Abstract
Introduction
Non-cigarette tobacco (NCT) represents a form of tobacco use with a misperceived significance in chronic disease events. Whether NCT use is sufficient to promote stroke events, especially among Africans, is yet to be understood. This study assessed the relationship between NCT use and stroke among indigenous Africans.
Methods
A total of 7617 respondents (NCT users: 41 vs. non-NCT: 7576) from the Stroke Investigative Research and Educational Network (SIREN) study were included in the current analysis. NCT use was defined as self-reported use of smoked (cigars or piper) or smokeless (snuff or chewed) tobacco in the past year preceding stroke events. Stroke was defined based on clinical presentation and confirmed with a cranial computed tomography/magnetic resonance imaging. Multivariable-adjusted logistic regression was applied to estimate the odds ratio (OR) and 95% confidence interval (CI) for the relationship of NCT with stroke at a two-sided p < .05.
Results
Out of the 41 (0.54%) who reported NCT use, 27 (65.9%) reported using smokeless NCT. NCT users were older than non-NCT users (62.8 ± 15.7 vs. 57.7 ± 14.8 years). Overall, NCT use was associated with first-ever stroke (OR: 2.08; 95% CI: 1.02, 4.23) in the entire sample. Notably, smokeless NCT use was independently associated with higher odds of stroke (OR: 2.74; 95% CI: 1.15, 6.54), but smoked NCT use (OR: 0.16; 95% CI: 0.02, 1.63) presented a statistically insignificant association after adjusting for hypertension and other covariates.
Conclusions
NCT use was associated with higher odds of stroke, and public health interventions targeting NCT use might be promising in reducing the burden of stroke among indigenous Africans.
Implications
A detailed understanding of the relationship between NCT use and stroke will likely inform well-articulated policy guidance and evidence-based recommendations for public health prevention and management of stroke on the African continent.
Introduction
Cigarette smoking is the most common form of tobacco used worldwide, and it remains the leading single preventable cause of chronic disease, including cancer, cardiovascular diseases, and respiratory diseases,1 with tobacco-related mortality occurring at least every 6 seconds.2 Of these deaths, 75% occur in low- and middle-income countries, where more than 80% of the world’s smokers live,3 and the pooled crude incidence of stroke is approximately 106 persons per 100, 000 population in Africa.4 The epidemic of smoking is being addressed by the World Health Organization through the Framework Convention on Tobacco Control—FCTC,5 which is an intervention strategy to control tobacco production and its uses. This initiative was primarily driven by an emphasis on smoked cigarette tobacco,6 with less attention to non-cigarette tobacco (NCT) products, particularly those commonly used in sub-Saharan Africa, such as cigars, pipers, snuff, or chewing tobacco, and pose significant health effects.7,8 However, public health interventions on preventing tobacco use by the FCTC exclusively target cigarette tobacco, devoting little attention to NCT products.
The association between smoked cigarette tobacco use and cardiovascular diseases (CVDs) such as stroke among Black Africans has been widely documented.9,10 However, few studies have explored the contribution of NCT use on the burden of stroke among Africans despite the growing use among Africans.11 NCT products are attracting new markets worldwide,12 but the reasons for these trends are not entirely clear, though concerns for smoking-related health effects may play a role.13,14 In sub-Saharan Africa, the decision to change from cigarette tobacco to NCT products is multifactorial but may not be farfetched from the erroneous misconceptions that they are less harmful or perhaps price minimization strategy.15 However, limited data are available on the health implications of using NCT products.16,17 The prevalence of NCT use among adults aged 15 years and above was reported to be 1.9% in Nigeria18 and 2.6% in Ghana.19 Of note, several studies have revealed severe health consequences related to NCT use, specifically oral health impairment,17,20 nutritional disorders,21 co-use of psychoactive substances, and CVD, including coronary heart diseases,22–24 but little is known about the significance of NCT use in the stroke events in this population.
A detailed understanding of the relationship between NCT use and the risk of stroke will likely inform well-articulated policy guidance and evidence-based recommendations for public health prevention and management of stroke on the African continent. As far as we know, this study is the largest and the first to characterize NCT use in relation to stroke epidemiology on the African continent. Given the increased morbidity and mortality due to stroke and the priority of its treatment and prevention, numerous studies have assessed the relationship between smoking and stroke with limited information on the significance of NCT use in stroke epidemiology, particularly among indigenous Africans. Therefore, this present study investigated the relationship of NCT use with the odds of first-ever stroke among adults in West Africa.
Methods
Study Design, Population, and Setting
Respondents for this current study were from the Stroke Investigative Research and Educational Network (SIREN) study. SIREN is a multicenter case–control study initiated in 2014 and carried out in Ghana and Nigeria to characterize the burden and risk factors of stroke among indigenous Africans. Ethical approval was obtained across 15 study sites in Ghana and Nigeria, and informed consent was obtained from respondents (≥18 years) before participation. The SIREN study recruited stroke cases with a first clinical stroke within eight days of current symptom onset or last seen without deficit, with neuroimaging confirmation on computed tomography (CT) or magnetic resonance imaging (MRI) scan within ten days of symptom onset. Population-based stroke-free controls were recruited primarily from catchment communities of the study sites. Stroke-free status was verified using the eight-item validated questionnaire for verifying stroke-free status.25 Details of methods and preliminary findings have been documented elsewhere.9,26 This current report included 3531 stroke cases and 4086 stroke-free population-based controls from the SIREN dataset who have never used cigarette tobacco in their lifetime. In-person interviews and physical examinations were conducted by trained medical personnel to extract data on demographic and lifestyle factors (including NCT use) and fasting blood samples (after an overnight fast) using validated instruments and uniform standard operating procedures across all study sites.
Definition of First-Ever Stroke (Outcome)
Based on clinical evaluation and brain imaging (CT or MRI), stroke cases were defined and phenotyped using electrocardiography, transthoracic echocardiography, and carotid Doppler ultrasound. Details of the definition and phenotyping procedure have been published elsewhere.9 Ischaemic stroke subtypes were defined using Oxfordshire community stroke project guidelines27 and the Trial of ORG 10172 in acute stroke treatment guidelines.28 Intracerebral haemorrhage was defined using structural, medication-related, amyloid angiopathy, systemic/other diseases, hypertension, and undetermined.9,29 Stroke-free controls were verified using a prevalidated eight-item scale to verify stroke-free status (with 98% negative predictive value) in three main local languages in West Africa (Ashanti Twi, Hausa, and Yoruba).25
NCT Use (Exposure)
The current study included respondents who reported having never used cigarette tobacco in a lifetime. NCT use was defined as self-reported use of at least one of the following NCTs: cigars or piper (for at least 50 times) or snuff or chewing tobacco (for at least 20 times) in the past year before the onset of stroke as described in the protocol.26 NCT use was classified as smoked (if respondents reported using cigars or piper) or smokeless (if respondents reported using snuff and chewed tobacco).12
Sociodemographic, Lifestyle, and Clinical Characteristics (Covariates)
Respondents provided information on country of residence (Ghana or Nigeria), sex (male or female), age in years, education status (classified as none, primary, secondary, or secondary school education and above), income (classified as “low” if respondent’s monthly earnings was <$100 or “high” if respondent’s monthly earnings was ≥$100)30, alcohol use (no or yes), and family history of CVD (no or yes). Physical activity was defined as regular involvement in moderate exercise (walking, cycling, or gardening) or strenuous exercise (jogging, football, and vigorous swimming) for at least 4 hours per week.30,31
Body mass index (BMI) was estimated from weight (in kg) divided by the square of height (in meters), and waist-to-hip ratio (WHR) was a function of the waist circumference divided by hip circumference (in cm). Obesity was defined as BMI ≥30 kg/m2, and WHR ≥0.90—males and ≥0.85—females used as the cutoff for respondents at risk of metabolic disorders.32 Diabetes mellitus (DM) was defined as any of the following conditions: self-reported DM diagnosis by a trained physician, current use of medications for DM, HbA1c ≥6.5%, or fasting blood glucose ≥7.0 mmol/L.33 Hypertension was defined as any of the following conditions: a one-off or sustained systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, a prior diagnosis of hypertension by a trained physician or use of antihypertensive medications.34 Dyslipidemia was defined as any of the following conditions: fasting total cholesterol ≥5.2 mmol/L, high-density lipoproteins ≤1.03 mmol/L, triglycerides ≥1.7 mmol/L, or low-density lipoproteins ≥3.4 mmol/L or use of statin prior before stroke onset.35
Statistical Analysis
Multiple imputation technique, enabled by the MICE package in the R statistical software, was used to fill in the missing data on NCT use in the dataset. A chi-square and independent-sample t test were used for categorical and continuous variables respectively to compare respondents’ characteristics according to NCT use status. Furthermore, we used logistic regression to estimate the odds ratio (OR) and 95% confidence intervals (CIs) for NCT use and first-ever stroke, controlling for covariates. We included covariates in the regression models based on whether they were of statistical significance in the bivariate analysis and our clinical understanding of stroke. The following covariates were included in the final regression model: age (in years, continuous), highest education completed (none, primary, or secondary school and above), monthly income (<$100 vs. ≥$100), current alcohol use (no vs. yes), family history of CVD (no vs. yes), physical inactivity (no vs. yes), obesity (<30 kg/m2 vs. ≥30 kg/m2), DM status (no vs. yes), dyslipidemia (no vs. yes), and hypertension (no or yes). Furthermore, we carried out subgroup analyses by NCT type, which included smoked and smokeless NCT use. All statistical analyses were performed using SPSS (version 25) and the R statistical program (version 3.6.2) at a two-sided p < .05.
Results
Characteristics of Respondents
The characteristics of respondents are presented in Table 1. NCT users were older than non-NCT users (62.8 ± 15.7 vs. 57.7 ± 14.8 years). Among NCT users, 65.9% were males while 34.1% were females and a higher proportion of respondents who earn ≥$100 reported NCT use compared to non-NCT users (57.5% vs. 44.5%). However, current alcohol use, family history of CVD, physical inactivity, mean WHR, and BMI differed insignificantly by NCT use status, but the prevalence of obesity was higher among NCT users (48.8%) than non-NCT users (23.5%). Similarly, the prevalence of dyslipidemia was higher among NCT users compared to non-NCT users (26.4% vs. 23.3%), and the proportion of NCT users 34 (0.6%) with hypertension was higher compared to non-NCT users 7 (0.4%).
Table 1.
Characteristics of All Nonsmokers According to NCT Use in the SIREN Study
| NCT use status | |||
|---|---|---|---|
| Characteristics | NCT (no) | NCT (yes) | p-value |
| Country | 7576 | 41 | |
| Ghana | 2220 (29.9) | 10 (24.4) | .491 |
| Nigeria | 5356 (70.1) | 31 (76.6) | |
| Sex | |||
| Females | 3865 (51.0) | 14 (34.1) | .02 |
| Males | 3711 (49.0) | 27 (65.9) | |
| Age (years), mean ± SD | 57.7 ± 14.8 | 62.8 ± 15.7 | .01 |
| <60 years | 4000 (52.8) | 13 (31.7) | .007 |
| ≥60 years | 3576 (47.2) | 28 (68.3) | |
| Education | |||
| None | 1569 (20.7) | 7 (17.5) | .87 |
| Primary school | 1678 (22.2) | 9 (22.5) | |
| Secondary school and above | 4317 (57.1) | 24 (60.0) | |
| Monthly income | |||
| <$100 | 4132 (55.5) | 17 (42.5) | .09 |
| ≥$100 | 3311 (44.5) | 23 (57.5) | |
| Lifestyle factors | |||
| Current alcohol use (yes) | 7334 (96.8) | 39(95.1) | .54 |
| Family history of CVD (yes) | 4623 (61.0) | 26 (63.4) | .75 |
| Physical inactivity (yes) | 251 (3.3) | 2 (4.9) | .57 |
| Anthropometric measurements | |||
| WHR, mean ± SD | 0.92 ± 0.8 | 0.93 ± 0.09 | .53 |
| WHR ≥ 0.90 (men) and 0.85 (women) | 5522 (72.9) | 28 (68.3) | .92 |
| BMI (kg/m2), mean ± SD | 26.5 ± 5.6 | 26.2 ± 5.2 | .79 |
| BMI ≥ 30 kg/m2 | 1781 (23.5) | 20 (48.8) | <.0001 |
| Blood pressure and metabolic factors | |||
| SBP (mm Hg), mean ± SD | 146.1 ± 30.0 | 152.1 ± 28.7 | .21 |
| DBP (mm Hg), mean ± SD | 89.1 ± 60.6 | 92.4 ± 15.1 | .73 |
| Hypertension (yes) | 7 (0.4) | 34 (0.6) | .20 |
| Diabetes (yes) | 3360 (44.4) | 24 (58.5) | .068 |
| Dyslipidemia (yes) | 1767 (23.3) | 11 (26.8) | <.0001 |
NCT: non-cigarette tobacco; CVD: cardiovascular diseases; WHR: waist-to-hip ratio; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure. Continuous data are presented as mean ± SD and compared using paired t test; categorical data are presented as n (%) and compared using the chi-square test.
NCT Use, Stroke Status, and Stroke Subtype
Of the total 41 (0.5%) who reported NCT use, 27/41 (65.9%) were stroke cases, while 14/41 (34.1%) were stroke-free controls. Among stroke cases that reported NCT use, 20 (0.8%) and 7 (0.7%) had ischaemic and hemorrhagic stroke subtypes, respectively.
NCT Use and Odds of First-Ever Stroke
The OR and 95% CI for the association of NCT use with all stroke events are presented in a single model (Table 2). In the unadjusted model, overall NCT use was significantly associated with higher odds of stroke (OR: 2.74; 95% CI: 1.35, 5.56). Overall, NCT use was associated with first-ever stroke (OR: 2.08; 95% CI: 1.02, 4.23) in the entire sample after adjusting for age, highest education completed, monthly income, current alcohol use, family history of CVD, physical inactivity, obesity status, DM status, and dyslipidaemia status—Table S1. However, the association was attenuated (OR: 1.98; 95% CI: 0.91, 4.28) after additionally adjusting for hypertension—Table S2. Notably, smokeless NCT was independently associated with higher odds of stroke (OR: 2.74; 95% CI: 1.15, 6.54), but smoked NCT use (OR: 0.16; 95% CI: 0.02, 1.63) presented a statistically insignificant association with stroke odds, even after adjusting for hypertension and other covariates (Figure 1).
Table 2.
Odds Ratio (95% Confidence Interval) of the Multivariate Adjustment for the Association of Smokeless and Smoked NCT Use by Odds of Stroke Using Non-NCT as Reference
| All stroke events | |
|---|---|
| NCT use (yes) | 2.74 (1.35, 5.56) |
| Crude odds | |
| Non-NCT (ref) | 1 |
| Smoked | 0.29 (0.03, 2.60) |
| Smokeless | 2.75 (1.36, 5.57) |
| Adjusted odds | 1.93 (0.89, 4.17) |
| Non-NCT (ref) | 1 |
| Smoked | 0.16 (0.02, 1.63) |
| Smokeless | 2.74 (1.15, 6.54) |
| Covariates | |
| Age (in years continuous) | 1.00 (0.99, 1.01) |
| Sex (males) | 1.34 (1.19, 1.50) |
| Secondary school and above | 1.24 (1.10, 1.40) |
| Monthly income (≥$100) | 0.1 (0.62, 0.80) |
| Current alcohol use (yes) | 1.29 (1.04, 1.61) |
| Family history of CVD (yes) | 1.40 (1.25, 1.56) |
| Physical inactivity (yes) | 2.33 (1.71, 3.19) |
| BMI (≥30 kg/m2) | 0.85 (0.72, 1.01) |
| Diabetes (yes) | 1.68 (1.50, 1.88) |
| Dyslipidemia (yes) | 3.66 (3.20, 4.18) |
| Hypertension (yes) | 15.45 (12.85, 18.58) |
NCT: non-cigarette tobacco; CVD: cardiovascular diseases; BMI: body mass index.
Figure 1.
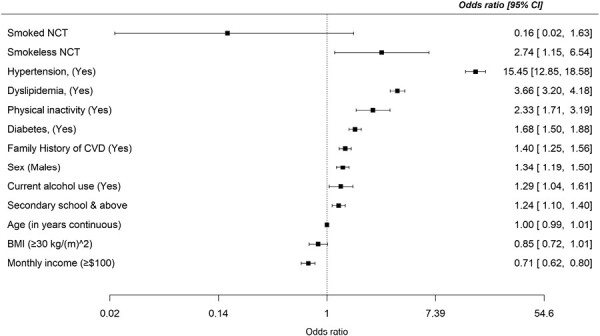
Multivariate adjustment for the association of smokeless and smoked non-cigarette tobacco (NCT) use by odds of stroke.
Discussion
In this study, we investigated the relationship between NCT use and first-ever stroke and found that NCT was independently associated with higher odds of stroke among indigenous Africans, with smokeless NCT presenting higher odds for all stroke events. Our study is the largest and the first to characterize NCT use, including smokeless NCT and its association with stroke, even after adjusting for vascular risk factors for stroke. NCT remains the main form of tobacco consumption by almost a quarter of all tobacco users worldwide.36 Nevertheless, its regulation and control lag behind cigarette tobacco use. Our findings will contribute to identifying the neglected environmental risk factors of stroke and necessitate its inclusion in designing culturally relevant primary prevention strategies for controlling stroke and other CVDs among Africans.
Several published reports have documented the significance of active and passive smoking in the epidemiology of stroke and other CVD events,37–39 with limited evidence on the implications of NCT use in stroke occurrence. Our findings corroborate reports from a few epidemiological studies.40,41 Two meta-analyses demonstrated a higher risk of stroke among current NCT users, especially for studies from the United States.42,43 A similar observational study in Iran reported a three-fold risk of first-ever ischaemic stroke among users of Hookah—an NCT product.44
Our findings revealed the significance of smokeless NCT use (chewing and snuffing tobacco) in stroke events. To date, a significant gap is the lack of observational studies on the risks associated with various types of smokeless NCT used within and between countries.36 The few epidemiological reports on the association between smokeless NCT use and stroke have been inconsistent, with some studies showing a slightly elevated risk of stroke and others reporting no association.45,46 Our study found that smokeless NCT was strongly and significantly associated with all stroke events, even after adjusting for related covariates, especially hypertension. Our report did not account for subgroup analysis by stroke type due to fewer stroke cases, but a recent study by Hergens et al.41 reported an increased risk of fatal ischaemic stroke subtype only and not overall stroke among current snuff users. A possible explanation could be the inclusion of snuffers and tobacco chewers in the current analysis as opposed to only snuffers in the study by Hergens et al.41
However, the apparent direct association between NCT use and stroke was not statistically significant after adjusting for hypertension, except for smokeless NCT use. Possible reasons could include the following. First, unlike smoked cigarettes, smokeless NCTs are consumed discretely without combustion, either nasally or orally, which aids regular consumption, thereby resulting in the absorption of nicotine and other chemicals across mucus membranes. Smokeless NCT products vary significantly in composition and contain high levels of free nicotine, total nicotine, and various carcinogens.47 In contrast to our findings, a meta-analysis has reported a significantly increased risk of stroke due to exposure to some toxicants and nicotine in smoked NCT.48,49 It is likely that misclassification of NCT status, particularly regarding underreporting of smoked NCT use, may partly explain the association toward the null for smoked NCT use in our study. Unlike smokeless NCT use, smoking is not considered socially desirable among African young adults due to cultural values and beliefs.50 Hence, smoked NCT use is likely to be underreported. Second, this might be due to the low statistical power to discriminate associations due to the few NCT users in the dataset. Third, it is likely that NCT use could trigger stroke events via hypertension risk due to early reports51,52 that have demonstrated a direct link between passive tobacco use and hypertension. A common ingredient in all tobacco products is nicotine, and the plasma level of nicotine in NCT is as high as in passive tobacco.53 Some authors have demonstrated an increase in heart rate and blood pressure measurements along with elevation of plasma epinephrine after administration of NCT.54 A secondary data analysis of the South African Demographic and Health Survey among 4092 South African women aged 20–75 years reported a significantly increased blood pressure to levels shown to increase CVD risk due to heavy snuff use.55 Also, combined lifetime use of both snuff and cigarettes has been reported to increase the odds of developing osteoporosis among women aged ≥40 years.56 Studies reporting a positive association of NCT use with hypertension postulated that frequent use of NCT could lead to continuous moderate levels of nicotine in the blood, causing sympathetic nervous system activation and a rise in blood pressure.57 The impact of NCT on stroke events may be attributable to the apparent effect of exposure to nicotine from tobacco products.45,58
Short-term NCT use has been linked to increased blood pressure and heart rate (primarily due to nicotine),59 which are risk factors for stroke events. Some studies have also linked smokeless NCT use with CVD.42,60 Biologic mechanisms by which NCT might result in stroke events are not implausible. Results from animal studies show that nicotine can induce cardiac arrhythmias.58 About 20% of all strokes are cardioembolic, and atrial fibrillation is a risk factor for this type of ischaemic stroke.61 Also, results from in vitro studies suggest that nicotine opens the blood-brain barrier, increasing the severity of the stroke by allowing post-ischaemic brain edema.62
There are some limitations in our study. The small number of stroke cases among non-cigarette smokers included in the study is a major limitation that could significantly underpower the study. A possibility is that NCT usage is underreported in the SIREN population because they are not generally acceptable products in Nigeria and Ghana. Also, NCT users might alongside NCT also smoke cigarette tobacco, and this might be underreported, making it challenging to exclusively isolate cigarette tobacco use in these associations. A larger sample of exclusive NCT users would improve the reliability of the associations between NCT use and stroke events, but this will be difficult to achieve in practice, given that most NCT users might have previously smoked cigarettes or used other forms of combustible tobacco. These limitations notwithstanding, this is one of the largest and most comprehensive efforts at delineating the association between NCT use and first-ever stroke among indigenous Africans. The physician-adjudicated stroke case ascertainment and multicenter setting are additional strengths of this study. Future longitudinal studies are necessary to infer causal associations between NCT use and stroke, including the contribution of the different forms of NCT.
Conclusions
NCT use, especially smokeless NCT, is associated with higher odds of stroke in this sample of Africans. Although NCT use is a relatively neglected aspect of tobacco use epidemiology, it might play a significant role in the global burden of stroke, especially among Africans. Tailored interventions directed against NCT use, might be necessary to manage the increasing burden of stroke among Africans.
Supplementary Material
Acknowledgments
The authors would like to thank all respondents in Ghana and Nigeria who volunteered to participate in the study.
Contributor Information
Adekunle Gregory Fakunle, Department of Medicine, College of Medicine, University of Ibadan, Ibadan, Nigeria; Department of Public Health, Osun State University, Osogbo, Nigeria.
Akinkunmi Paul Okekunle, Department of Medicine, College of Medicine, University of Ibadan, Ibadan, Nigeria; Department of Food and Nutrition, Seoul National University, Seoul, Korea.
Osahon Jeffery Asowata, Department of Epidemiology and Medical Statistics, University of Ibadan, Ibadan, Nigeria.
Onoja Akpa, Department of Epidemiology and Medical Statistics, University of Ibadan, Ibadan, Nigeria; Institute of Cardiovascular Diseases, College of Medicine, University of Ibadan, Ibadan, Nigeria.
Fred S Sarfo, Department of Medicine, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
Albert Akpalu, Department of Medicine, University of Ghana Medical School, Accra, Ghana.
Kolawole Wahab, Department of Medicine, University of Ilorin Teaching Hospital, Ilorin, Nigeria.
Reginald Obiako, Department of Radiology, Ahmadu Bello University, Zaria, Nigeria.
Morenikeji Komolafe, Department of Medicine, Obafemi Awolowo University Teaching Hospital, Ile-Ife, Nigeria.
Lukman Owolabi, Department of Medicine, Aminu Kano Teaching Hospital, Kano, Nigeria.
Godwin O Osaigbovo, Department of Medicine, Jos University Teaching Hospital, Jos, Nigeria.
Abiodun M Adeoye, Department of Medicine, College of Medicine, University of Ibadan, Ibadan, Nigeria.
Hemant K Tiwari, Department of Biostatistics, University of Alabama at Birmingham, Birmingham, AL, USA.
Ezinne O Uvere, Department of Medicine, College of Medicine, University of Ibadan, Ibadan, Nigeria.
Joshua Akinyemi, Department of Epidemiology and Medical Statistics, University of Ibadan, Ibadan, Nigeria.
Carolyn Jenkins, Department of Nursing, Medical University of South Carolina, Charleston, SC, USA.
Oyedunni Arulogun, Department of Health Promotion and Education, University of Ibadan, Ibadan, Nigeria.
Philip Ibinaiye, Department of Radiology, Ahmadu Bello University, Zaria, Nigeria.
Lambert T Appiah, Department of Medicine, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
Temilade Bello, Department of Public Health, Osun State University, Osogbo, Nigeria.
Arti Singh, Department of Medicine, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
Joseph Yaria, Department of Medicine, College of Medicine, University of Ibadan, Ibadan, Nigeria.
Benedict Calys-Tagoe, Department of Community Health, University of Ghana Medical School, Accra, Ghana.
Godwin Ogbole, Department of Radiology, University of Ibadan, Ibadan, Nigeria.
Ijezie Chukwuonye, Department of Medicine, Federal Medical Centre, Umahia, Nigeria.
Chidinma Melikam, Department of Radiology, Ahmadu Bello University, Zaria, Nigeria.
Philip Adebayo, Department of Internal Medicine, Aga-Khan University, Dar es Salaam, Tanzania.
Yaw Mensah, Department of Radiology, University of Ghana Medical School, Accra, Ghana.
Oladimeji Adebayo, Department of Medicine, College of Medicine, University of Ibadan, Ibadan, Nigeria; Institute of Cardiovascular Diseases, College of Medicine, University of Ibadan, Ibadan, Nigeria.
Sunday Adeniyi, Department of Medicine, University of Ilorin Teaching Hospital, Ilorin, Nigeria.
Wisdom Oguike, Department of Radiology, Ahmadu Bello University, Zaria, Nigeria.
Arnett Donna, College of Public Health, University of Kentucky, Lexington, KY, USA.
Rufus Akinyemi, Department of Medicine, Federal Medical Centre, Abeokuta, Nigeria; Neuroscience and Ageing Research Unit, Institute for Advanced Medical Research and Training, College of Medicine, University of Ibadan, Ibadan, Nigeria; Center for Genomic and Precision Medicine, College of Medicine, University of Ibadan, Ibadan, Nigeria.
Bruce Ovbiagele, Weill Institute for Neurosciences, University of California San Francisco, San Francisco, CA, USA.
Mayowa Owolabi, Department of Medicine, College of Medicine, University of Ibadan, Ibadan, Nigeria; Center for Genomic and Precision Medicine, College of Medicine, University of Ibadan, Ibadan, Nigeria; Lebanese American University, Beirut, Lebanon.
Funding
The study and investigators were supported by the National Institutes of Health grants SIREN (U54HG007479), SIBS Genomics (R01NS107900), African Neurobiobank for Precision Stroke Medicine ELSI project (U01HG010273), SIBS Gen Gen (R01NS107900‐02S1), ARISES (R01NS115944‐01), H3Africa CVD Supplement (3U24HG009780‐03S5), and CaNVAS (1R01NS114045-01), sub-Saharan Africa Conference on Stroke Conference (1R13NS115395-01A1), and Training Africans to Lead and Execute Neurological Trials & Studies (TALENTS; D43TW012030) and and Growing Data-science Research in Africa to Stimulate Progress (1UE5HL172183-01). APO is a recipient of the Brain Pool Fellowship of the National Research Foundation of Korea (2020H1D3A1A04081265). OJA is a recipient of the SIGHPC Computational and Data Science Fellowship in the United States. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Declaration of Interests
None declared.
Ethical Approval
The Stroke Investigative Research and Educational Network (SIREN) study is a multicenter study, and the Institutional Review Board (IRB) at all study sites provided ethical approval for the study. The overall coordinating IRB for the SIREN study was the University of Ibadan/University College Hospital Ibadan, Nigeria (IRB Approval No.: UI/EC/13/0105).
Author Contributions
Adekunle Fakunle (Conceptualization [lead], Data curation [lead], Formal analysis [supporting], Investigation [equal], Methodology [equal], Writing—original draft [lead]), Akinkunmi Okekunle (Conceptualization [lead], Data curation [lead], Formal analysis [supporting], Methodology [lead], Supervision [lead], Writing—original draft [lead]), Osahon Asowata (Formal analysis [lead], Writing—review & editing [supporting]), Onoja Akpa (Conceptualization [lead], Formal analysis [supporting], Funding acquisition [supporting], Investigation [lead], Writing—review & editing [lead]), Fred Sarfo (Funding acquisition [supporting], Investigation [lead], Writing—review & editing [supporting]), Albert Akpalu (Investigation [lead], Writing—review & editing [supporting]), Kolawole Wahab (Investigation [lead], Writing—review & editing [supporting]), Reginald Obiako (Investigation [lead], Writing—review & editing [supporting]), Morenikeji Komolafe (Investigation [lead], Writing—review & editing [supporting]), Lukman Owolabi (Investigation [lead], Writing—review & editing [supporting]), Godwin Osaigbovo (Investigation [lead], Writing—review & editing [supporting]), Abiodun Adeoye (Investigation [equal], Writing—review & editing [supporting]), Hemant Tiwari (Investigation [supporting], Writing—review & editing [supporting]), Ezinne Uvere (Investigation [supporting], Writing—review & editing [supporting]), Joshua Akinyemi (Formal analysis [supporting], Writing—review & editing [supporting]), Carolyn Jenkins (Investigation [supporting], Writing—review & editing [supporting]), Oyedunni Arulogun (Funding acquisition [supporting], Investigation [supporting], Writing—review & editing [supporting]), Philip Ibinaiye (Writing—review & editing [supporting]), Lambert Appiah (Writing—review & editing [supporting]), Temilade Bello (Writing—review & editing [supporting]), Arti Singh (Writing—review & editing [supporting]), Joseph Yaria (Writing—review & editing [supporting]), Benedict Calys-Tagoe (Writing—review & editing [supporting]), Godwin Ogbole (Writing—review & editing [supporting]), Innocent Chukwuonye (Writing—review & editing [supporting]), Chimdinma Melikam (Data curation [supporting], Writing—review & editing [supporting]), Philip Adebayo (Writing—review & editing [supporting]), Yaw Mensah (Writing—review & editing [supporting]), Olademiji Adebayo (Writing—review & editing [supporting]), Adeniyi Sunday (Data curation [supporting], Writing—review & editing [supporting]), Wisdom Oguike (Data curation [supporting], Writing—review & editing [supporting]), Arnett Donna (Writing—review & editing [supporting]), Rufus Akinyemi (Funding acquisition [supporting], Investigation [lead], Writing—review & editing [supporting]), Bruce Ovbiagele (Funding acquisition [equal], Supervision [equal], Writing—review & editing [equal]), and Mayowa Owolabi (Funding acquisition [lead], Investigation [lead], Project administration [lead], Supervision [lead], Writing—review & editing [lead])
Data Availability
Individual participant data that underlie the results reported in this article (text, tables, and figures) has been deidentified. The joint dataset is available upon reasonable request, and a proposal to access the data should be directed to MO (PI: mayowaowolabi@yahoo.com). Data requestors will need to sign a data access agreement.
References
- 1. Alberg AJ, Shopland DR, Cummings KMT. 2014 Surgeon General’s report: commemorating the 50th anniversary of the 1964 report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am J Epidemiol. 2014;179(4):403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sheet TF. World Health Organization; 2013. http://www.who.int/mediacentre/factsheets/fs339/en/. Accessed July 13, 2021. [Google Scholar]
- 4. Okekunle, AP, Jones, S, Adeniji, O, et al. ; Stroke in Africa: A systematic review and meta-analysis of the incidence and case-fatality rates. International Journal of Stroke. 2022;18(6):634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO. WHO Framework Convention on Tobacco Control. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- 6. Desalu OO, Iseh KR, Olokoba AB, Salawu FK, Danburam A. Smokeless tobacco use in adult Nigerian population. Niger J Clin Pract. 2010;13(4):382–387. [PubMed] [Google Scholar]
- 7. Gupta R, Gupta N, Khedar RS. Smokeless tobacco and cardiovascular disease in low and middle income countries. Indian Heart J. 2013;65(4):369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nahhas GJ, Cummings KM, Halenar MJ, et al. Smokeless tobacco use and prevalence of cardiovascular disease among males in the Population Assessment of Tobacco and Health (PATH) study, waves 1–4. Prev Med Rep. 2022;25(1):101650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Owolabi MO, Sarfo F, Akinyemi R, et al. ; SIREN Team. Dominant modifiable risk factors for stroke in Ghana and Nigeria (SIREN): a case-control study. Lancet Glob Health. 2018;6(4):e436–e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oshunbade AA, Yimer WK, Valle KA, et al. Cigarette smoking and incident stroke in blacks of the Jackson Heart Study. J Am Heart Assoc. 2020;9(12):e014990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. James PB, Bah AJ, Kabba JA, Kassim SA, Dalinjong PA. Prevalence and correlates of current tobacco use and non-user susceptibility to using tobacco products among school-going adolescents in 22 African countries: a secondary analysis of the 2013-2018 global youth tobacco surveys. Arch Public Health. 2022;80(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O’Connor RJ. Non-cigarette tobacco products: what have we learnt and where are we headed? Tob Control. 2012;21(2):181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nasim A, Khader Y, Blank MD, Cobb CO, Eissenberg T. Trends in alternative tobacco use among light, moderate, and heavy smokers in adolescence, 1999-2009. Addict Behav. 2012;37(7):866–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weglicki LS, Templin TN, Rice VH, Jamil H, Hammad A. Comparison of cigarette and water-pipe smoking by Arab and non-Arab-American youth. Am J Prev Med. 2008;35(4):334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nkosi L, Agaku IT, Ayo-Yusuf O. Prevalence and correlates of roll-your-own cigarette smoking among South African adults during 2010-2011 and 2017-2018. Tob Induc Dis. 2022;20(3):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pednekar MS, Gupta PC, Yeole BB, Hébert JR. Association of tobacco habits, including bidi smoking, with overall and site-specific cancer incidence: results from the Mumbai cohort study. Cancer Causes Control. 2011;22(6):859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Awan KH, Patil S. Association of smokeless tobacco with oral cancer - evidence from the South Asian studies: a systematic review. J Coll Physicians Surg Pak. 2016;26(9):775–780. [PubMed] [Google Scholar]
- 18. Onoh I, Owopetu O, Olorukooba AA, et al. Prevalence, patterns and correlates of smokeless tobacco use in Nigerian adults: an analysis of the Global Adult Tobacco Survey. PLoS One. 2021;16(1):e0245114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singh PK, Singh L, Wehrmeister FC, et al. Prevalence of smoking and smokeless tobacco use during breastfeeding: a cross-sectional secondary data analysis based on 032 million sample women in 78 low-income and middle-income countries. eClinicalMedicine. 2022;53(10):101660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Critchley JA, Unal B. Health effects associated with smokeless tobacco: a systematic review. Thorax. 2003;58(5):435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nonterah EA, Debpuur C, Agongo G, et al. ; as members of AWI-Gen and the H3Africa Consortium. Socio-demographic and behavioural determinants of body mass index among an adult population in rural Northern Ghana: the AWI-Gen study. Glob Health Action. 2018;11(2):1467588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benowitz NL, Porchet H, Sheiner L, Jacob P III. Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther. 1988;44(1):23–28. [DOI] [PubMed] [Google Scholar]
- 23. Baig S, Rubab Z, Farooq W. Molecular pathogenesis of chewable tobacco. J Coll Physicians Surg Pak. 2018;28(5):381–385. [DOI] [PubMed] [Google Scholar]
- 24. Gupta R, Gupta S, Sharma S, Sinha DN, Mehrotra R. Risk of coronary heart disease among smokeless tobacco users: results of systematic review and meta-analysis of global data. Nicotine Tob Res. 2019;21(1):25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sarfo F, Gebregziabher M, Ovbiagele B, et al. ; Stroke Investigative Research Educational Networks. Multilingual validation of the questionnaire for verifying stroke-free status in West Africa. Stroke. 2016;47(1):167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akpalu A, Sarfo FS, Ovbiagele B, et al. ; SIREN as part of the H3Africa Consortium. Phenotyping stroke in sub-Saharan Africa: Stroke Investigative Research and Education Network (SIREN) Phenomics Protocol. Neuroepidemiology. 2015;45(2):73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337(8756):1521–1526. [DOI] [PubMed] [Google Scholar]
- 28. Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria. Stroke. 2001;32(12):2735–2740. [DOI] [PubMed] [Google Scholar]
- 29. Sarfo FS, Ovbiagele B, Gebregziabher M, et al. ; SIREN. Stroke among young West Africans. Stroke. 2018;49(5):1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Akpa, OM, Okekunle, AP, Sarfo, FS, et al. ; Sociodemographic and behavioural risk factors for obesity among community-dwelling older adults in Ghana and Nigeria: A secondary analysis of data from the SIREN study. Chronic Illness. 2021;19(1):40–55. [DOI] [PubMed] [Google Scholar]
- 31. Akpa OM, Okekunle AP, Ovbiagele B, et al. ; SIREN Study as part of the H3Africa Consortium. Factors associated with hypertension among stroke-free indigenous Africans: findings from the SIREN study. J Clin Hypertens (Greenwich). 2021;23(4):773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. World Health Organization. Obesity and Overweight: Fact Sheet. 2017. http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed November 28, 2017. [Google Scholar]
- 33. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. [DOI] [PubMed] [Google Scholar]
- 34. Chobanian AV, Bakris GL, Black HR, et al. ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. [DOI] [PubMed] [Google Scholar]
- 35. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 36. Siddiqi K, Husain S, Vidyasagaran A, et al. Global burden of disease due to smokeless tobacco consumption in adults: an updated analysis of data from 127 countries. BMC Med. 2020;18(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheng Y-C, Reyes-Guzman CM, Christensen CH, et al. Biomarkers of exposure among adult smokeless tobacco users in the population assessment of tobacco and health study (wave 1, 2013–2014). Cancer Epidemiol Biomarkers Prev. 2020;29(3):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fisher MT, Tan-Torres SM, Gaworski CL, Black RA, Sarkar MA. Smokeless tobacco mortality risks: an analysis of two contemporary nationally representative longitudinal mortality studies. Harm Reduct J. 2019;16(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Okekunle AP, Asowata JO, Adedokun B, Akpa OM. Secondhand smoke exposure and dyslipidemia among non-smoking adults in the United States. Indoor Air. 2022;32(1):e12914. [DOI] [PubMed] [Google Scholar]
- 40. Tabrizi R, Borhani-Haghighi A, Lankarani KB, et al. Hookah smoking: a potentially risk factor for first-ever ischemic stroke. JSCVD. 2020;29(10):105138. [DOI] [PubMed] [Google Scholar]
- 41. Hergens M-P, Lambe M, Pershagen G, Terent A, Ye W. Smokeless tobacco and the risk of stroke. Epidemiology. 2008;19(6):794–799. [DOI] [PubMed] [Google Scholar]
- 42. Boffetta P, Straif K. Use of smokeless tobacco and risk of myocardial infarction and stroke: systematic review with meta-analysis. BMJ. 2009;339(2):b3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gupta R, Gupta S, Sharma S, Sinha DN, Mehrotra R. A systematic review on association between smokeless tobacco & cardiovascular diseases. Indian J Med Res. 2018;148(1):77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tabrizi R, Borhani-Haghighi A, Lankarani KB, et al. Hookah smoking: a potentially risk factor for first-ever ischemic stroke. J Stroke Cerebrovasc Dis. 2020;29(10):105138. [DOI] [PubMed] [Google Scholar]
- 45. Rostron BL, Chang CM, van Bemmel DM, et al. Smokeless tobacco users: results from 1999 to 2012 National Health and Nutrition Examination Survey data. Cancer Epidemiol Biomarkers Prev. 2015;24(12):1829–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Timberlake DS, Nikitin D, Johnson NJ, Altekruse SF. A longitudinal study of smokeless tobacco use and mortality in the United States. Int J Cancer. 2017;141(2):264–270. [DOI] [PubMed] [Google Scholar]
- 47. Public Health: A Global Perspective. USA: National Cancer Institute. Centers for Disease Control and Prevention. U.S. Bethesda MUSDoHaHSC. [Google Scholar]
- 48. Klein AP, Yarbrough K, Cole JW. Stroke, smoking and vaping: the no-good, the bad and the ugly. Ann Public Health Res. 2021;8(1):1104. [PMC free article] [PubMed] [Google Scholar]
- 49. Pan B, Jin X, Jun L, et al. The relationship between smoking and stroke: a meta-analysis. Medicine (Baltimore). 2019;98(12):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Egbe CO, Petersen I, Meyer-Weitz A, Oppong Asante K. An exploratory study of the socio-cultural risk influences for cigarette smoking among Southern Nigerian youth. BMC Public Health. 2014;14(1):1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Akpa OM, Okekunle AP, Asowata JO, Adedokun B. Passive smoking exposure and the risk of hypertension among non-smoking adults: the 2015–2016 NHANES data. Clin Hypertens. 2021;27(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim BJ, Kang JG, Kim JH, et al. Association between secondhand smoke exposure and hypertension in 106,268 Korean self-reported never-smokers verified by cotinine. J Clin Med. 2019;8(8):1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Holm H, Jarvis MJ, Russell MA, Feyerabend C. Nicotine intake and dependence in Swedish snuff takers. Psychopharmacology (Berl). 1992;108(4):507–511. [DOI] [PubMed] [Google Scholar]
- 54. Wolk R, Shamsuzzaman AS, Svatikova A, et al. Hemodynamic and autonomic effects of smokeless tobacco in healthy young men. J Am Coll Cardiol. 2005;45(6):910–914. [DOI] [PubMed] [Google Scholar]
- 55. Ayo-Yusuf O, Omole O. Snuff use and the risk of hypertension among black South African women. S Afr Acad Fam Pract/Prim Care. 2008;50(2):64. [Google Scholar]
- 56. Ayo-Yusuf OA, Olutola BG. Epidemiological association between osteoporosis and combined smoking and use of snuff among South African women. Niger J Clin Pract. 2014;17(2):174–177. [DOI] [PubMed] [Google Scholar]
- 57. Hergens MP, Lambe M, Pershagen G, Ye W. Risk of hypertension amongst Swedish male snuff users: a prospective study. J Intern Med. 2008;264(2):187–194. [DOI] [PubMed] [Google Scholar]
- 58. Hawkins BT, Brown RC, Davis TP. Smoking and ischemic stroke: a role for nicotine? Trends Pharmacol Sci. 2002;23(2):78–82. [DOI] [PubMed] [Google Scholar]
- 59. Broadstock M. Systematic Review of the Health Effects of Modified Smokeless Tobacco Products. New Zealand: New Zealand Health Technology Assessment (NZHTA); 2007. [Google Scholar]
- 60. Rodu B, Godshall WT. Tobacco harm reduction: an alternative cessation strategy for inveterate smokers. Harm Reduct J. 2006;3(3):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kamel H, Healey JS. Cardioembolic stroke. Circ Res. 2017;120(3):514–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shah KK, Boreddy PR, Abbruscato TJ. Nicotine pre-exposure reduces stroke-induced glucose transporter-1 activity at the blood–brain barrier in mice. Fluids Barriers CNS. 2015;12(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data that underlie the results reported in this article (text, tables, and figures) has been deidentified. The joint dataset is available upon reasonable request, and a proposal to access the data should be directed to MO (PI: mayowaowolabi@yahoo.com). Data requestors will need to sign a data access agreement.


