ABSTRACT
Kidney fibrosis is a common outcome of a wide variety of chronic kidney diseases, in which virtually all kinds of renal resident and infiltrating cells are involved. As such, well-orchestrated intercellular communication is of vital importance in coordinating complex actions during renal fibrogenesis. Cell–cell communication in multicellular organisms is traditionally assumed to be mediated by direct cell contact or soluble factors, including growth factors, cytokines and chemokines, through autocrine, paracrine, endocrine and juxtacrine signaling mechanisms. Growing evidence also demonstrates that extracellular vesicles, lipid bilayer–encircled particles naturally released from almost all types of cells, can act as a vehicle to transfer a diverse array of biomolecules including proteins, mRNA, miRNA and lipids to mediate cell–cell communication. We recently described a new mode of intercellular communication via building a special extracellular niche by insoluble matricellular proteins. Kidney cells, upon injury, produce and secrete different matricellular proteins, which incorporate into the local extracellular matrix network, and regulate the behavior, trajectory and fate of neighboring cells in a spatially confined fashion. This extracellular niche–mediated cell–cell communication is unique in that it restrains the crosstalk between cells within a particular locality. Detailed delineation of this unique manner of intercellular communication will help to elucidate the mechanism of kidney fibrosis and could offer novel insights in developing therapeutic intervention.
Keywords: cell–cell communication, EVs, fibrogenic niche, kidney fibrosis, matricellular protein
INTRODUCTION
Kidney fibrosis, the final common sequela of a wide variety of chronic kidney diseases (CKD), is characterized by sustained fibroblast activation leading to excessive deposition of extracellular matrix (ECM). This results in scar formation, structural damage of renal parenchyma and kidney dysfunction [1]. Kidney fibrogenesis is also accompanied by tubular atrophy, tissue inflammation and microvascular rarefaction, suggesting complex and intimate interplays among fibroblasts, tubular epithelial cells, infiltrating inflammatory cells and vascular endothelial cells in CKD. Currently, there is no specific remedy that directly targets kidney fibrosis per se [2], largely because of an insufficient understanding of its underlying mechanisms.
Extensive studies demonstrate that virtually all kinds of kidney resident cells and infiltrating inflammatory cells participate in renal fibrogenesis after injury. As such, proper cell–cell communication is paramount and vital for coordinating complex cellular actions in this process. Notably, renal fibrotic lesions are unevenly distributed in kidney parenchyma, often with clean boundaries that separate them from the surrounding area with normal structure. These findings led us to propose the concept of the fibrogenic niche, a specialized tissue microenvironment that drives fibroblast activation and ECM production, leading to subsequent formation of the fibrotic foci [3]. The existence of such fibrotic foci suggests that there might be specialized mechanisms by which multiple cell–cell communications are spatially restrained to a particular locality.
Cell–cell communication in multicellular organisms is traditionally presumed to be mediated by direct cell contact or soluble factors, including growth factors, cytokines and chemokines. Accumulating evidence suggests that extracellular vesicles (EVs) also play a crucial role in mediating intercellular communication by transferring a diverse array of biomolecules including proteins, mRNA, miRNA and lipids between cells. Apart from the intercellular crosstalk mediated by direct contact, soluble factors and EVs, we recently described a new mode of intercellular communication via building a special extracellular niche by insoluble matricellular proteins [4–6]. This extracellular niche-mediated cell–cell communication is unique in that it confines the crosstalk between cells within a particular setting (Table 1). In this review, we concisely discuss the three modes of cell–cell communication mediated by soluble factors, EVs and insoluble matricellular proteins, respectively, in kidney fibrosis. Emphasis is placed on delineating the role and mechanism of the extracellular niche–mediated cell–cell communication in kidney fibrogenesis.
Table 1:
Modes and characteristic features of intercellular communication in kidney fibrosis.
| Modes of cell–cell communication | Mediators | Characteristic features | Routes | Relationship between sending and receiving cells | Mechanisms of action |
|---|---|---|---|---|---|
| Cell contact mediated | Specialized apparatus such as gap junction | Non-diffusional, only between neighboring cells | Physical contact | Neighboring cells: one-to-one | Biomolecules can directly pass from one cell to its neighboring cell without going through extracellular space |
| Soluble factors mediated | Growth factors, cytokines, chemokines | Diffusional, gradient dependent | Autocrine, paracrine, endocrine, juxtacrine | Ligand/receptor pair: one-to-one | Ligand secreted by sending cell binds to the specific receptor on the plasma membrane of receiving cell; this triggers a cascade of intracellular signaling, leading to the expression of target genes |
| EV mediated | EV-encapsulated proteins, miRNA, lncRNA mRNA, lipids | Diffusional, gradient dependent | Autocrine, paracrine, endocrine | One-to-many or many-to-many | EVs produced by sending cells can be taken up by receiving cells through direct fusion, endocytosis or receptor-mediated process; EVs elicit their actions by releasing the contents of the cargo |
| Extracellular niche mediated | Insoluble matricellular proteins | Non-diffusional, within spatially confined location | Autocrine, paracrine | One-to-many or many-to-many | Matricellular proteins secreted by producing cells are incorporated into the ECM network, orchestrating the formation of special niche; this regulates the activity and fate of receiving cells in the vicinity through binding to membrane receptors, recruiting and presenting soluble factors |
CELL–CELL COMMUNICATION MEDIATED BY SOLUBLE FACTORS
Cell–cell communication involves the transmission of a signal from a sending cell to a receiving cell. Adjacent cells can crosstalk with each other through direct cell contact via gap junctions, tight junctions and desmosomes. Besides this type of signal exchanges by cell contact, the vast majority of cell–cell communication is mediated by extracellular cues, which typically are soluble factors including cytokines, chemokines and growth factors or other molecules. These cues are often produced by a sending cell and released into the extracellular space [3]. There are four routes of intercellular communication by soluble factors, including autocrine, paracrine, endocrine and juxtacrine routes. The main distinction between the different categories of signaling routes is the distance that the cues travel through the tissues or organs to reach the target cells.
Soluble factors spread through the extracellular space easily by diffusion, thereby generating concentration gradients according to the distance from their cellular source. In order to respond to soluble factors, the receiving cells must have specific and dedicated receptors in the plasma membrane to transduce the signal intracellularly, thereby establishing the one-to-one relationship between producing and responding cells (Table 1).
After kidney injury, many kinds of cells, such as damaged tubular epithelial cells, can become secretory, releasing a diverse array of soluble factors, triggering the activation of fibroblasts and facilitating inflammatory responses in an autocrine or paracrine manner [7–10]. Among them, the chief one is transforming growth factor-β (TGF-β), a master profibrotic factor driving kidney fibrosis via activation of both canonical Smad and non-canonical signaling [8]. TGF-β is one the most potent cues activating fibroblasts to transform into myofibroblasts, the predominant matrix-producing effector cells in fibrotic tissue. TGF-β also impairs the integrity of tubular cells and endothelial cells by promoting them to undergo dedifferentiation and cell cycle arrest [8]. In addition, TGF-β also triggers cellular senescence and apoptosis of tubular epithelial cells, podocytes and endothelial cells [8, 11]. Other potent profibrotic factors include platelet-derived growth factor (PDGF) and fibroblast growth factor-2 (FGF-2) [12, 13]. In contrast, hepatocyte growth factor (HGF), produced mainly by interstitial fibroblasts, elicits a strong anti-fibrotic effect primarily by antagonizing TGF-β actions [14, 15].
There are several developmental signal pathways that are reactivated and play a critical role in kidney fibrogenesis through autocrine, paracrine and juxtacrine routes. In particular, Wnt/β-catenin signaling has been shown to be instrumental in driving fibroblast activation and kidney fibrosis. Genetic studies using conditional knockout approach show that blockade of Wnts secretion from kidney tubular cells, rather than interstitial fibroblasts, ameliorates kidney fibrosis, suggesting a predominant role of tubule-derived Wnts in fibroblast activation through paracrine mechanism [10]. Wnt9b promotes tubular cell injury by the autocrine route and further stimulates fibroblasts activation in an paracrine fashion [16]. Wnt9a accelerates tubular cell senescence through upregulating the expression of p16, p19, p53 and p21 [16]. Besides Wnt/β-catenin, overexpression of sonic hedgehog (Shh) in tubular epithelial cells also selectively promotes fibroblasts proliferation and leads to the excessive deposition of ECM, apparently by the paracrine mechanism [7, 17], whereas Notch signaling promotes tubular cell dedifferentiation and fibroblast activation via juxtacrine signaling [18].
Kidney fibrosis is often preceded by renal infiltration of inflammatory cells, in which injured tubular cells play a critical role by secreting a variety of pro-inflammatory cytokines, chemokines and components of the renin–angiotensin system (RAS). For example, tumor necrosis factor-α (TNF-α) from tubular cells can stimulate cytokines release and facilitate renal infiltration of inflammatory cells under pathological conditions [19]. Interleukin-1β (IL-1β) and IL-6 bind to their respective receptors to induce cytokines and activates different types of leukocytes [20, 21]. In addition, IL-18, similar to other inflammatory factors, is also activated by inflammasome complex and induces a diverse array of inflammatory factors [22]. Angiotensin II (Ang II), the principal bioactive component of RAS, can induce oxidative stress and stimulate inflammatory response through Toll-like receptors (TLRs) and upregulates the expression of inflammatory cytokines, such as TNF-α, IL-1β and monocyte chemotactic protein-1 (MCP-1) [23, 24]. Notably, CKD also causes lesions in other organs such as heart in cardiorenal syndrome via an endocrine mechanism [25]. Together, virtually all cell–cell interactions mediated by growth factors, cytokines and chemokines in CKD are carried out in autocrine, paracrine, endocrine and juxtacrine manners [26, 27].
EXTRACELLULAR VESICLES AS A VEHICLE TO MEDIATE INTERCELLULAR COMMUNICATION
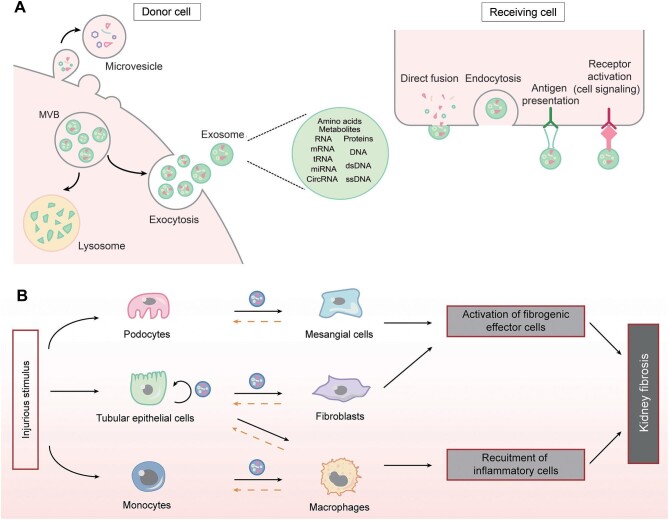
Apart from soluble factors, an increasing number of studies show that EVs play a fundamental role in mediating cell–cell communication in CKD (Fig. 1). Based on their size and biogenesis, EVs can be broadly classified into three main subgroups. EVs of endosomal origin, ranging in size from 30 to 150 nm in diameter, released into the extracellular space by fusion of multi-vesicular bodies with the plasma membrane, are termed exosomes. In contrast, microvesicles are released by budding of the plasma membrane and range in size from 50 nm to 1 µm in diameter (Fig. 1A). Apoptotic bodies are shed from the plasma membrane of cells undergoing apoptosis and range from 200 nm to 5 mm in diameter. EVs also include vesicles of mitochondrial origin (mitovesicles) [28, 29].
Figure 1:
The role of extracellular vesicles in mediating cell–cell communication. (A) Biosynthesis, release and uptake of EVs, and their travel routes between donor and receiving cells. (B) The routes of EVs in mediating cell–cell communication in kidney fibrosis. Solid lines indicate the principal direction of EVs from donor to receiving cells, while dashed lines denote the potential routes of EVs movement. MVB, multivesicular bodies.
Although EVs were initially thought to be a mechanism by which cells remove unwanted or degraded protein components during maturation, a growing number of studies in recent years have shown that EVs can facilitate the delivery of a multitude of endogenous substances from the original cell, including miRNAs, mRNA, tRNA, circRNA, DNA, proteins, lipids, amino acids and metabolites. EVs can affect the cell in the close proximity or distant cells without the need for direct cell contact [28–30]. In addition, all of the above bioactive cargoes from the parent cell are enriched in EVs, and these different categories of EVs are determined by variances in cellular sources, physiological or pathological states, and extracellular microenvironments [29]. Most importantly, in contrast to secreted soluble factors, which require the target cell to possess their corresponding receptor, EVs do not require the recipient cell to have a corresponding receptor for intercellular signaling, as EVs can deliver both ligands and receptors to the target cell, a property that makes EVs-mediated signaling more efficient and robust. Recent data suggest that most EVs can traverse many biological barriers to complete the transfer of substances and messages, including the endothelial basement membrane of the glomeruli, supporting the idea that EVs act as integral messengers for intercellular communication.
Almost all kidney cells can secrete EVs, including renal tubular epithelial cells, podocytes and fibroblasts. The most common sources of EVs are tubular epithelial cells, glomerular podocytes and monocytes/macrophages (Fig. 1B), respectively. Using fluorescently labeled exosomes and time-lapse video, it was found that EVs released from proximal tubular epithelial cells can be taken up by downstream distal tubular epithelial cells and collecting duct cells, revealing a new mode of long-distance intercellular communication between different regions of the kidney. Further analysis showed that miR-21-encapsulated EVs released from proximal tubular epithelial cells promoted Akt signaling in downstream distal tubular epithelial cells and collecting duct cells, facilitating phenotypic transformation [31].
It has also been shown that exosomes secreted by damaged tubular epithelial cells can act on interstitial fibroblasts by carrying TGF-β1 mRNA, Shh ligand, osteopontin (OPN) and TNF-α-induced protein 8 (TNFAIP8) to induce their proliferation, activation and ECM production and prevent against apoptosis [31–34]. Furthermore, damaged tubular cells promote the release of miR-23a, MCP-1 mRNA-rich EVs, which target macrophages, promote their activation and amplify the inflammatory cascade of responses, thereby exacerbating tubulointerstitial inflammation [29, 35]. In addition, EVs released from damaged podocytes can also influence the course of renal fibrosis by transporting miRNAs, p38 MAPK and CD36, inducing apoptosis or directly provoking a fibrotic response in proximal tubular epithelial cells [29].
BUILDING A NICHE: NOVEL MECHANISM OF CELL–CELL COMMUNICATION
Kidney fibrotic lesions are not homogeneous and usually initiate at certain sites with clear boundaries. This suggests a determining role of local tissue microenvironment, which contains an assortment of soluble factors, EVs and insoluble ECM proteins including matricellular proteins, in kidney fibrogenesis [4, 11, 36]. We recently proposed the existence of the fibrogenic niche [3], a term coined for describing the specialized extracellular microenvironment that spontaneously promotes fibroblasts proliferation. During the evolution of kidney fibrosis, ECM proteins in the fibrogenic niche undergo qualitative and quantitative changes [37], dynamically regulate different signaling in various kidney resident cells, and alter their phenotypes and fates. Because ECM and its associated proteins are stationary in nature, their actions are restricted to a particular location.
Extracellular matrix scaffold as a platform of cell–cell communication
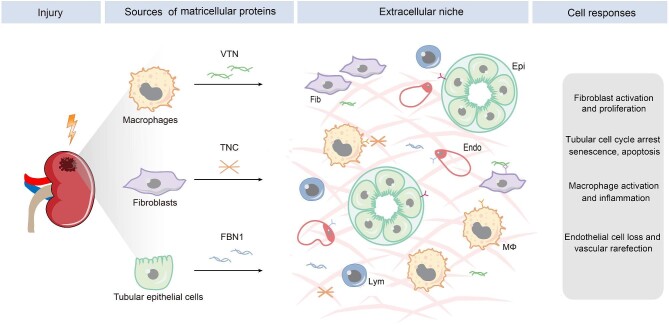
The fibrogenic niche is composed of several structural elements, including cellular components, soluble factors and EVs, and ECM scaffold. Among them, the ECM components are particularly important, as they are physically stationary and define a spatially restricted setting. After kidney injury, different kinds of renal cells such as fibroblasts, tubular epithelial cells and infiltrated macrophages are stimulated to produce ECM and its associated proteins (Fig. 2). They contribute to the formation of the ECM scaffold in the fibrogenic niche. One can envision that the ECM produced by a particular cell will influence the cellular activities of other kinds of cells in the vicinity, including cell proliferation, differentiation, migration, senescence and apoptosis, thereby ultimately creating a circuit of cell–ECM–cell communication (Fig. 2). In this regard, the ECM scaffold acts as a signal exchange hub and a platform of cell–cell communication.
Figure 2:
The sources and effector cells of matricellular proteins in kidney fibrosis. Diverse types of cells contribute to the formation of the fibrogenic niche by secreting distinct matricellular proteins such as TNC, FBN1 and VTN. Different cells in the fibrogenic niche are primed to respond in distinct manners, leading to fibroblast activation and proliferation, tubular cell cycle arrest, senescence and apoptosis, macrophage activation, and endothelial depletion. Fib, fibroblasts; Epi, epithelial cells; endo, endothelial cells; Mϕ, macrophage, lym, lymphocytes.
Unlike soluble factors, the actions of ECM proteins in mediating the interaction between cells are through binding to a variety of other growth factors and cell-surface receptors such as integrins, Toll-like receptors (TLRs) and low-density lipoprotein receptor–related protein 6 (LRP6), forming one-to-many and many-to-many relationships to profoundly affect cellular behaviors. The ECM proteins can roughly be divided into four major categories: structural ECM proteins, matricellular proteins, proteoglycans and matrix-modifying proteins [3]. Of them, matricellular proteins are particularly appealing, as this group of structurally unrelated, ECM-associated glycoproteins are often incorporated into ECM network. They are not required for maintaining the structural integrity of ECM scaffold but possess imperative regulatory functions via mediating intercellular communication. In contrast to soluble factors and EVs whose actions are dependent on their concentration gradients, the mode of the cell–ECM–cell communication mediated by ECM components is quite unique in that it is spatially confined to a particular site.
Matricellular proteins as a mediator of cell–cell communication
Matricellular proteins are a group of heterogenous, non-structural ECM glycoproteins. They usually possess numerous structural motifs and facilitate ECM–cell interaction through binding to cell-surface receptors and other growth factors. The expression of matricellular proteins is low, if any, in adult tissues under normal physiological conditions, but markedly upregulated in different cells after tissue injury. Matricellular proteins often have a high turnover rate, exhibit regulatory roles and incorporate into ECM network. As such, they serve as an indispensable component of the fibrogenic niche and act as a mediator of cell–cell communication. After kidney injury, a diverse array of matricellular proteins is induced in different cells, secreted to extracellular space in a spatially restricted manner. They then target the responding cells and mediate a broad range of cellular behaviors (Fig. 2).
Tenascin C (TNC) is a large ECM glycoprotein produced by activated fibroblasts. It contains multiple functional domains, including N-terminal assembly domain, epidermal growth factor (EGF)-like repeats, fibronectin type-III like repeats and a C-terminal fibrinogen-like globe domain. TNC is secreted by fibroblasts, but it can influence the activities of other types of cells nearby, primarily by interacting with specific cell-surface receptors, such as integrins, EGF receptor (EGFR) and TLR4. Recent studies show that TNC is an integral constituent of the fibrogenic niche and promotes renal fibroblasts proliferation and impairs tubular integrity through integrin/FAK/ERK signaling [4, 5], or signal transducer and activator of transcription 3 (STAT3) [38]. TNC also induces tubular cells and fibroblasts to secrete profibrotic factors, such as TGF-β, Shh and Wnt ligands, which in turn reinduce fibroblasts to secrete more TNC, forming a vicious circle during kidney fibrogenesis. In view of the fact that TNC is induced rapidly and focally after kidney injury, it has been proposed that it functions as an organizer of the fibrogenic niche [4], which coordinates a multitude of cell–cell communications in kidney fibrosis.
Fibrillin-1 (FBN1) is another large, cysteine-rich, calcium binding, ECM glycoprotein in elastic and non-elastic connective tissue. Mutations in FBN1 gene can cause a wide variety of genetic diseases such as Marfan syndrome, a disorder characterized by ocular, skeletal and cardiovascular abnormalities [39]. Structurally, FBN1 consists of 47 EGF-like motifs and numerous domains homologous with TGF-β binding protein and a proline-rich region. FBN1 is normally produced by fibroblasts, but it is markedly upregulated in renal tubular epithelial cells in CKD [11], suggesting that kidney injury causes its ectopic expression. Tubule-derived FBN1 is released to peritubular extracellular space and incorporated into ECM network, rendering it to orchestrate a hostile microenvironment for endothelial cell survival and proliferation [11]. Indeed, FBN1 can directly provoke endothelial cell apoptosis via integrin αvβ6/TGF-β1/Smad3 signaling pathway [11].
Vitronectin (VTN) is a multifunctional glycoprotein present in blood circulation and in the ECM scaffold. It is a major component of the fibrogenic niche in the fibrotic kidney [6]. VTN plays an important role in various biological processes, such as cell adhesion, migration and invasion, proliferation and tissue remodeling. Recent studies indicate that VTN is induced specifically in macrophages and targets interstitial fibroblasts in CKD [6]. Genetic knockout of VTN gene inhibits fibroblast activation and ameliorates kidney fibrotic lesions, while overexpression of VTN aggravates kidney lesions. Furthermore, VTN is upregulated in models of sclerosis and fibrosis in various disorders, including liver and lung fibrosis, degenerative skin disease or degenerative central nervous system disease [6, 40]. These findings suggest that VTN mediates the crosstalk between infiltrated macrophages and interstitial fibroblasts through building a VTN-enriched tissue microenvironment in tissue fibrosis.
Connective tissue growth factor (CTGF), also known as cellular communication network factor 2 (CCN2), is a well-studied profibrotic matricellular protein [12]. It drives kidney fibrosis by a multitude of ways such as impairing tubular cell integrity, promoting fibroblast activation and proliferation, and inducing the production and secretion of ECM components [41]. In addition, CTGF is also induced by many extracellular cues, including TGF-β, Ang II, high glucose and cellular stress [42]. The induction of CTGF and its secretion to extracellular space facilitate the formation of the fibrogenic microenvironment.
Several other ECM-associated, matricellular proteins play a pivotal role in kidney fibrogenesis. For example, periostin (POSTN), an ECM protein involved in tissue remodeling, also has multiple domains to bind to profibrotic factors and integrins. POSTN is predominantly produced and secreted into the injured sites, promoting tubular cell injury and renal inflammation in CKD [43]. As a matricellular glycoprotein, secreted protein acidic and rich in cysteine (SPARC) interacts with several profibrotic factors, with its expression being induced by TGF-β1 and Ang II. SPARC mediates a battery of cellular activities, including cell adhesion, proliferation and ECM production [44]. Similarly, thrombospondin 1 (TSP1) is another matricellular protein that exhibits multiple roles in the development of CKD. With the different structural domains that bind to various cell-surface receptors and growth factors, TSP1 regulates an assortment of cellular behaviors and pathological processes, such as activation of TGF-β, cell adhesion and migration, inflammation, capillary density reduction and ECM remodeling [45, 46]. TSP1 is induced in tubular epithelial cells and podocytes by high level of glucose and Ang II, and then activates TGF-β signaling, resulting in a worsened prognosis of CKD [45].
As an insoluble mediator of cell–cell communication, matricellular proteins, as a whole, modulate cell function by participating and orchestrating a specialized ECM niche, in which they interact with different growth factors, cell surface receptors, cytokines and other molecules. This extracellular niche–mediated intercellular communication possesses several unique characteristic features, including that: (i) it occurs in a spatially confined setting, because the mediators are insoluble and non-diffusional; (ii) it renders the signal-sending cell to communicate with multiple cellular partners at the same time in the same location, as long as these cells are in the close vicinity; (iii) it works through matricellular protein binding to many cell-surface receptors, growth factors and other molecules in one-to-many and many-to-many relationship manners, resulting in diverse outcomes; and (iv) the sequel of such a mode of cell–cell communication is context-dependent, because it depends on which binding partners come into contact in that particular local microenvironment.
Mechanism of matricellular proteins–mediated cell–cell communication
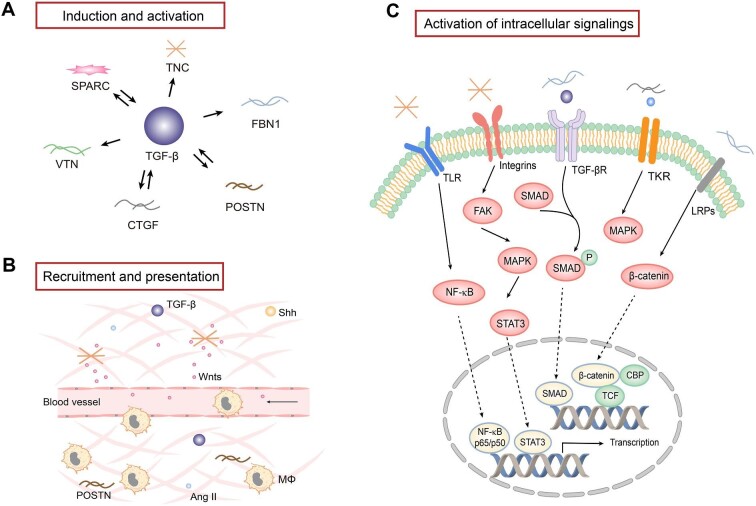
While soluble factors mediate intercellular crosstalk via a one-to-one paired ligand/receptor signaling, the routes by which matricellular proteins facilitate cell–cell communication are complex and context-dependent. Matricellular proteins are composed of various structural domains, which mediate the interaction with a diverse array of growth factors, such as TGF-β, PDGF and Wnt ligands. In addition, matricellular proteins bind to different cell surface receptors, such as integrins, TLRs, LRPs and growth factor receptors. Through these interactions within a confined space, matricellular proteins regulate a wide range of intracellular signaling and modulate a host of cellular processes, such as cell survival, adhesion, proliferation, migration and differentiation (Fig. 3).
Figure 3:
The regulation, role and mechanism of matricellular proteins in the fibrogenic niche. (A) TGF-β plays a central role in regulating the expression of a variety of matricellular proteins, while some matricellular proteins are also instrumental in TGF-β expression and activation. (B) Matricellular proteins play a key role in recruiting and presenting various soluble factors through binding to ligands and receptors. Some matricellular proteins also play a role in recruiting the infiltration of inflammatory cells such as macrophages. (C) Matricellular proteins are capable of binding to various plasma membrane receptors and activate intracellular signaling. Major signal pathways are highlighted. Mϕ, macrophage; TKR, tyrosine kinase receptor; NF-κB, nuclear factor-κB; FAK, focal adhesion kinase; MAKP, mitogen-activated protein kinase; CBP, cAMP response element-binding protein (CREB)-binding protein; TCF, T cell factor.
Induction, activation and presentation of growth factors
Matricellular proteins are instrumental in regulating the expression, activation and presentation of growth factors and cytokines. TGF-β1 is a central regulator in renal fibrosis and inflammation in CKD. TGF-β induces many matricellular proteins such as CTGF, TNC, FBN1, VTN, POSTN and SPARC after kidney injury, and CTGF, POSTN and SPARC can reciprocally upregulate TGF-β expression, forming a positive feedback loop (Fig. 3A). CTGF acts with TGF-β in a cooperative manner to promote fibrosis and binds TGF-β receptors to promote mesangial cell proliferation and ECM production [42]. Similar to CTGF, POSTN also induces TGF-β synthesis and regulate TGF-β signaling, which in turn amplifies POSTN production and secretion [43]. In addition to TGF-β, SPARC is upregulated in response to the stimulation of Ang II, serving a dual role in mediating matrix remodeling [47]. SPARC also interacts with PDGF or vascular endothelial growth factor (VEGF), inhibiting their binding to corresponding receptors and affecting PDGF or VEGF signaling [48].
In addition to modulating growth factors expression, matricellular proteins also play a critical role in the posttranslational activation of certain growth factors. For example, FBN1 has been shown to promote TGF-β1 posttranslational activation in endothelial cells via integrin αvβ6 signaling, although it does not affect TGF-β1 protein abundance. This leads to endothelial cell apoptosis and vascular rarefaction in CKD [11]. Some matricellular proteins such as TNC can bind Wnt ligand and its membrane coreceptor LRP6 simultaneously, thereby presenting the ligand to its receptor and facilitating its signal transduction.
Building a fibrogenic niche by recruiting soluble factors and inflammatory cells
With the ability to interact with soluble factors, matricellular proteins are able to bind to and recruit soluble factors from the surrounding milieu, creating a special microenvironment in which profibrotic factors are enriched and concentrated (Fig. 3B). For example, we have previously shown that TNC-enriched decellularized kidney tissue scaffold (KTS) can recruit Wnt ligands from surrounding media, generating an ECM niche with high level of Wnt proteins, which facilitates tubular cell repair and regeneration after acute kidney injury [49]. In addition, POSTN has been demonstrated to recruit macrophages through binding to integrin αvβ3 and promotes macrophages proliferation and polarization [50]. POSTN-overexpressing mice had more F4/80+ macrophages after acute kidney injury, which promotes injury repair and kidney regeneration after ischemia–reperfusion injury [51].
Activation of intracellular signaling via membrane receptors
Matricellular proteins can activate intracellular signaling through directly binding to cell surface receptors, such as integrins. Integrins are αβ heterodimers, consisting of 18 α subunits and 8 β subunits, and commonly recognized by TNC, FBN1, VTN, CTGF, POSTN and SPARC [52]. The specificity and selectivity of integrins to be involved are largely dependent on many factors, such as different matricellular proteins, target cell types, cellular context and pathophysiological setting. For example, TNC initiates tubular cell dedifferentiation via integrin αvβ6/FAK/ERK1/2 pathway [5]. VTN promotes fibroblast activation and proliferation through integrin αvβ5/Src tyrosine kinase signaling [6], whereas FBN1 activates integrin αvβ6/TGF-β1/Smad3 to regulate endothelial cell apoptosis [11]. CTGF promotes collagen deposition via integrin α6β1 and regulates cell adhesion and migration via integrin α5β1 or integrin αvβ3 [53]. POSTN binds to integrin αv to activate tissue repair pathways [54], while SPARC may activate TGF-β signaling through binding to integrin β1 [44].
Other membrane receptors are also involved in transducing the signaling triggered by matricellular proteins (Fig. 3C). LRPs, including LRP1 and LRP6, are an essential receptor for CTGF. CTGF competitively binds to LRP with other LRP ligands. CTGF can induce phosphorylation of both LRP1 and LRP6 to activate ERK1/2 signaling and β-catenin, respectively [42]. With multiple functions in inflammation and fibrosis, TLR4 is also a receptor for TNC. TNC binding to TLR4 can promote collagen synthesis and mediate M1/M2 macrophage polarization, serving as a dual role in mediating tissue fibrosis [3]. In addition, EGFR activation is significantly associated with CTGF overexpression, suggesting a possible role of EGFR in mediating CTGF-induced ECM production in fibroblasts [55]. Furthermore, studies suggest that matricellular proteins can also bind to CD36, CD47 and CD44 [45, 56], through which extracellular signals trigger a cascade of intracellular actions, leading to various cellular responses.
EXTRACELLULAR NICHE–MEDIATED INTERCELLULAR COMMUNICATION: FUTURE IMPLICATIONS
While the cell–cell communication mediated by soluble factors and EVs has been well studied, the new mode of extracellular niche–mediated intercellular signal exchange is just beginning to be recognized. This mode of cellular crosstalk possesses several characteristic features (Table 1) and enables cell–cell communication to take place in a spatially confined setting. Such an intercellular communication mediated by insoluble matricellular proteins will reshape our thinking about the pathophysiology of kidney fibrosis and have wide implications in elucidating the mechanisms and developing new remedies for fibrotic CKD.
At this stage, the relative contribution and importance of this new mode of cell–cell communication mediated by matricellular proteins, compared with other types of intercellular communication, in driving kidney fibrosis remains largely unknown. However, in view of the fact that fibrotic foci are not evenly distributed across kidney parenchyma, this implies a possibly vital importance of such a spatially confined cell–cell communication in the pathogenesis of CKD. Furthermore, matricellular proteins can recruit soluble factors and EVs from surrounding milieu, thereby creating a local microenvironment with high concentrations of diffusional mediators. In this regard, insoluble matricellular proteins could bring the soluble factors and EVs together, in which three modes of intercellular communication work in concert.
There are many questions waiting to be addressed for better understanding the importance of the extracellular niche–mediated cell–cell communication in kidney fibrosis. For example, while the proteomic landscape of the ECM in the fibrotic kidney has recently been revealed [37], our knowledge on how kidney cells in the fibrogeneic niche behave differently is limited. Furthermore, the spatial gene expression of kidney cells should be deciphered at single cell level within 3D context [57–59]. It is also unexplored to what extent different etiologies of CKD affect the expression and dynamic of the matricellular proteins. Finally, it remains a challenge how to disrupt the extracellular niche–mediated intercellular communication for developing an effective strategy for therapeutic intervention of fibrotic CKD.
CONCLUSION
Kidney fibrosis involves the coordinated action of a wide variety of renal resident and infiltrated cells, which relies on relevant cell–cell communication. Traditionally, intercellular communication is thought to be mediated primarily by direct cell contact, soluble factors or EVs. However, a growing body of evidence perceives a novel mode of cell–cell communication, which is mediated by insoluble matricellular proteins through contributing to the formation of a unique extracellular microenvironment. This extracellular niche–mediated intercellular communication is unique in that it occurs in a spatially restricted setting because the mediators are non-diffusional. These findings represent a paradigm shift in our understanding of the modes and mechanism of cell–cell communication and have profound implications in comprehending the complexity and spatial biology of CKD.
Contributor Information
Meizhi He, State Key Laboratory of Organ Failure Research, Division of Nephrology, Nanfang Hospital, Southern Medical University; National Clinical Research Center of Kidney Disease, Guangdong Provincial Institute of Nephrology, Guangzhou, China.
Zhao Liu, State Key Laboratory of Organ Failure Research, Division of Nephrology, Nanfang Hospital, Southern Medical University; National Clinical Research Center of Kidney Disease, Guangdong Provincial Institute of Nephrology, Guangzhou, China.
Li Li, State Key Laboratory of Organ Failure Research, Division of Nephrology, Nanfang Hospital, Southern Medical University; National Clinical Research Center of Kidney Disease, Guangdong Provincial Institute of Nephrology, Guangzhou, China.
Youhua Liu, State Key Laboratory of Organ Failure Research, Division of Nephrology, Nanfang Hospital, Southern Medical University; National Clinical Research Center of Kidney Disease, Guangdong Provincial Institute of Nephrology, Guangzhou, China.
FUNDING
This work was supported by the National Key R&D Program of China grant (2022YFC2502504), National Natural Science Foundation of China (NSFC) grants (82230020 and 81920108007), Key Technologies R&D Program of Guangdong Province (2023B1111030004), and funds from the Guangdong Provincial Key Laboratory of Renal Failure Research and Guangdong Provincial Clinical Research Center for Kidney Disease.
DATA AVAILABILITY STATEMENT
No new data were generated or analyzed in support of this research.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1. Webster AC, Nagler EV, Morton RL et al. Chronic kidney disease. Lancet 2017;389:1238–52. 10.1016/S0140-6736(16)32064-5 [DOI] [PubMed] [Google Scholar]
- 2. Himmelfarb J, Vanholder R, Mehrotra R et al. The current and future landscape of dialysis. Nat Rev Nephrol 2020;16:573–85. 10.1038/s41581-020-0315-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li L, Fu H, Liu Y. The fibrogenic niche in kidney fibrosis: components and mechanisms. Nat Rev Nephrol 2022;18:545–57. 10.1038/s41581-022-00590-z [DOI] [PubMed] [Google Scholar]
- 4. Fu H, Tian Y, Zhou L et al. Tenascin-C is a major component of the fibrogenic niche in kidney fibrosis. J Am Soc Nephrol 2017;28:785–801. 10.1681/ASN.2016020165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu H, Liao J, Zhou X et al. Tenascin-C promotes acute kidney injury to chronic kidney disease progression by impairing tubular integrity via αvβ6 integrin signaling. Kidney Int 2020;97:1017–31. 10.1016/j.kint.2020.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peng Y, Li L, Shang J et al. Macrophage promotes fibroblast activation and kidney fibrosis by assembling a vitronectin-enriched microenvironment. Theranostics 2023;13:3897–913. 10.7150/thno.85250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou D, Li Y, Zhou L et al. Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. J Am Soc Nephrol 2014;25:2187–200. 10.1681/ASN.2013080893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol 2016;12:325–38. 10.1038/nrneph.2016.48 [DOI] [PubMed] [Google Scholar]
- 9. Wu CF, Chiang WC, Lai CF et al. Transforming growth factor β-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am J Pathol 2013;182:118–31. 10.1016/j.ajpath.2012.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou D, Fu H, Zhang L et al. Tubule-derived Wnts are required for fibroblast activation and kidney fibrosis. J Am Soc Nephrol 2017;28:2322–36. 10.1681/ASN.2016080902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li L, Liao J, Yuan Q et al. Fibrillin-1-enriched microenvironment drives endothelial injury and vascular rarefaction in chronic kidney disease. Sci Adv 2021;7:eabc7170. 10.1126/sciadv.abc7170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kok HM, Falke LL, Goldschmeding R et al. Targeting CTGF, EGF and PDGF pathways to prevent progression of kidney disease. Nat Rev Nephrol 2014;10:700–11. 10.1038/nrneph.2014.184 [DOI] [PubMed] [Google Scholar]
- 13. Livingston MJ, Shu S, Fan Y et al. Tubular cells produce FGF2 via autophagy after acute kidney injury leading to fibroblast activation and renal fibrosis. Autophagy 2023;19:256–77. 10.1080/15548627.2022.2072054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vargas GA, Hoeflich A, Jehle PM. Hepatocyte growth factor in renal failure: promise and reality. Kidney Int 2000;57:1426–36. 10.1046/j.1523-1755.2000.00987.x [DOI] [PubMed] [Google Scholar]
- 15. Liu Y. Hepatocyte growth factor in kidney fibrosis: therapeutic potential and mechanisms of action. Am J Physiol Renal Physiol 2004;287:F7–16. 10.1152/ajprenal.00451.2003 [DOI] [PubMed] [Google Scholar]
- 16. Schunk SJ, Floege J, Fliser D et al. WNT-β-catenin signalling - a versatile player in kidney injury and repair. Nat Rev Nephrol 2021;17:172–84. 10.1038/s41581-020-00343-w [DOI] [PubMed] [Google Scholar]
- 17. Zhou D, Tan RJ, Liu Y. Sonic hedgehog signaling in kidney fibrosis: a master communicator. Sci China Life Sci 2016;59:920–9. 10.1007/s11427-016-0020-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Edeling M, Ragi G, Huang S et al. Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nat Rev Nephrol 2016;12:426–39. 10.1038/nrneph.2016.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Al-Lamki RS, Mayadas TN. TNF receptors: signaling pathways and contribution to renal dysfunction. Kidney Int 2015;87:281–96. 10.1038/ki.2014.285 [DOI] [PubMed] [Google Scholar]
- 20. Anders HJ. Of inflammasomes and alarmins: IL-1β and IL-1α in kidney disease. J Am Soc Nephrol 2016;27:2564–75. 10.1681/ASN.2016020177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang J, Xiong M, Fan Y et al. Mecp2 protects kidney from ischemia-reperfusion injury through transcriptional repressing IL-6/STAT3 signaling. Theranostics 2022;12:3896–910. 10.7150/thno.72515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thomas JM, Huuskes BM, Sobey CG et al. The IL-18/IL-18R1 signalling axis: diagnostic and therapeutic potential in hypertension and chronic kidney disease. Pharmacol Ther 2022;239:108191. 10.1016/j.pharmthera.2022.108191 [DOI] [PubMed] [Google Scholar]
- 23. Majumder S, Pushpakumar S, Juin SK et al. Toll-like receptor 4 mutation protects the kidney from Ang-II-induced hypertensive injury. Pharmacol Res 2022;175:106030. 10.1016/j.phrs.2021.106030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simões e Silva AC, Silveira KD, Ferreira AJ et al. ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. Br J Pharmacol 2013;169:477–92. 10.1111/bph.12159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao Y, Wang C, Hong X et al. Wnt/beta-catenin signaling mediates both heart and kidney injury in type 2 cardiorenal syndrome. Kidney Int 2019;95:815–29. 10.1016/j.kint.2018.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tan RJ, Zhou D, Liu Y. Signaling crosstalk between tubular epithelial cells and interstitial fibroblasts after kidney injury. Kidney Dis (Basel) 2016;2:136–44. 10.1159/000446336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu BC, Tang TT, Lv LL et al. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int 2018;93:568–79. 10.1016/j.kint.2017.09.033 [DOI] [PubMed] [Google Scholar]
- 28. Erdbrügger U, Blijdorp CJ, Bijnsdorp IV et al. Urinary extracellular vesicles: a position paper by the Urine Task Force of the International Society for Extracellular Vesicles. J Extracell Vesicles 2021;10:e12093. 10.1002/jev2.12093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grange C, Bussolati B. Extracellular vesicles in kidney disease. Nat Rev Nephrol 2022;18:499–513. 10.1038/s41581-022-00586-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y, Kim MS, Jia B et al. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 2017;548:52–7. 10.1038/nature23282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gildea JJ, Seaton JE, Victor KG et al. Exosomal transfer from human renal proximal tubule cells to distal tubule and collecting duct cells. Clin Biochem 2014;47:89–94. 10.1016/j.clinbiochem.2014-06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen S, Zhang M, Li J et al. β-catenin-controlled tubular cell-derived exosomes play a key role in fibroblast activation via the OPN-CD44 axis. J Extracell Vesicles 2022;11:e12203. 10.1002/jev2.12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu X, Miao J, Wang C et al. Tubule-derived exosomes play a central role in fibroblast activation and kidney fibrosis. Kidney Int 2020;97:1181–95. 10.1016/j.kint.2019.11.026 [DOI] [PubMed] [Google Scholar]
- 34. Liu X, Liu Z, Wang C et al. Kidney tubular epithelial cells control interstitial fibroblast fate by releasing TNFAIP8-encapsulated exosomes. Cell Death Dis 2023;14:672. 10.1038/s41419-023-06209-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lv LL, Feng Y, Wen Y et al. Exosomal CCL2 from tubular epithelial cells is critical for albumin-induced tubulointerstitial inflammation. J Am Soc Nephrol 2018;29:919–35. 10.1681/ASN.2017050523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li L, Lu M, Peng Y et al. Oxidatively stressed extracellular microenvironment drives fibroblast activation and kidney fibrosis. Redox Biol 2023;67:102868. 10.1016/j.redox.2023.102868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li L, He M, Tang X et al. Proteomic landscape of the extracellular matrix in the fibrotic kidney. Kidney Int 2023;103:1063–76. 10.1016/j.kint.2023.01.021 [DOI] [PubMed] [Google Scholar]
- 38. Xie Q, Zhang M, Mao X et al. Matrix protein Tenascin-C promotes kidney fibrosis via STAT3 activation in response to tubular injury. Cell Death Dis 2022;13:1044. 10.1038/s41419-022-05496-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zeigler SM, Sloan B, Jones JA. Pathophysiology and pathogenesis of Marfan syndrome. Adv Exp Med Biol 2021;1348:185–206. 10.1007/978-3-030-80614-9_8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shin K, Kent JE, Singh C et al. Calcium and hydroxyapatite binding site of human vitronectin provides insights to abnormal deposit formation. Proc Natl Acad Sci USA 2020;117:18504–10. 10.1073/pnas.2007699117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rayego-Mateos S, Morgado-Pascual JL, Lavoz C et al. CCN2 binds to tubular epithelial cells in the kidney. Biomolecules 2022;12:252. 10.3390/biom12020252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yin Q, Liu H. Connective tissue growth factor and renal fibrosis. Adv Exp Med Biol 2019;1165:365–80. 10.1007/978-981-13-8871-2_17 [DOI] [PubMed] [Google Scholar]
- 43. Mael-Ainin M, Abed A, Conway SJ et al. Inhibition of periostin expression protects against the development of renal inflammation and fibrosis. J Am Soc Nephrol 2014;25:1724–36. 10.1681/ASN.2013060664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Toba H, Ikemoto MJ, Kobara M et al. Secreted protein acidic and rich in cysteine (SPARC) and a disintegrin and metalloproteinase with thrombospondin type 1 motif (ADAMTS1) increments by the renin-angiotensin system induce renal fibrosis in deoxycorticosterone acetate-salt hypertensive rats. Eur J Pharmacol 2022;914:174681. 10.1016/j.ejphar.2021.174681 [DOI] [PubMed] [Google Scholar]
- 45. Murphy-Ullrich JE. Thrombospondin 1 and its diverse roles as a regulator of extracellular matrix in fibrotic disease. J Histochem Cytochem 2019;67:683–99. 10.1369/0022155419851103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yao M, Rogers NM, Csányi G et al. Thrombospondin-1 activation of signal-regulatory protein-α stimulates reactive oxygen species production and promotes renal ischemia reperfusion injury. J Am Soc Nephrol 2014;25:1171–86. 10.1681/ASN.2013040433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Socha MJ, Manhiani M, Said N et al. Secreted protein acidic and rich in cysteine deficiency ameliorates renal inflammation and fibrosis in angiotensin hypertension. Am J Pathol 2007;171:1104–12. 10.2353/ajpath.2007.061273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol 2001;19:816–27. 10.1016/S0945-053X(00)00133-5 [DOI] [PubMed] [Google Scholar]
- 49. Chen S, Fu H, Wu S et al. Tenascin-C protects against acute kidney injury by recruiting wnt ligands. Kidney Int 2019;95:62–74. 10.1016/j.kint.2018.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhou W, Ke SQ, Huang Z et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol 2015;17:170–82. 10.1038/ncb3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kormann R, Kavvadas P, Placier S et al. Periostin promotes cell proliferation and macrophage polarization to drive repair after AKI. J Am Soc Nephrol 2020;31:85–100. 10.1681/ASN.2019020113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takada Y, Ye X, Simon S. The integrins. Genome Biol 2007;8:215. 10.1186/gb-2007-8-5-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen Z, Zhang N, Chu HY et al. Connective tissue growth factor: from molecular understandings to drug discovery. Front Cell Dev Biol 2020;8:593269. 10.3389/fcell.2020.593269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Raman A, Parnell SC, Zhang Y et al. Periostin overexpression in collecting ducts accelerates renal cyst growth and fibrosis in polycystic kidney disease. Am J Physiol Renal Physiol 2018;315:F1695–707. 10.1152/ajprenal.00246.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rayego-Mateos S, Morgado-Pascual JL, Rodrigues-Diez RR et al. Connective tissue growth factor induces renal fibrosis via epidermal growth factor receptor activation. J Pathol 2018;244:227–41. 10.1002/path.5007 [DOI] [PubMed] [Google Scholar]
- 56. Vianello E, Kalousová M, Dozio E et al. Osteopontin: the molecular bridge between fat and cardiac-renal disorders. Int J Mol Sci 2020;21:5568. 10.3390/ijms21155568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sun Y, Wu L, Zhong Y et al. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell 2021;184:404–21.16. 10.1016/j.cell.2020.11.041 [DOI] [PubMed] [Google Scholar]
- 58. Wilson PC, Humphreys BD. Single-cell genomics and gene editing: implications for nephrology. Nat Rev Nephrol 2019;15:63–4. 10.1038/s41581-018-0094-3 [DOI] [PubMed] [Google Scholar]
- 59. Kuppe C, Ibrahim MM, Kranz J et al. Decoding myofibroblast origins in human kidney fibrosis. Nature 2021;589:281–6. 10.1038/s41586-020-2941-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.