Abstract
The fusion (F) protein of the paramxyovirus simian parainfluenza virus 5 (SV5) promotes virus-cell and cell-cell membrane fusion. Previous work had indicated that removal of the SV5 F protein cytoplasmic tail (F Tail− or FΔ19) caused a block in fusion promotion at the hemifusion stage. Further examination has shown that although the F Tail− mutant is severely debilitated in promotion of fusion as measured by using two reporter gene assays and is debilitated in the formation of syncytia relative to the wild-type F protein, the F Tail− mutant is capable of promoting the transfer of small aqueous dyes. These data indicate that F Tail− is fully capable of promoting formation of small fusion pores. However, enlargement of fusion pores is debilitated, suggesting that either the cytoplasmic tail of the F protein plays a direct role in pore expansion or that it interacts with other components which control pore growth.
Membrane fusion is an essential step in the life cycle of enveloped viruses, allowing viral entry into the host cell and release of the viral genome (for a review, see reference 16). For members of the Paramyxoviridae family, such as the Rubulavirus Simian parainfluenza virus 5 (SV5), fusion is promoted by the fusion (F) protein.
Examination of the fusion promoted by a number of viral glycoproteins has produced a working model for the stages of membrane fusion, with the most extensive data to date coming from study of the influenza virus hemagglutinin (HA) protein. For HA, triggering of fusion occurs through the low pH found in endosomes. This leads to a major protein refolding event, during which the fusion peptide is relocated by approximately 100 Å (5, 42). Conformational changes such as these are proposed to occur for other fusion proteins and allow the fusion peptide to interact with the target membrane (reviewed in reference 16). Further conformational changes are thought to lead to formation of a complex between heptad repeat regions flanking the fusion peptide and transmembrane (TM) domains, an event which has been hypothesized to drive initial fusion of the membrane bilayers (4, 25).
The membrane fusion event itself can be broken down into several stages. First, mixing of the outer leaflets occurs, a stage that is known as restricted hemifusion. Next, a fusion pore is formed, a process that can be measured at its earliest stages by electrophysiological techniques. Subsequently, as the fusion pore enlarges, mixing of aqueous contents can be detected. Several mutations in fusion proteins have been reported to block fusion promotion at the hemifusion stage, including mutations in, or shortening of, the TM domain of HA (1, 24), mutations in the TM domain of the vesicular stomatitis virus G protein (8), or mutation of the N terminus of the fusion peptide of HA (35). In addition, replacement of the HA TM domain with a glycosylphosphatidylinositol anchor (GPI-HA) has been reported to lead to a hemifusion phenotype (20, 26). However, recent electrophysiological experiments have demonstrated that GPI-HA can form small, nonenlarging pores under certain conditions (22).
Several studies have been conducted on the role of the cytoplasmic tail of the paramxyovirus F proteins in promotion of membrane fusion. Truncation of the cytoplasmic tail of Newcastle disease virus (NDV) F protein led to greatly reduced syncytium formation (41), and removal of the cytoplasmic tail of human parainfluenza virus type 3 (HPIV3) F protein caused a severe defect in oligomerization (43). However, deletion of the cytoplasmic tail of HPIV2 F protein did not affect folding or promotion of membrane fusion (43). For SV5, removal of 19 residues of the cytoplasmic tail of F protein (FΔ19 or F Tail−), leaving a single charged lysine residue to abut the presumptive TM domain, resulted in an F protein which was blocked in fusion promotion at the hemifusion stage (3). Stimulated by the more recent work with GPI-HA, we performed a more detailed analysis of fusion properties of the F Tail− mutant.
BHK 21F cells, Vero cells, and CV-1 cells were grown as described previously (33). The recombinant vaccinia virus vTF7-3, which expresses T7 RNA polymerase, was grown in CV-1 cells as described previously (12). The SV5 F and hemagglutinin-neuraminidase (HN) cDNAs were expressed from pGEM plasmids or from the eukaryotic expression plasmid pCAGGS (17, 29, 31, 34). The F Tail− plasmid, originally designated FΔ19, was that used previously (3) or was expressed using pCAGGS. The pIntT7βgal plasmid was kindly provided by Edward Berger and Bernard Moss (National Institutes of Health, Bethesda, Md.). Plasmid pBH82 contains the chloramphenicol acetyltransferase (CAT) gene under the control of the T7 RNA polymerase promoter (15), and the plasmid pCAGGS-T7 contains the T7 RNA polymerase gene (34). Wild-type (wt) F, F Tail−, and HN proteins were expressed transiently by using either the recombinant vaccinia virus-T7 (vac-T7) RNA polymerase expression system (12) or by use of the pCAGGS vector (29) as described previously (9, 34). For quantification of surface expression by flow cytometry, HeLa T4 cells transfected with pCAGGS vectors were prepared and analyzed as described previously (9, 18).
Fresh human erythrocytes were singly labeled with the aqueous probe 6-carboyxfluorescein (CF) or dually labeled with the lipid probe octadecyl rhodamine B (R18) and CF as described previously (2, 26, 27). Analysis of membrane fusion by confocal microscopy was performed as described previously (34). The β-galactosidase assay for content mixing (2, 10) and the CAT assay for content mixing (14, 34) were performed as described previously. Syncytium formation assays were performed with BHK 21F cells or Vero cells that were transiently transfected using a total of 2 μg of the pCAGGS F, F Tail−, and HN plasmids.
Removal of the cytoplasmic tail of the SV5 F protein does not change surface expression levels.
The original study with the F Tail− mutant was performed when transfection efficiencies were only about 20% (3). Since that time, transfection efficiencies have increased to 50 to 80% positive cells and the pCAGGS expression system has become available for comparison with the vac-T7 expression system. Quantification of the surface levels of pCAGGS-expressed wt F and F Tail−, by flow cytometry, indicated that both percent positive cells and mean fluorescent intensities (MFI) were similar for the two proteins: wt F, 75.5% transfected, with an MFI of 150.4, and F Tail−, 71.4% transfected, with an MFI of 129.6. That the surface density of the F protein is not greatly changed by removal of the cytoplasmic tail simplifies the interpretation of the experiments described below because it has been shown that changes in surface density affect fusion promotion (10). The aberrant glycosylation of the F Tail− protein noted previously (3) was still observed when the F Tail− protein was expressed using pCAGGS vectors (data not shown).
The F Tail− mutant promotes both lipid mixing and transfer of a small aqueous dye.
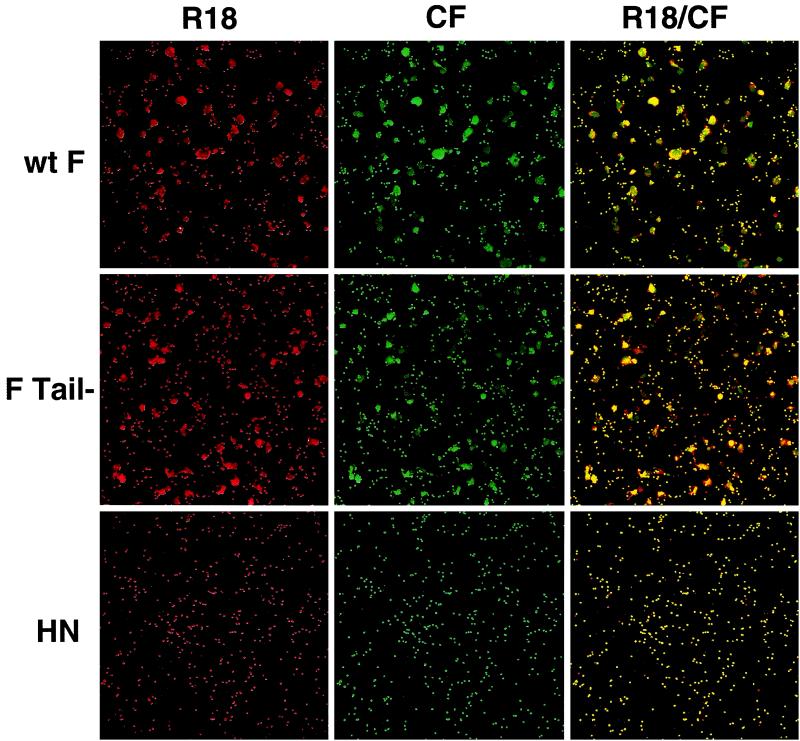
To examine the ability of F Tail− to promote membrane fusion, wt F and F Tail−, along with the SV5 HN protein to provide target cell binding, were expressed using the vac-T7 expression system in CV-1 cells. Fusion reactions were monitored by binding to the CV-1 cells (at 4°C) fresh human red blood cells (RBCs) labeled with both the lipid probe R18 and the small aqueous dye CF. Fusion was initiated by the addition of phosphate-buffered saline (PBS) prewarmed to 37°C, plates were incubated at 37°C for 10 min, and cells were examined by confocal microscopy. No transfer of either dye was seen in the control cells expressing only HN (Fig. 1, bottom). As expected, wt F promoted coincident transfer of both the lipid probe R18 and the aqueous dye CF, indicating that fusion events resulting in lipid spread also resulted in spread of an aqueous probe (Fig. 1, top). Interestingly, though previously thought to be blocked in fusion promotion at the hemifusion stage, F Tail− promoted transfer of both probes at levels similar to those of wt F (Fig. 1, middle). Transfer of both probes by F Tail− was also seen when the pCAGGS expression system was used in Vero cells (data not shown), indicating that this result is not system specific. Transfer of the aqueous probe CF was seen only when R18 transfer was also detected (yellow, merge of CF and R18 fluorescence), suggesting that nonspecific leakage is not the cause of the CF transfer. Instead, the concomitant transfer of lipid and aqueous probes by F Tail− indicates that this mutant is capable of promoting formation of fusion pores and is not blocked at the hemifusion stage.
FIG. 1.
Transfer of lipid and aqueous dyes by wt F and F Tail−. SV5 HN and either wt F or F Tail− were coexpressed in CV-1 cells by using the recombinant vac-T7 polymerase expression system. At 24 h p.t., human RBCs double labeled with the lipid probe R18 and aqueous probe CF were bound to CV-1 cells at 4°C. Fusion was initiated by replacement of cold PBS with PBS prewarmed to 37°C, and cells were incubated at 37°C for 10 min. Fusion was stopped by replacement with ice-cold PBS. Cells were examined using a confocal microscope (Zeiss LSM 410; Carl Zeiss, Inc., Thornwood, N.Y.), with dual images recorded on both fluorescein and rhodamine channels.
Time course of transfer of CF is similar for WT F and F Tail−.
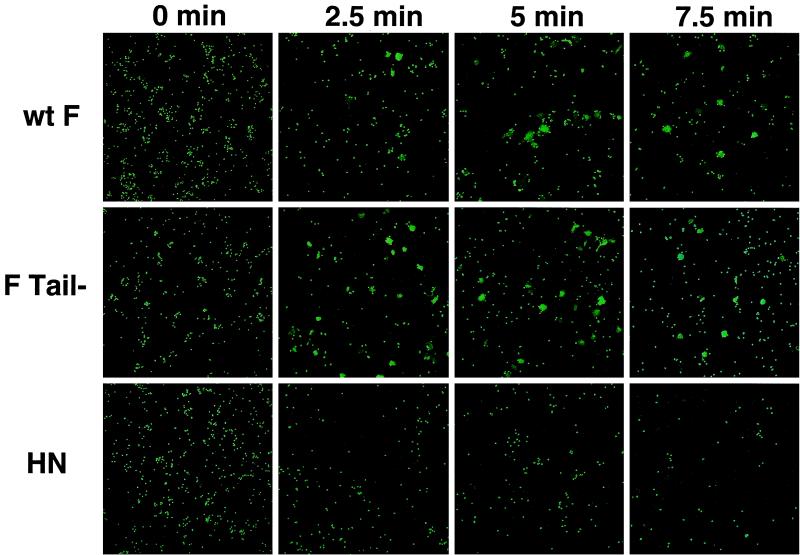
An examination of the time course of transfer of CF mediated by wt F and F Tail− was conducted using the vac-T7 expression system. Singly labeled RBCs were used to monitor fusion, as it has been demonstrated that the presence of lipid dyes in the target membrane can influence membrane fusion (22). No transfer of CF was seen by confocal microscopy when HN was expressed alone, though loss of RBCs was detected over the time course (Fig. 2, bottom), consistent with previous observations that RBCs release from HN when not coexpressed with the F protein (10). Transfer of the aqueous probe CF was seen by 2.5 min with both wt F and F Tail−, with maximal fusion reached by 5 min (Fig. 2), suggesting that pore opening to allow transfer of the small dye CF is not slowed for F Tail−. Indeed, F Tail−routinely showed a greater percentage of cells containing CF at 2.5 min, raising the possibility that this mutant is faster in the opening of small fusion pores. In addition, similar experiments with the larger aqueous dye tetramethylrhodamine-tagged dextran (Mr of ≈40,000 compared to an Mr of 376 for CF) showed no significant difference between the wt F and F Tail−, indicating that pores large enough to permit transfer of this larger aqueous dye are formed by F Tail− (data not shown).
FIG. 2.
Time course of transfer of the aqueous probe CF. Human RBCs labeled with the aqueous probe CF were bound to CV-1 cells coexpressing HN and either wt F or F Tail−, as described in the legend to Fig. 1. The cells were incubated at 37°C for various times, and the results were analyzed by confocal microscopy.
Reporter gene assays indicate F Tail− is debilitated in fusion promotion.
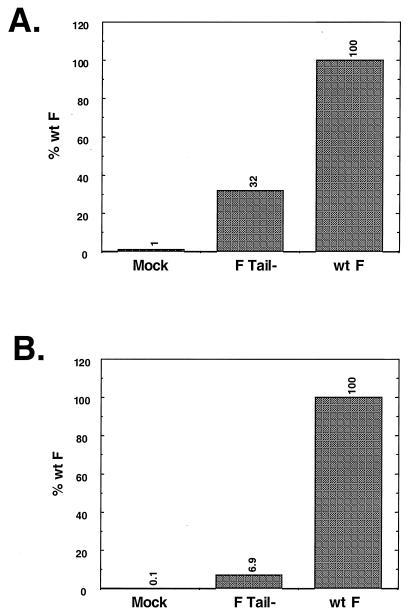
As F Tail− is not compromised in its ability to transfer small aqueous dyes, the fusion promotion of F Tail− was examined by reporter gene assays. First, a β-galactosidase reporter gene assay using the vac-T7 expression system and CV-1 cells was used. As shown in Fig. 3A, F Tail− promoted fusion at only 32% of that seen for wt F, a result that was seen consistently over several experiments. To examine further the extent to which F Tail− was debilitated in content mixing, a second expression system and a second reporter assay were used. wt F, F Tail−, and HN were expressed in Vero cells using the pCAGGS eukaryotic expression vector, and fusion was assessed by measuring using a reporter assay, expression of the CAT gene (14, 34). As shown in Fig. 3B, F Tail− was greatly impaired for content mixing, with only 6.9% of the fusion seen for wt F. Results from subsequent experiments confirmed this, with F Tail− giving a range of 6 to 13% of the fusion seen with the wt F protein.
FIG. 3.
Reporter gene assays of wt F and F Tail−. (A) β-Galactosidase assay. CV-1 cells infected with the vac-T7 polymerase recombinant and coexpressing HN and either wt F or F Tail− were incubated with a second population of CV-1 cells infected with wt vaccinia virus and transfected with the plasmid pINTT7 β-gal, which encodes β-galactosidase under the control of the T7 polymerase promoter. After incubation at 37°C for 4 h, samples were analyzed by a colorimetric lysate assay. Results shown are the average of triplicate samples and are representative of two separate experiments. (B) CAT assay. Vero cells were cotransfected with pCAGGS expressing HN and either wt F or F Tail−. In addition, these cells were transfected with the plasmid encoding CAT under control of the T7 polymerase promoter. A second set of Vero cells was transfected with pCAGGS expressing T7 polymerase. After overnight incubation, the T7 polymerase-expressing cells were overlaid on the cells expressing the F and HN proteins. Membrane fusion between the cell populations allows the T7 polymerase to transcribe the CAT gene, and subsequent CAT activity was assayed as described previously (14). Samples are an average of duplicates and are representative of three separate experiments.
For fusion content mixing to be measured by these assays, a pore of sufficient size to allow transfer of either the plasmid DNA or the T7 polymerase is required. These data therefore suggest that removal of the cytoplasmic tail of the SV5 F protein affects enlargement of the fusion pore. The differing levels of inhibition seen between the two assays may be due either to differences in the expression systems used or to the difference in cell types examined.
F Tail− causes a reduction in the extent of syncytium formation.
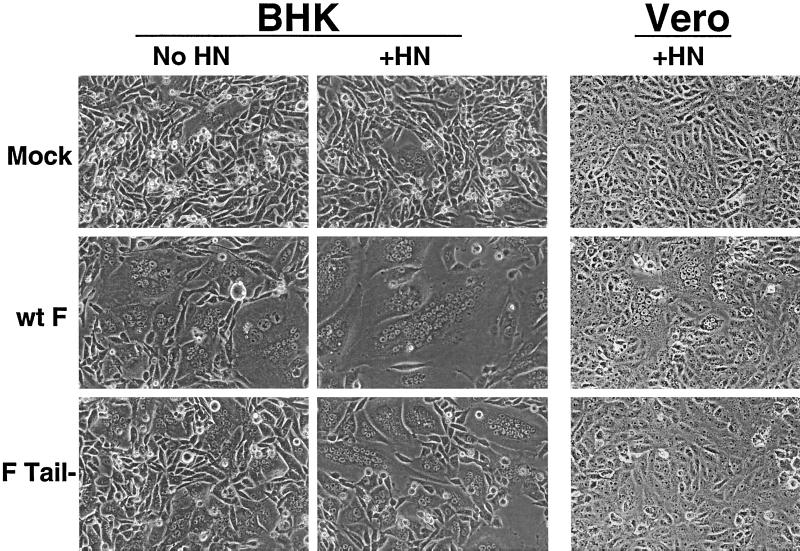
Expression of the SV5 F protein from cDNA can promote formation of syncytia (19, 32). As this fusion assay requires large expansion of the fusion pore, the ability of F Tail− to promote syncytia was examined. BHK cells, which are known to be highly fusogenic in this assay, were transfected with pCAGGS expressing wt F or F Tail−, with or without coexpression of HN, and syncytium formation was examined 18 to 24 h posttransfection (p.t.). A very small number of syncytia, probably forming spontaneously, were seen in BHK cells either transfected with vector alone or those expressing HN (Fig. 4). Expression of wt F led to the formation of numerous multinucleated cells. When HN was coexpressed with wt F, the average number of nuclei in these giant cells increased as observed previously (19). Much smaller syncytia were observed when F Tail− was expressed, though the size of the syncytia also increased in when F Tail− was coexpressed with HN. These data indicate that F Tail− is capable of forming syncytia in BHK cells, though not to the extent seen with wt F.
FIG. 4.
Syncytium formation assay. BHK 21F cells or Vero cells were transfected using a total of 2 μg of pCAGGS wt F, F Tail−, or HN plasmid. At 18 to 24 h p.t. (BHK) or 36 h p.t. (Vero), monolayers were examined using a Nikon Diaphot inverted phase-contrast microscope (Nikon Inc., Garden City, N.Y.). Photographs were taken using a Kodak DCS 420 digital camera (Eastman Kodak Company, Rochester, N.Y.).
The ability of wt F and F Tail− to form syncytia in Vero cells was also examined. Vero cells were transfected with pCAGGS vectors, and fusion was examined at 36 h p.t. No spontaneous syncytia were seen in Vero cells, even when HN was expressed (Fig. 4). Multinucleated cells, averaging 8 to 10 nuclei per giant cell, were seen throughout the wells expressing wt F. No syncytia were seen in cells expressing F Tail− at this time point. Upon further incubation of the plates (to 48 h p.t.), a few giant cells containing two to three nuclei could be seen in Vero cells expressing F Tail−. These data support the conclusion that F Tail− is debilitated in syncytium formation.
The data described here indicates that F Tail− is capable of promoting efficiently transfer of the small aqueous dye CF between target RBCs and cells expressing F Tail− (Fig. 2 and 3), whereas previously it was thought that fusion promoted by F Tail− was blocked at the hemifusion stage (3). Colabeling experiments demonstrate that aqueous dye transfer occurred only in cells also transferring the lipid probe R18 (Fig. 2), as would be expected if CF transfer is due to the formation of a fusion pore. In addition, a time course of CF transfer indicated that fusion promoted by F Tail− was not slower than that for wt F, with detectable transfer seen for both proteins by 2.5 min after the triggering of fusion (Fig. 3). Indeed, at the 2.5-min time point, the percentage of cells transferring CF was reproducibly higher for F Tail−, suggesting that pore opening may be faster when the cytoplasmic tail of the SV5 F protein is removed. Thus, our data indicate that the SV5 F protein cytoplasmic tail does not play a direct role in the hemifusion diaphragm to fusion pore transition nor does its removal adversely affect regions such as the TM domain which are known to be important in this process.
Whereas CF transfer by the SV5 F protein is not impaired by removal of the cytoplasmic tail, two reporter gene assays and also syncytium formation assays (Fig. 4) indicate a significant defect in promotion of membrane fusion. A positive signal in the reporter gene assay requires transfer of either T7 polymerase or of the plasmid containing the reporter gene, both of which are considerably larger than CF. Thus, these results suggest that pore enlargement is compromised in the absence of an F protein cytoplasmic tail.
A number of mutations have been identified in other viral fusion proteins that affect pore enlargement. Fusion peptide mutations in HA were found to affect both syncytium formation (13) and transfer of large aqueous dyes (40). Changes in the TM domain can also affect pore expansion. GPI-anchored HA can form pores, but no enlargement of these pores is seen (22). In addition, fusion pore enlargement promoted by HA mutants, as measured by transfer of various-sized aqueous dyes, was found to vary with the combination of the TM domain and the cytoplasmic tail (24).
Modifications to the cytoplasmic tail of fusion proteins have also been implicated in pore enlargement defects. A fowl plague virus HA protein chimera with the cytoplasmic tail of CD4 was found to be strongly impaired in enlargement of fusion pores, as judged by dye transfer (21). In addition, either deacylation of the cytoplasmic tail of the fowl plague virus HA (H7 subtype) (11) or addition of histidine residues to the C terminus of its cytoplasmic tail (30) were found to affect pore enlargement. These data led to the hypothesis that the cytoplasmic tail affects TM domain mobility such that changes that enlarge the cytoplasmic tail or affect its hydrophobicity impair the ability of the fusion protein to enlarge the fusion pore. This hypothesis is consistent with findings that removal of the cytoplasmic tail of retroviral Env proteins results in enhanced syncytium formation (28, 36), as mobility of the TM domain should be increased by removal of the cytoplasmic tail.
Removal of the cytoplasmic tail, however, does not consistently result in fusion enhancement. A mutated HA (subtype 3) protein lacking its cytoplasmic tail was found to have no differences in pore enlargement (23). For paramxyovirus fusion proteins, removal of the cytoplasmic tail of the HPIV2 F protein had no effect on the promotion of membrane fusion (43), while removal of the HPIV3 F protein cytoplasmic tail led to defects in oligomerization that made fusion assays impossible (43). However, an NDV F protein mutant lacking its cytoplasmic tail was severely affected in formation of syncytia (41), a result that is consistent with our finding of a fusion pore enlargement defect for SV5 F Tail−. It is possible that removal of the NDV and SV5 cytoplasmic tails adversely affects the ability of the TM domain to play its required role in pore enlargement. Alternatively, for these fusion proteins, the cytoplasmic tail may play a more direct role in enlargement of the pore.
Last, the data emphasize that the different cell types used affect both the threshold level of detectable fusion and the extent of fusion observed. In Vero cells, F Tail− did not promote detectable syncytium formation, whereas in BHK cells syncytium formation was detected (Fig. 4). The lipid composition of cells has been shown to affect both fusion induced by polyethylene glycol (37, 38) and fusion induced by viral proteins (39). Addition of lipids of various spontaneous curvature has also been seen to affect membrane fusion promoted by viral fusion proteins (6, 7). Thus, it is possible that the necessity for a cytoplasmic tail in pore enlargement is affected by the lipid composition of the cell types examined. Alternatively, cytoskeletal proteins may interact with the cytoplasmic tail region and thus play a role in pore expansion, and these proteins may vary according to the cell types examined. Further studies are clearly needed to delineate the role of these components in the expansion of the fusion pore.
Acknowledgments
This work was supported in part by research grant AI-23173 from the National Institute of Allergy and Infectious Disease. R.E.D. was supported in part by Public Health Service grant NRSA F32 AI-09607. R.A.L. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Armstrong R T, Kushnir A S, White J M. The transmembrane domain of influenza hemagglutinin exhibits a stringent length requirement to support the hemifusion to fusion transition. J Cell Biol. 2000;151:425–437. doi: 10.1083/jcb.151.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagai S, Lamb R A. Quantitative measurement of paramyxovirus fusion: differences in requirements of glycoproteins between simian virus 5 and human parainfluenza virus 3 or Newcastle disease virus. J Virol. 1995;69:6712–6719. doi: 10.1128/jvi.69.11.6712-6719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagai S, Lamb R A. Truncation of the COOH-terminal region of the paramyxovirus SV5 fusion protein leads to hemifusion but not complete fusion. J Cell Biol. 1996;135:73–84. doi: 10.1083/jcb.135.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker K A, Dutch R E, Lamb R A, Jardetzky T S. Structural basis for paramyxovirus-mediated membrane fusion. Mol Cell. 1999;3:309–319. doi: 10.1016/s1097-2765(00)80458-x. [DOI] [PubMed] [Google Scholar]
- 5.Bullough P A, Hughson F M, Skehel J J, Wiley D C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 6.Chernomordik L V, Frolov V A, Leikina E, Bronk P, Zimmerberg J. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J Cell Biol. 1998;140:1369–1382. doi: 10.1083/jcb.140.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chernomordik L V, Leikina E, Frolov V, Bronk P, Zimmerberg J. An early stage of membrane fusion mediated by the low pH conformation of influenza hemagglutinin depends upon membrane lipids. J Cell Biol. 1997;136:81–93. doi: 10.1083/jcb.136.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleverley D Z, Lenard J. The transmembrane domain in viral fusion: essential role for a conserved glycine residue in vesicular stomatitis virus G protein. Proc Natl Acad Sci USA. 1998;95:3425–3430. doi: 10.1073/pnas.95.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutch R E, Hagglund R N, Nagel M A, Paterson R G, Lamb R A. Paramyxovirus fusion (F) protein: a conformational change on cleavage-activation. Virology. 2001;281:138–150. doi: 10.1006/viro.2000.0817. [DOI] [PubMed] [Google Scholar]
- 10.Dutch R E, Joshi S B, Lamb R A. Membrane fusion promoted by increasing surface densities of the paramyxovirus F and HN proteins: comparison of fusion reactions mediated by simian virus 5 F, human parainfluenza virus type 3 F, and influenza virus HA. J Virol. 1998;72:7745–7753. doi: 10.1128/jvi.72.10.7745-7753.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer C, Schroth-Diez B, Herrman A, Garten W, Klenk H-D. Acylation of the influenza hemagglutinin modulates fusion activity. Virology. 1998;248:284–294. doi: 10.1006/viro.1998.9286. [DOI] [PubMed] [Google Scholar]
- 12.Fuerst T R, Niles E G, Studier F W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gething M-J, Doms R W, York D, White J M. Studies on the mechanism of membrane fusion: site-specific mutagenesis of the hemagglutinin of influenza virus. J Cell Biol. 1986;102:11–23. doi: 10.1083/jcb.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He B, Leser G P, Paterson R G, Lamb R A. The paramyxovirus SV5 small hydrophobic (SH) protein is not essential for virus growth in tissue culture cells. Virology. 1998;250:30–40. doi: 10.1006/viro.1998.9354. [DOI] [PubMed] [Google Scholar]
- 15.He B, McAllister W T, Durbin R K. Phage RNA polymerase vectors that allow efficient gene expression in both prokaryotic and eukaryotic cells. Gene. 1995;164:75–79. doi: 10.1016/0378-1119(95)00475-l. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez L D, Hoffman L R, Wolfsberg T G, White J M. Virus-cell and cell-cell fusion. Annu Rev Cell Develop Biol. 1996;12:627–661. doi: 10.1146/annurev.cellbio.12.1.627. [DOI] [PubMed] [Google Scholar]
- 17.Hiebert S W, Paterson R G, Lamb R A. Hemagglutinin-neuraminidase protein of the paramyxovirus simian virus 5: nucleotide sequence of the mRNA predicts an N-terminal membrane anchor. J Virol. 1985;54:1–6. doi: 10.1128/jvi.54.1.1-6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horvath C M, Lamb R A. Studies of the fusion peptide of a paramyxovirus fusion glycoprotein: roles of conserved residues in cell fusion. J Virol. 1992;66:2443–2455. doi: 10.1128/jvi.66.4.2443-2455.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horvath C M, Paterson R G, Shaughnessy M A, Wood R, Lamb R A. Biological activity of paramyxovirus fusion proteins: factors influencing formation of syncytia. J Virol. 1992;66:4564–4569. doi: 10.1128/jvi.66.7.4564-4569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemble G W, Danieli T, White J M. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell. 1994;76:383–391. doi: 10.1016/0092-8674(94)90344-1. [DOI] [PubMed] [Google Scholar]
- 21.Kozerski C, Ponimaskin E, Schroth-Diez B, Schmidt M F, Herrman A. Modification of the cytoplasmic domain of influenza virus hemagglutinin affects enlargement of the fusion pore. J Virol. 2000;74:7529–7537. doi: 10.1128/jvi.74.16.7529-7537.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markosyan R M, Cohen F S, Melikyan G B. The lipid-anchored ectodomain of influenza virus hemagglutinin (GPI-HA) is capable of inducing nonenlarging fusion pores. Mol Biol Cell. 2000;11:1143–1152. doi: 10.1091/mbc.11.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melikyan G B, Jin H, Lamb R A, Cohen F S. The role of the cytoplasmic tail regions of influenza virus hemagglutinin in formation and growth of fusion pores. Virology. 1997;235:118–128. doi: 10.1006/viro.1997.8686. [DOI] [PubMed] [Google Scholar]
- 24.Melikyan G B, Lin S, Roth M G, Cohen F S. Amino acid sequence requirements of the transmembrane and cytoplasmic domains of influenza virus hemagglutinin for viable membrane fusion. Mol Biol Cell. 1999;10:1821–1836. doi: 10.1091/mbc.10.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melikyan G B, Markosyan R M, Hemmati H, Delmedico M K, Lambert D M, Cohen F S. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J Cell Biol. 2000;151:413–423. doi: 10.1083/jcb.151.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melikyan G B, White J M, Cohen F S. GPI-anchored influenza hemagglutinin induces hemifusion to both red blood cell and planar bilayer membranes. J Cell Biol. 1995;131:679–691. doi: 10.1083/jcb.131.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris S J, Sarkar D P, White J M, Blumenthal R. Kinetics of pH-dependent fusion between 3T3 fibroblasts expressing influenza hemagglutinin and red blood cells. Measurement by dequenching of fluorescence. J Biol Chem. 1989;264:3972–3978. [PubMed] [Google Scholar]
- 28.Mulligan M J, Yamshchikov G V, Ritter G D, Jr, Gao F, Jin M J, Nail C D, Spies C P, Hahn B H, Compans R W. Cytoplasmic domain truncation enhances fusion activity by the exterior glycoprotein complex of human immunodeficiency virus type 2 in selected cell types. J Virol. 1992;66:3971–3975. doi: 10.1128/jvi.66.6.3971-3975.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants by a novel eukaryotic vector. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 30.Ohuchi M, Fischer C, Ohuchi R, Herwig A, Klenk H-D. Elongation of the cytoplasmic tail interferes with the fusion activity of influenza virus hemagglutinin. J Virol. 1998;72:3554–3559. doi: 10.1128/jvi.72.5.3554-3559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paterson R G, Harris T J R, Lamb R A. Fusion protein of the paramyxovirus simian virus 5: nucleotide sequence of mRNA predicts a highly hydrophobic glycoprotein. Proc Natl Acad Sci USA. 1984;81:6706–6710. doi: 10.1073/pnas.81.21.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paterson R G, Hiebert S W, Lamb R A. Expression at the cell surface of biologically active fusion and hemagglutinin-neuraminidase proteins of the paramyxovirus simian virus 5 from cloned cDNA. Proc Natl Acad Sci USA. 1985;82:7520–7524. doi: 10.1073/pnas.82.22.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paterson R G, Lamb R A. The molecular biology of influenza viruses and paramyxoviruses. In: Davidson A, Elliott R M, editors. Molecular virology: a practical approach. Oxford, England: IRL Oxford University Press; 1993. pp. 35–73. [Google Scholar]
- 34.Paterson R G, Russell C J, Lamb R A. Fusion protein of the paramyxovirus SV5: destabilizing and stabilizing mutants of fusion activation. Virology. 2000;270:17–30. doi: 10.1006/viro.2000.0267. [DOI] [PubMed] [Google Scholar]
- 35.Qiao H, Armstrong R T, Melikyan G B, Cohen F S, White J M. A specific point mutation at position 1 of the influenza hemagglutinin fusion peptide displays a hemifusion phenotype. Mol Biol Cell. 1999;10:2759–2769. doi: 10.1091/mbc.10.8.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritter G D, Jr, Mulligan M J, Lydy S L, Compans R W. Cell fusion activity of the simian immunodeficiency virus envelope protein is modulated by the intracytoplasmic domain. Virology. 1993;197:255–264. doi: 10.1006/viro.1993.1586. [DOI] [PubMed] [Google Scholar]
- 37.Roos D S, Choppin P W. Biochemical studies on cell fusion. I. Lipid composition of fusion-resistant cells. J Cell Biol. 1985;101:1578–1590. doi: 10.1083/jcb.101.4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roos D S, Choppin P W. Biochemical studies on cell fusion. II. Control of fusion response by lipid alteration. J Cell Biol. 1985;101:1591–1598. doi: 10.1083/jcb.101.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roos D S, Duchala C S, Stephensen C B, Holmes K V, Choppin P W. Control of virus-induced cell fusion by host cell lipid composition. Virology. 1990;175:345–357. doi: 10.1016/0042-6822(90)90419-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoch C, Blumenthal R. Role of the fusion peptide sequence in initial stages of influenza hemagglutinin-induced cell fusion. J Biol Chem. 1993;268:9267–9274. [PubMed] [Google Scholar]
- 41.Sergel T, Morrison T G. Mutations in the cytoplasmic domain of the fusion glycoprotein of Newcastle disease virus depress syncytia formation. Virology. 1995;210:264–272. doi: 10.1006/viro.1995.1343. [DOI] [PubMed] [Google Scholar]
- 42.Wilson I A, Skehel J J, Wiley D C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature. 1981;289:366–375. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- 43.Yao Q, Compans R W. Differences in the role of the cytoplasmic domain of human parainfluenza virus fusion proteins. J Virol. 1995;69:7045–7053. doi: 10.1128/jvi.69.11.7045-7053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]