Abstract
Sarcomyxa edulis, a notable edible and medicinal mushroom indigenous to northeast China, is celebrated for its high nutritional value and delightful taste. In this study, white light as a control and examined the effects of red, green, and blue light on the agronomic traits, antioxidant capabilities, and nutritional composition of S. edulis. The results showed that different monochromatic light qualities affected the color of S. edulis pileus, with blue light demonstrating particular efficacy. Furthermore, blue light also regulated pileus length, whereas red light was instrumental in significantly increasing stalk length. Regarding antioxidant capacity, compared with red and green light, the activities of POD, SOD, and CAT were significantly improved by blue light irradiation, decreased MDA levels, and improved free radical scavenging potential. Additionally, blue light exposure led to an increase in the contents of 15 amino acids. Green light treatment reduced the crude fat content. For the first time, we found that light quality is a key factor in controlling the color of S. edulis. Blue light is an effective way to regulate the color and pileus size of S. edulis, and improve the antioxidant properties. The photobiological characteristics and the response of nutritional quality to light environment of S. edulis were clarified.
Subject terms: Fungi, Fungal biology, Fungal ecology
Introduction
Sarcomyxa edulis, widely known as “Yuan mo,” “yellow mushroom,” and “frozen mushroom,” predominantly thrives in the cold regions of Northeast China, South Korea, Japan, and Russia’s Far East. S. edulis grows on broad-leaved trees such as elm, linden, and birch, particularly on standing deadwood, belonging to the low-temperature edible fungus1. S. edulis has a variety of colors, mostly yellow, yellow-green, or brown, the fruit body is tender and fragrant, the meat is full, can be eaten fresh, but also can be dried to eat2. Nutritionally, it is a rich source of proteins, vitamins, minerals, and other essential nutrients3. Previous studies have suggested that the consumption of S. edulis is associated with potential benefits, such as preventing arteriosclerosis, improving immunity, and inhibiting tumor growth4. In addition, there are many unknown natural products in S. edulis. Our laboratory obtained a new highly degraded sterol and a new β-carboline alkaloid from S. edulis for the first time, and cell experiments showed that β-carboline alkaloid had good anti-inflammatory activity5.
LED is the fourth generation of new lighting sources, offering significant benefits for industrial lighting design, including energy efficiency, environmental friendliness, compact size, and longevity6. The emission wavelengths of LEDs are well-suited to plant photoreceptors, optimizing crop yield and quality by influencing crop morphology and metabolism7. Light is one of the environmental conditions necessary to induce the differentiation of mushroom primordium and regulate the normal growth of fruiting bodies8. Although edible fungi can not perform photosynthesis9, they possess a light-sensing system analogous to the blue, red, and green light systems found in plants. This system allows them to detect and respond to environmental light signals10. The quality of light is known to have varied effects on plant morphology and development11. Among the spectrum of light, blue light is one of the most important environmental signals for various organisms because it regulates their development and physiological processes through photoreceptors12. For plants, the blue (425–490 nm) and red light (610–700 nm) spectra are optimally absorbed for photosynthesis, while very small amounts of green light (520–610 nm) are absorbed13. Red light is mainly involved in photomorphogenesis and the development of photosynthetic organs, while blue light is crucial for morphogenesis and stomatal opening14. In fungi, the effect of blue light on the fruiting body is particularly prominent. Blue light significantly influences the development of Flammulina filiformis15. Four blue light receptors FfWC-1, FfWC-2, FfCpl, and FfCry-DASH were identified in the genome of F. filiformis, further supporting the importance of blue light on the growth and development of F. filiformis from the molecular level16. Coprinopsis cinerea is often used as a model fungus to study the molecular mechanisms of photomorphogenesis17. The blue light can stimulate the development of the fruiting body of C. cinerea18. Moreover, blue light has been shown to enlarge and thicken Pleurotus ostreatus pileus and shorten stalk length19. Numerous edible mushrooms, including Lentinula edodes, Grifola frondosa, and Pleurotus eryngii exhibit heightened sensitivity to blue light, highlighting its broad significance across fungal species20.
The interest in natural antioxidants has significantly increased among consumers and researchers in recent years21, with findings indicating that fungi are rich sources of antioxidants and possess antibacterial properties22. The quality of light plays a pivotal role in the production of reactive oxygen species and antioxidant capacity23. Compared with composite light, monochromatic light has a significant impact on antioxidant activity. Blue light can significantly increase the activity of antioxidant enzymes and enhance the antioxidant defense system in organisms24. The ethanol extract from the fruit body and mycelium of P. ostreatus, exposed to ultraviolet B light, shows a high content of vitamin D2, suggesting that both the fruit body and mycelium could serve as potential antioxidants and be developed into novel dietary supplements25. Furthermore, blue light has been found to stimulate the accumulation of non-enzymatic antioxidants, such as carotenoids, flavonoids, and total phenols in plants, effectively enhancing their resilience to extreme environmental conditions26.
Light quality elicits varied responses in the biosynthesis of carotenoids, carbohydrates, fatty acids, nucleotide metabolism, and other secondary metabolites within edible fungi27. For instance, blue light irradiation has been shown to elevate the ergosterol content in Hypsizigus marmoreus28. Additionally, the yield of Ganoderma lucidum spore powder is influenced by different light qualities, with blue light notably enhancing the polysaccharide levels in G. lucidum29. The abundance and content of amino acids were also affected under different light quality irradiation. For example, after blue light irradiation, the abundance of amino acids in mycelia changed, significantly increasing phenylalanine while reducing tyrosine levels30. In F. filiformis, blue light has been found to upregulate the expression of genes within the lysine biosynthesis pathway, thereby fostering lysine production, enriching nutritional value, and improving taste31. Furthermore, light is an important cause of pigment production in Cordyceps militaris32, with blue light inducing carotenoid accumulation, and pink light augmenting cordycepin content in the fruit body33.
Currently, the effect of light quality on the cultivation of S. edulis has not been studied. This study aims to investigate how different light qualities affect the agronomic characteristics, antioxidant capacity, and nutrient composition of S. edulis. Three monochromatic light qualities of red light, green light, and blue light were chosen for the experiment, with white light was used as the control, and three S. edulis strains were exposed to light. The ultimate goal of this study was to identify the optimal monochromatic light condition that promotes the growth of S. edulis, enhancing its antioxidant properties and nutritional value. This research will provide to theoretical basis for subsequent research on the effects of blue light intensity and photoperiod on the fruity body of S. edulis.
Materials and methods
Strains and medium
Based on previous laboratory studies, S. edulis was divided into 7 colors, including yellow-white, yellow, yellow-green, gray-green, yellow-brown, brown-gray and dark green34. Yellow strain H1, yellowish-brown strain H2 and brownish-gray strain H3 were selected as experimental strains. All strains were stored in the Engineering Research Center of Edible and Medicinal Fungi, Ministry of Education, Jilin Agricultural University. The strains were inoculated in potato dextrose agar (PDA) medium, prepared by mixing 200 g of potato, 20 g of glucose, and 20 g of agar in 1 L RO water. PDA medium was placed in a 25℃ incubator for constant temperature culture. The activated strains were inoculated in 1 L conical bottle of 400 mL liquid medium. The liquid culture medium consisted of 200 g of potato, 8 g of glucose, 5 g of yeast extract, 5 g of peptone, 3 g of KH2PO4, and 1.5 g of MgSO4 in 1 L RO water. The liquid culture was incubated in a 25 °C shaker (120 rpm) under dark conditions for 12 days.
Cultivation experiments
S. edulis cultivation place was located in the mushroom room No. 22 of the mushroom vegetable base of Jilin Agricultural University. 1 kg of cultivation material was put into polypropylene bag and autoclaved at 121℃ for 90 min. The culture material formula was oak sawdust 78%, wheat bran 20%, lime 1%, light calcium carbonate 1%, pH = 7, and water content 65%. After cooling, 15 mL of liquid strains were inoculated in a bag, placed in a culture chamber, and incubated at 25 °C in the dark for 110 days. Once the mycelium had matured, a sterile knife was used to make a cross-shaped opening on the mushroom bag. Ten mushroom bags were placed under each light treatment. The mushroom bags treated with the same light quality is placed on a mushroom shelf, and the LED light belt (Shenzhen Fangde Lighting Co., LTD.) is installed above each mushroom shelf and surrounded by a blackout cloth. After 30 days of light treatment, mature fruiting bodies were harvested, and agronomic traits were measured. In the light time of 8 h light /16 h dark, at the temperature of 17 ± 1℃, humidity is 85–90% under the conditions of S. edulis management.
Light quality design
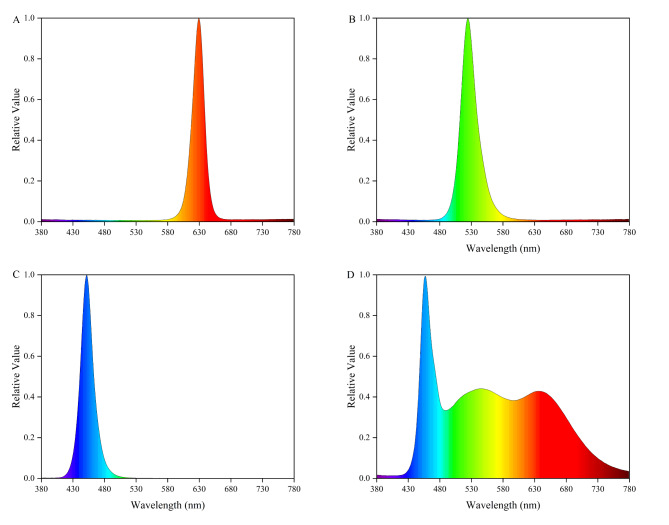
The experiment set up four kinds of light quality treatment, respectively red light (R), green light (G), blue light (B), and white light (CK). Spectral distribution of light quality: R (peak at 629 nm; 5 W), G (peak at 525 nm; 5 W), B (peak at 451 nm; 5 W), and CK (B + R + G), the spectrum was measured in the range of 300–800 nm using the LI-180 plant spectrometer of LI-COR in the United States. Each LED lamp has 105 lamp beads, and the spectral characteristics of different light treatments are shown in Fig. 1.
Fig. 1.
Relative spectral distribution of different LED strips. (A) red light treatment, (B) green light treatment, (C) blue light treatment, (D) white light treatment.
Determination of agronomic traits
There was some variability in the growth state of the fruiting bodies. Therefore, in order to more accurately reflect the overall agronomic traits of each strain under each light treatment, samples of each strain under each light quality were randomly selected from 10 mushroom bags to determine 10 fruit bodies. Agronomic parameters including yield, pileus length, pileus width, pileus thickness, stalk length, stalk width, and stalk thickness were recorded. The fresh weight was measured by electronic scale, and the index of pileus and stalk was measured by vernier caliper, and each treatment was measured 10 times. After harvesting, S. edulis were dried by a fruit and vegetable dryer at 50 °C for the determination of nutritional content. (Sichuan Changhong Electric Co., Ltd., China).
To quantify the color of S. edulis pileus, reference was made to the method determination in the published literature35, and some modifications were made. To avoid the influence of different light sources on the color of S. edulis pileus, a unified digital camera was used to take pictures in the LED photography lightbox (Canon EOS 700D). The shooting conditions were set as follows ISO value 400, exposure time 1/50 second, aperture value f /5.6, and no flash. By using Adobe Photoshop CS6 software (Adobe Systems, SAN Jose, California, USA), L*, a*, and b* values were extracted from the primary colors of S. edulis photos by the L*a*b* model. Where the L* value represents the lightness value, the a* value (+ redness /− green), and the b* value (+ yellow) /− blue). The determination was six replicates to ensure the accuracy of the results and improve the representativeness of the measurement.
Determination of antioxidant capacity
According to Vattem’s method36, the free radical scavenging rate (DPPH) was calculated. Weighed about 0.5 g fresh mushroom tissue, added 1 mL sodium acetate buffer, homogenized in an ice bath at 12,000 rpm, centrifuged at 4℃ for 10 min. Took the supernatant and placed it on ice for measurement. Took 20 µL supernatant, added 380 µL DPPH solution. Mixed thoroughly, and allowed it to react at room temperature for 20 min. Took 200 µL to determine the absorption value of 515 nm. DPPH free radical clearance (%) =[(absorbance of blank solution - sample absorbance)/absorbance of blank solution]× 100%.
Malondialdehyde content (MDA) was slightly modified according to Spitz37, weighed about 0.5 g fresh mushroom tissue, added 1 mL phosphate buffer, homogenized at room temperature, and centrifuged at 10,000 rpm for 10 min. Mix 100 µL supernatant with 300 µL 10% trichloroacetic acid containing 0.6% thiobarbituric acid. The mixture was placed in a 92℃ water bath for 30 min, cooled and centrifuged (12000 rpm, 25℃, 10 min). The light absorption values at 532 nm and 600 nm were measured with supernatant.
According to the determination of superoxide anion (OFR) by Jiao’s method38, about 0.5 g fresh mushroom tissue was weighed, 1 mL dipotassium hydrogen phosphate and potassium dihydrogen phosphate solution were added and homogenized in an ice bath. Then centrifugation was performed, and the centrifugation condition was 12,000 rpm, 4℃, 20 min, and the supernatant ice was taken for determination. 200 µL supernatant was added to 160 µL hydroxylamine hydrochloride solution and mixed, a water bath at 37℃ for 20 min. Then, it was mixed with 120 µL p-aminobenzenesulfonic acid solution and 120 µL of alpha-naphthylamine solution, mixed and bathed in 37℃ water for 20 min. Measured the absorption value at 530 nm.
Weighed 0.5 g of fresh fruit bodies and placed them into a centrifuge tube. Added 1 mL of extracted phosphate buffer and centrifuged the mixture at 4 ◦C at 12,000 rpm for 10 min. Finally, the supernatant was collected to detect the activity of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD). Determination of SOD, POD, and CAT activity reference reagent test box (Suzhou Michy Biomedical Technology Co., Ltd., Suzhou, China, Catalog nos. M0102A, M0103A, M0105A). SOD activity was measured by assessing the reaction between O2−. and WST-8, which produced the water-soluble formazan dye that absorbed at 450 nm. SOD scavenged O2−., thereby inhibiting formazan formation. A deeper yellow color in the reaction solution indicated lower SOD activity, whereas a lighter color corresponded to higher SOD activity39.
POD activity catalyzed the oxidation of guaiacol in the presence of hydrogen peroxide, producing a brownish substance. This substance exhibited maximum absorbance at 470 nm, which was used to measure POD activity40.
The determination of CAT activity was based on the ability of CAT to decompose H2O2, which has a characteristic absorption peak at 240 nm, calculated by measuring the rate of change of absorbance at 240 nm. Each strain was tested three replicates for each enzyme under each light quality41.
Nutrient composition analysis
The dried mushroom was ground with a grinder for 1.5 min and screened with a 200-mesh sieve. The crude fiber content was determined by filter bag technology, and the total sugar content was determined by the phenol-sulfuric acid method. The crude fat content was determined using the “Determination of Fat in Food under National Standards for Food Safety” (GB 5009.6–2016). The total amino acid content was reacted with ninhydrin hydrate using α-amino42, and the amino acid content calculated by measuring the absorbance at 570 nm. The reaction of proline and hydroxyproline with ninhydrin has no absorption peak at 570 nm, so the determination results at 570 nm do not contain these two amino acids. For each strain, each nutrient component was three replicates under each light quality.
Determination of 17 free amino acid components: 17 amino acid standards, HPLC-grade acetonitrile (OCEANPAK, purity ≥ 99.9%), hydrochloric acid, n-leucine, triethylamine, n-hexane, anhydrous sodium acetate (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), phenyl isothiocyanate (sigma, purity ≥ 99.9%). Weighed about 0.1 g of S. edulis, added 10 ml 0.1% phenol with 6 mol/L hydrochloric acid, and ground it into pulp. Then, placed it in a 110 ℃ oven for hydrolysis for 24 h. Removed and cooled, took 1 mL of hydrolysate, and dried it with a nitrogen blower. Added 1 mL 0.1 M hydrochloric acid aqueous solution to dissolve, and obtained the solution to be tested. Agilent 1100 high performance liquid chromatography was used to measure amino acids, UV detector wavelength at 254 nm. The analytical column was a Amethyst C18-H (4.6 mm×250 mm, 5 μm) from Sepax Technologies, Inc. The mobile phases were: A, weighed 7.6 g of anhydrous sodium acetate, added 925 mL of water, dissolved it, and adjusted the pH to 6.5 with glacial acetic acid. Then, 70 mL of acetonitrile was added and mixed thoroughly. Filtered with a 0.45 μm membrane. B, 80% acetonitrile aqueous solution. The elution condition was shown in Table 1. The flow rate was 1 mL/min at 40 °C.
Table 1.
Mobile phase gradient employed for the detect amino acids.
| Time(min) | Phase A(%) | Phase A(%) |
|---|---|---|
| 0 | 100 | 0 |
| 2 | 100 | 0 |
| 15 | 90 | 10 |
| 25 | 70 | 30 |
| 33 | 55 | 45 |
| 33.1 | 0 | 100 |
| 38 | 0 | 100 |
| 38.1 | 100 | 0 |
| 45 | 100 | 0 |
Statistical analysis
The experimental data were statistically analyzed and plotted using Microsoft Excel 2022, SPSS 24.0, and Origin 2022 software. All data were analyzed by one-way analysis of variance (ANOVA). Duncan’s test method was used for multiple comparisons and difference significance analysis. Mean and standard errors were used to draw histograms.
Results
Effect of different light qualities on the color of the pileus of S. Edulis
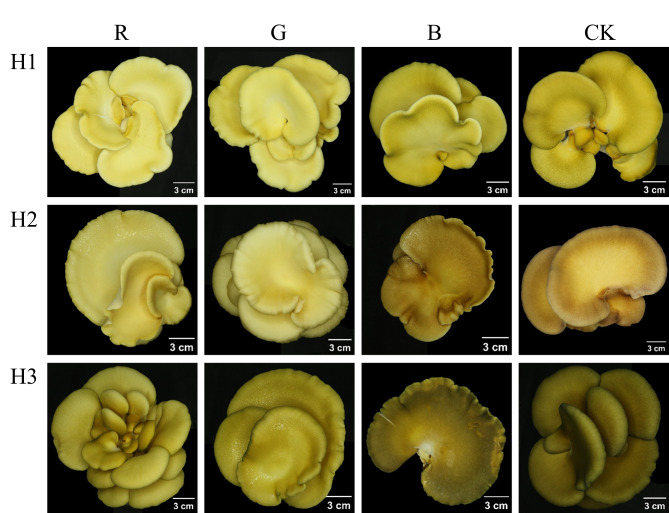
The influence of varying light qualities on the pileus color of S. edulis was depicted in Fig. 2, illustrating a noticeable color variation across three strains. The pileus appeared lighter under red and green light, whereas blue light and CK treatment conditions resulted in a darker hue. The color difference results were shown in Table 2. The color difference values of S. edulis in different light quality are L* (36.67–84.33), a* (7.83–7.33) and b* (35.67–64.33), respectively. Notably, in the dimension of L* value, the lightness value of three S. edulis strains was higher under red light and green light, and the difference was significant, which indicated that red light and green light promoted the color brightness of S. edulis pileus, and red light was the most significant effect. In the H1 and H3 strains, there was no significant difference between the L* value of CK and blue light, and the L* value of blue light in H2 was slightly lower than that of CK. On the a* value, a* of the H2 strain was positive under CK and blue light, and the light quality of other strains was negative. The b* values were all positive, reflecting that the pileus of three S. edulis strains was yellow, and the b* value of H1 was the highest. Through the analysis of the color parameters of the S. edulis pileus, compared with red light and green light, blue light deepened the color of S. edulis pileus, which was consistent with the appearance of S. edulis.
Fig. 2.
Comparison of the coloration of fruiting bodies of S. edulis under different light illumination. Light quality treatment from left to right (R-red light, G-green light, B-blue light, CK-white light), the strains were H1, H2, and H3 from top to bottom.
Table 2.
Color difference value of S. Edulis pileus under different light quality. Note: values are means ± SD of six replicate experiments. Different letters indicate significant differences (p < 0.05) between treatment groups according to Duncan’s test. Different letters indicate that a strain is significant between different light quality treatments.
| L* | a* | b* | ||
|---|---|---|---|---|
| H1 | R | 84.33 ± 0.94a | -7.33 ± 0.75c | 50.50 ± 2.75c |
| G | 75.50 ± 2.5b | -5.50 ± 0.76b | 59.00 ± 1.73b | |
| B | 70.00 ± 1.29c | -5.83 ± 1.07b | 61.17 ± 1.95b | |
| CK | 71.67 ± 2.13c | -2.83 ± 1.07a | 64.33 ± 2.29a | |
| H2 | R | 80.00 ± 1.41a | -5.83 ± 0.37b | 46.83 ± 1.57a |
| G | 74.00 ± 1.15b | -6.83 ± 0.69b | 41.50 ± 3.35b | |
| B | 48.33 ± 1.6d | 6.17 ± 1.34a | 47.83 ± 1.34a | |
| CK | 50.50 ± 2.06c | 7.83 ± 2.19a | 43.5 ± 4.92ab | |
| H3 | R | 70.17 ± 2.67a | -6.00 ± 0.58a | 53.50 ± 2.87a |
| G | 51.67 ± 1.8b | -3.17 ± 1.21b | 51.33 ± 1.11a | |
| B | 36.67 ± 1.37c | -2.33 ± 0.94ab | 35.67 ± 2.69b | |
| CK | 37.33 ± 1.7c | -1.83 ± 0.69a | 36.83 ± 1.34b | |
Effect of different light qualities on growth characteristics of S. Edulis
The agronomic properties of S. edulis under different light treatments were shown in Table 3. S. edulis showed different growth responses to different light treatments. Strain H3 yielded the highest, while strain H2 showed the lowest yield. There was no significant difference in yield between blue light and CK treatment in H1 strain. The yield decreased under red and green light in H1 strain. There was no significant difference in yield between H2 and H3 strains under different light quality. Compared to CK, there was no significant difference in pileus length under blue light, while red light and green light reduced pileus length. Similarly, blue light had little effect on pileus width, while either green or red light caused a reduction in pileus width. In H1 and H3 strains, compared with red and green light, the pileus thickness under blue light was the largest, and the difference was significant. Under the red light treatment, the stalk length was the longest. Compared with CK, the stalk width of H1 increased significantly under the three monochromatic lights. There was no significant difference in stalk width between H2 and H3 under each light treatment. There was no significant difference in stalk thickness under different light treatments. In conclusion, after the blue light treatment, the appearance of S. edulis is most in line with the higher economic value of the market.
Table 3.
Comparison of agronomic traits of S. edulis under different light quality. Note: values are means ± SD of ten replicate experiments. Different letters indicate significant differences (p < 0.05) between treatment groups according to Duncan’s test. Different letters indicate that a strain is significant between different light quality treatments.
| Yield | Pileus length | Pileus width | Pileus thickness | Stalk length | Stalk width | Stalk thickness | ||
|---|---|---|---|---|---|---|---|---|
| (g) | (mm) | (mm) | (mm) | (mm) | (mm) | (mm) | ||
| H1 | R | 124.5 ± 20.71b | 90.79 ± 5.79b | 50.25 ± 4.8c | 16.41 ± 2.34bc | 35.93 ± 5.72a | 18.56 ± 1.14a | 14.24 ± 1.45a |
| G | 127.13 ± 25.16b | 85.79 ± 7.89b | 54.08 ± 3.5bc | 15.64 ± 2.38c | 33.46 ± 5.71ab | 18.29 ± 1.58a | 15.63 ± 1.48a | |
| B | 158.18 ± 38.17a | 102.72 ± 9.25a | 57.33 ± 6.86b | 19.63 ± 2.2a | 26.5 ± 3.03c | 18.56 ± 1.86a | 15.45 ± 2.27a | |
| CK | 160.07 ± 22.2a | 111.1 ± 12.93a | 66.09 ± 5.48a | 18.6 ± 3.84ab | 29.02 ± 5.54bc | 16.29 ± 1.42b | 15.18 ± 1.98a | |
| H2 | R | 87.17 ± 15.51a | 76.35 ± 11.53b | 54.98 ± 10.21b | 14.33 ± 1.45b | 26.4 ± 6.34a | 12.77 ± 2.42a | 11.53 ± 2.51a |
| G | 109.37 ± 24.84a | 83.31 ± 8.65b | 55.61 ± 3.77b | 15.34 ± 1.48ab | 23.24 ± 3.96ab | 14.36 ± 3.27a | 12.23 ± 2.96a | |
| B | 89.73 ± 27.81a | 92.86 ± 4.01a | 61.81 ± 5.54ab | 16.94 ± 2.27ab | 20.77 ± 3.66b | 14.08 ± 1.91a | 12.3 ± 3.13a | |
| CK | 93.57 ± 28.17a | 98.43 ± 12.32a | 64.39 ± 7.44a | 17.79 ± 1.98a | 19.88 ± 2.93b | 13.51 ± 0.9a | 11.31 ± 1.15a | |
| H3 | R | 235.7 ± 40.16a | 87.09 ± 5.72c | 55.87 ± 10.22b | 17.63 ± 8.21b | 36.86 ± 2.36a | 17.19 ± 2.12a | 16.00 ± 1.65a |
| G | 240.38 ± 32.1a | 97.99 ± 9.21b | 58.29 ± 6.95b | 16.33 ± 2.53b | 34.56 ± 2.46b | 18.68 ± 1.02a | 17.77 ± 1.77a | |
| B | 249.5 ± 43.31a | 113.83 ± 11.85a | 62.29 ± 6.76ab | 22.5 ± 2.28a | 27.89 ± 2.56c | 17.24 ± 1.28a | 16.32 ± 2.53a | |
| CK | 254.35 ± 38.59a | 117.82 ± 7.91a | 69.66 ± 6.83a | 19.34 ± 2.07ab | 26.63 ± 1.89c | 17.06 ± 2.02a | 16.24 ± 3.21a | |
To further explore the effects of different light quality on the agronomic traits of S. edulis, the correlation among agronomic and color traits under different light qualities was studied (Fig. 3). The L* value was positively correlated with stalk length, and the correlation coefficients were as follows: H3 (0.83) > H2 (0.56) > H1(0.5). The L* value was negatively correlated with the length and width of the pileus, indicating that the size of the pileus decreased with the increase of the brightness value. In H1 and H2 strains, a* value was positively correlated with pileus length, pileus width, and pileus thickness.
Fig. 3.
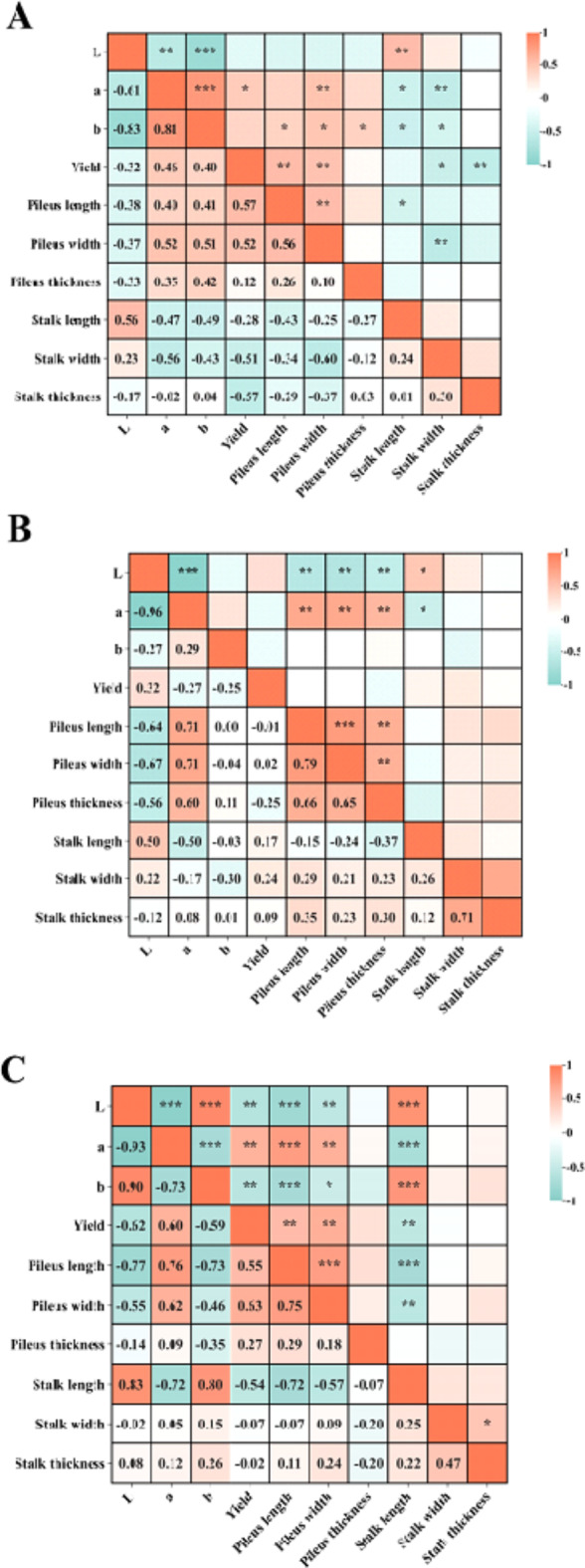
Correlation of agronomic traits of S. edulis under different light quality. (A)represents the H1 strain, (B)represents the H2 strain, (C) represents the H3 strain, correlations were significance. ***P < 0.001, **P < 0.01, *P < 0.05).
Effect of different light qualities on antioxidant enzyme activity, MDA, DPPH and OFR of S. Edulis
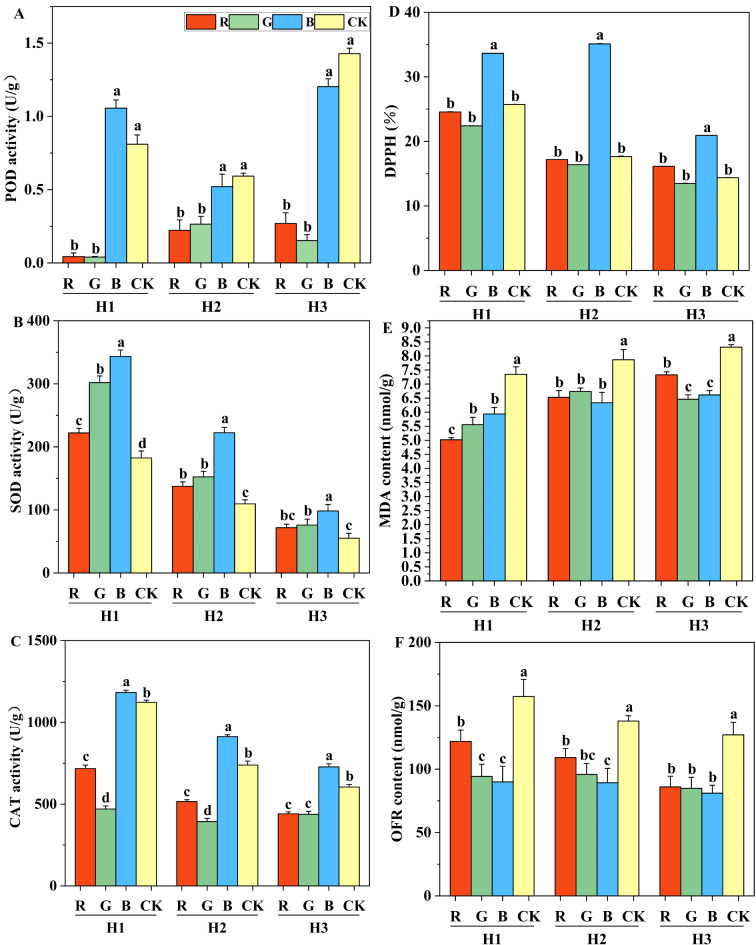
The antioxidant capacity of S. edulis was significantly influenced by light quality (Fig. 4). POD activity in S. edulis was higher under blue light and CK treatment. The POD activity in red and green light was significantly different from that in the CK treatment. Red and green light significantly reduced POD activity, while there was no significant difference between blue light and CK treatment. Compared with CK treatment, the use of monochromatic light increased the SOD activity in general, and the SOD activity under blue light was the highest(Fig. 4B). The CAT activity under blue light was the highest. The CAT activity under red and green light was significantly reduced. Under different light quality treatments, blue light treatment had the highest DPPH free radical clearance rate (Fig. 4D). Compared with CK treatment, blue light treatment increased the free radical clearance rate of H1, H2 and H3 strains by 30.78%, 98.98% and 45.55%, respectively. There was no significant difference in free clearance between red light and green light treatment. Compared with the CK treatment, the contents of MDA and OFR were significantly reduced under the three monochromatic light qualities (Fig. 4E and F). In summary, compared with red light and green light, blue light treatment improved the activity of antioxidant enzymes in the fruit bodies of S. edulis, monochromic light was beneficial in reducing the degree of membrane lipid peroxidation and remove a large number of superoxide anion free radicals.
Fig. 4.
Antioxidant capacity of S. edulis treated with different light quality. (A) peroxidase (POD) activity, (B) superoxide dismutase (SOD) activity, (C) catalase (CAT) activity, (D) radical scavenging capacity (DPPH), (E) Malondialdehyde (MDA) content, (F) superoxide anion (OFR) content. Different letters indicate significant differences (p < 0.05) between treatment groups according to Duncan’s test. Different letters indicate that a strain is significant between different light quality treatments.
Effect of different light qualities on the content of various nutrients in S. Edulis
The organic nutrients of S. edulis were significantly affected by different light treatments Table 4. Under the three light quality treatments, the crude fiber content in green light is the highest. Compared to CK, red and green light increased crude fiber content, while blue light either reduces crude fiber content in H1 strain and H2 strain.
Table 4.
Content of organic compounds in S. edulis treated with different light quality. Values are means ± SD of three replicate experiments. Different letters indicate significant differences (p < 0.05) between treatment groups according to Duncan’s test. Different letters indicate that a strain is significant between different light quality treatments.
| Crude fiber | Total sugar | Crude fat | Total amino acids | ||
|---|---|---|---|---|---|
| (g/100 g) | (g/100 g) | (g/100 g) | (g/100 g) | ||
| H1 | R | 31.46 ± 0.31b | 38.61 ± 0.34a | 1.46 ± 0.03b | 1.5 ± 0.04c |
| G | 34.54 ± 0.29a | 33.86 ± 1.96b | 0.97 ± 0.03c | 1.57 ± 0.05bc | |
| B | 27.79 ± 0.29d | 29.95 ± 0.61c | 1.41 ± 0.01b | 1.72 ± 0.01a | |
| CK | 29.81 ± 0.17c | 24.14 ± 0.52d | 1.78 ± 0.02a | 1.62 ± 0.04b | |
| H2 | R | 28.38 ± 0.36b | 31.42 ± 0.12a | 1.58 ± 0.02b | 0.93 ± 0.01c |
| G | 30.63 ± 0.24a | 29.16 ± 1.11b | 1.13 ± 0.05c | 1.11 ± 0.02b | |
| B | 26.14 ± 0.28c | 26.84 ± 0.89d | 1.81 ± 0.03a | 1.41 ± 0.04a | |
| CK | 26.61 ± 0.13c | 28.08 ± 0.46bc | 1.82 ± 0.02a | 1.11 ± 0.03b | |
| H3 | R | 32.58 ± 0.21ab | 28.6 ± 1.49a | 1.22 ± 0.02c | 1.5 ± 0.03c |
| G | 32.84 ± 0.11a | 28.75 ± 0.95a | 0.59 ± 0.01d | 1.94 ± 0.07b | |
| B | 28.25 ± 0.18c | 26.77 ± 0.27a | 1.33 ± 0.02b | 2.27 ± 0.04a | |
| CK | 32.36 ± 0.25b | 27.44 ± 0.16a | 1.64 ± 0.01a | 1.91 ± 0.03b | |
The crude fiber content of H1 strain was significantly different among different light quality treatments. In the H2 strain, there was no significant difference in crude fiber content between CK and blue light. In the H3 strain, there was no significant difference in crude fiber content between CK and red light. Compared with CK, red light significantly increased total sugar content by 60% and 12% in H1 and H2 strains, respectively. The crude fat content of the S. edulis was between 0.59 and 1.82 g/100 g. Green light produced the highest reduction, also red light reduced crude fat compared to CK, while blue light did or did not depending on the strain. Compared with CK, there were significant differences in total free amino acid contents under red and blue light treatment, but no significant differences under green light. Compared with CK, total amino acid contents of H1, H2 and H3 under blue light treatment were increased by 6%, 27% and 19%, respectively. In contrast, red light reduced the total amino acid content of H1, H2 and H3 by 7%, 16% and 21%, respectively.
In this study, the composition of free amino acids of S. edulis under different light quality was further analyzed. The content of 17 amino acids, including 6 essential amino acids and 9 non-essential amino acids, in the fruit body of S. edulis were detected by analysis. The concentration of methionine and cysteine was low, so it was not analyzed. Glu is the most abundant amino acid in S. edulis, followed by Asp. In the CK treatment, Glu accounts for 21%, 20% and 20% of the total free acid content of H1, H2 and H3, respectively.
In strain H1, amino acid content changes under different light qualities were shown in Table 5. Blue light had the most significant effect on amino acid content, followed by red light. Blue light significantly increased the content of 15 amino acids. Compared with CK, His content was 8.3 times higher under blue light. There was no significant difference in the content of His between CK and green light. The contents of Ala, Leu, Phe and Lys were not significantly affected under red and green light irradiation.
Table 5.
Effect of different light quality on free amino acids of H1 strain. Values are means ± SD of three replicate experiments. Different letters indicate significant differences between treatments for each FAA (p < 0.05) according to Duncan’s test.
| FAA | H1 | |||
|---|---|---|---|---|
| (g/100 g DW) | R | G | B | CK |
| Asp | 1.57 ± 0.025b | 0.91 ± 0.016c | 1.63 ± 0.012a | 0.8 ± 0.011d |
| Glu | 2.32 ± 0.038b | 2.42 ± 0.022a | 2.43 ± 0.05a | 1.8 ± 0.03c |
| Ser | 0.83 ± 0.014b | 0.73 ± 0.006c | 0.86 ± 0.007a | 0.65 ± 0.011d |
| Gly | 0.52 ± 0.009b | 0.5 ± 0.009c | 0.56 ± 0.007a | 0.48 ± 0.01c |
| His | 0.24 ± 0.006b | 0.03 ± 0.001c | 0.25 ± 0a | 0.03 ± 0.001c |
| Arg | 0.61 ± 0.011b | 0.55 ± 0.01c | 0.66 ± 0.006a | 0.5 ± 0.011d |
| Ala | 0.66 ± 0.01b | 0.66 ± 0.012b | 0.69 ± 0.008a | 0.59 ± 0.011c |
| Pro | 0.51 ± 0.008b | 0.48 ± 0.01c | 0.54 ± 0.008a | 0.46 ± 0.01d |
| Tyr | 0.35 ± 0.006b | 0.33 ± 0.005c | 0.38 ± 0.003a | 0.31 ± 0.006d |
| Val | 0.45 ± 0.009b | 0.48 ± 0.006a | 0.5 ± 0.004a | 0.41 ± 0.005c |
| Ile | 0.45 ± 0.009b | 0.47 ± 0.003a | 0.47 ± 0.003a | 0.42 ± 0.004c |
| Leu | 0.78 ± 0.011b | 0.77 ± 0.012b | 0.82 ± 0.009a | 0.69 ± 0.014c |
| Phe | 0.46 ± 0.007b | 0.45 ± 0.007b | 0.49 ± 0.006a | 0.41 ± 0.006c |
| Lys | 0.72 ± 0.012b | 0.45 ± 0.007b | 0.75 ± 0.005a | 0.57 ± 0.012c |
| Thr | 0.64 ± 0.01b | 0.61 ± 0.009c | 0.69 ± 0.007a | 0.52 ± 0.009d |
In H2 and H3 strains, the amino acid content changes under different light quality, as shown in Tables 6 and 7.The content of 15 amino acids under blue light was the highest. Compared with CK, the content of Glu, Ser, Gly, Arg, Ala, Pro, Tyr, Val, Ile, Leu, Phe, Lys and Thr 13 amino acids were decreased under red light, and the content was the lowest. Under green light, Asp and His contents were the lowest.
Table 6.
Effect of different light quality on free amino acids of H2 strain. Values are means ± SD of three replicate experiments. Different letters indicate significant differences between treatments for each FAA (p ≤ 0.05) according to Duncan’s test.
| FAA | H2 | |||
|---|---|---|---|---|
| (g/100 g DW) | R | G | B | CK |
| Asp | 1.22 ± 0.008b | 0.94 ± 0.003c | 1.62 ± 0.016a | 1.25 ± 0.016b |
| Glu | 1.64 ± 0.008d | 2.01 ± 0.01c | 2.27 ± 0.018a | 2.06 ± 0.024b |
| Ser | 0.62 ± 0.003d | 0.68 ± 0.005c | 0.82 ± 0.007a | 0.79 ± 0.009b |
| Gly | 0.47 ± 0.001d | 0.49 ± 0.001c | 0.62 ± 0.002a | 0.54 ± 0.007b |
| His | 0.19 ± 0.001b | 0.04 ± 0.001d | 0.3 ± 0.002a | 0.18 ± 0.003c |
| Arg | 0.53 ± 0.002d | 0.59 ± 0.004c | 0.71 ± 0.005a | 0.63 ± 0.006b |
| Ala | 0.58 ± 0.002d | 0.69 ± 0.005b | 0.76 ± 0.006a | 0.63 ± 0.008c |
| Pro | 0.44 ± 0.001d | 0.47 ± 0.002c | 0.59 ± 0.008a | 0.52 ± 0.006b |
| Tyr | 0.29 ± 0b | 0.31 ± 0.003b | 0.46 ± 0.063a | 0.37 ± 0.031b |
| Val | 0.4 ± 0.001d | 0.51 ± 0.005b | 0.54 ± 0.006a | 0.46 ± 0.006c |
| Ile | 0.41 ± 0.004d | 0.47 ± 0.001b | 0.55 ± 0.006a | 0.46 ± 0.004c |
| Leu | 0.67 ± 0.002c | 0.77 ± 0.003b | 0.88 ± 0.008a | 0.76 ± 0.01b |
| Phe | 0.4 ± 0.002c | 0.45 ± 0.003b | 0.52 ± 0.005a | 0.45 ± 0.005b |
| Lys | 0.55 ± 0.004c | 0.67 ± 0.017b | 0.72 ± 0.008a | 0.65 ± 0.009b |
| Thr | 0.53 ± 0.003c | 0.62 ± 0.003b | 0.71 ± 0.008a | 0.61 ± 0.007b |
Table 7.
Effect of different light quality on free amino acids of H3 strain. Values are means ± SD of three replicate experiments. Different letters indicate significant differences between treatments for each FAA (p < 0.05) according to Duncan’s test.
| FAA | H3 | |||
|---|---|---|---|---|
| (g/100 g DW) | R | G | B | CK |
| Asp | 1.3 ± 0.023c | 1.05 ± 0.018d | 1.73 ± 0.028a | 1.61 ± 0.012b |
| Glu | 2.1 ± 0.034d | 2.77 ± 0.042b | 2.85 ± 0.022a | 2.43 ± 0.017c |
| Ser | 0.7 ± 0.009d | 0.79 ± 0.008c | 0.94 ± 0.013a | 0.86 ± 0.007b |
| Gly | 0.52 ± 0.006d | 0.58 ± 0.01c | 0.72 ± 0.003a | 0.66 ± 0.006b |
| His | 0.25 ± 0.004b | 0.04 ± 0.001c | 0.35 ± 0.003a | 0.25 ± 0.005b |
| Arg | 0.65 ± 0.01d | 0.74 ± 0.012c | 0.96 ± 0.013a | 0.82 ± 0.011b |
| Ala | 0.61 ± 0.008c | 0.78 ± 0.011b | 0.85 ± 0.007a | 0.77 ± 0.005b |
| Pro | 0.49 ± 0.004d | 0.55 ± 0.01c | 0.69 ± 0.003a | 0.64 ± 0.005b |
| Tyr | 0.3 ± 0.002d | 0.36 ± 0.005c | 0.43 ± 0.003a | 0.38 ± 0.004b |
| Val | 0.43 ± 0.006c | 0.55 ± 0.035a | 0.56 ± 0.035a | 0.5 ± 0.004b |
| Ile | 0.44 ± 0.438d | 0.54 ± 0.005b | 0.59 ± 0.589a | 0.51 ± 0.515c |
| Leu | 0.71 ± 0.01c | 0.88 ± 0.011b | 0.99 ± 0.01a | 0.9 ± 0.01b |
| Phe | 0.41 ± 0.006c | 0.51 ± 0.008b | 0.57 ± 0.005a | 0.51 ± 0.005b |
| Lys | 0.65 ± 0.011d | 0.87 ± 0.007b | 0.95 ± 0.021a | 0.8 ± 0.006c |
| Thr | 0.56 ± 0.009c | 0.7 ± 0.011b | 0.79 ± 0.007a | 0.72 ± 0.013b |
Discussion
S. edulis is distinguished by its considerable nutritional value, ranking second only to Hericium erinaceus. Its cultivation has now started to be systematically developed. According to Traditional Chinese Medicine, S. edulis possesses properties that promote blood circulation, as well as soothe meridians and alleviate wind43. In this study, the light quality experiment was carried out with three representative colors of S. edulis. The results indicated that blue light played a pivotal role in the development of the pileus color of S. edulis, effectively inducing its color formation. This suggests the presence of a light-responsive substance within S. edulis, warranting further investigation to identify this specific compound. This phenomenon aligns with findings from studies on Morchella sextelata44, and P. eryngii45, which propose that blue light exerts similar regulatory effects across various edible fungi species. The yields of three S. edulis strains differed, with H3 producing the highest yield. Between the H2 and H3 strains, yield did not significantly vary under three distinct monochromatic light conditions. This could be attributed to the fact that, although individual fungi exposed to red and green light weighed less than those exposed to blue light, the overall yield under red and green light matched that under blue light, owing to the higher number of primordia and fruiting bodies.
Light quality not only influences the coloration but also the morphology of S. edulis. Specifically, blue light facilitated the regulation of the pileus length and morphology in S. edulis, aligning with findings from Wang Huan’s research, which suggests blue light might enhance the expansion of the fungus pileus by up regulating genes related to the synthesis of growth regulatory substances46. Edible fungi, being extensively cultivated worldwide with high yields throughout the year, demonstrate a critical sensitivity to light quality, which is pivotal in their production. This study reveals that blue light is an effective way to improve the market value of S. edulis, which may be related to blue light receptors. Moreover, different light quality has a great influence on plant agronomic traits47. Red light can significantly promote the growth of the stalk length of S. edulis, potentially due to the wavelength of red light’s ability to penetrate deeply into cells and influence stalk cell elongation48. The correlation analyses revealed a positive relationship between the L* value and stalk length, while the a* value was associated with three characteristics of the pileus. This underscores the significant connection between agronomic traits and light quality, with variations among strains indicating diverse light perception capabilities across different edible fungi. The findings of this study suggest that the morphology of S. edulis fruit bodies under blue light aligns with market preferences, avoids interference from other wavelengths, reduces energy consumption, and lowers production costs.
Edible mushrooms are a delicious and economical source of antioxidants in the diet, and in recent years, people have begun to seek functional foods and nutritional supplements with antioxidant properties49. This study discovered a distinct specificity in the antioxidant system under varying light qualities. Notably, blue light treatment significantly improved the activities of POD, SOD and CAT in S. edulis, compared with red light and green light. These findings align with existing literature that underscores the role of blue light in bolstering the activity of antioxidant enzymes in plants50. Furthermore, blue light may stimulate the expression and activation of these enzymes through the activation of specific photoreceptive receptors or signal transduction pathways, thereby augmenting cellular capacity to scavenge reactive oxygen species51. Notably, under monochromatic light treatments, MDA content was reduced, particularly with blue and green light, suggesting these treatments effectively shield cell membranes from oxidative harm. DPPH is a stable free radical, and the DPPH scavenging efficiency of plant antioxidant substances can reflect the strength of its antioxidant capacity52. S. edulis exhibited the highest DPPH free radical scavenging rate under blue light. The blue light was observed to reduce the content of OFR. These findings indicate that the antioxidant molecules in S. edulis are significantly influenced by specific light qualities, with blue light potentially enhancing the S. edulis overall antioxidant capacity. This enhancement is attributed not only to the activation of the antioxidant enzyme system but also to the increase in non-enzymatic antioxidant components53, making blue light an optimal choice for boosting the antioxidant properties of S. edulis.
The light quality had an impact on crude fiber content, and studies have shown that the proportion of red light in LED regulated the crude fiber content in lettuce54. Consequently, this study further investigated the impact of light quality on the crude fiber content in S. edulis. It was observed that green light significantly enhances the crude fiber content of S. edulis, suggesting that its metabolic pathways and physiological responses may be particularly responsive to certain wavelengths. This implies that specific wavelengths could modulate metabolic activities via mechanisms yet to be elucidated.
Notably, the crude fiber content in S. edulis was all above 20 g/100 g. Food containing at least 6 g of fiber in 100 g could be considered as fiber-rich food55. Consequently, S. edulis examined in this study qualifies as a high fiber food. Exposure to red light was found to significantly elevate the total sugar content in H1 and H2 strains, indicating that light might influence intracellular signaling molecules responsible for the synthesis of sugars and other metabolites56. However, the total sugar content in the H3 strain remained unaffected by light quality, hinting at the possibility of genetic and physiological variances across strains. These disparities could be attributed to differences in intracellular photoreceptors, cell wall compositions, and metabolic pathway specificity57. As a nutrient rich food source, edible fungi offer vitamin D, minerals, dietary fiber and protein, earning the title of “superfood” from both the World Health Organization and the Food and Agriculture Organization of the United Nations58. This study found that the crude fat content in S. edulis ranged between 0.59 and 1.82 g/100 g, with green light significantly diminishing the crude fat content. Typically, the crude fat content in dried edible mushrooms varies between 2 and 9%. For instance, P. ostreatus exhibits a crude fat range from 0.2 to 8 g per 100 g of dried mushrooms59. Despite varying light conditions, the crude fat content in the H1, H2, and H3 strains of S. edulis remained low, positioning it as an ideal component of a health conscious diet, particularly for individuals seeking low fat options60.
Amino acids, crucial compounds influenced by the light environment, were significantly affected in this study61. Compared with CK, distinct differences in the content of total free amino acids under various light qualities were observed, with an increase under blue light treatment. This result might be attributed to blue light’s specific regulatory influence on the metabolic pathways of the strain, enhancing amino acid synthesis, an observation that aligns with findings on the impact of light on amino acid content in plants62. The response to red light varied among S. edulis strains: it positively affected 13 amino acids in H1, whereas it reduced the same in H2 and H3 strains, indicating strain specific amino acid metabolism. Glu and Asp are the two most abundant amino acids in the S. edulis, and are known for their unique umami flavor, with Glu being particularly noted for its strong umami taste and high nutritional value63. A thorough analysis of amino acid composition provides deeper insight into how light quality influences the synthesis and accumulation of amino acids in S. edulis. These insights underscore the potential of utilizing specific light conditions to enhance the nutritional profile of S. edulis in cultivation, offering a novel approach to its cultivation and nutritional enhancement.
Conclusion
In summary, this study demonstrated for the first time that blue light possesses a regulatory effect on the color of S. edulis, introducing a novel perspective for selecting light quality in its production. The findings unequivocally establish blue light as the optimal choice for S. edulis cultivation, underscoring the necessity for further studies on factors such as blue light intensity in the later stage. Employing blue light in place of white light not only enhances the color depth and increases the size of the S. edulis pileus but also boosts its antioxidant capabilities. While green and red lights can also reduce MDA content and OFR content, blue light uniquely fosters the accumulation of total free amino acids, aligning with market demands. This research fills a critical knowledge gap regarding the impact of light quality on the agronomic traits and quality of S. edulis, laying the groundwork for the production of high quality S. edulis based on scientific evidence.
Acknowledgements
Thanks to teacher Chuanwen Jia, and students Yukun Ma and Jingyue Yao from the Engineering Research Center of Edible and Medicinal Fungi, Ministry of Education, Jilin Agricultural University, for their help in the cultivation experiment. Thanks to the China Agricultural Research System (CARS-20) and edible and medicinal mushrooms in Jilin Province (202300601) project for their support for this study.
Author contributions
Tian Tian: Writing – original draft, Writing – review & editing. Huiyue Hu: Data curation. YongSheng Ma: Formal analysis. JiaWen Qin: Software. Changtian Li: Conceptualization, Supervision, Writing – review & editing. Yu Li: Conceptualization.
Data availability
All data used for this study are contained in this article.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chang-Tian Li, Email: lct@jlau.edu.cn.
Yu Li, Email: fungi966@126.com.
References
- 1.Dai, Y. C., Niemelä, T. & Qin, G. F. Changbai wood-rotting fungi 14. A new pleurotoid species Panellus Edulis. Ann. Bot. Fenn. 40, 107–112 (2003). [Google Scholar]
- 2.Wang, J. R., Liu, Y., Lu, T., Wang, Q. Z. & Bau, T. Cultivation of Pleurotus ostreatus and Panellus edulis using dry stems of Triarrhena sacchariflora. Acta Edulis Fungi. 20, 24-26 (2013).
- 3.Woo, S. I. et al. Mycelial culture and fruiting analysis of Panellus edulis strains collected in Korea. Korean J. Mycol. 46, 281–294 (2018). [Google Scholar]
- 4.Tian, F., Li, C. & Li, Y. Genomic Analysis of Sarcomyxa Edulis reveals the basis of its Medicinal properties and Evolutionary relationships. Front. Microbiol. 12, 652324 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao, L. et al. Two new compounds from edible mushroom Sarcomyxa Edulis. Nat. Prod. Res. 37, 1491–1497 (2023). [DOI] [PubMed] [Google Scholar]
- 6.Rahman, M. H. et al. The Growth and Tuber Yield of Potatoes (Solanum tuberosum L.) under Varying LED Light Spectrums in Controlled Greenhouse Conditions. Horticulturae. 10, 254 (2024).
- 7.Feng, Y. et al. Effect of light on quality of preharvest and postharvest edible mushrooms and its action mechanism: a review. Trends Food Sci. Technol. 139, 104119 (2023). [Google Scholar]
- 8.Yue, Z. et al. Effect of different light qualities and intensities on the yield and quality of facility-grown Pleurotus eryngii. J. fungi. 8, 1244 (2022). [DOI] [PMC free article] [PubMed]
- 9.Fuller, K. K., Loros, J. J. & Dunlap, J. C. Fungal photobiology: visible light as a signal for stress, space and time. Curr. Genet. 61, 275–288 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Purschwitz, J., Müller, S., Kastner, C. & Fischer, R. Seeing the rainbow: light sensing in fungi. Curr. Opin. Microbiol. 9, 566–571 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Sabzalian, M. R. et al. High performance of vegetables, flowers, and medicinal plants in a red-blue LED incubator for indoor plant production. Agron. Sustain. Dev. 34, 879–886 (2014). [Google Scholar]
- 12.Cashmore, A. R., Jarillo, J. A., Wu, Y. J. & Liu, D. J. S. Cryptochromes: blue light receptors for plants and animals. Science. 284, 760–765 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Shafiq, I. et al. Crop photosynthetic response to light quality and light intensity. J. Integr. Agric. 20, 4–23 (2021). [Google Scholar]
- 14.Shao, M. J., Liu, W. K., Zhou, C. B., Wang, Q. & Li, B. S. Alternation of temporally overlapped red and blue light under continuous irradiation affected yield, antioxidant capacity and nutritional quality of purple-leaf lettuce. Sci. Hort. 295, 110864 (2022). [Google Scholar]
- 15.Sakamoto, Y., Ando, A., Tamai, Y. & Yajima, T. Pileus differentiation and pileus-specific protein expression in Flammulina velutipes. Fungal Genet. Biol. 44, 14–24 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Liu, J. et al. Genome-wide characterization and expression analysis of the photoreceptors encoded in Flammulina Filiformis. Mycosystema. 42, 759–769 (2023). [Google Scholar]
- 17.Kamada, T., Sano, H., Nakazawa, T. & Nakahori, K. Regulation of fruiting body photomorphogenesis in Coprinopsis Cinerea. Fungal Genet. Biol. 47, 917–921 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto, Y. et al. Blue light exposure and nutrient conditions influence the expression of genes involved in simultaneous hyphal knot formation in Coprinopsis Cinerea. Microbiol. Res. 217, 81–90 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Li, J. et al. Blue light and its receptor white collar complex (FfWCC) regulates mycelial growth and fruiting body development in Flammulina Filiformis. Sci. Hort. 309, 111623 (2023). [Google Scholar]
- 20.Huang, M. Y. et al. The intensity of blue light-emitting diodes influences the antioxidant properties and sugar content of oyster mushrooms (Lentinus sajor-caju). Sci. Hort. 218, 8–13 (2017). [Google Scholar]
- 21.Gao, S. et al. Effect of different LED light quality combination on the content of vitamin C, soluble sugar, organic acids, amino acids, antioxidant capacity and mineral elements in green onion (Allium fistulosum L). Food Res. Int. 156, 111329 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Doğan, H. H. Evaluation of phenolic compounds, antioxidant activities and fatty acid composition of Amanita Ovoidea (Bull.) Link. In Turkey. J. Food Compos. Anal. 31, 87–93 (2013). [Google Scholar]
- 23.Sanchez, F. et al. Spectral light distribution affects photosynthesis, leaf reflective indices, antioxidant activity and growth of Vanillaplanifolia. Plant. Physiol. Biochemistry: PPB. 182, 145–153 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Wu, X. L. et al. Effects of UV-C on antioxidant activity, total phenolics and main phenolic compounds of the melanin biosynthesis pathway in different tissues of button mushroom. Postharvest Biol. Technol. 118, 51–58 (2016). [Google Scholar]
- 25.Huang, S. J., Lin, C. P. & Tsai, S. Y. Vitamin D2 content and antioxidant properties of fruit body and mycelia of edible mushrooms by UV-B irradiation. J. Food Compos. Anal. 42, 38–45 (2015). [Google Scholar]
- 26.Zhang, R. et al. Effects of long-term blue light irradiation on carotenoid biosynthesis and antioxidant activities in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Food research international (Ottawa, Ont.). 174, 113661 (2023). [DOI] [PubMed]
- 27.Zhang, Y., Wu, X., Huang, C., Zhang, Z. & Gao, W. Isolation and identification of pigments from oyster mushrooms with black, yellow and pink caps. Food Chem. 372, 131171 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Tsai, S. Y., Huang, E. W. & Chen, Z. Y. Nutritional qualities and antioxidant properties of Hypsizygus marmoreus as affected by light source on cultivation. J. Adv. Agricultural Technol. 6, 9-13 (2019).
- 29.Hao, J., Chen, X. & Lan, J. Effect of light quality on growth and polysaccharides content of Ganoderma Lucidum. China J. Chin. Materia Med. 35, 2242–2245 (2010). [PubMed] [Google Scholar]
- 30.Kim, J. Y., Kim, D. Y., Park, Y. J. & Jang, M. J. Transcriptome analysis of the edible mushroom Lentinula edodes in response to blue light. PloS One. 15, e0230680 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao, Y. X. et al. Identification of genes in Flammulina filiformis L-lysine biosynthesis pathway and their expression in response to light conditions. Acta Edulis Fungi. 25, 1–8 (2018). [Google Scholar]
- 32.Chiang, S. S., Liang, Z. C., Wang, Y. C. & Liang, C. H. Effect of light-emitting diodes on the production of cordycepin, mannitol and adenosine in solid-state fermented rice by Cordyceps Militaris. J. Food Compos. Anal. 60, 51–56 (2017). [Google Scholar]
- 33.Zhang, J. J. et al. Heat and light stresses affect metabolite production in the fruit body of the medicinal mushroom cordyceps militaris. Appl. Microbiol. Biotechnol. 102, 4523–4533 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Tian, F. H. Evaluation of germplasm resources and genes related to triterpene synthetic path way of Sarcomyxa edulis in Northeast China. (2019).
- 35.Wu, Y. et al. The color difference of rubus fruits is closely related to the composition of flavonoids including anthocyanins. LWT. 149, 111825 (2021). [Google Scholar]
- 36.Vattem, D. A., Lin, Y. T., Labbe, R. G. & Shetty, K. Phenolic antioxidant mobilization in cranberry pomace by solid-state bioprocessing using food grade fungus Lentinus edodes and effect on antimicrobial activity against select food borne pathogens. Innovative Food Sci. Emerg. Technol. 5, 81–91 (2004). [Google Scholar]
- 37.Spitz, D. R. & Oberley, L. W. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 179, 8–18 (1989). [DOI] [PubMed] [Google Scholar]
- 38.Jiao, C. J. et al. β-ODAP accumulation could be related to low levels of superoxide anion and hydrogen peroxide in Lathyrus sativus L. Food Chem. Toxicology: Int. J. Published Br. Industrial Biol. Res. Association. 49, 556–562 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Ukeda, H., Kawana, D. & &., M. S. Spectrophotometric Assay for Superoxide Dismutase based on the reduction of highly Water-soluble tetrazolium salts by Xanthine-Xanthine Oxidase. J. Agricultural Chem. Soc. Japan. 63, 485–488 (1999). [DOI] [PubMed] [Google Scholar]
- 40.Doerge, D. R., Divi, R. L. & Churchwell, M. I. Identification of the colored guaiacol oxidation product produced by peroxidases. Anal. Biochem. 250, 10–17 (1997). [DOI] [PubMed] [Google Scholar]
- 41.Johansson, L. H. & Borg, L. A. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 174, 331–336 (1988). [DOI] [PubMed] [Google Scholar]
- 42.Chen, Y. et al. Proteolysis of chloroplast proteins is responsible for accumulation of free amino acids in dark-treated tea (Camellia sinensis) leaves. J. Proteom. 157, 10–17 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Duan, C. et al. Artificial cultivation and evaluation of two late fall oyster strains (Sarcomyxa Edulis) from Jilin, China. Mycological Progress. 22, 47 (2023). [Google Scholar]
- 44.Qiu, Z. et al. Synthesis and structural characteristics analysis of melanin pigments induced by blue light in Morchella sextelata. Front. Microbiol. 14, 1276457 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du, F., Zou, Y., Hu, Q., Zhang, H. & Ye, D. Comparative transcriptomic analysis reveals molecular processes involved in pileus morphogenesis in Pleurotus eryngii under different light conditions. Genomics. 112, 1707–1715 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Wang, H. et al. Transcriptomic profiling sheds light on the blue-light and red-light response of oyster mushroom (Pleurotus Ostreatus). AMB Express. 10, 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang, Y., Dong, Y., Yang, Q., Urano, D. & Wang, Z. Interactive effects of light quality and nitrate supply on growth and metabolic processes in two lettuce cultivars (Lactuca sativa L). Environ. Exp. Bot. 213, 105443 (2023). [Google Scholar]
- 48.Paradiso, R. & Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: the state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 41, 742–780 (2022). [Google Scholar]
- 49.Borbély, P. et al. Light intensity and Spectrum Dependent Redox Regulation of Plant Metabolism. Antioxid. (Basel Switz.). 11, 1311 (2022). [DOI] [PMC free article] [PubMed]
- 50.Zhu, H. et al. Effects of low light on photosynthetic properties, antioxidant enzyme activity, and anthocyanin accumulation in purple pak-choi (Brassica campestris Ssp. Chinensis Makino). PloS One. 12, e0179305 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun, B. et al. Effect of light on sensory quality, health-promoting phytochemicals and antioxidant capacity in post-harvest baby mustard. Food Chem. 339, 128057 (2021). [DOI] [PubMed] [Google Scholar]
- 52.Iordănescu, O. A. et al. A DPPH· Kinetic Approach on the antioxidant activity of various parts and ripening levels of papaya (Carica papaya L.) ethanolic extracts. Plants (Basel Switz.). 10, 1679 (2021). [DOI] [PMC free article] [PubMed]
- 53.Lai, C. C. et al. Light quality modulates growth, triggers differential accumulation of phenolic compounds, and changes the total antioxidant capacity in the red callus of Vitis Davidii. J. Agric. Food Chem. 70, 13264–13278 (2022). [DOI] [PubMed] [Google Scholar]
- 54.Chen, X. L., Li, Y. L., Wang, L. & Guo, W. Z. Red and blue wavelengths affect the morphology, energy use efficiency and nutritional content of lettuce (Lactuca sativa L). Sci. Rep. 11, 8374 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siyame, P., Kassim, N. & Makule, E. Effectiveness and suitability of Oyster mushroom in improving the nutritional value of maize flour used in complementary foods. International journal of food science. 8863776 (2021). (2021). [DOI] [PMC free article] [PubMed]
- 56.Li, Y. et al. Effects of red and blue light on leaf anatomy, CO(2) assimilation and the photosynthetic electron transport capacity of sweet pepper (Capsicum annuum L.) seedlings. BMC Plant Biol. 20, 318 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bayram, Ö. & Bayram, Ö. S. An anatomy of Fungal Eye: fungal photoreceptors and signalling mechanisms. J. Fungi. 9, 591 (2023). [DOI] [PMC free article] [PubMed]
- 58.Zied, D. C., Pardo, J. E. & Noble, R. Pardo-Giménez, A. Efficiency of mushrooms for food production-fundamental strategic decision-making. J. Food Compos. Anal. 125, 105734 (2024). [Google Scholar]
- 59.Zhou, S., Ma, F., Zhang, X. & Zhang, J. Carbohydrate changes during growth and fruiting in Pleurotus Ostreatus. Fungal Biology. 120, 852–861 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Dawadi, E. et al. Nutritional and post-harvest quality preservation of mushrooms: a review. Heliyon. 8, e12093 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu, D. et al. In situ neutral desorption-extractive electrospray ionization mass spectrometry reveals red-blue light promoted the accumulation of amino acids and polyphenols in Anoectochilus Roxburghii. J. Food Compos. Anal. 125, 105761 (2024). [Google Scholar]
- 62.Monostori, I. et al. LED lighting - modification of growth, metabolism, yield and flour composition in wheat by Spectral Quality and Intensity. Front. Plant Sci. 9, 605 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xue, L. X. et al. Evaluation of the umami in edible fungi and study on umami extraction of Agaricus Bisporus. J. Food Compos. Anal. 128, 106069 (2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used for this study are contained in this article.