odor onset, with the darker shading corresponding to the inhalation period. Bottom: corresponding peri-event time histograms (PETHs). Odors: (a) 1-hexanal; (b) ethyl tiglate; (c) butyraldehyde; (d) R-(-)-2-octanol.
Just as a single M/T responds to many odorants, a specific odorant can activate an ensemble of M/Ts (Uchida et al., 2014). Thus, the response dynamics from an ensemble of M/Ts can provide abundant information on odor identity. With prolonged odor stimulation, the dynamics of M/T ensembles can change immediately after odor onset. The temporal dynamics vary among odorants and can be used to decode the identity of the odorants. This coding strategy has been confirmed in the principal neurons of locusts and in M/Ts of behaving rats and mice (Stopfer et al., 2003; Bathellier et al., 2008; Gschwend et al., 2015).
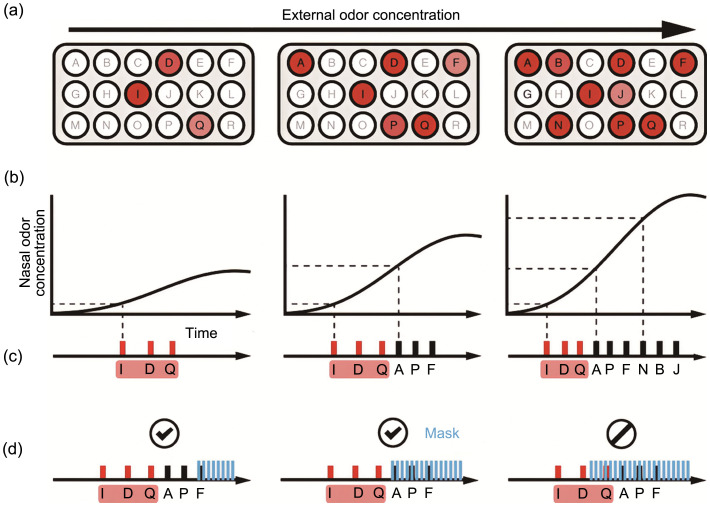
Finally, a new coding strategy has been proposed for concentration-invariant odor identification, termed “primacy coding” (Fig. 5) (Wilson et al., 2017). In this strategy, an initial weak concentration of a specific odorant will only activate a few glomeruli that receive inputs from the most sensitive olfactory receptors for this odorant. These first few activated glomeruli thus encode the odor identity. As the concentration increases, other less sensitive glomeruli are also activated but with longer response latencies. Therefore, while the earlier or primary set of glomeruli encodes the odor identity, the activity in the later-recruited glomeruli provides information only on other aspects of the odorant, such as concentration. This coding strategy has been confirmed by optogenetic masking of the signals in the few earliest activated glomeruli versus later-recruited glomeruli (Chong et al., 2020). It is found that neurons activated earlier in a sequence have a more significant impact on perception than those activated later. This suggests a “primacy effect” where the initial part of the sensory input carries more weight in the perceptual process. Interestingly, the perceptual relevance of neuron activation timings is more related to their sequence relative to each other rather than to external rhythms such as the sniffing cycle (Chong et al., 2020). Finally, a template-matching computational model that effectively predicts perceptual outcomes based on the timing and sequence of neural activations demonstrates that the brain matches incoming sensory information against learned templates, focusing on the relative timing within activation sequences (Chong et al., 2020).
Fig. 5. Schematic diagram illustrating the primacy coding of odor identity. Reprinted from Wilson et al. (2017). (a) Example of the activity in glomeruli for three different odor concentrations. The total number of active glomeruli increases as the odor concentration increases. (b) Temporal profiles of the odor concentration in the nose during inhalation for three different odor concentrations. (c) Temporal sequence of glomerulus activation for the three different odor concentrations. (d) Schematic diagram demonstrating the effect of an optogenetic mask on temporal sequences when presented late and early in the odor-evoked temporal pattern.
6. Concluding remarks and future directions
As the first processing center in the olfactory system, the OB plays a crucial role in the encoding, processing, and transmission of odor information. It has the ability to precisely encode odor information in different internal states and external environments. Although the spatial coding strategy is supported by solid anatomical and imaging data, it is unclear how these maps are interpreted by downstream cortical regions since, to date, no evidence suggests that such an odor map exists in the piriform cortex or the anterior olfactory nucleus, which are the major output targets of the OB. The temporal coding strategy does not use the firing rate but focuses on temporal features of spiking, such as the latency, pattern, and trajectory of neuron ensembles with respect to a specific temporal framework, such as the respiration/sniffing cycle or LFP oscillations. Although this strategy can faithfully encode odor information, such as odor identity, under some circumstances, how the OB uses this strategy to accurately represent different odorants under ever-changing states remains elusive. Therefore, more work is needed to investigate how these coding strategies are implemented in specific situations. Here, we present some points regarding possible future developments.
As discussed above, a distributive representation of neurons in the sensory epithelium is converted into a topographical map in the OB, which is the basis of the spatial coding in the OB. However, downstream of the OB, the piriform cortex discards this spatial order, as it has been extensively reported that axons from individual glomerulus project diffusely to the piriform without apparent spatial preference (Ghosh et al., 2011; Miyamichi et al., 2011; Sosulski et al., 2011), and odor information is encoded by ensembles of coordinately active neurons distributed across the piriform cortex without topographic organization (Stettler and Axel, 2009; Roland et al., 2017). This raises the question of how the downstream piriform cortex, without topography, transforms information from the OB that has well-defined topography. It has been suggested that at the output level of the OB, after being processed by the circuits both within the OB and from other centers, such as the feedback and centrifugal innervations, the odor-evoked neural activity of M/Ts that contain critical information about odor identity shows pattern decorrelation (Laurent, 2002; Wiechert et al., 2010; Friedrich and Wiechert, 2014; Gschwend et al., 2015). Such a pattern decorrelation can be easily detected by distributed clusters in the piriform cortex and benefits the classification and storage of information by associative networks in the piriform cortex (Friedrich and Wiechert, 2014). Recently, a tabula rasa model for cortical olfactory processing has been established, in which odor representations in the piriform cortex only achieve behavioral significance through learning and neural plasticity (Choi et al., 2011; Meissner-Bernard et al., 2019; Schoonover et al., 2021). In addition, numerous computational models have suggested that the transformation of encoding from the OB to the piriform cortex occurs when distributed neurons in the piriform cortex process information from the OB based on the piriform cortex’s complex neural circuits (Lysetskiy et al., 2002; Kepple et al., 2019). Interestingly, recent studies using high-throughput tracing have revealed that neural projections to and from the piriform cortex do exhibit spatially structured connectivity (Wang et al., 2021; Chen et al., 2022; Diaz et al., 2023). While the details of this connectivity need further evidence, its function should be further identified in future studies.
Since a given odorant often activates a large number of M/Ts, odorant-related information is likely encoded by many neurons rather than a few. However, in most studies, spikes are sampled from only a very small number of M/Ts (usually 10–50 cells in each animal) by 16- or 32-channel electrodes. Therefore, it will become important to utilize advanced large arrays of electrodes to sample spikes from a greater number of M/Ts simultaneously (e.g., 100 or more) to provide better information on how the M/Ts encode a specific odorant. Furthermore, if a large number of M/Ts are sampled from a single animal, we can evaluate how they encode odorants within that animal rather than needing to pool cells across a group of animals. By evaluating the decoding performance in each animal, we can assess the reliability of the coding strategy in individuals.
To evaluate the decoding performance of the M/Ts, a specific decoding model, such as a support vector machine (SVM) or logistic regression (LR), is used (Cury and Uchida, 2010). However, whether these models reliably reflect the real situation needs further confirmation. It will be helpful to develop new and better mathematical models in the future. Furthermore, while decoding performance is evaluated offline in most studies, it is important to also calculate performance online with only a small delay. Such online calculations are also critical for the development of a bioelectronic nose, which is the subject of pioneering work in the field (Zhuang et al., 2015, 2021; Gao et al., 2018; Liu MX et al., 2023). Recent technical advances have allowed online decoding to be performed within a few milliseconds of odor delivery (Wu YJ et al., 2023).
For a full understanding of the olfactory system, we must also elucidate how odor information is encoded beyond the OB in the downstream olfactory cortex. Although some studies have shed light on the circuits and coding strategy in the piriform cortex (Bolding and Franks, 2018), more studies should be performed in other olfactory centers, such as the anterior olfactory nucleus and lateral entorhinal cortex (Liu PL et al., 2023). Finally, recent studies have explored neural activity in human olfactory centers via intracranial electroencephalogram (EEG) recordings in patients and have demonstrated a relationship between specific oscillations, such as theta oscillations, and olfactory perception (Jiang et al., 2017; Yang et al., 2022). Further studies are required to investigate how spikes in these centers encode odor information in humans and nonhuman primates.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 32271055 and 32070995), the Jiangsu Province Innovative and Entrepreneurial Team Program, and the Guangdong Medical University (No. GDMUB2022048), China.
Author contributions
Panke WANG, Shan LI, and An’an LI contributed to concept of the design and manuscript preparation. Panke WANG and An’an LI contributed to the collection of literature. An’an LI provided substantive guidance and financial support for this work. All authors have read and approved the final manuscript.
Compliance with ethics guidelines
Panke WANG, Shan LI, and An'an LI declare that they have no conflict of interest.
This review does not contain any studies with human or animal subjects performed by any of the authors.
References
- Abraham NM, Spors H, Carleton A, et al. , 2004. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron, 44(5): 865-876. 10.1016/j.neuron.2004.11.017 [DOI] [PubMed] [Google Scholar]
- Abraham NM, Egger V, Shimshek DR, et al. , 2010. Synaptic inhibition in the olfactory bulb accelerates odor discrimination in mice. Neuron, 65(3): 399-411. 10.1016/j.neuron.2010.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham NM, Vincis R, Lagier S, et al. , 2014. Long term functional plasticity of sensory inputs mediated by olfactory learning. eLife, 3: e02109. 10.7554/eLife.02109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackels T, Erskine A, Dasgupta D, et al. , 2021. Fast odour dynamics are encoded in the olfactory system and guide behaviour. Nature, 593(7860): 558-563. 10.1038/s41586-021-03514-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian ED, 1950. The electrical activity of the mammalian olfactory bulb. Electroencephalogr Clin Neurophysiol, 2(1-4): 377-388. 10.1016/0013-4694(50)90075-7 [DOI] [PubMed] [Google Scholar]
- Alonso M, Lepousez G, Wagner S, et al. , 2012. Activation of adult-born neurons facilitates learning and memory. Nat Neurosci, 15(6): 897-904. 10.1038/nn.3108 [DOI] [PubMed] [Google Scholar]
- Aqrabawi AJ, Kim JC, 2020. Olfactory memory representations are stored in the anterior olfactory nucleus. Nat Commun, 11: 1246. 10.1038/s41467-020-15032-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzi A, Rozenkrantz L, Gorodisky L, et al. , 2020. Olfactory sniffing signals consciousness in unresponsive patients with brain injuries. Nature, 581(7809): 428-433. 10.1038/s41586-020-2245-5 [DOI] [PubMed] [Google Scholar]
- Astic L, Saucier D, 1986. Anatomical mapping of the neuroepithelial projection to the olfactory bulb in the rat. Brain Res Bull, 16(4): 445-454. 10.1016/0361-9230(86)90172-3 [DOI] [PubMed] [Google Scholar]
- Bagur S, Lacroix MM, de Lavilléon G, et al. , 2018. Harnessing olfactory bulb oscillations to perform fully brain-based sleep-scoring and real-time monitoring of anaesthesia depth. PLoS Biol, 16(11): e2005458. 10.1371/journal.pbio.2005458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathellier B, Buhl DL, Accolla R, et al. , 2008. Dynamic ensemble odor coding in the mammalian olfactory bulb: sensory information at different timescales. Neuron, 57(4): 586-598. 10.1016/j.neuron.2008.02.011 [DOI] [PubMed] [Google Scholar]
- Bhattacharjee AS, Konakamchi S, Turaev D, et al. , 2019. Similarity and strength of glomerular odor representations define a neural metric of sniff-invariant discrimination time. Cell Rep, 28(11): 2966-2978.e5. 10.1016/j.celrep.2019.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee AS, Joshi SV, Naik S, et al. , 2020. Quantitative assessment of olfactory dysfunction accurately detects asymptomatic COVID-19 carriers. eClinicalMedicine, 28: 100575. 10.1016/j.eclinm.2020.100575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmik R, Pardasani M, Mahajan S, et al. , 2023. Persistent olfactory learning deficits during and post-COVID-19 infection. Curr Res Neurobiol, 4: 100081. 10.1016/j.crneur.2023.100081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billesbølle CB, de March CA, van der Velden WJC, et al. , 2023. Structural basis of odorant recognition by a human odorant receptor. Nature, 615(7953): 742-749. 10.1038/s41586-023-05798-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm E, Brunert D, Rothermel M, 2020. Input dependent modulation of olfactory bulb activity by HDB GABAergic projections. Sci Rep, 10: 10696. 10.1038/s41598-020-67276-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolding KA, Franks KM, 2018. Recurrent cortical circuits implement concentration-invariant odor coding. Science, 361(6407): eaat6904. 10.1126/science.aat6904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd AM, Sturgill JF, Poo C, et al. , 2012. Cortical feedback control of olfactory bulb circuits. Neuron, 76(6): 1161-1174. 10.1016/j.neuron.2012.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunert D, Rothermel M, 2021. Extrinsic neuromodulation in the rodent olfactory bulb. Cell Tissue Res, 383(1): 507-524. 10.1007/s00441-020-03365-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunert D, Tsuno Y, Rothermel M, et al. , 2016. Cell-type-specific modulation of sensory responses in olfactory bulb circuits by serotonergic projections from the raphe nuclei. J Neurosci, 36(25): 6820-6835. 10.1523/JNEUROSCI.3667-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunert D, Medinaceli Quintela R, Rothermel M, 2023. The anterior olfactory nucleus revisited ‒ An emerging role for neuropathological conditions? Prog Neurobiol, 228: 102486. 10.1016/j.pneurobio.2023.102486 [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R, 1991. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell, 65(1): 175-187. 10.1016/0092-8674(91)90418-x [DOI] [PubMed] [Google Scholar]
- Burton SD, 2017. Inhibitory circuits of the mammalian main olfactory bulb. J Neurophysiol, 118(4): 2034-2051. 10.1152/jn.00109.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case DT, Burton SD, Gedeon JY, et al. , 2017. Layer- and cell type-selective co-transmission by a basal forebrain cholinergic projection to the olfactory bulb. Nat Commun, 8: 652. 10.1038/s41467-017-00765-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae H, Banerjee A, Dussauze M, et al. , 2022. Long-range functional loops in the mouse olfactory system and their roles in computing odor identity. Neuron, 110(23): 3970-3985.e7. 10.1016/j.neuron.2022.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FJ, Liu W, Liu PL, et al. , 2021. α-Synuclein aggregation in the olfactory bulb induces olfactory deficits by perturbing granule cells and granular-mitral synaptic transmission. npj Parkinsons Dis, 7: 114. 10.1038/s41531-021-00259-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YS, Chen XY, Baserdem B, et al. , 2022. High-throughput sequencing of single neuron projections reveals spatial organization in the olfactory cortex. Cell, 185(22): 4117-4134.e28. 10.1016/j.cell.2022.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GB, Stettler DD, Kallman BR, et al. , 2011. Driving opposing behaviors with ensembles of piriform neurons. Cell, 146(6): 1004-1015. 10.1016/j.cell.2011.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong E, Moroni M, Wilson C, et al. , 2020. Manipulating synthetic optogenetic odors reveals the coding logic of olfactory perception. Science, 368(6497): eaba2357. 10.1126/science.aba2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu MW, Li WL, Komiyama T, 2016. Balancing the robustness and efficiency of odor representations during learning. Neuron, 92(1): 174-186. 10.1016/j.neuron.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cury KM, Uchida N, 2010. Robust odor coding via inhalation-coupled transient activity in the mammalian olfactory bulb. Neuron, 68(3): 570-585. 10.1016/j.neuron.2010.09.040 [DOI] [PubMed] [Google Scholar]
- Dasgupta D, Warner TPA, Erskine A, et al. , 2022. Coupling of mouse olfactory bulb projection neurons to fluctuating odor pulses. J Neurosci, 42(21): 4278-4296. 10.1523/JNEUROSCI.1422-21.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz C, Franks KM, Blazing RM, 2023. Neuroscience: seq-ing maps in the olfactory cortex. Curr Biol, 33(7): R266-R269. 10.1016/j.cub.2023.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette W, Gire DH, Whitesell J, et al. , 2011. Associative cortex features in the first olfactory brain relay station. Neuron, 69(6): 1176-1187. 10.1016/j.neuron.2011.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann A, Shykind BM, Sosulski DL, et al. , 2008. Mice with a “monoclonal nose”: perturbations in an olfactory map impair odor discrimination. Neuron, 60(6): 1068-1081. 10.1016/j.neuron.2008.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher ML, Chen WR, 2010. Neural correlates of olfactory learning: critical role of centrifugal neuromodulation. Learn Mem, 17(11): 561-570. 10.1101/lm.941510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich RW, Wiechert MT, 2014. Neuronal circuits and computations: pattern decorrelation in the olfactory bulb. FEBS Lett, 588(15): 2504-2513. 10.1016/j.febslet.2014.05.055 [DOI] [PubMed] [Google Scholar]
- Fukunaga I, Berning M, Kollo M, et al. , 2012. Two distinct channels of olfactory bulb output. Neuron, 75(2): 320-329. 10.1016/j.neuron.2012.05.017 [DOI] [PubMed] [Google Scholar]
- Gadziola MA, Tylicki KA, Christian DL, et al. , 2015. The olfactory tubercle encodes odor valence in behaving mice. J Neurosci, 35(11): 4515-4527. 10.1523/JNEUROSCI.4750-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao KQ, Li SM, Zhuang LJ, et al. , 2018. In vivo bioelectronic nose using transgenic mice for specific odor detection. Biosens Bioelectron, 102: 150-156. 10.1016/j.bios.2017.08.055 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Larson SD, Hefzi H, et al. , 2011. Sensory maps in the olfactory cortex defined by long-range viral tracing of single neurons. Nature, 472(7342): 217-220. 10.1038/nature09945 [DOI] [PubMed] [Google Scholar]
- Greer PL, Bear DM, Lassance JM, et al. , 2016. A family of non-GPCR chemosensors defines an alternative logic for mammalian olfaction. Cell, 165(7): 1734-1748. 10.1016/j.cell.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwend O, Abraham NM, Lagier S, et al. , 2015. Neuronal pattern separation in the olfactory bulb improves odor discrimination learning. Nat Neurosci, 18(10): 1474-1482. 10.1038/nn.4089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo LL, Cheng J, Lian S, et al. , 2023. Structural basis of amine odorant perception by a mammal olfactory receptor. Nature, 618(7963): 193-200. 10.1038/s41586-023-06106-4 [DOI] [PubMed] [Google Scholar]
- Hanchate NK, Kondoh K, Lu ZH, et al. , 2015. Single-cell transcriptomics reveals receptor transformations during olfactory neurogenesis. Science, 350(6265): 1251-1255. 10.1126/science.aad2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CW, Yuan Q, 2021. Locus coeruleus optogenetic modulation: lessons learned from temporal patterns. Brain Sci, 11(12): 1624. 10.3390/brainsci11121624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HD, Schuele S, Rosenow J, et al. , 2017. Theta oscillations rapidly convey odor-specific content in human piriform cortex. Neuron, 94(1): 207-219.e4. 10.1016/j.neuron.2017.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Leon M, 2007. Chemotopic odorant coding in a mammalian olfactory system. J Comp Neurol, 503(1): 1-34. 10.1002/cne.21396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Leon M, 1998. Spatial coding of odorant features in the glomerular layer of the rat olfactory bulb. J Comp Neurol, 393(4): 457-471. [DOI] [PubMed] [Google Scholar]
- Jordan R, Fukunaga I, Kollo M, et al. , 2018. Active sampling state dynamically enhances olfactory bulb odor representation. Neuron, 98(6): 1214-1228.e5. 10.1016/j.neuron.2018.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junek S, Kludt E, Wolf F, et al. , 2010. Olfactory coding with patterns of response latencies. Neuron, 67(5): 872-884. 10.1016/j.neuron.2010.08.005 [DOI] [PubMed] [Google Scholar]
- Kapoor V, Provost AC, Agarwal P, et al. , 2016. Activation of raphe nuclei triggers rapid and distinct effects on parallel olfactory bulb output channels. Nat Neurosci, 19(2): 271-282. 10.1038/nn.4219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato HK, Chu MW, Isaacson JS, et al. , 2012. Dynamic sensory representations in the olfactory bulb: modulation by wakefulness and experience. Neuron, 76(5): 962-975. 10.1016/j.neuron.2012.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato HK, Gillet SN, Peters AJ, et al. , 2013. Parvalbumin-expressing interneurons linearly control olfactory bulb output. Neuron, 80(5): 1218-1231. 10.1016/j.neuron.2013.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, 2022. COVID-19 and olfactory dysfunction: a looming wave of dementia? J Neurophysiol, 128(2): 436-444. 10.1152/jn.00255.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Beshel J, Brea J, et al. , 2009. Olfactory oscillations: the what, how and what for. Trends Neurosci, 32(4): 207-214. 10.1016/j.tins.2008.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepple DR, Giaffar H, Rinberg D, et al. , 2019. Deconstructing odorant identity via primacy in dual networks. Neural Comput, 31(4): 710-737. 10.1162/neco_a_01175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AG, Sarangi M, Bhalla US, 2012. Rats track odour trails accurately using a multi-layered strategy with near-optimal sampling. Nat Commun, 3: 703. 10.1038/ncomms1712 [DOI] [PubMed] [Google Scholar]
- Kim H, Kim H, Nguyen LT, et al. , 2022. Amplification of olfactory signals by Anoctamin 9 is important for mammalian olfaction. Prog Neurobiol, 219: 102369. 10.1016/j.pneurobio.2022.102369 [DOI] [PubMed] [Google Scholar]
- Kiyokage E, Pan YZ, Shao ZY, et al. , 2010. Molecular identity of periglomerular and short axon cells. J Neurosci, 30(3): 1185-1196. 10.1523/JNEUROSCI.3497-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruppath P, Xue L, Pouille F, et al. , 2023. Hyperexcitability in the olfactory bulb and impaired fine odor discrimination in the Fmr1 KO mouse model of fragile X syndrome. J Neurosci, 43(48): 8243-8258. 10.1523/JNEUROSCI.0584-23.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G, 2002. Olfactory network dynamics and the coding of multidimensional signals. Nat Rev Neurosci, 3(11): 884-895. 10.1038/nrn964 [DOI] [PubMed] [Google Scholar]
- Leong LM, Storace DA, 2024. Imaging different cell populations in the mouse olfactory bulb using the genetically encoded voltage indicator arclight. Neurophotonics, 11(3): 033402. 10.1117/1.NPh.11.3.033402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AN, Gong L, Xu FQ, 2011. Brain-state-independent neural representation of peripheral stimulation in rat olfactory bulb. Proc Natl Acad Sci USA, 108(12): 5087-5092. 10.1073/pnas.1013814108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AN, Gire DH, Bozza T, et al. , 2014. Precise detection of direct glomerular input duration by the olfactory bulb. J Neurosci, 34(48): 16058-16064. 10.1523/JNEUROSCI.3382-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AN, Gire DH, Restrepo D, 2015. ϒ spike-field coherence in a population of olfactory bulb neurons differentiates between odors irrespective of associated outcome. J Neurosci, 35(14): 5808-5822. 10.1523/JNEUROSCI.4003-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AN, Rao XP, Zhou Y, et al. , 2020. Complex neural representation of odour information in the olfactory bulb. Acta Physiol, 228(1): e13333. 10.1111/apha.13333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Swerdloff M, She TY, et al. , 2023. Robust odor identification in novel olfactory environments in mice. Nat Commun, 14: 673. 10.1038/s41467-023-36346-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Cleland TA, 2016. Neuromodulation of olfactory transformations. Curr Opin Neurobiol, 40: 170-177. 10.1016/j.conb.2016.07.006 [DOI] [PubMed] [Google Scholar]
- Liu MX, Jiang N, Shi YQ, et al. , 2023. Spatiotemporal coding of natural odors in the olfactory bulb. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 24(11): 1057-1061. 10.1631/jzus.B2300249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PL, Cao TT, Xu JS, et al. , 2020. Plasticity of sniffing pattern and neural activity in the olfactory bulb of behaving mice during odor sampling, anticipation, and reward. Neurosci Bull, 36(6): 598-610. 10.1007/s12264-019-00463-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PL, Gao C, Wu J, et al. , 2023. Negative valence encoding in the lateral entorhinal cortex during aversive olfactory learning. Cell Rep, 42(10): 113204. 10.1016/j.celrep.2023.113204 [DOI] [PubMed] [Google Scholar]
- López-Mascaraque L, de Carlos JA, Valverde F, 1986. Structure of the olfactory bulb of the hedgehog (Erinaceus europaeus): description of cell types in the granular layer. J Comp Neurol, 253(2): 135-152. 10.1002/cne.902530202 [DOI] [PubMed] [Google Scholar]
- Lyons-Warren AM, Tantry EK, Moss EH, et al. , 2023. Co-transmitting interneurons in the mouse olfactory bulb regulate olfactory detection and discrimination. Cell Rep, 42(12): 113471. 10.1016/j.celrep.2023.113471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysetskiy M, Lozowski A, Zurada JM, 2002. Temporal-to-spatial dynamic mapping, flexible recognition, and temporal correlations in an olfactory cortex model. Biol Cybern, 87(1): 58-67. 10.1007/s00422-002-0319-0 [DOI] [PubMed] [Google Scholar]
- Ma M, Luo MM, 2012. Optogenetic activation of basal forebrain cholinergic neurons modulates neuronal excitability and sensory responses in the main olfactory bulb. J Neurosci, 32(30): 10105-10116. 10.1523/JNEUROSCI.0058-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manella LC, Petersen N, Linster C, 2017. Stimulation of the locus ceruleus modulates signal-to-noise ratio in the olfactory bulb. J Neurosci, 37(48): 11605-11615. 10.1523/JNEUROSCI.2026-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markopoulos F, Rokni D, Gire DH, et al. , 2012. Functional properties of cortical feedback projections to the olfactory bulb. Neuron, 76(6): 1175-1188. 10.1016/j.neuron.2012.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiros N, Kapoor V, Kim SE, et al. , 2022. Distinct representation of cue-outcome association by D1 and D2 neurons in the ventral striatum’s olfactory tubercle. eLife, 11: e75463. 10.7554/eLife.75463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazo C, Nissant A, Saha S, et al. , 2022. Long-range GABAergic projections contribute to cortical feedback control of sensory processing. Nat Commun, 13: 6879. 10.1038/s41467-022-34513-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner-Bernard C, Dembitskaya Y, Venance L, et al. , 2019. Encoding of odor fear memories in the mouse olfactory cortex. Current Biology, 29(3): 367-380.e4. 10.1016/j.cub.2018.12.003 [DOI] [PubMed] [Google Scholar]
- Menni C, Valdes AM, Freidin MB, et al. , 2020. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med, 26(7): 1037-1040. 10.1038/s41591-020-0916-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamichi K, Amat F, Moussavi F, et al. , 2011. Cortical representations of olfactory input by trans-synaptic tracing. Nature, 472(7342): 191-196. 10.1038/nature09714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamichi K, Shlomai-Fuchs Y, Shu M, et al. , 2013. Dissecting local circuits: parvalbumin interneurons underlie broad feedback control of olfactory bulb output. Neuron, 80(5): 1232-1245. 10.1016/j.neuron.2013.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moein ST, Hashemian SM, Tabarsi P, et al. , 2020. Prevalence and reversibility of smell dysfunction measured psychophysically in a cohort of COVID-19 patients. Int Forum Allergy Rhinol, 10(10): 1127-1135. 10.1002/alr.22680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JD, Kleinfeld D, Wang F, 2014. How the brainstem controls orofacial behaviors comprised of rhythmic actions. Trends Neurosci, 37(7): 370-380. 10.1016/j.tins.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Sakano H, 2011. How is the olfactory map formed and interpreted in the mammalian brain? Annu Rev Neurosci, 34: 467-499. 10.1146/annurev-neuro-112210-112917 [DOI] [PubMed] [Google Scholar]
- Mori K, Sakano H, 2021. Olfactory circuitry and behavioral decisions. Annu Rev Physiol, 83: 231-256. 10.1146/annurev-physiol-031820-092824 [DOI] [PubMed] [Google Scholar]
- Mori K, Nowycky MC, Shepherd GM, 1981. Electrophysiological analysis of mitral cells in the isolated turtle olfactory bulb. J Physiol, 314(1): 281-294. 10.1113/jphysiol.1981.sp013707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Kishi K, Ojima H, 1983. Distribution of dendrites of mitral, displaced mitral, tufted, and granule cells in the rabbit olfactory bulb. J Comp Neurol, 219(3): 339-355. 10.1002/cne.902190308 [DOI] [PubMed] [Google Scholar]
- Mori K, Nagao H, Yoshihara Y, 1999. The olfactory bulb: coding and processing of odor molecule information. Science, 286(5440): 711-715. 10.1126/science.286.5440.711 [DOI] [PubMed] [Google Scholar]
- Mori K, Takahashi YK, Igarashi KM, et al. , 2006. Maps of odorant molecular features in the mammalian olfactory bulb. Physiol Rev, 86(2): 409-433. 10.1152/physrev.00021.2005 [DOI] [PubMed] [Google Scholar]
- Najac M, Diez AS, Kumar A, et al. , 2015. Intraglomerular lateral inhibition promotes spike timing variability in principal neurons of the olfactory bulb. J Neurosci, 35(10): 4319-4331. 10.1523/JNEUROSCI.2181-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocera S, Simon A, Fiquet O, et al. , 2019. Somatostatin serves a modulatory role in the mouse olfactory bulb: neuroanatomical and behavioral evidence. Front Behav Neurosci, 13: 61. 10.3389/fnbeh.2019.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinski BL, Kim A, Xiao WX, et al. , 2018. Pharmacological manipulation of the olfactory bulb modulates beta oscillations: testing model predictions. J Neurophysiol, 120(3): 1090-1106. 10.1152/jn.00090.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardasani M, Ramakrishnan AM, Mahajan S, et al. , 2023. Perceptual learning deficits mediated by somatostatin releasing inhibitory interneurons of olfactory bulb in an early life stress mouse model. Mol Psychiatry, 28(11): 4693-4706. 10.1038/s41380-023-02244-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson MA, Lagier S, Carleton A, 2013. Odor representations in the olfactory bulb evolve after the first breath and persist as an odor afterimage. Proc Natl Acad Sci USA, 110(35): E3340-E3349. 10.1073/pnas.1303873110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peace ST, Johnson BC, Werth JC, et al. , 2024. Coherent olfactory bulb gamma oscillations arise from coupling independent columnar oscillators. J Neurophysiol, 131(3): 492-508. 10.1152/jn.00361.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poplawsky AJ, Cover C, Reddy S, et al. , 2023. Odor-evoked layer-specific fMRI activities in the awake mouse olfactory bulb. NeuroImage, 274: 120121. 10.1016/j.neuroimage.2023.120121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler RT, Strowbridge BW, 2006. Blanes cells mediate persistent feedforward inhibition onto granule cells in the olfactory bulb. Neuron, 49(6): 889-904. 10.1016/j.neuron.2006.02.019 [DOI] [PubMed] [Google Scholar]
- Pressler RT, Strowbridge BW, 2022. Extraglomerular excitation of rat olfactory bulb mitral cells by depolarizing GABAergic synaptic input. J Neurosci, 42(36): 6878-6893. 10.1523/JNEUROSCI.0094-22.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB, 1993. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell, 73(3): 597-609. 10.1016/0092-8674(93)90145-g [DOI] [PubMed] [Google Scholar]
- Restrepo D, Doucette W, Whitesell JD, et al. , 2009. From the top down: flexible reading of a fragmented odor map. Trends Neurosci, 32(10): 525-531. 10.1016/j.tins.2009.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A, 2006. Sparse odor coding in awake behaving mice. J Neurosci, 26(34): 8857-8865. 10.1523/JNEUROSCI.0884-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland B, Deneux T, Franks KM, et al. , 2017. Odor identity coding by distributed ensembles of neurons in the mouse olfactory cortex. eLife, 6: e26337. 10.7554/eLife.26337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel M, Carey RM, Puche A, et al. , 2014. Cholinergic inputs from basal forebrain add an excitatory bias to odor coding in the olfactory bulb. J Neurosci, 34(13): 4654-4664. 10.1523/JNEUROSCI.5026-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BD, Katz LC, 1999. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron, 23(3): 499-511. 10.1016/S0896-6273(00)80803-X [DOI] [PubMed] [Google Scholar]
- Schafer JR, Kida I, Rothman DL, et al. , 2005. Adaptation in the rodent olfactory bulb measured by fMRI. Magn Reson Med, 54(2): 443-448. 10.1002/mrm.20588 [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, Clancy AN, Forbes WB, et al. , 1994. The spatial organization of the peripheral olfactory system of the hamster. Part I: receptor neuron projections to the main olfactory bulb. Brain Res Bull, 34(3): 183-210. 10.1016/0361-9230(94)90059-0 [DOI] [PubMed] [Google Scholar]
- Schoonover CE, Ohashi SN, Axel R, et al. , 2021. Representational drift in primary olfactory cortex. Nature, 594(7864): 541-546. 10.1038/s41586-021-03628-7 [DOI] [PubMed] [Google Scholar]
- Schoppa NE, 2006. A novel local circuit in the olfactory bulb involving an old short-axon cell. Neuron, 49(6): 783-784. 10.1016/j.neuron.2006.03.005 [DOI] [PubMed] [Google Scholar]
- Sharp FR, Kauer JS, Shepherd GM, 1975. Local sites of activity-related glucose metabolism in rat olfactory bulb during olfactory stimulation. Brain Res, 98(3): 596-600. 10.1016/0006-8993(75)90377-7 [DOI] [PubMed] [Google Scholar]
- Shayya HJ, Kahiapo JK, Duffié R, et al. , 2022. ER stress transforms random olfactory receptor choice into axon targeting precision. Cell, 185(21): 3896-3912.e22. 10.1016/j.cell.2022.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff A, Pandolfi G, Nguyen VS, et al. , 2021. Long-range respiratory and theta oscillation networks depend on spatial sensory context. J Neurosci, 41(48): 9957-9970. 10.1523/JNEUROSCI.0719-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley MT, Ennis M, 1996. Functional organization of olfactory system. J Neurobiol, 30(1): 123-176. [DOI] [PubMed] [Google Scholar]
- Smear M, Shusterman R, O'Connor R, et al. , 2011. Perception of sniff phase in mouse olfaction. Nature, 479(7373): 397-400. 10.1038/nature10521 [DOI] [PubMed] [Google Scholar]
- Smear M, Resulaj A, Zhang JJ, et al. , 2013. Multiple perceptible signals from a single olfactory glomerulus. Nat Neurosci, 16(11): 1687-1691. 10.1038/nn.3519 [DOI] [PubMed] [Google Scholar]
- Snitz K, Andelman-Gur M, Pinchover L, et al. , 2021. Proof of concept for real-time detection of SARS CoV-2 infection with an electronic nose. PLoS ONE, 16(6): e0252121. 10.1371/journal.pone.0252121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosulski DL, Bloom ML, Cutforth T, et al. , 2011. Distinct representations of olfactory information in different cortical centres. Nature, 472(7342): 213-216. 10.1038/nature09868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spors H, Wachowiak M, Cohen LB, et al. , 2006. Temporal dynamics and latency patterns of receptor neuron input to the olfactory bulb. J Neurosci, 26(4): 1247-1259. 10.1523/JNEUROSCI.3100-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler DD, Axel R, 2009. Representations of odor in the piriform cortex. Neuron, 63(6): 854-864. 10.1016/j.neuron.2009.09.005 [DOI] [PubMed] [Google Scholar]
- Stopfer M, Jayaraman V, Laurent G, 2003. Intensity versus identity coding in an olfactory system. Neuron, 39(6): 991-1004. 10.1016/j.neuron.2003.08.011 [DOI] [PubMed] [Google Scholar]
- Szyszka P, Stierle JS, Biergans S, et al. , 2012. The speed of smell: odor-object segregation within milliseconds. PLoS ONE, 7(4): e36096. 10.1371/journal.pone.0036096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyszka P, Emonet T, Edwards TL, 2023. Extracting spatial information from temporal odor patterns: insights from insects. Curr Opin Insect Sci, 59: 101082. 10.1016/j.cois.2023.101082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Tsuji C, Lozic M, et al. , 2019. Coding of odors in the anterior olfactory nucleus. Physiol Rep, 7(22): e14284. 10.14814/phy2.14284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Mainen ZF, 2003. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci, 6(11): 1224-1229. 10.1038/nn1142 [DOI] [PubMed] [Google Scholar]
- Uchida N, Poo C, Haddad R, 2014. Coding and transformations in the olfactory system. Annu Rev Neurosci, 37: 363-385. 10.1146/annurev-neuro-071013-013941 [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Baker KL, Vasan G, et al. , 2023. Odor encoding by signals in the olfactory bulb. J Neurophysiol, 129(2): 431-444. 10.1152/jn.00449.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, 2011. All in a sniff: olfaction as a model for active sensing. Neuron, 71(6): 962-973. 10.1016/j.neuron.2011.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, Economo MN, Díaz-Quesada M, et al. , 2013. Optical dissection of odor information processing in vivo using gcamps expressed in specified cell types of the olfactory bulb. J Neurosci, 33(12): 5285-5300. 10.1523/JNEUROSCI.4824-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DJ, Liu PL, Mao XF, et al. , 2019. Task-demand-dependent neural representation of odor information in the olfactory bulb and posterior piriform cortex. J Neurosci, 39(50): 10002-10018. 10.1523/JNEUROSCI.1234-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DJ, Wang XJ, Liu PL, et al. , 2020. Serotonergic afferents from the dorsal raphe decrease the excitability of pyramidal neurons in the anterior piriform cortex. Proc Natl Acad Sci USA, 117(6): 3239-3247. 10.1073/pnas.1913922117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DJ, Wu J, Liu PL, et al. , 2022. Vip interneurons regulate olfactory bulb output and contribute to odor detection and discrimination. Cell Rep, 38(7): 110383. 10.1016/j.celrep.2022.110383 [DOI] [PubMed] [Google Scholar]
- Wang PY, Sun Y, Axel R, et al. , 2021. Evolving the olfactory system with machine learning. Neuron, 109(23): 3879-3892.e5. 10.1016/j.neuron.2021.09.010 [DOI] [PubMed] [Google Scholar]
- Wesson DW, 2013. Sniffing behavior communicates social hierarchy. Curr Biol, 23(7): 575-580. 10.1016/j.cub.2013.02.012 [DOI] [PubMed] [Google Scholar]
- Wesson DW, 2020. The tubular striatum. J Neurosci, 40(39): 7379-7386. 10.1523/jneurosci.1109-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiechert MT, Judkewitz B, Riecke H, et al. , 2010. Mechanisms of pattern decorrelation by recurrent neuronal circuits. Nat Neurosci, 13(8): 1003-1010. 10.1038/nn.2591 [DOI] [PubMed] [Google Scholar]
- Wilson CD, Serrano GO, Koulakov AA, et al. , 2017. A primacy code for odor identity. Nat Commun, 8: 1477. 10.1038/s41467-017-01432-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Liu PL, Geng C, et al. , 2023. Principal neurons in the olfactory cortex mediate bidirectional modulation of seizures. J Physiol, 601(16): 3557-3584. 10.1113/JP284731 [DOI] [PubMed] [Google Scholar]
- Wu TT, Li S, Du DL, et al. , 2023. Olfactory-auditory sensory integration in the lateral entorhinal cortex. Prog Neurobiol, 221: 102399. 10.1016/j.pneurobio.2022.102399 [DOI] [PubMed] [Google Scholar]
- Wu YJ, Li BZ, Wang LY, et al. , 2023. An unsupervised real-time spike sorting system based on optimized osort. J Neural Eng, 20(6): 066015. 10.1088/1741-2552/ad0d15 [DOI] [PubMed] [Google Scholar]
- Xiong A, Wesson DW, 2016. Illustrated review of the ventral striatum’s olfactory tubercle. Chem Senses, 41(7): 549-555. 10.1093/chemse/bjw069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu FQ, Greer CA, Shepherd GM, 2000. Odor maps in the olfactory bulb. J Comp Neurol, 422(4): 489-495. [DOI] [PubMed] [Google Scholar]
- Xu FQ, Liu N, Kida I, et al. , 2003. Odor maps of aldehydes and esters revealed by functional MRI in the glomerular layer of the mouse olfactory bulb. Proc Natl Acad Sci USA, 100(19): 11029-11034. 10.1073/pnas.1832864100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang QH, Zhou GY, Noto T, et al. , 2022. Smell-induced gamma oscillations in human olfactory cortex are required for accurate perception of odor identity. PLoS Biol, 20(1): e3001509. 10.1371/journal.pbio.3001509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Liu PL, Zhang Y, et al. , 2022. The response dynamics and function of cholinergic and GABAergic neurons in the basal forebrain during olfactory learning. Front Cell Neurosci, 16: 911439. 10.3389/fncel.2022.911439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu KW, Burton SD, Nagai MH, et al. , 2022. Decoding the olfactory map through targeted transcriptomics links murine olfactory receptors to glomeruli. Nat Commun, 13: 5137. 10.1038/s41467-022-32267-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang LJ, Guo TT, Cao DX, et al. , 2015. Detection and classification of natural odors with an in vivo bioelectronic nose. Biosens Bioelectron, 67: 694-699. 10.1016/j.bios.2014.09.102 [DOI] [PubMed] [Google Scholar]
- Zhuang LJ, Wei XW, Jiang N, et al. , 2021. A biohybrid nose for evaluation of odor masking in the peripheral olfactory system. Biosens Bioelectron, 171: 112737. 10.1016/j.bios.2020.112737 [DOI] [PubMed] [Google Scholar]