Abstract
Snakebite envenoming is currently considered a neglected tropical disease, which affects over 5 million people worldwide, and causes almost 150 000 deaths every year, as well as severe injuries, amputations and other sequelae. Snakebite envenoming in children, although proportionally less frequent, is generally more severe, and represents an important challenge for pediatric medicine, since they often result in worse outcomes. In Brazil, given its ecological, geographic and socioeconomic characteristics, snakebites are considered an important health problem, presenting approximately 30 000 victims per year, approximately 15% of them in children. Even with low snakebite incidence, children tend to have higher snakebite severity and complications due to the small body mass and same venom volume inoculated in comparison to adults, even though, due to the lack of epidemiological information about pediatric snakebites and induced injuries, it is difficult to measure the treatment effectiveness, outcomes and quality of emergency medical services for snakebites in children. In this review, we report how Brazilian children are affected by snakebites, describing the characteristics of this affected population, clinical aspects, management, outcomes and main challenges.
Keywords: venom, snakebite, envenomation, infants, child, disabilities
INTRODUCTION
Snakebite envenoming (SBE) is a Category A neglected tropical disease that results from the inoculation of toxic venoms by a snake into humans, usually under accidental conditions [1, 2]. Snake venoms present high variability and complexity. Since they are rich sources of proteins and peptides targeting a wide range of tissue receptors, they result in a gamut of clinical effects [2, 3]. The events derived from SBEs range from local tissue damage, which eventually results in permanent sequelae, to systemic effects that include hemostatic abnormalities, acute kidney injury, rhabdomyolysis, neuroparalysis, hypotension, bradycardia and shock [1, 4–6]. Local and systemic alterations can be severe and require different health services, including hospitalization for long periods, surgical procedures and follow-up for rehabilitation [4, 7, 8]. In addition to the physical disabilities that can be permanent, SBE can lead to significant psychological trauma [9, 10].
SBEs prevail among young male adults from rural regions of low- and middle-income countries, with certain significant risks and poor access to medical care [8, 11–13]. However, children living in rural areas are also potential victims when participating in agricultural labor, or when exploring different environments while playing or walking around [14]. Indeed, the global burden of SBEs is large and, disproportionately, affects children living in low-income environments, resulting in long-term effects (e.g. amputations) and post-traumatic stress [4, 11–13]. Due to the smaller bodies and large proportional volume of injected venom, children have more severe presentations, such as faster and more severe neurotoxicity, coagulopathy and more severe local tissue damage even though, due to the lack of epidemiological information about pediatric snakebites and induced injuries, it is a challenge to measure the treatment effectiveness, outcomes and quality of emergency medical services for snakebites in children [14–19]. In this article, we review SBEs in Brazilian children and present information on clinical aspects, management and outcomes.
EPIDEMIOLOGY
Annually, approximately 5.4 million people worldwide are bitten by venomous snakes, which results in 2.7 million envenomings and up to 138 000 deaths [9]. As a problem with global and widespread impact, in 2017, SBE was designated by the World Health Organization (WHO) as a Category A disease in its list of neglected tropical diseases [1, 20, 21]. Despite their importance, the real burden remains unknown due to a remarkable underreporting [22]. Studies suggest that the number of people suffering from permanent sequelae could be as high as 400 000, and the associated number of disability-adjusted life years may add up to over 6 million [1, 15, 23–26]. Moreover, there are significant changes in the incidence of snakebites between different regions, being the highest in rural areas.
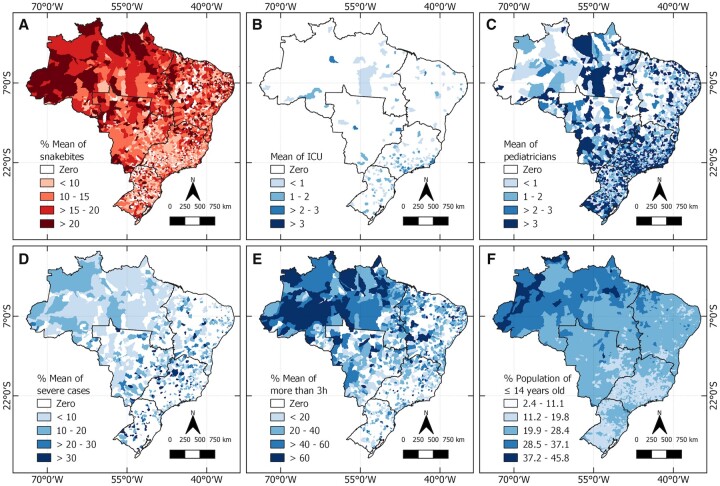
Ninety-five percent of snakebites happen in Africa, Asia and Latin America [15]. Because of their characteristic ecology and biogeography, tropical and subtropical developing countries, such as those in Latin America, have high incidence and mortality rates [1]. In Brazil, since 2010, all envenomings caused by venomous animals have been considered mandatory notification events. From 2012 to 2020, 404 311 cases of SBEs were reported to the Ministry of Health (MoH) [27], with 63 869 (15.8%) occurring in children below 14 years old [27]. Although there is a lack of epidemiological studies, the Brazilian incidence of snakebites is similar to other reports in the world showing around 15–30% of the total occurs in children [14, 16, 28]. In Brazil, the SBE incidence is much higher in the Amazon region compared to the central and southern regions of the country [29]. Figure 1 shows SBEs spatial distribution in Brazil and its relation to severity and access to health facilities. Regardless of the noteworthy higher incidence observed in the Amazon region, the number of intensive care units (ICUs) and pediatricians is proportionately lower in this region [30]. These characteristics and variations within countries must be considered when formulating public health policies and priorities at the national, regional and local levels.
Figure 1.
Snakebite envenomings in children and health facilities in Brazil. The mean percentage of snakebites (A), pediatric intensive care units (B), mean numbers of pediatricians (C), severe cases (D) and cases in which the patient took >3 h from health care to receive appropriate care were calculated (E). Finally, the distribution of the mean percentage of children up to 14 years old in Brazil was established (F). Data regarding the number of pediatricians and pediatric intensive care units (ICU-ped) in Brazil were obtained from the National Register of Health Establishments (CNES, http://cnes.datasus.gov.br/), while the estimated population up to 14 years old was obtained from the Department of Informatics of the Unified Health System (DATASUS, https://datasus.saude.gov.br/). Information regarding snakebites involving individuals aged up to 14 years old from 2012 to 2020, cases classified as severe, and time to reach health care facilities were collected from the Notifiable Diseases Information System [27]. The cartographic bases of Brazil and the macro-regions were obtained from the Brazilian Institute of Geography and Statistics (IBGE, https://www.ibge.gov.br/geociencias/downloads-geociencias.html). All descriptive analyses were performed using the R Studio software Version 1.4.1106, and the results were plotted on choropleth maps using the QGIS software version 3.18.
CIRCUMSTANCES Of SNAKEBITES AMONG CHILDREN
Although children are proportionally less exposed to snakebites than adult male individuals because of work-related exposure, records of envenoming in children occurred indoors, while playing or sleeping [31–33], and outdoors, when fishing or farming [34, 35]. Also, children are less likely to defend themselves or understand the potential risks of venomous snakes [36].
Children living in conservation units and indigenous lands are more susceptible to SBEs because of the overlap between these environments and those where venomous snakes are more prevalent [37, 38], leading to envenomation occurring in typical places and situations such as the backyards, routes to schools, forestry activities and fishing [34, 36, 39–42]. Moreno, et al. [41] reported that 18.7% of SBEs occurred in Rio Branco (Acre) students on their way to or from school, reflecting a permanent risk of being bitten by snakes during daily activities [37, 38].
Although more prevalent in rural areas, venomous snakes can also be found in certain places among the urban zones such as forest fragments, rivers, streams and wasteland, where they benefit from finding natural prey such as rodents and amphibians [43–45]. In Rio Branco (Acre), as in many other municipalities in the Amazonian region, there was an increase in snakebites in households [46]. Oliveira, et al. [46] observed a higher incidence of cases in children under 10 years old in the urban (23.94% of cases) compared to the rural zones (8.5%) in this state [46].
SPECIES INVOLVED IN SNAKEBITE ENVENOMING
In Brazil, Bothrops, Crotalus, Lachesis and Micrurus are the medically important snake genera [47]. Envenoming triggered by Bothrops genus corresponds to most cases of SBEs in Brazil due to the wide distribution of Bothrops snake genus throughout the national territory, represented by 29 species [48]. Moreover, of all snakes, Bothrops spp. appear to have a more defensive behavior, which makes them more likely to strike out, contributing to the high frequency of bites by this genus [34, 38, 47, 49]. Hence, the majority of clinical reports and case series studies of snakebites involving Brazilian children cite Bothrops as the cause (Table 1) [31, 34, 42].
Table 1.
Snakebite envenoming involving children according to snake species
| Snake | Age | Gender | Location of bite | State | Symptoms | Outcome | Reference |
|---|---|---|---|---|---|---|---|
| Sub-family Dipsadidae | |||||||
| Boiruna maculata | 1.3 | F | Ankle | RS | Pain, swelling, local erythema and mild cyanosis | Discharged | [33] |
| Helicops angulatus | 8 | M | Foot | AC | No | Discharged | [66] |
| Helicops angulatus | 9 | F | Foot | AC | No | Discharged | [66] |
| Philodryas olfersii (11 cases, 25.6%) | <9 | ? | ? | SP | ? | ? | [64] |
| Philodryas patagoniensis | 5 | M | Leg | MG | Swelling and warmth on the bitten limb | ? | [62] |
| Philodryas patagoniensis (60 cases) | <9 | ? | ? | SP | ? | No sequelae | [65] |
| Sub-family Elapidae | |||||||
| Micrurus averyi | 7 | F | Foot | AM | Intense local pain, paresthesia, edema, erythema, nausea and drooling | Discharged | [89] |
| Micrurus corallinus | 1 | M | Finger | RS | Acute myasthenia, ptosis, salivation and muscular weakness | ? | [36] |
| Micrurus corallinus | 2 | M | Forearm | SP | ? | ? | [60] |
| Micrurus corallinus | 4 | M | Finger | SP | ? | ? | [60] |
| Micrurus corallinus | 6 | M | Foot | SP | ? | ? | [60] |
| Micrurus corallinus | 11 | M | Hand | SP | ? | ? | [60] |
| Micrurus frontalis | 9 | M | Finger | SP | ? | ? | [60] |
| Sub-family Viperidae | |||||||
| Bothrops spp. (73 cases) | – | – | – | SP | Edema, pain, ecchymosis, blisters, necrosis, local and/or systemic bleeding, local infection, compartment syndrome, gangrene and acute renal failure | Two fibular nerve palsy and one amputation | [90] |
| Bothrops atrox | 10 | M | Arm | AC | ? | [34] | |
| Bothrops bilineatus | 13 | M | Chest | AC | ? | ? | [39] |
| Bothrops sp. | 13 | M | Foot | AC | ? | ? | [39] |
| Bothrops sp. | 14 | M | Foot | AC | ? | [39] | |
| Bothrops jararaca | 13 | M | Leg | SP | ? | [34] | |
| Botrops jararaca (11 cases) | – | – | – | SP | ? | ? | [90] |
| Bothrops jararacuçu | 3 | F | Thigh | SP | ? | [34] | |
| Bothrops jararacuçu | 11 | M | Foot | SP | ? | ? | [34] |
| Bothrops marajoensis | 10 | M | Foot | PA | Local pain edema, ecchymosis, blisters, somnolence, hematuria and a comatose state | Sequelae, right side flaccid hemiplegia | [42] |
| Bothrops marajoenis | 15 | M | Leg | PA | Intense local pain, edema, ecchymosis, local bleeding, nausea, vomiting, epigastralgia and altered urine color | Discharged | [35] |
| Bothrops moojeni | 5 | F | Eye | MG | Gross facial swelling, bilateral periorbital ecchymosis, exophthalmus and local bleeding | Eye enucleation | [31] |
| Bothrops neuwiedi | – | – | – | SP | ? | ? | [90] |
| Crotalus durissus (31 cases) | 1–14 | 25 M/6 F | Feet 12, legs 9 and ankles 7 | SP | Palpebral ptosis, myalgia, weakness, slight edema and erythema | ? | [52] |
| Crotalus durissus | 12 | M | ? | SP | ? | ? | [34] |
| Crotalus durissus | 13 | M | ? | SP | ? | ? | [34] |
, unknown information.
Bothrops atrox species cause most of the SBEs in the Amazon region with a significant, detrimental, economic and public health impact on rural communities [50, 51]. Campbell and Lamar reported a case of a child bitten by B. atrox while fishing in Rio Branco (Acre) whose outcome was arm amputation [34]. A 5-year-old girl was bitten in the eye by a Bothrops moojeni while sleeping in her bed in Campina Verde (Minas Gerais) and the enucleation of her eyeball was needed [31]. Pardal, et al. [42] and Silva and Pardal [35] reported two boys (10 and 15 years old, respectively) who were bitten by Bothrops marajoensis in the foot and in the leg, respectively, while carrying out açaí (the fruit from Euterpe oleracea—a palm tree) on Marajó Island (Pará). Another case of an SBE from an arboreal snake, Bothrops bilineatus, involved a 13-year-old boy bitten on the chest while climbing the açaí palm tree at the height of 4 m [39].
Rattlesnake (Crotalus durissus) envenomings are less common than those from Bothrops snakes, probably due to their geographic distribution, more frequent in open areas such as Cerrado and Caatinga biomes. There are no reports of envenoming from this species in forested areas of most of the Amazon and Atlantic Forests, since they usually colonize deforested areas [47]. Compared to Bothrops, rattlesnakes are less aggressive and usually make a sound with their rattle at the tip of their tail, thus alerting people to their presence [47]. In a case series published by Bucaretchi, et al. [52], in the region of Campinas (São Paulo), 31 cases involving children were described over a period of 16 years (1984–99). The authors reported rattlesnake envenoming involving children aged 1–14 years (mean age 8 years), mostly male [25], and in rural areas, including in backyards.
Bushmaster (Lachesis muta) envenoming is also less frequent due to the natural geographical distribution (Amazon and Atlantic Forests from the northeast of Brazil to Rio de Janeiro), overlapping with low population density forested areas. Also, their large size which is poorer to sight together with the less aggressive behavior contributes to the lower incidence [47]. Reports in the literature show that envenoming by Lachesis muta occurs mainly in adults who are generally more exposed due to activities within forests or due to keeping specimens in captivity [53, 54]. However, caution must be taken with the underestimated envenoming induced by Lachesis, including in children (3.9%) [55]. Because of the popular names, health professionals mistakenly attribute to B. atrox, envenoming possibly caused by L. muta [56, 57]; only one proven case of a 13-year-old child bitten in Recife (Pernambuco) is available in the literature [58], with no details on the circumstances involved.
Coral snakes (Micrurus and Leptomicrurus) present aposematic (warning) colors and secretive habits. Although the species are widely found in Brazil [47], elapid-derived envenomings represent less than 1% of Brazilian SBEs. The smallmouth and their proteroglyph dentition (functional tooth in front of their jaws) make it challenging to inoculate toxins, and most bites occur when these snakes are handled [47, 59, 60]. However, their color can be attractive [58], especially for younger children unaware of the danger. Mota-da-Silva, et al. [55] observed elapid envenoming in children in 6% of envenoming cases under 4 years old in Brazil, which corresponds to twice that recorded for other groups of snakes (Bothrops, Crotalus and Lachesis) in the same age group.
SBEs caused by Colubridae and Dipsadidae families, with opistoglyph dentition (functional tooth behind in their jaws), are less frequent and are generally milder cases, even though they may account for 40–56% of snakebites in some regions [45, 61]. In Brazil, snakes of the genus Philodryas (mainly Philodryas olfersii and Philodryas patagoniensis) are among the main species causing snakebites and can cause pain and edema like Bothrops envenoming [61–65]. Because of that and the likeness with that species, snakebites by Colubridae and Dipsadidae are sometimes mistakenly reported as Bothrops-derived envenoming [66]. The brown-banded water snake (Helicops angulatus) is the most involved in snakebites in urban areas in Rio Branco [41] and in Cruzeiro do Sul (Acre). There is scarce literature on details to understand the circumstances of SBEs in children (Table 1).
CLINICAL ASPECTS AND OUTCOMES
Overall clinical aspects
The snake venom inoculated by snakes induces a variety of pathophysiological events in victims. In Brazil, envenoming caused by the Viperidae family (i.e. Bothrops and Lachesis) is characterized by exacerbated local symptoms (swelling and pain) and coagulopathies [67, 68]. Also, envenoming from the Brazilian Crotalus genus has mostly systemic myotoxic and pre-synaptic neurotoxic action, with mild local symptoms. On the other hand, the Elapidae family-derived venom presents neurotoxins that affect the neuromuscular junction causing acute neuromuscular weakness with respiratory involvement [69]. Since the severity classification depends on medical assessment and snake identification, the clinical management depends on health professional’s ability to recognize them.
Differences between adults and children
The global burden of snakebite disproportionately affects low-income children and often leads to significant permanent physical and psychological sequelae [16, 17, 20, 26, 70, 71]. Although long-term sequelae in a child’s life are relevant, few studies describe the type of sequelae, details of causes, and frequency [71]. Bites to the hands or feet cause more disabilities in children than adults due to local tissue necrosis associated with compartment syndrome [72]. Edema causes blood vessel compression preventing extremity vascularization (compartment syndrome), exacerbated by vascular lesions caused by hemorrhages, inadequate treatments (e.g. tourniquets, which are commonly used in Brazil) or severe anemia caused by bleeding [71]. Amputation or loss of limb in children after snakebite is commonly caused by suspected compartment syndrome [73]. Capillary occlusion can occur at a pressure lower than that of the axial artery, so the diagnosis requires monitoring of intra-tissue pressure [74]. The interruption of the microcirculation in children causes a drop in blood pressure that can sometimes occur with compartment pressure thresholds higher than those observed in adults [75].
Lethality
Brazilian MoH data from 2007 to 2020 show that lethality and clotting abnormalities are similar between adults and children, except for children under 3 years old, for whom lethality rates are higher and unclottable blood is more usual. Other studies also show higher lethality rates in children and the elderly than adults [55].
Risk factors for severity
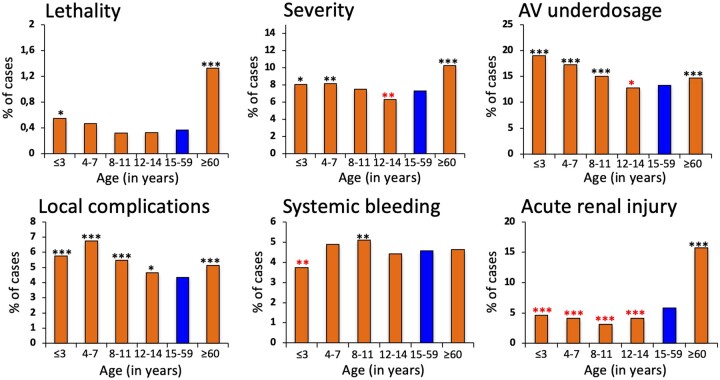
Severity and antivenom underdosing are more frequent in children over 7 and 11 years, respectively; however, it is less prevalent between 12 and 14 years. Local complications prevail among children of all ages, and delayed medical care is more frequent in children aged 4–14. Systemic bleeding is relevant in children between 8 and 11 years old but is significantly less frequent in children under three years old. Acute kidney injury is considerably less frequent in children (Figure 2 and Table 2).
Figure 2.
Distinct clinical aspects compared between children (under 14 years old), adults (between 15 and 59 years old) and the elderly (over 60 years old) in Brazil from 2007 to 2020. Comparison of outcome rates between age groups was made using Chi-square test, corrected by Fisher’s exact test if necessary. Group of adults (15–59 years old, in blue) was the reference for comparisons. Statistical significance was considered if p < 0.05 in statistical tests. The analysis was performed using STATA software (StataCorp. 2013: Release 13. College Station, TX, USA). Red asterisks indicate that the group had a significantly lower frequency than the reference group. Black asterisks indicate that the group had a significantly higher frequency than the reference group. *p < 0.01; **p < 0.001; ***p < 0.0001.
Table 2.
Factors associated with 42 257 Bothrops envenoming affecting children in Brazil, from 2007 to 2021, according to the epidemiological surveillance database
| Variable (completeness) | Total (%) | OR (95% CI) | p | aOR (95% CI) | p |
|---|---|---|---|---|---|
| Age (years) (100%) | |||||
| ≤3 | 6034 (14.3) | 1 | 1 | ||
| 4–7 | 8295 (19.6) | 1.01 (0.89–1.14) | 0.828 | … | … |
| 8–11 | 13 720 (32.5) | 0.93 (0.83–1.04) | 0.189 | 0.90 (0.71–1.14) | 0.393 |
| 12–14 | 14 208 (33.6) | 0.77 (0.68–0.86) | <0.001 | 0.71 (0.56–0.92) | 0.008 |
| Gender (99.9%) | |||||
| Male | 29 198 (69.1) | 1 | 1 | ||
| Female | 13 051 (30.9) | 0.89 (0.82–0.97) | 0.007 | 0.90 (0.75–1.06) | 0.207 |
| Area of occurrence (98.1%) | |||||
| Urban | 6453 (15.6) | 1 | 1 | ||
| Rural | 35 001 (84.4) | 1.56 (1.38–175) | <0.001 | 1.31 (1.03–1.67) | 0.030 |
| Ethnicity (93.2%) | |||||
| White | 8305 | 1 | 1 | ||
| Black | 2735 | 0.71 (0.60–0.84) | <0.001 | 1.06 (0.75–1.51) | 0.730 |
| Asian | 371 | 0.88 (0.60–1.29) | 0.505 | … | … |
| Brown | 24 943 | 0.67 (0.61–0.73) | <0.001 | 1.03 (0.83–1.29) | 0.766 |
| Amerindian | 3015 | 1.11 (0.96–1.28) | 0.140 | 1.27 (0.91–1.77) | 0.158 |
| Site of the bite (99%) | |||||
| Lower limbs | 36 047 | 1 | 1 | ||
| Upper limbs and trunk | 5796 | 1.38 (1.25–1.52) | <0.001 | 1.16 (0.93–1.43) | 0.167 |
| Blood clottability (49.4%) | |||||
| Clottable blood | 12 422 | 1 | 1 | ||
| Unclottable blood | 8467 | 3.71 (3.35–4.11) | <0.001 | 2.17(1.84–2.56) | <0.001 |
| Antivenom dosagea (99.9%) | |||||
| Correct dosage | 35 792 | 1 | 1 | ||
| Underdosage | 6458 | 5.61 (5.20–6.06) | <0.001 | 2.24 (2.16–2.32) | <0.001 |
| Time to care (94.6%) | |||||
| <3 h | 25 388 | 1 | 1 | ||
| >3 h | 14 590 | 1.61 (1.49–1.73) | <0.001 | 1.56 (1.32–1.84) | <0.001 |
Proportions of severe cases and deaths were compared using a Chi-square test (corrected by Fisher’s test if necessary); differences were considered statistically significant for p < 0.05. The crude odds ratio (OR) with its respective 95% confidence interval (95% CI) was determined considering severity as the dependent variable. Logistic regression was used for the multivariable analyses and the adjusted OR with 95% CI was also calculated. All variables associated with the outcomes at a significance level of p < 0.20 in the univariate analysis were included in the multivariate analysis. Statistical significance was considered if p < 0.05.
aOR, adjusted odds ratio.
Bold values indicate significant differences.
According to the recommendations of the Brazilian MoH.
In Brazil, surveillance data from 2007 to 2020 show that living in rural areas (OR 1.56, p < 0.001), unclottable blood (OR 3.72, p < 0.001), antivenom underdosing (OR 5.61, p < 0.001) and time to care >3 h (OR 1.61, p < 0.001) are considered risk factors for children envenoming severity following Bothrops accidents (Table 2). Nonetheless, the age group 12 to 14 years old presented a lower risk for severity compared to ≤3-year-old children. Living in rural areas, which is probably a proxy for insufficient access to the health system and antivenom unavailability, is considered a risk factor for snakebite severity. In addition, younger ages and male sex are risk factors for severity and more adverse outcomes. It is believed that young men are more prone to try to catch, kill or otherwise interfere with snakes while exploring the environments, which poses greater exposure to various snakes [76].
The time between the bite and medical care is a well-known risk factor for complications and fatalities. In Brazil, delays in medical assistance greater than 6 h after envenoming are frequent. Child mortality was approximately 3-times higher regardless of that time and the snake genera [27]. According to a study in Australia, 20% of children treated with antivenom with a confirmed SBE have not seen the snake [77].
Sequelae in children
In Brazil, there is a massive shortage of data regarding the disabilities in children caused by SBEs, mainly because the mandatory Notifiable Diseases Information System (SINAN) notification is filled out during hospitalization, and some sequelae can appear later. Of the few articles reporting SBE involving children in Brazil (Table 1), the main outcomes and sequelae were peroneal nerve palsy, amputation, flaccid hemiplegia and eye enucleation. A study in Costa Rica documented these sequelae in children who had suffered snakebites deforming scars, skin grafts, limited functional mobility, affected limb and amputation [21]. In India, long-term complications and sequelae in children related to hemotoxic snakebite include amputations and limb deformities, hypopituitarism, osteomyelitis, squamous cell carcinoma in non-healing ulcer sites, sequelae of acute coronary syndromes, such as left ventricular dysfunction and stroke sequelae, such as limb weakness or cognitive impairment [78].
Disabilities, ranging from hypertrophic scars to amputations, translate into long-term transient or permanent disability [73]. In children, the physical and psychosocial implications are difficult to define, but children who develop sequelae, as well as their families, undergo suffering and limitations, not only physical but also psychological. Children have reported that SBE impacts community, social and civic life, self-care, education, activities of daily living and their ability to communicate [79]. In addition, the costs of monitoring and the consequences of envenoming are high, both for health services and for the injured person [71, 80]. When it comes to children, it is necessary to consider the economic and educational impacts. SBEs with severe sequelae, such as amputations, cause the individual's impairments, preventing work and school activities. This seems to have a more significant impact on children than adults, given their longer life expectancy.
THERAPY
The World Health Organization (WHO) recommends the same protocol for adults and children under the same circumstances, although the amount of inoculated venom is not proportionally equal [81]. In Brazil, no specific guideline is available for snakebite envenoming in children.
Mainly in the Amazon rural areas, incisions, suction, tourniquets and other traditional therapies are typical, which can delay adequate clinical management and worsen the case, especially in children. Thus, it is essential to alert and educate the population about the risks associated with these practices. In addition, parents must be able to recognize the snake by bringing part of it or a photograph to the hospital to help the medical staff provide the appropriate management [69].
During the transfer to the health unit, the child must be kept calm and comfortable to reduce the hyperdynamic response that can accelerate the spread of the venom [82]. According to Parker-Cote and Meggs [82], immobilization of the bitten body part can lessen the lymphatic absorption of the venom. However, the clinical management is based on symptomatic medication, immunotherapy and adjuvant surgery. Symptomatic drugs include a range of analgesics, anti-inflammatory and antibiotics. Although the use of antibiotics is controversial, broad-spectrum antibiotics may be recommended for bacterial infections [83].
Antivenom serum is the specific treatment to reverse or prevent the effects of SBEs, being highly effective if administered promptly [82]. Essafti, et al. [84] demonstrated the benefit of antivenom therapy in children, decreasing the need for blood transfusion, fasciotomy and length of stay at ICU. Mortality and systemic complications were lower with specific immunotherapy but were not statistically significant due to the small sample size.
Antivenom should be administered intravenously by infusion or slow intravenous impulse. For intravenous infusion, pediatric burettes of 100 ml normal saline infusion bags are useful for dose dilution and volume-controlled administration in the absence of mechanical infusion pumps. If clinical manifestations do not resolve after antivenom infusion, a second dose should be considered [83]. However, acute allergic reactions are associated with antivenom occurring in 2–50% of cases depending on the type of antivenom and dose. Acute hypersensitivity reactions to Crotalidae Polyvalent Immune Fab (CPIF) are rare with the use of CPIF in pediatric patients. Two studies showed a prevalence of 7 and 19% of acute hypersensitivity reactions [85]. The frequency of hypersensitivity reaction in adults and children to antivenoms produced in Brazil is not known.
The treatment of early reactions in children includes interrupting antivenom (AV) infusion and administration of an initial dose of 0.01 mg/kg of epinephrine via the intramuscular route. In addition, hydrocortisone, at a dose of 4 mg/kg every 6 h, and promethazine are often used following epinephrine. To treat late reactions, oral antihistamine for 5 days, or a corticosteroid, such as prednisone, at a dose of 1 mg/kg per day for 5–7 days are recommended [69]. Thus, clinical trials are needed to better understand the appropriate treatment for children, due to their particularities.
THE LONG WAY FORWARD
As a medical emergency, SBEs require a quick response, with treatment using antivenoms within the first 6 h after the bite [11, 86]. However, for populations living in rural areas of Brazil, especially in the Amazon region, access to health centers and the antivenom can take days, with consequent complications and increased morbidity and lethality. In addition to the poor availability of antivenom, patients’ families may opt to search for traditional healers for first aid and treatment [37]. Traditional measures to deal with SBE include local incision(s), herbal medicine(s), tourniquet and suction application to the bite site; all of which are ineffective and can be harmful, leading to tendon, nerve or vascular injury, tissue necrosis, bleeding or infection. In addition, such measures can further delay access to antivenoms, thus resulting in increased severity and amputations, which can be more significant in children, and even death [14].
In Brazil, Butantan Institute is one of the institutions that manufactures antivenoms used to treat SBEs. The Institute has been recently modernized and currently produces five different antivenoms. However, there is still great difficulty in getting the antivenom for places far from the large cities, where most SBEs happen. In addition, antivenom preservation demands refrigerated storage, requiring electrical energy, which is not always available in rural healthcare units. Health professionals must be trained to diagnose SBE correctly, prescribe the proper type and amount of antivenom, and monitor adverse reactions. Considering the difficulties for patients to access the treatment, the antivenom supply system must be decentralized to reach the people who need it [87], which poses one of the biggest challenges in Brazil for managing and treating snakebites.
Prevention of SBEs is better than treating once a patient is envenomed. Therefore, SBEs must be avoided. Childhood is a period of life when knowledge and skills are learned, and the social context in which children are living can determine knowledge transmission and acquisition [1, 2]; thus, there is a need to educate children not to approach snakes and not to put their hands in holes or nests where snakes could be. Additionally, children must be taught preventive measures such as shoe use and the need to use lights in dark environments and at work. Although these are important recommendations, it is worth noting that they are not always in line with the socio-economic reality of some communities, as well as their cultural context. Therefore, recommendations for managing snakebites, including pediatric cases, must consider socio-economic, demographic, and cultural aspects. As a strategy for snakebite prevention and control, the WHO has used community involvement in the development of solutions for SBEs as well as ensure that programs instill community empowerment [14, 76, 81].
In rural and riverine communities, hunting and food gathering skills are essential for survivorship, especially for the poor. Children and adolescents constitute a necessary workforce within the family context of riverine populations that economically depend on the extraction of piassava (Attalea funifera). Therefore, these children are constantly exposed to a higher risk for malaria, leishmaniasis and SBEs [4, 71]. Child labor in Brazil is a harmful reality far from eradication, and such work activities generally occur in rural areas, the leading site of SBEs. Thus, it is necessary to combat this practice in addition to promoting personal protective equipment use, which is sometimes less frequently used by children.
In addition to socioeconomic issues, another critical point is the geographic distribution of physicians in Brazil. In November 2020, Brazil reached the historic milestone of having 500 000 physicians, thus representing a ratio of 2.4 physicians per 1000 inhabitants. However, the inequalities in the distribution of those professionals persist and are evident. As seen in Figure 1, the number of pediatricians and ICU beds in the northern region is lower, although the number of children and SBEs is much more significant. Thus, more equity of health professional’s distribution in different regions is necessary. Pediatricians are essential in the Amazon region, especially for managing snakebites in children [88].
CONCLUSIONS
SBEs in children tend to have higher severity and complications due to the small body mass and same venom volume inoculated in comparison to adults. In Brazil, well-identified risk factors for severity of envenomation include envenoming caused by pit vipers (e.g. Bothrops spp.), living in rural areas, bites occurring in upper limbs and trunk, having unclottable blood, receiving less than the required amount of antivenom and being more than 3 h away from health system facilities. Since they cause significant lethality, morbidity, and physical and psychological sequelae, SBEs have drastic economic and social impacts and further foster the vicious cycle of poverty and disease, especially in children affected at the very beginning of their lives.
Contributor Information
Isadora S Oliveira, Department of BioMolecular Sciences, School of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo, São Paulo 14040-903, Brazil.
Manuela B Pucca, Medical School, Federal University of Roraima, Boa Vista, Roraima 69310-000, Brazil; Health Sciences Postgraduate Program, Federal University of Roraima, Boa Vista, Roraima 69310-000, Brazil.
Felipe A Cerni, Health Sciences Postgraduate Program, Federal University of Roraima, Boa Vista, Roraima 69310-000, Brazil.
Samuel Vieira, Medical School, Federal University of Roraima, Boa Vista, Roraima 69310-000, Brazil.
Jacqueline Sachett, School of Health Sciences, Amazonas State University, Manaus, Amazonas 69065-001, Brazil; Department of Teaching and Research, Alfredo da Matta Foundation, Manaus, Amazonas 69065-130, Brazil.
Altair Seabra de Farias, School of Health Sciences, Amazonas State University, Manaus, Amazonas 69065-001, Brazil.
Marcus Lacerda, Instituto Leônidas & Maria Deane, Fiocruz Amazônia, Manaus, Amazonas 69040-000, Brazil; Department of Teaching and Research, Dr. Heitor Vieira Dourado Tropical Medicine Foundation, Manaus, Amazonas 69040-000, Brazil.
Felipe Murta, Department of Teaching and Research, Dr. Heitor Vieira Dourado Tropical Medicine Foundation, Manaus, Amazonas 69040-000, Brazil.
Djane Baia-da-Silva, School of Health Sciences, Amazonas State University, Manaus, Amazonas 69065-001, Brazil; Department of Teaching and Research, Dr. Heitor Vieira Dourado Tropical Medicine Foundation, Manaus, Amazonas 69040-000, Brazil.
Thiago Augusto Hernandes Rocha, Duke Global Health Institute, Duke University, Durham, NC, USA.
Lincoln Luís Silva, Graduate Program in Biosciences and Physiopathology, State University of Maringá, Paraná, Brazil.
Quique Bassat, ISGlobal, Hospital Clínic - Universitat de Barcelona, Barcelona, Spain; Centro de Investigação em Saúde de Manhiça (CISM), Maputo, Mozambique; ICREA, Barcelona 08010, Spain; Pediatrics Department, Hospital Sant Joan de Déu, Universitat de Barcelona, Barcelona, Spain; Consorcio de Investigación Biomédica en Red de Epidemiología y Salud Pública (CIBERESP), Madrid, Spain.
João Ricardo Nickenig Vissoci, Duke Global Health Institute, Duke University, Durham, NC, USA; Graduate Program in Biosciences and Physiopathology, State University of Maringá, Paraná, Brazil; Department of Emergency Medicine, Duke University School of Medicine, Durham, NC, USA.
Charles J Gerardo, Duke Global Health Institute, Duke University, Durham, NC, USA; Graduate Program in Biosciences and Physiopathology, State University of Maringá, Paraná, Brazil.
Vanderson Souza Sampaio, School of Health Sciences, Amazonas State University, Manaus, Amazonas 69065-001, Brazil; Department of Teaching and Research, Dr. Heitor Vieira Dourado Tropical Medicine Foundation, Manaus, Amazonas 69040-000, Brazil; Instituto Todos pela Saúde, São Paulo, Brazil.
Fan Hui Wen, Instituto Butantan, São Paulo, Brazil.
Paulo S Bernarde, Laboratório de Herpetologia, Campus Floresta, Federal University of Acre, Cruzeiro do Sul CEP, Acre, Brazil.
Wuelton M Monteiro, School of Health Sciences, Amazonas State University, Manaus, Amazonas 69065-001, Brazil; Department of Teaching and Research, Dr. Heitor Vieira Dourado Tropical Medicine Foundation, Manaus, Amazonas 69040-000, Brazil.
FUNDING
Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), São Paulo Research Foundation [scholarship to ISO No. 2020/13176-3] and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), The National Council for Scientific and Technological Development [scholarship to MP No. 307184/2020-0, WM No. 309207/2020-7, PB 311509/2020-7, ML No. 314424/2021-0 and VS No. 303146/2021-4]. W.M. acknowledges funding support from Fundação de Amparo à Pesquisa do Estado do Amazonas [PAPAC 005/2019, PRO-ESTADO and POSGRAD calls].
REFERENCES
- 1. Gutiérrez JM, Calvete JJ, Habib AG, et al. Snakebite envenoming. Nat Rev Dis Primers 2017;3:17063. [DOI] [PubMed] [Google Scholar]
- 2. Calvete JJ. Proteomic tools against the neglected pathology of snake bite envenoming. Expert Rev Proteomics 2011;8:739–58. [DOI] [PubMed] [Google Scholar]
- 3. Warrell DA. Snake bite. Lancet 2010;375:77–88. [DOI] [PubMed] [Google Scholar]
- 4. Gutiérrez JM, Theakston RDG, Warrell DA. Confronting the neglected problem of snake bite envenoming: the need for a global partnership. PLoS Med 2006;3:e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Islam K, Seth S, Roy A, et al. Predictors of renal complications in children with hematotoxic snakebite. Indian Pediatr 2020;57:427–30. [PubMed] [Google Scholar]
- 6. Shin Y, Jang Y, Borzée A. Snakebite envenomings in the Republic of Korea from the 1970s to the 2020s: a review. Toxicon 2021;196:8–18. [DOI] [PubMed] [Google Scholar]
- 7. Burki T. Resolution on snakebite envenoming adopted at the WHA. Lancet 2018;391:2311. [DOI] [PubMed] [Google Scholar]
- 8. Magalhães SFV, Peixoto HM, Sachett JAG, et al. Snakebite envenomation in the Brazilian Amazon: a cost-of-illness study. Trans R Soc Trop Med Hyg 2020;114:635–42. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization. Snakebite envenoming [Internet]. 2021. [cited 24 December 2021]. https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming (20 March 2022, date last accessed).
- 10. Habib AG, Brown NI. The snakebite problem and antivenom crisis from a health-economic perspective. Toxicon 2018;150:115–23. [DOI] [PubMed] [Google Scholar]
- 11. Feitosa EL, Sampaio VS, Salinas JL, et al. Older age and time to medical assistance are associated with severity and mortality of snakebites in the Brazilian Amazon: a case-control study. PLoS One 2015;10:e0132237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Graciano S, Coelho M, Teixeira A, et al. Perfil epidemiológico dos acidentes ofídicos em homens. Rev Enf Ref 2013;III Série:89–98. [Google Scholar]
- 13. Albuquerque PLMM, Jacinto CN, Silva GB Jr, et al. Acute kidney injury caused by Crotalus and Bothrops snake venom: a review of epidemiology, clinical manifestations and treatment. Rev Inst Med Trop Sao Paulo 2013;55:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pach S, Le Geyt J, Gutiérrez JM, et al. Paediatric snakebite envenoming: the world’s most neglected “Neglected Tropical Disease.” Arch Dis Child 2020;105:1135–9. [DOI] [PubMed] [Google Scholar]
- 15. Mohapatra B, Warrell DA, Suraweera W, et al. ; Million Death Study Collaborators. Snakebite mortality in India: a nationally representative mortality survey. PLoS Negl Trop Dis 2011;5:e1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kshirsagar VY, Ahmed M, Colaco SM. Clinical profile of snake bite in children in rural India. Iran J Pediatr 2013;23:632–6. [PMC free article] [PubMed] [Google Scholar]
- 17. Le Geyt J, Pach S, Gutiérrez JM, et al. Paediatric snakebite envenoming: recognition and management of cases. Arch Dis Child 2021;106:14–9. [DOI] [PubMed] [Google Scholar]
- 18. Janes DN, Bush SP, Kolluru GR. Large snake size suggests increased snakebite severity in patients bitten by rattlesnakes in Southern California. Wilderness Environ Med 2010;21:120–6. [DOI] [PubMed] [Google Scholar]
- 19. Gerardo CJ, Vissoci JRN, Evans CS, et al. Does this patient have a severe snake envenomation?: The rational clinical examination systematic review. JAMA Surg 2019;154:346–54. [DOI] [PubMed] [Google Scholar]
- 20. Chippaux JP. Snakebite envenomation turns again into a neglected tropical disease!. J Venom Anim Toxins Incl Trop Dis 2017;23:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brenes-Chacón H, Gutiérrez JM, Camacho-Badilla K, et al. Snakebite envenoming in children: a neglected tropical disease in a Costa Rican pediatric tertiary care center. Acta Trop 2019;200:105176. [DOI] [PubMed] [Google Scholar]
- 22. Gutiérrez JM, Solano G, Pla D, et al. Assessing the preclinical efficacy of antivenoms: from the lethality neutralization assay to antivenomics. Toxicon 2013;69:168–79. [DOI] [PubMed] [Google Scholar]
- 23. Habib AG, Kuznik A, Hamza M, et al. Snakebite is under appreciated: appraisal of burden from West Africa. PLoS Negl Trop Dis 2015;9:e0004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knudsen C, Jürgensen JA, Føns S, et al. Snakebite envenoming diagnosis and diagnostics. Front Immunol 2021;12:1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fox S, Rathuwithana AC, Kasturiratne A, et al. Underestimation of snakebite mortality by hospital statistics in the Monaragala District of Sri Lanka. Trans R Soc Trop Med Hyg 2006;100:693–5. [DOI] [PubMed] [Google Scholar]
- 26. Kasturiratne A, Wickremasinghe AR, de Silva N, et al. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med 2008;5:1591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ministério da Saúde. Acidentes por animais peçonhentos - Notificações registradas no Sistema de Informação de Agravos de Notificação - Brasil [Internet]. 2022. [cited 26 April 2022]. http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sinannet/cnv/animaisbr.def (20 March 2022, date last accessed).
- 28. Paudel K, Sharma S. Study of clinico-epidemiological profile and outcome of poisonous snake bites in children. J Nepal Paedtr Soc 1970;32:47–52. [Google Scholar]
- 29. Oliveira RS, Wen FH, Sifuentes DN. Epidemiologia dos acidentes por animais peconhentos. In: Cardoso JLC, França FOS, Fan HW, Málaque CMS, Haddad Jr. V. (eds.) Animais Peconhentos No Brasil Biología, Clínica E Terapeutica Dos Acidentes. Sao Paulo, Brasil: Sarvier Editora, 2009, 6–21. [Google Scholar]
- 30.Ministério da Saúde. Cadastro Nacional de Estabeleciemntos de Saúde [Internet]. 2022. [cited 9 October 2022]. http://cnes.datasus.gov.br.
- 31. Brandão ÉO, de Bastos HC, de Nishioka SA, et al. Lance-headed viper (Bothrops moojeni) bite wounding the eye. Rev Inst Med Trop Sao Paulo 1993;35:381–3. [DOI] [PubMed] [Google Scholar]
- 32. Fritts TH, McCoid MJ, Haddock RL. Risks to infants on Guam from bites of the brown tree snake (Boiga irregularis). Am J Trop Med Hyg 1990;42:607–11. [DOI] [PubMed] [Google Scholar]
- 33. dos Santos-Costa MC, Outeiral AB, D'Agostini FM, et al. Envenomation by the neotropical colubrid Boiruna maculata (Boulenger, 1896): a case report. Rev Inst Med Trop Sao Paulo 2000;42:283–6. [DOI] [PubMed] [Google Scholar]
- 34. Campbell JA, Lamar WW. The Venomous Reptiles of the Western Hemisphere. New York, USA: Cornell University Press, Ithaca, 2004. [Google Scholar]
- 35. EO da Silva, Pardal PP, de O. Envenenamento por serpente Bothrops no município de Afuá, Ilha de Marajó, estado do Pará, Brasil. Revista Pan-Amazônica de Saúde [Internet]. 2018;9:57–62. http://scielo.iec.gov.br/scielo.php?script=sci_arttext&pid=S2176-62232018000300007&lng=pt&nrm=iso. [Google Scholar]
- 36. Bucaretchi F, Capitani EMD, Vieira RJ, et al. Coral snake bites (Micrurus spp.) in Brazil: a review of literature reports. Clin Toxicol (Phila) 2016;54:222–34. [DOI] [PubMed] [Google Scholar]
- 37. Mota-da-Silva A, Colombini M, Moura-da-Silva AM, et al. Ethno-knowledge and attitudes regarding snakebites in the Alto Juruá region, Western Brazilian Amazonia. Toxicon 2019;171:66–77. [DOI] [PubMed] [Google Scholar]
- 38. da Silva JL, da Siva AM, do Amaral GLG, et al. The deadliest snake according to ethnobiological perception of the population of the Alto Juruá region, western Brazilian Amazonia. Rev Soc Bras Med Trop [Internet]. 2020;53:e20190305. http://www.scielo.br/j/rsbmt/a/FMPTvd6ZBH4fsJCSvt5MpRF/abstract/?lang=en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mota-da-Silva A, Sachett J, Monteiro WM, et al. Extractivism of palm tree fruits: a risky activity because of snakebites in the state of Acre. Rev Soc Bras Med Trop 2019;52:e20180195. [DOI] [PubMed] [Google Scholar]
- 40. Martins BF, Campos AP dos S, Seleghim MR, et al. Accidents caused by snakes (Bothrops spp. and Crotallus spp.) in children: report of two cases. Rev Rene 2012;13:693–703. [Google Scholar]
- 41. Moreno E, Queiroz-Andrade M, Lira-da-Silva RM, et al. Características clínicoepidemiológicas dos acidentes ofídicos em Rio Branco, Acre. Rev Soc Bras Med Trop 2005;38:15–21. [DOI] [PubMed] [Google Scholar]
- 42. Pardal PP de O, Pinheiro ACJ, da S, et al. Hemorrhagic stroke in children caused by Bothrops marajoensis envenoming: a case report. J Venom Anim Toxins Incl Trop Dis [Internet]. 2015; 21:53. http://www.scielo.br/j/jvatitd/a/ZnxHSn6DFFF4cjsJX54vYyr/?lang=en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Puorto G, Laporta-Ferreira IL, Sazima I. Serpentes na selva de pedra. Ciência Hoje 1991;13:66–7. [Google Scholar]
- 44. Brites VLC, Bauab FA. Fauna ofidiana do município de Uberlândia, Minas Gerais - Brasil. I. Ocorrência na área urbana. Rev Cent Ciên Bioméd Univ Fed Uberlândia 1988;4:3–8. [Google Scholar]
- 45. de Carvalho MA, Nogueira F. Serpentes da área urbana de Cuiabá, Mato Grosso: aspectos ecológicos e acidentes ofídicos associados. Cad Saude Publica 1998;14:753–63. [DOI] [PubMed] [Google Scholar]
- 46. Oliveira LP de M, JG do V, Sachett J de AG, et al. Snakebites in Rio Branco and surrounding region, Acre, Western Brazilian Amazon. Rev Soc Bras Med Trop [Internet]. 2020; 53:e20200214. https://www.scielo.br/j/rsbmt/a/KWHk9TvwK7QqsC5QxtFjXGQ/?lang=en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bernarde PS. Serpentes Peçonhentas e Acidentes Ofídicos no Brasil. São Paulo: Anolis Books, 2014, 223 p. [Google Scholar]
- 48. Costa HC, Guedes TB, Bérnils RS. Lista de Répteis do Brasil: padrões e tendências. Herpetologia Brasileira [Internet] 2022. [cited 9 February 2022]; 10. https://zenodo.org/record/5838950. [Google Scholar]
- 49. Martins M, Oliveira ME. Natural history of snakes in forests of the Manaus region, Central Amazonia, Brazil. Herpetolog Natural Hist 1998;6:78–150. [Google Scholar]
- 50. Magalhães SFV, Peixoto HM, Moura N, et al. Snakebite envenomation in the Brazilian Amazon: a descriptive study. Trans R Soc Trop Med Hyg 2019;113:143–51. [DOI] [PubMed] [Google Scholar]
- 51. Feitosa ES, Sampaio V, Sachett J, et al. Snakebites as a largely neglected problem in the Brazilian Amazon: highlights of the epidemiological trends in the State of Amazonas. Rev Soc Bras Med Trop 2015;48(Suppl. 1):34–41. [DOI] [PubMed] [Google Scholar]
- 52. Bucaretchi F, Herrera SRF, Hyslop S, et al. Snakebites by Crotalus durissus spp in children in Campinas, São Paulo, Brazil. Rev Inst Med Trop Sao Paulo 2002;44:133–8. [DOI] [PubMed] [Google Scholar]
- 53. Jorge MT, Sano-Martins IS, Tomy SC, et al. Snakebite by the bushmaster (Lachesis muta) in Brazil: case report and review of the literature. Toxicon 1997;35:545–54. [DOI] [PubMed] [Google Scholar]
- 54. Souza RCG. Aspectos clínicos do acidente laquético. In: Animais Peçonhentos No Brasil: Biologia, Clínica e Terapêutica Dos acidentes. 2nd ed. São Paulo, Brazil: Editora Sarvier, 2009, 96–107. [Google Scholar]
- 55. Mota-da-Silva A, Bernarde PS, de Abreu LC. Accidents with poisonous animals in Brazil by age and sex. J Hum Growth Dev 2015;25:54. [Google Scholar]
- 56. Bernarde PS, Gomes JO. Serpentes peçonhentas e ofidismo em Cruzeiro do Sul, Alto Juruá, Estado do Acre, Brasil. Acta Amaz 2012;42:65–72. [Google Scholar]
- 57. Mota-da-Silva A, Monteiro WM, Bernarde PS. Popular names for bushmaster (Lachesis muta) and lancehead (Bothrops atrox) snakes in the Alto Juruá region: repercussions for clinical-epidemiological diagnosis and surveillance. Rev Soc Bras Med Trop 2019;52:e-20180140. [DOI] [PubMed] [Google Scholar]
- 58. de Lima PHS, Haddad Junior V. A snakebite caused by a bushmaster (Lachesis muta): report of a confirmed case in State of Pernambuco. Rev Soc Bras Med Trop 2015;48:636–7. [DOI] [PubMed] [Google Scholar]
- 59. Melgarejo AR. Serpentes peçonhentas do Brasil. In: Animais Peçonhentos No Brasil: Biologia, Clínica e Terapêutica Dos acidentes. 2nd ed. São Paulo - Brazil: Sarvier Editora, 2009, 42–70. [Google Scholar]
- 60. Risk JY, Cardoso JLC, Sueiro LR, et al. Acidentes com cobras-corais e o Instituto Butantan. In: As Cobras-Corais Do Brasil - Biologia, Taxonomia, Venenos e envenenamentos. Goiânia - Brazil: Editora PUC, 2016, 382–415. [Google Scholar]
- 61. Albolea ABP, Salomão MG, Almeida-Santos SM. Why do non-poisonous snakes cause snakebites? Toxicon 2000;38:567–8. [Google Scholar]
- 62. Nishioka SA, Silveira PV. Philodryas patagoniensis bite and local envenoming. Rev Inst Med Trop Sao Paulo 1994;36:279–81. [DOI] [PubMed] [Google Scholar]
- 63. Ribeiro LA, Puorto G, Jorge MT. Acidentes por serpentes do gênero Philodryas: avaliação de 132 casos. Revista da Sociedade Brasileira de Medicina Tropical 1994;27(Suppl. I):87.8073157 [Google Scholar]
- 64. Ribeiro LA, Puorto G, Jorge MT. Bites by the colubrid snake Philodryas olfersii: a clinical and epidemiological study of 43 cases. Toxicon 1999;37:943–8. [DOI] [PubMed] [Google Scholar]
- 65. Medeiros CR, Hess PL, Nicoleti AF, et al. Bites by the colubrid snake Philodryas patagoniensis: a clinical and epidemiological study of 297 cases. Toxicon 2010;56:1018–24. [DOI] [PubMed] [Google Scholar]
- 66. Mota-da-Silva A, Mendes VK da G, Monteiro WM, et al. Non-venomous snakebites in the Western Brazilian Amazon. Rev Soc Bras Med Trop [Internet]. 2019. [cited 10 November 2021]; 52. http://www.scielo.br/j/rsbmt/a/3pjyw57bfnDJPb3PCJqrFPr/abstract/?lang=en. [DOI] [PubMed] [Google Scholar]
- 67. Oliveira FN, Brito MT, de Morais ICO, et al. Accidents caused by Bothrops and Bothropoides in the State of Paraiba: epidemiological and clinical aspects. Rev Soc Bras Med Trop 2010;43:662–7. [DOI] [PubMed] [Google Scholar]
- 68. Monteiro WM, Contreras-Bernal JC, Bisneto PF, et al. Bothrops atrox, the most important snake involved in human envenomings in the Amazon: how venomics contributes to the knowledge of snake biology and clinical toxinology. Toxicon: X 2020;6:100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fundação Nacional de Saúde (Brazil). Manual de Diagnóstico e Tratamento de Acidentes Por Animais Peçonhentos. Brasília: Ministério da Saúde, Fundação Nacional de Saúde, 2001. [Google Scholar]
- 70. Harrison RA, Hargreaves A, Wagstaff SC, et al. Snake envenoming: a disease of poverty. PLoS Negl Trop Dis 2009;3:e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gras S, Plantefève G, Baud F, et al. Snakebite on the hand: lessons from two clinical cases illustrating difficulties of surgical indication. J Venom Anim Toxins Incl Trop Dis 2012;18:467–77. [Google Scholar]
- 72. Quiroga M, Avila-Agüero ML, Faingezicht I. Abscess secondary to facial snakebite. J Venom Anim Toxins 2000;6:261–70. [Google Scholar]
- 73. Mars M, Hadley GP. Raised compartmental pressure in children: a basis for management. Injury 1998;29:183–5. [DOI] [PubMed] [Google Scholar]
- 74. Hachimi K, Fnini S, EL Andaloussi Y, et al. Envenimations par morsure de serpents et syndrome de loge. Chirurgie de la Main 2005;24:184–6. [DOI] [PubMed] [Google Scholar]
- 75. Berdai MA, Labib S, Harandou M. Snake bites in children at the Fez University Hospital in Morocco. Med Sante Trop 2013;23:427–32. [DOI] [PubMed] [Google Scholar]
- 76. Schulte J, Domanski K, Smith EA, et al. Childhood victims of snakebites: 2000-2013. Pediatrics 2016;138:e20160491. [DOI] [PubMed] [Google Scholar]
- 77. Mead HJ, Jelinek GA. Suspected snakebite in children: a study of 156 patients over 10 years. Med J Aust 1996;164:467–70. [DOI] [PubMed] [Google Scholar]
- 78. Menon JC, Joseph JK. Complications of hemotoxic snakebite in India. In: Gopalakrishnakone P, Faiz A, Fernando R, Gnanathasan CA, Habib AG, Yang CC (eds) Clinical Toxinology in Asia Pacific and Africa. Dordrecht: Springer Netherlands, 2015. [cited 27 December 2021], 209–32. http://link.springer.com/10.1007/978-94-007-6386-9_22. [Google Scholar]
- 79. Tupetz A, Phillips AJ, Kelly PE, et al. Contextualizing the impact of snakebite envenoming on patients: a qualitative content analysis of patient-specific functional scale activities using the international classification of functioning, disability and health. Int J Environ Res Public Health 2021;18:9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jayawardana S, Arambepola C, Chang T, et al. Long-term health complications following snake envenoming. J Multidiscip Healthc 2018;11:279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. World Health Organization. Guidelines for the Management of Snake Bites. [Internet]. Regional Office for South-East Asia: World Health Organization, 2010. https://apps.who.int/iris/handle/10665/204464. [Google Scholar]
- 82. Parker-Cote J, Meggs WJ. First aid and pre-hospital management of venomous snakebites. Trop Med Infect Dis 2018;3:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Avau B, Borra V, Vandekerckhove P, et al. The treatment of snake bites in a first aid setting: a systematic review. PLoS Negl Trop Dis 2016;10:e0005079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Essafti M, Fajri M, Rahmani C, et al. Snakebite envenomation in children: an ongoing burden in Morocco. Ann Med Surg (Lond) 2022;77:103574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Corbett B, Otter J, Masom CP, et al. Prevalence of acute hypersensitivity reactions in pediatric patients receiving Crotalidae polyvalent immune fab. J Med Toxicol 2021;17:48–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cristino JS, Salazar GM, Machado VA SR, et al. A painful journey to antivenom: the therapeutic itinerary of snakebite patients in the Brazilian Amazon (The QUALISnake Study) . PLoS Negl Trop Dis 2021;15:e0009245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wen FH, Monteiro WM. History and perspectives on how to ensure antivenom accessibility in the most remote areas in Brazil. Toxicon 2018;151:15–23. [DOI] [PubMed] [Google Scholar]
- 88. Scheffer M, Cassenote A, Guerra A, et al. Demografia Médica no Brasil 2020. São Paulo, SP: Faculdade de Medicina da USP, Conselho Federal de Medicina, 2020, 312 p. [Google Scholar]
- 89. da Silva IM, Bernal JC, Gonçalves Bisneto PF, et al. Snakebite by Micrurus averyi (Schmidt, 1939) in the Brazilian Amazon basin: case report. Toxicon 2018;141:51–4. [DOI] [PubMed] [Google Scholar]
- 90. Bucaretchi F, Herrera SRF, Hyslop S, et al. Snakebites by Bothrops spp in children in Campinas, São Paulo. Brazil. Rev Inst Med Trop Sao Paulo 2001;43:329–33. [DOI] [PubMed] [Google Scholar]