Abstract
The goal of this narrative review is to summarize the effects of prolonged fasting on various metabolic health measures, including body weight, blood pressure, plasma lipids, and glycemic control. Prolonged fasting is characterized by consciously eating little to no food or caloric beverages for several days to weeks. Results reveal that prolonged fasting for 5–20 days produces potent increases in circulating ketones, and mild to moderate weight loss of 2–10%. Approximately two-thirds of the weight lost is lean mass, and one-third is fat mass. The excessive lean mass loss suggests that prolonged fasting may increase the breakdown of muscle proteins, which is a concern. Systolic and diastolic blood pressure consistently decreased with prolonged fasting. However, the impact of these protocols on plasma lipids is less clear. While some trials demonstrate decreases in LDL cholesterol and triglycerides, others show no benefit. With regard to glycemic control, reductions in fasting glucose, fasting insulin, insulin resistance, and glycated hemoglobin (HbA1c) were noted in adults with normoglycemia. In contrast, these glucoregulatory factors remained unchanged in patients with type 1 or type 2 diabetes. The effects of refeeding were also examined in a few trials. It was shown that 3–4 months after the fast was completed, all metabolic benefits were no longer observed, even when weight loss was maintained. With regard to adverse events, metabolic acidosis, headaches, insomnia, and hunger were observed in some studies. In summary, prolonged fasting appears to be a moderately safe diet therapy that can produce clinically significant weight loss (>5%) over a few days or weeks. However, the ability of these protocols to produce sustained improvements in metabolic markers warrants further investigation.
Keywords: body weight, blood pressure, Buchinger fasting, cholesterol, insulin sensitivity, ketones, metabolic disease, prolonged fasting, safety, water fasting
INTRODUCTION
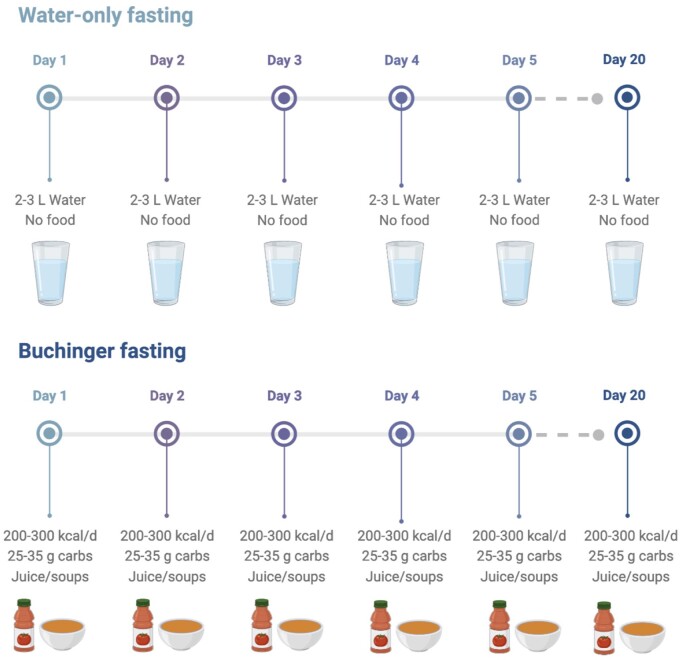
In recent decades, there has been a tremendous interest in the health benefits of fasting.1 Intermittent fasting, which involves short periods of food abstention for 16 to 36 hours, is the most popular form of fasting used today.2 However, more recently, “prolonged fasting” has gained substantial interest.3 Prolonged fasting is characterized by consciously eating little to no food or caloric beverages for several days to several weeks. The main types of prolonged fasting are water-only fasting and Buchinger fasting (Figure 1). During water-only fasting, participants consume only mineral water or distilled water daily (∼2–3 L) without any food intake. Buchinger fasting, on the other hand, is a medically supervised program that is popular in central Europe. The protocol was developed by Dr. Otto Buchinger in the 1920s. During this fasting protocol, small amounts of food are permitted daily. Specifically, participants consume 250 mL of fruit or vegetable juice as a lunch and 250 mL of vegetable soup as a dinner, leading to an average total calorie intake of 200–300 kcal and 25–35 g of carbohydrates per day.3 Although the data are limited, accumulating evidence shows that water-only fasting and Buchinger fasting produce several health benefits, including weight loss, abdominal fat loss, blood pressure reductions, and insulin sensitivity improvements after 5 to 20 days.3–5
Figure 1.
Types of prolonged fasting. Water-only fasting generally involves consuming 2–3 L of mineral water or distilled water per day, with no food intake. The Buchinger fasting program involves consuming 250 mL of fruit or vegetable juice as a lunch and 250 mL of vegetable soup as a dinner, leading to an average total calorie intake of 200–300 kcal and 25–35 g of carbohydrates per day. These protocols can last for several days to several weeks. Abbreviation: carbs, carbohydrates.
The mechanism responsible for these beneficial effects most likely involves flipping the metabolic switch.6,7 After 12 to 36 hours of fasting, the body switches from utilizing glucose (from glycogen stores) to fatty acids, and fatty-acid–derived ketones for energy. Adipocytes liberate free fatty acids, which are then transported to the liver and converted into ketones (ie, beta-hydroxybutyrate and acetoacetate). Ketone levels generally reach a plateau after 5 to 10 days of prolonged fasting and serve as the primary energy source for the body for up to 90 days. In addition to acting as a primary energy source, ketones also have direct physiological benefits. Augmented levels of circulating ketones can decrease blood pressure and increase vascular function.8,9 High levels of ketones can also reduce cardiac inflammation and blunt the development of heart failure.10 Ketones may also improve insulin sensitivity by increasing insulin receptor activity through the action of activating AMP-activated protein kinase (AMPK) and downregulating mammalian target of rapamycin (mTOR).11 In addition, high levels of ketones can potentially decrease appetite, which can further promote weight loss.12
Although several favorable effects of prolonged fasting have been observed, these benefits must be weighed against the risks. Most medically supervised fasting programs have reported only minor adverse events, including hunger, headaches, nausea, vomiting, dry mouth, and fatigue.4,5 However, more severe events have also been documented, including edema,13 abnormal liver function tests,14 decreased bone density,14 and metabolic acidosis.15 Thus, an in-depth examination into the safety of these fasting protocols is warranted.
Accordingly, the goal of this narrative review is to summarize the effects of prolonged fasting on various metabolic health measures, including body weight, blood pressure, plasma lipids, fatty liver index, and glycemic control. The effects of refeeding, after the fast is completed, will also be discussed. Pertinent safety considerations will also be reviewed.
METHODS—HUMAN TRIAL SELECTION
A PubMed, Embase, and Cochrane Library search was conducted using the following key words: “prolonged fasting,” “water fasting,” “Buchinger fasting,” “weight loss,” “body weight,” “body composition,” “visceral fat,” “cholesterol,” “lipids,” “blood pressure,” “glucose,” “insulin,” “glucoregulatory factors,” “insulin resistance,” “insulin sensitivity,” “safety,” “fatty liver,” and “adverse events.” Inclusion criteria for research articles were as follows: (1) randomized trials or nonrandomized trials; (2) adult participants with normal weight, overweight, or obesity; and (3) endpoints that included changes in body weight and at least 1 relevant metabolic disease risk marker. The following exclusion criteria were applied: (1) fasting performed as a religious practice (Islam or Seventh-Day Adventist); (2) intermittent fasting protocols, such as alternate-day fasting or time-restricted eating; (3) periodic fasting protocols, such as the fasting mimicking diet; and (4) trials that did not standardize the duration of fasting. Our search retrieved 8 human trials16–23 of prolonged fasting, which are summarized in Table 1.16–23 The trial by Wilhelmi de Toledo23 tested the effects of various fasting durations (5 d, 10 d, 15 d, and 20 d). To allow for easier comparison between fasting durations, the results of this study have been separated by intervention arm in Table 1.
Table 1.
Effect of prolonged fasting on body weight and cardiometabolic risk markers
| Reference | Subjects | Fast duration, d | Design and intervention groups | Body weight | Body composition |
BP | Plasma lipids |
Glucoregulatory factors |
Ketones | Adverse events | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FM | FFM | WC | LDL | HDL | TG | FBG | Fasting insulin | IR | A1c | ||||||||
| Wilhelmi de Toledo, 201823 | n = 656; M, F; age: 54 ± 1 y; overweight; no diabetes | 5 | Nonrandomized trial; inpatient clinic; Buchinger fast; ∼250 kcal/d intake | ↓4.0%* | — | — | ↓* | ↓S*, ↓D* | ↓* | ↓* | ↓* | ↓* | — | — | ↓* |
|
|
| Jiang, 202118 | n = 45; M, F; age: ND; normal weight; no diabetes | 5 | Nonrandomized trial; outpatient; water-only fast | ↓6.4%* | — | — | ↓* | ↓S*, ↓D* | ↑* | ↓* | ↑* | — | ↓* | — | — |
|
|
| Berger, 202116 | n = 20; M, F; age: 56 ± 6 y; overweight; T1DM | 7 | Nonrandomized trial; inpatient clinic; Buchinger fast; ∼250 kcal/d intake | ↓2.1%* | — | — | — | ∅ S, ∅ D | ∅ | ↓* | — | ∅ | — | — | ∅ |
|
|
| Li, 201319 | n = 30; F; age: 50 ± 8 y; obese; no diabetes | 7 | Nonrandomized trial; outpatient; Buchinger fast; ∼250 kcal/d intake | ↓6.7%* | — | — | — | ↓S*, ↓D* | ↓* | ↓* | ∅ | — | ↓* | — | — | — | — |
| Li, 201720 | n = 32; M, F ; age: 65 ± 2 y ; obese; T2DM | 7 | RCT; outpatient; (1) Buchinger fast, ∼250 kcal/d intake; (2) Control—Med diet | (1) ↓3.9%*,**; (2) ↓2.1%* | — | — | (1) ↓*,**; (2) ∅ | (1) ↓S, ↓D; (2) ∅ | (1) ∅; (2) ∅ | (1) ∅; (2) ∅ | (1) ∅; (2)∅ | (1) ∅; (2) ↓* | (1) ∅; (2) ↓* | (1) ∅; (2)∅ | (1) ∅; (2) ∅ | — |
|
| Ogłodek, 202121 | n = 12; M; age: 50 ± 2 y; normal weight; no diabetes | 8 | Nonrandomized trial; outpatient; water-only fast | ↓7.4%* | BIA↓* | BIA↓* | — | — | — | — | — | — | — | — | — |
|
|
| Dai, 202217 | n = 13; M; age: 39 ± 3 y; overweight; no diabetes | 10 | Nonrandomized trial; inpatient clinic; water-only fast | ↓9.8%* | DXA↓* | DXA↓* | ↓* | ↓S*, ∅D | ↑* | ∅ | ∅ | ∅ | ↓* | ↓* | ↓* |
|
|
| Wilhelmi de Toledo, 201823 | n = 530; M, F; age: 56 ± 1 y; overweight; no diabetes | 10 | Nonrandomized trial; inpatient clinic; Buchinger fast; ∼250 kcal/d intake | ↓5.3%* | — | — | ↓* | ↓S*, ↓D* | ↓* | ↓* | ↓* | ↓* | — | — | ↓* |
|
|
| Wilhelmi de Toledo, 201823 | n = 196; M, F; age: 54 ± 1 y; overweight; no diabetes | 15 | Nonrandomized trial; inpatient clinic; Buchinger fast; ∼250 kcal/d intake | ↓7.0%* | — | — | ↓* | ↓S*, ↓D* | ↓* | ↓* | ↓* | ↓* | — | — | ↓* |
|
|
| Scharf, 202222 | n = 54; M, F; age: 57 ± 3 y; obese; no diabetes | 17 | Nonrandomized trial; inpatient clinic; water-only fast | ↓10.2%* | — | — | ↓* | ↓S*, ↓D* | ↓* | ∅ | ↑* | ↓* | ↓* | ↓* | — | — | — |
| Wilhelmi de Toledo, 201823 | n = 37; M, F; age: 54 ± 2 y; obese; no diabetes | 20 | Nonrandomized trial; inpatient clinic; Buchinger fast; ∼250 kcal/d intake | ↓7.3%* | — | — | ↓* | ↓S*, ↓D* | ↓* | ↓* | ↓* | ↓* | — | — | ↓* |
|
|
P < .05, significantly different from baseline (within-group effect).
P < .05, significantly different from the control group (between-group effect).
Abbreviations: A1c, glycated hemoglobin; AA, acetoacetic acid; BHB, beta-hydroxybutyrate; BIA, bioelectrical impedance analysis; BP, blood pressure; D, diastolic blood pressure; DXA, dual-energy X-ray absorptiometry; F, female; FBG, fasting blood glucose; FFM, fat-free mass; FM, fat mass; HDL, high-density lipoprotein cholesterol; IR, insulin resistance; LDL, low-density lipoprotein cholesterol; M, male; Med diet, Mediterranean diet; ND, not disclosed; RCT, randomized controlled trial; S, systolic blood pressure; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; T3, triiodothyronine; TG, triglycerides; TSH, thyroid-stimulating hormone; WC, waist circumference; –, not measured; ∅, no statistically significant change.
EFFECTS OF PROLONGED FASTING ON BODY WEIGHT AND BODY COMPOSITION
Body weight: effects of fasting
Weight loss of 5–10% can improve several metabolic measures, including blood pressure, plasma lipids, and fasting insulin.24 Changes in body weight during 5 to 20 days of prolonged fasting are shown in Table 1. Longer fasting durations generally produced more pronounced weight loss than shorter fasts, but findings were variable. For instance, 5 days of water fasting or Buchinger fasting lowered weight by 4–6% from baseline.18,23 Moderate durations of fasting (7–10 d) typically produced greater weight loss (2–10%),16,19–21 while very long durations of fasting (15–20 d) produced the greatest weight loss (7–10%).17,22,23 Prolonged fasting appears to be effective for weight reduction in a variety of population groups, including individuals with normal weight,18,21 overweight,16,17,23 obesity,19,20,22,23 type 1 diabetes,16 and type 2 diabetes.20 The degree of weight loss did not appear to be age dependent, as younger (40 y),17 middle-aged (55 y),16,18,19,21–23 and older (65 y)20 participants achieved similar reductions in body weight. However, since most studies16,18,19,21–23 enrolled middle-aged adults (50–60 y old), more research will be needed to explore how these effects vary in younger and older adults. Only 1 study23 examined if weight loss differed by sex. These findings show that weight reductions were significantly higher in men, compared with women, with various durations of fasting (5 d, 10 d, 15 d, and 20 d).23 Interestingly, outpatient interventions18–21 produced reductions in weight similar to inpatient programs.16,17,22,23 Thus, it is possible that the weight-loss benefits of prolonged fasting can be attained under free-living conditions.
Body weight: effects of refeeding
A few of these trials16,18,20,22 included follow-up periods to investigate refeeding effects. In the study by Jiang et al18 normal-weight adults lost 6% of their body weight after 5 days of water-only fasting, but then gained it all back after 3 months of eating regularly. In contrast, the trials by Berger et al,16 Li et al,20 and Scharf et al22 demonstrated only small weight regains (1–2%) 2–4 months after the finishing the fast. However, these trials 16,20,22 advised participants to follow a calorie-restricted diet during the refeeding period, which could explain why weight regain was minimal.
Fat mass and lean mass: effects of fasting
Only 2 studies17,21 measured changes in fat mass and lean mass during prolonged fasting (Table 1). In the study by Ogłodek et al21 changes in body composition were measured by bioelectrical impedance analysis (BIA). After 8 days of water-only fasting, body weight decreased by 6 kg, fat mass by 2 kg, and lean mass by 4 kg. 21 In the study by Dai et al17 changes in body composition were quantified by dual-energy X-ray absorptiometry (DXA). Following a 10-day water fast, body weight decreased by 7 kg, fat mass by 3 kg, and lean mass by 4 kg.17 Thus, these data suggest that approximately two-thirds of the weight loss may be lean mass. This finding is concerning as lean mass is a key predictor of resting metabolic rate.25 Reductions in lean mass can translate into lower resting metabolic rate after fasting, which can put individuals at risk for future weight regain.25 Moreover, the reductions in lean mass suggest that prolonged fasting may increase the breakdown of muscle proteins. This makes sense from a mechanistic standpoint, as degraded proteins provide the carbon chains necessary for the synthesis of newly formed glucose. However, these data are limited in that BIA was used in 1 of the studies21 to assess body composition. It is well known that BIA measurements are greatly influenced by hydration status.26 Specifically, higher water intake results in marked overestimations of fat mass, and underestimations of lean mass.26 This methodological limitation should be considered when interpreting these findings.
Fat mass and lean mass: effects of refeeding
The 2 studies17,21 that assessed changes in fat mass and lean mass during prolonged fasting did not involve a follow-up period. Thus, the effects of refeeding on these body composition parameters remain unknown. Since prolonged fasting may have deleterious effects on lean mass, future trials in this area should prioritize measuring changes in body composition using robust techniques, such as magnetic resonance imaging (MRI) or DXA. This will help inform whether these marked reductions in lean mass are maintained weeks or months after the fast is completed.
Waist circumference: effects of fasting
Visceral fat mass is highly metabolically active and constantly releases free fatty acids into the portal circulation.27 In consequence, visceral fat acts as a key contributor to many metabolic abnormalities, including hyperinsulinemia, dyslipidemia, and atherosclerosis.27–29 Reductions in visceral fat mass, via dietary restriction and weight loss, can help reverse these metabolic disorders.30,31 MRI is the gold standard for assessing changes in visceral fat mass.32 However, this technique is expensive and not always available in the clinic or at research centers. Measuring waist circumference serves as a less expensive and more accessible tool for measuring visceral fat mass. This technique is considered an accurate and reproducible measure of abdominal fat, when compared with MRI.33
Many trials17,18,20,22,23 included in this review measured waist circumference as an indirect marker of visceral obesity (Table 1). Waist circumference significantly decreased in all studies by 3 to 9 cm when compared with baseline.17,18,20,22,23 Greater decreases in waist circumference were observed in the trials that produced higher weight loss (>5%)17,18,22,23 versus the trials that produced only mild weight loss (<5%).20,23
Men are more likely to accumulate fat in the abdominal region, whereas women have a higher tendency to deposit fat subcutaneously on their lower extremities.34 In view of this, we were interested in seeing if changes in waist circumference varied by biological sex. The only study to examine sex-based differences was conducted by Wilhelmi de Toledo et al.23 Results from this trial23 showed that waist circumference reductions were more pronounced in men, compared with women, after 5 days, 10 days, 15 days, and 20 days of Buchinger fasting.23
Waist circumference: effects of refeeding
None of the studies that included a refeeding period assessed changes in waist circumference, so we are unable to comment on how this parameter changes after the fast is over.
EFFECTS OF PROLONGED FASTING ON BLOOD PRESSURE AND PLASMA LIPIDS
Blood pressure: effects of fasting
Body-weight reductions greater than 5% are associated with decreases in systolic and diastolic blood pressure in individuals with obesity.24 Changes in blood pressure with prolonged fasting are reported in Table 1. Consistent reductions in systolic (ranging from 9 to 14 mm Hg) and diastolic (ranging from 6 to 13 mm Hg) blood pressure were observed in the trials that achieved greater than 4% weight loss.17–20,22,23 Only the trial by Berger et al16 showed no change in blood pressure with prolonged fasting. However, the degree of weight loss in this study16 was minimal (2%), which may explain why blood pressure remained unaffected. It was also noted that longer fasting durations produced more pronounced reductions in both systolic and diastolic blood pressure.23 Participants with higher blood pressure values at baseline experienced greater decreases in blood pressure.23 However, reductions in blood pressure did not vary according to sex.23
The mechanism by which prolonged fasting improves blood pressure most likely involves decreased dietary sodium intake, enhanced production of natriuretic peptides, and the activation of the parasympathetic nervous system after 2–3 days of fasting.4 In addition, increased circulating levels of ketones may play a role. In preclinical studies, beta-hydroxybutyrate, and its precursor 1,3 butanediol, have been shown to produce potent vasodilation via their ability to activate potassium channels and nitric oxide synthase.35,36 In addition, exogenous ketone supplementation (resulting in plasma ketone levels of 1–3 mmol/L) has been shown to decrease blood pressure and increase vascular function in small clinical studies.8,9 Thus, the increased levels of circulating ketones, and their precursors, may have contributed to the blood pressure improvements noted here.
Blood pressure: effects of refeeding
A few studies16,18,20,22 examined whether the improvements in blood pressure were sustained 3–4 months after the fasting intervention was discontinued. Results reveal that blood pressure returned to baseline levels upon refeeding, even when weight loss was maintained.
Plasma lipids: effects of fasting
Changes in low-density lipoprotein (LDL)–cholesterol levels with prolonged fasting were highly variable (Table 1). For instance, 3 studies reported decreases,19,22,23 2 studies observed increases,17,18 and 2 others showed no change.16,20 In the studies reporting reductions (ranging from 10% to 25%), it was shown that longer durations of fasting produced more potent reductions in this lipid marker.19,22,23 However, no sex-based differences were noted for this improvement.23
High-density lipoprotein (HDL)–cholesterol levels either decreased by 7–18%,16,18,19,23 or remained unchanged17,20,22 (Table 1). Reductions in HDL cholesterol were related to the duration of fasting, with longer fasts (15–20 d) producing greater decreases and shorter fasts (5–10 d) producing smaller decreases.23 Gender-based differences were also noted. At baseline, women tended to have higher HDL-cholesterol levels than men. Consequently, decreases in HDL cholesterol were higher in females than in males.23
The effects of fasting on triglyceride concentrations were mixed (Table 1). For example, 1 study23 reported decreases, 2 trials18,22 demonstrated increases, and 3 studies17,19,20 observed no change. The study by Wilhelmi de Toledo et al23 compared the effects of various fasting durations on triglycerides. They found that longer periods of Buchinger fasting (15–20 d) did not produce greater reductions in triglycerides versus shorter periods (5–10 d), suggesting a floor effect.23 It is unclear why the changes in triglycerides varied so much between trials. From a physiological standpoint, it would be assumed that triglycerides would decrease with prolonged fasting. After a few days of prolonged fasting, circulating levels of insulin decrease, leading to lipolysis in adipose tissue and the release of free fatty acids into plasma.37 The free fatty acids are then picked up by the liver and used to produce ketone bodies. This results in less very-low-density lipoprotein (VLDL) being synthesized, and consequently, lower triglyceride levels in the circulation (as VLDL serves as 1 of the main carriers for triglycerides).37 The reason why this did not occur in some of the studies reviewed here17,19,20 remains unknown. However, these equivocal findings highlight the need for more studies to examine how water fasting impacts lipid metabolism.
Plasma lipids: effects of refeeding
A handful of trials 16,18,20,22 investigated the effect of refeeding on plasma lipids. Results from these studies suggest that LDL cholesterol, HDL cholesterol, and triglycerides return to baseline levels upon refeeding, even when body-weight reductions are maintained.
EFFECTS OF PROLONGED FASTING ON GLUCOREGULATORY FACTORS
Fasting glucose: effects of fasting
Moderate weight loss of 3–10% from baseline is associated with improvements in several glycemic measures, including fasting glucose, fasting insulin, and insulin resistance.24 In patients with obesity, but without diabetes, fasting glucose levels decreased with prolonged fasting 22,23 (Table 1). In these individuals, blood glucose at baseline was approximately 90–100 mg/dL and decreased to approximately 85 mg/dL after 5 days of fasting.22,23 Interestingly, longer fasts (10–20 d) did not produce greater reductions in fasting glucose.23
In patients with type 1 diabetes,16 glucose levels remained constant throughout the trial. However, this most likely occurred because mean daily insulin dosage decreased from 24 IU (on the last day before fasting) to 8 IU (on day 7, the last day of fasting), which helped to maintain target glucose control values.16 In patients with type 2 diabetes,20 mean fasting glucose levels did not change significantly after 7 days of outpatient Buchinger fasting. The lack of change in fasting glucose levels in patients with diabetes is somewhat surprising. However, it should be noted that glucose levels were measured at only 1 point in the day, instead of continuously throughout the day (using a continuous glucose monitor). Continuous glucose monitor data provides a more accurate reflection of glycemic control by reporting overall time in euglycemic range and mean glucose levels over 24 hours. It will be important for future trials of prolonged fasting to implement continuous glucose monitoring for these assessments.
Fasting insulin and insulin resistance: effects of fasting
In patients with obesity, fasting insulin decreased in all studies that measured this parameter17–19,22 (Table 1). Specifically, insulin levels were reduced by 65–80% after 5 to 17 days of prolonged fasting.17–19 Insulin reached a nadir in all studies by day 3–5, and these low levels of insulin were maintained throughout the duration of the study.17–19 Two trials17,22 that assessed insulin also measured insulin resistance using the homeostatic model assessment for insulin resistance (HOMA-IR). Complementary to the findings for insulin, HOMA-IR decreased from approximately 1.5 to 1.0 (33% decrease) after 10 to 17 days of prolonged fasting.17,22 Like insulin, HOMA-IR reached its lowest values after 3–5 days of fasting, and these low levels were maintained until the fast was completed.17,22 The reductions in insulin resistance may have been partly mediated by increased circulating ketones. Ketones increase insulin receptor activity by activating AMPK and downregulating mTOR.11 In obese diabetic mice, ketogenic diets improve glucose tolerance, even in the absence of weight loss.38,39 In humans with diabetes, ketogenic diets may produce greater improvements in glycemic control compared with calorie-restricted diets.40 However, it is difficult to tease apart the effects of weight loss versus ketones, as weight reduction is almost always noted with ketogenic diets.41
In individuals with type 2 diabetes, fasting insulin and insulin resistance (measured by HOMA-IR) remained unchanged after 7 days of Buchinger fasting.20 However, the authors noted that the small sample size of the study (n = 32) may have limited their ability to detect significant changes in these glycemic control variables.20 Moreover, fasting insulin and insulin resistance are more likely to decrease with more than 5% weight loss.42 Since the average weight reduction in this study was only 3.9%, this may also explain why these markers of glucoregulation did not change.
Fasting glucose, fasting insulin, and insulin resistance: effects of refeeding
The impact of refeeding on fasting glucose, fasting insulin, and insulin resistance was only evaluated in the study by Scharf et al.22 In this study,22 subjects participated in a 17-day water fast, and then followed a plant-based refeeding diet for 2 months. By the end of the refeeding period, fasting glucose, fasting insulin, and insulin resistance returned to baseline levels, even though participants maintained the majority of their weight loss.
Glycated hemoglobin: effects of fasting
In patients with obesity, but without diabetes,17,23 glycated hemoglobin (HbA1c) levels consistently decreased with 5 to 20 days of prolonged fasting (Table 1). In the study by Wilhelmi de Toledo et al23 it was shown that longer fasting durations (15–20 d) produced greater decreases in HbA1c, compared with shorter fasts (5–10 d). Overall, HbA1c was reduced by approximately 0.2–0.5% with prolonged fasting (in participants without diabetes).17,23 This finding is somewhat surprising as HbA1c is generally only ameliorated after 3 months of intervention.43,44 The reason why prolonged fasting produced these improvements within such a short period of time remains unclear.
The effect of prolonged fasting on HbA1c levels was also examined in patients with type 1 or type 2 diabetes.16,20 After 7 days of water-only fasting or Buchinger fasting, HbA1c levels did not change from baseline in adults with diabetes.16,20
HbA1c: effects of refeeding
The effect of refeeding on HbA1c levels was evaluated in the trials by Dai et al17 and Wilhelmi de Toledo et al.23 Results showed that HbA1c rebounded back to pretreatment levels during the refeeding period, even when weight loss was maintained.17,23
Fatty liver index: effects of fasting
Nonalcoholic fatty liver disease (NAFLD) is defined as the presence of 5% or more fat in the liver.45 The global prevalence of NAFLD is approximately 30% of adults.46 Individuals with NAFLD are at greater risk of developing insulin resistance and type 2 diabetes, compared with people who do not have the disorder.47 Weight loss of 5–10% can help reverse fatty liver disease.45 The gold standard for measuring the amount of fat in the liver is biopsy or MRI.48 However, since these procedures are expensive and invasive, the fatty liver index49 was created to quickly assess one’s risk of having NAFLD. The fatty liver index is calculated based on body mass index (BMI), waist circumference, triglycerides, and gamma-glutamyl-transferase levels. Scores range from 0 to 100. Scores below 30 suggest that the patient does not have NAFLD, whereas scores above 60 are highly suggestive of NAFLD diagnosis.49
In the study by Wilhelmi de Toledo et al23 a subanalysis was performed to see how fatty liver index changes in response to Buchinger fasting.50 At total of 697 participants from the original study23 were enrolled. After 8–9 days of fasting, body weight was reduced by approximately 4 kg, BMI by 1.5 kg/m2, and fatty liver index from 47 to 33.50 Greater reductions in fatty liver index were associated with longer fasting duration and greater reductions in BMI.50 These pilot findings suggest that the weight loss induced by prolonged fasting may decrease the degree of hepatic steatosis in people at risk for having NAFLD. However, these findings will need to be confirmed by more robust techniques, such as biopsy or MRI, before a solid conclusion can be reached.
Fatty liver index: effect of refeeding
No study to date has examined how refeeding impacts the fatty liver index, or other parameters of fatty liver disease, so we are unable to comment on this at present.
SAFETY CONSIDERATIONS
Ketones
Levels of beta-hydroxybutyrate and acetoacetic acid were measured in several trials reviewed here (Table 1). In patients without diabetes, plasma levels of beta-hydroxybutyrate increased to 4–5 mmol/L after 8 days of fasting,21 and then stayed constant for up to 10 days.17 Concentrations of acetoacetic acid in urine reached maximum levels of 50 mg/dL after 5 days of fasting18,23 and did not change with longer fasts (10–20 d).23 Thus, plateau levels of ketones are reached after 5–8 days of either water-only or Buchinger fasting in adults who do not have diabetes.
Ketones were also assessed in individuals with type 1 diabetes.16 Water fasting is typically contraindicated for patients with type 1 diabetes due to the risk of developing ketoacidosis. Diabetic ketoacidosis is a severe and life-threatening disease caused by a deficiency of insulin.51 When insulin levels are low, the body starts to convert free fatty acids into ketone bodies for energy.52 The initial formation of ketones acts as a protective mechanism. However, if ketones are overproduced, this can overwhelm the buffering capacity of the body, leading to metabolic acidosis.51 In the study by Berger et al16 adults with type 1 diabetes participated in a 7-day Buchinger fasting protocol. To prevent ketoacidosis, participants monitored blood glucose and ketone levels daily. If ketone values were above 6 mmol/L, participants were advised to administer 2 to 4 IU of insulin and consume carbohydrates (eg, apple juice).16 By the end of the 7-day fast, no episodes of diabetic ketoacidosis were reported. Additionally, glucose levels remained in the normal range and plasma beta-hydroxybutyrate only increased to 2.8 mmol/L.16 Thus, these preliminary findings suggest that prolonged fasting may be safe in people with type 1 diabetes, as long as ketogenesis is controlled by a sufficient insulin supply. Nonetheless, these data require confirmation by larger randomized controlled trials specifically designed to assess the safety of prolonged fasting in people with type 1 diabetes.
Circulating ketone levels were not assessed the study that enrolled patients with type 2 diabetes20; thus, we are not able to comment on changes in ketones during fasting in this population.
Adverse events
No serious adverse events or deaths were reported in any of the studies reviewed here.16–23 However, mild adverse events, such as insomnia, fatigue, dizziness, dry mouth, and headaches, were frequently observed.16,18,20,23 Liver enzymes, such as alanine transaminase (ALT) and aspartate aminotransferase (AST), remained unaffected after 5–10 days of fasting.17,18 With regard to thyroid hormones, circulating levels of triiodothyronine (T3) and thyroid-stimulating hormone (TSH) decreased with 5 days of water fasting. However, low T3 levels, in the absence of impaired thyroid function, are strongly associated with longevity,53,54 so this change can be considered beneficial. No adverse changes in urine creatinine, protein, or urea were observed, indicating that renal function was preserved.17,18,21 Fat-soluble and water-soluble vitamin levels remained unchanged.17 However, circulating levels of sodium and chloride decreased to just below the acceptable limit after 8–10 days of water fasting.17,21 Thus, individuals should consider taking sodium chloride supplements to prevent electrolyte loss during water fasting.
Hunger
Increased hunger levels are also frequently reported during prolonged fasting.18,23 In the study by Jiang et al18 hunger levels increased approximately 4-fold after water fasting for 5 days. Feelings of hunger may interfere with patient compliance and may lead to high attrition rates, so hunger should be closely monitored. No study to date has examined how levels of hunger change during the refeeding period. If feelings of hunger do indeed surge after fasting, the patient may quickly gain back all the weight they lost, which would undo the metabolic benefits observed. It will be important for future trials to measure how appetite and body weight change in the days and weeks following the fast.
OPTIMAL DURATION OF THE FAST
The optimal number of days that a person should engage in prolonged fasting remains largely unknown. From the studies reviewed here, longer fasts (15–20 d) produced more pronounced decreases in body weight,17,22,23 systolic and diastolic blood pressure,23 LDL cholesterol,19,22,23 and HbA1c.23 However, longer fasts were also associated with more potent reductions in the cardioprotective lipoprotein HDL cholesterol.23 Thus, the benefits of fasting for longer durations should be weighed against the disadvantages. Moreover, the safety and tolerability of longer fasts have only been measured in a few studies to date. Much more research will be required to answer this important question.
LIMITATIONS TO THE CURRENT BODY OF EVIDENCE
There are several limitations to the current body of evidence. First, we were only able to retrieve a small amount of prolonged fasting trials (8 total) that assessed both body weight and metabolic disease risk. Second, most studies had small sample sizes. Thus, it is likely that many of these trials were not adequately powered to detect significant changes in secondary outcomes (ie, metabolic disease risk factors). Third, most trials did not include a control group in their design. Thus, it is difficult to confirm if these results are due to the fasting intervention instead of other extraneous variables. Fourth, many studies did not include a refeeding period or a follow-up period. As such, it remains uncertain how body weight and metabolic markers rebound after the fasting intervention is terminated. Fifth, occurrences of edema, abnormal liver function, decreased bone density, malnutrition, and metabolic acidosis were not recorded in most trials reviewed here. Thus, whether prolonged fasting results in more severe adverse health effects is still unclear. Last, the generalizability of the findings to other races or ethnicities is limited, as most studies were conducted in individuals of European descent.
DIRECTIONS FOR FUTURE RESEARCH
It will be important for future studies to implement randomized controlled designs with larger sample sizes. These types of trials will be instrumental in helping to clarify if prolonged fasting is indeed a viable treatment option for metabolic disturbances. It will also be essential for future studies to test the efficacy of different follow-up interventions (eg, time-restricted eating, daily calorie restriction, or ketogenic diets) after the prolonged fast is over. This may help identify which diet strategy is effective for sustaining the metabolic benefits noted during the active weight-loss period. More studies are also warranted in patients with diabetes and other obesity-related comorbidities. Last, future trials should implement robust techniques to measure metabolic endpoints—that is, MRI or DXA for body composition, MRI for liver fat, and continuous glucose monitoring for glycemic control.
CONCLUSION
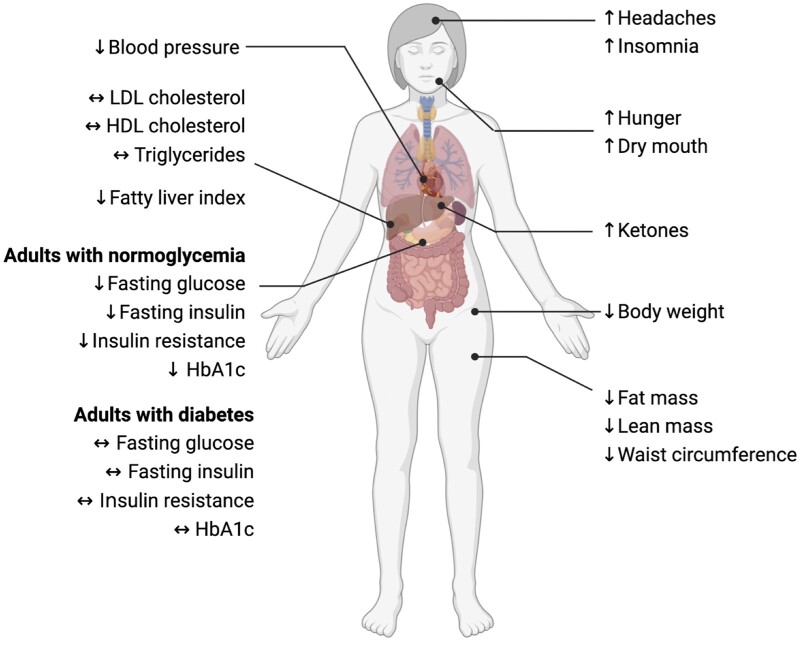
Our findings show that water-only or Buchinger fasting for 5–20 days produces potent increases in circulating ketones and mild to moderate weight loss (2–10% of baseline) (Figure 2). Approximately two-thirds of the weight lost is lean mass, and one-third is fat mass. Systolic and diastolic blood pressure consistently decreased with prolonged fasting. However, the impact of these protocols on plasma lipids is still unclear. While some trials demonstrate decreases in LDL cholesterol and triglycerides, others show no benefit. Fatty liver index, a surrogate marker of hepatic steatosis, was reduced in people at risk for having NAFLD. With regard to glycemic control, reductions in fasting glucose, fasting insulin, insulin resistance, and HbA1c were noted in adults with normoglycemia. In contrast, these glucoregulatory factors remained unchanged in patients with type 1 or type 2 diabetes. The effects of refeeding were also examined. It was shown that 3–4 months after the fast was completed, all metabolic benefits were no longer observed, even when weight loss was maintained. No serious adverse events, metabolic acidosis, or deaths were reported. However, mild side effects, such as headaches, insomnia, hunger, and dry mouth, were frequently observed. In summary, prolonged fasting appears to be a safe diet therapy that can produce clinically significant weight loss (>5%) over a few days or weeks. However, the ability of these protocols to produce sustained improvements in these health markers warrants further investigation, since most benefits disappear upon refeeding.
Figure 2.
Effects of prolonged fasting on metabolic risk factors. Prolonged fasting for 5–20 days produced potent increases in circulating ketones and mild to moderate weight loss (2–10% of baseline). Reductions in fat mass, lean mass, and abdominal fat were also observed. Systolic and diastolic blood pressure generally decreased, but changes in plasma lipids were highly variable. The fatty liver index was also improved in patients at risk of having NAFLD. Reductions in fasting glucose, fasting insulin, insulin resistance, and HbA1c were noted in adults with normoglycemia, but not in patients with diabetes. With regard to adverse events, headaches, insomnia, hunger, and dry mouth were frequently reported. Abbreviations: HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NAFLD, nonalcoholic fatty liver disease.
Acknowledgments
Author contributions. Conception, design, data collection or analysis: M.E., S.C., S.L., V.P., K.G., K.A.V. Writing or revision: M.E., S.C., S.L., V.P., K.G., K.A.V. Final reading and approval of the manuscript: M.E., S.C., S.L., V.P., K.G., K.A.V.
Funding. No external funding was received to support this work.
Declaration of interest. K.A.V. received author fees from Hachette Book Group for the book, The Every Other Day Diet, and from Pan Macmillan Press for the book, The Fastest Diet. The other authors have no conflicts of interest to disclose.
Contributor Information
Mark Ezpeleta, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago, Illinois, USA.
Sofia Cienfuegos, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago, Illinois, USA.
Shuhao Lin, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago, Illinois, USA.
Vasiliki Pavlou, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago, Illinois, USA.
Kelsey Gabel, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago, Illinois, USA.
Krista A Varady, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago, Illinois, USA.
References
- 1. Varady KA, Cienfuegos S, Ezpeleta M, et al. Clinical application of intermittent fasting for weight loss: progress and future directions. Nat Rev Endocrinol. 2022;18:309–321. doi: 10.1038/s41574-022-00638-x. [DOI] [PubMed] [Google Scholar]
- 2. Anderson K. Popular fad diets: an evidence-based perspective. Prog Cardiovasc Dis. 2023;77:78–85. doi: 10.1016/j.pcad.2023.02.001. [DOI] [PubMed] [Google Scholar]
- 3. Wilhelmi de Toledo F, Grundler F, Sirtori CR, et al. Unravelling the health effects of fasting: a long road from obesity treatment to healthy life span increase and improved cognition. Ann Med. 2020;52:147–161. doi: 10.1080/07853890.2020.1770849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Michalsen A, Li C. Fasting therapy for treating and preventing disease—current state of evidence. Forsch Komplementmed. 2013;20:444–453. doi: 10.1159/000357765. [DOI] [PubMed] [Google Scholar]
- 5. Wilhelmi de Toledo F, Buchinger A, Burggrabe H, et al. ; Medical Association for Fasting and Nutrition (Ärztegesellschaft für Heilfasten und Ernährung, ÄGHE). Fasting therapy—an expert panel update of the 2002 consensus guidelines. Forsch Komplementmed. 2013;20:434–443. doi: 10.1159/000357602. [DOI] [PubMed] [Google Scholar]
- 6. Anton SD, Moehl K, Donahoo WT, et al. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity (Silver Spring). 2018;26:254–268. doi: 10.1002/oby.22065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381:2541–2551. doi: 10.1056/NEJMra1905136. [DOI] [PubMed] [Google Scholar]
- 8. Costa TJ, Linder BA, Hester S, et al. The Janus face of ketone bodies in hypertension. J Hypertens. 2022;40:2111–2119. doi: 10.1097/HJH.0000000000003243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walsh JJ, Neudorf H, Little JP. 14-Day ketone supplementation lowers glucose and improves vascular function in obesity: a randomized crossover trial. J Clin Endocrinol Metab. 2021;106:1738–1754. doi: 10.1210/clinem/dgaa925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Byrne NJ, Soni S, Takahara S, et al. Chronically elevating circulating ketones can reduce cardiac inflammation and blunt the development of heart failure. Circ: Heart Failure. 2020;13:e006573. doi: 10.1161/CIRCHEARTFAILURE.119.006573. [DOI] [PubMed] [Google Scholar]
- 11. Zhu H, Bi D, Zhang Y, et al. Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Signal Transduct Target Ther. 2022;7:11. doi: 10.1038/s41392-021-00831-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deemer SE, Plaisance EP, Martins C. Impact of ketosis on appetite regulation-a review. Nutr Res. 2020;77:1–11. doi: 10.1016/j.nutres.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 13. Spencer IO. Death during therapeutic starvation. Lancet. 1968;2:679–680. doi: 10.1016/s0140-6736(68)92530-0. [DOI] [PubMed] [Google Scholar]
- 14. Fasting and obesity. Br Med J. 1978;1:673. [PMC free article] [PubMed] [Google Scholar]
- 15. Ross SK, Macleod A, Ireland JT, et al. Acidosis in obese fasting patients. Br Med J. 1969;1:380–381. doi: 10.1136/bmj.1.5640.380-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berger B, Jenetzky E, Koblos D, et al. Seven-day fasting as a multimodal complex intervention for adults with type 1 diabetes: feasibility, benefit and safety in a controlled pilot study. Nutrition. 2021;86:111169. doi: 10.1016/j.nut.2021.111169. [DOI] [PubMed] [Google Scholar]
- 17. Dai Z, Zhang H, Wu F, et al. Effects of 10-day complete fasting on physiological homeostasis, nutrition and health markers in male adults. Nutrients. 2022;14:3860. doi: 10.3390/nu14183860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang Y, Yang X, Dong C, et al. Five-day water-only fasting decreased metabolic-syndrome risk factors and increased anti-aging biomarkers without toxicity in a clinical trial of normal-weight individuals. Clin Transl Med. 2021;11:e502. doi: 10.1002/ctm2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li C, Ostermann T, Hardt M, et al. Metabolic and psychological response to 7-day fasting in obese patients with and without metabolic syndrome. Forsch Komplementmed. 2013;20:413–420. doi: 10.1159/000353672. [DOI] [PubMed] [Google Scholar]
- 20. Li C, Sadraie B, Steckhan N, et al. Effects of a one-week fasting therapy in patients with type-2 diabetes mellitus and metabolic syndrome—a randomized controlled explorative study. Exp Clin Endocrinol Diabetes. 2017;125:618–624. doi: 10.1055/s-0043-101700. [DOI] [PubMed] [Google Scholar]
- 21. Ogłodek E, Pilis Prof W. Is water-only fasting safe? Glob Adv Health Med. 2021;10:21649561211031178. doi: 10.1177/21649561211031178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scharf E, Zeiler E, Ncube M, et al. The effects of prolonged water-only fasting and refeeding on markers of cardiometabolic risk. Nutrients. 2022;14:1183. doi: 10.3390/nu14061183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilhelmi de Toledo F, Grundler F, Bergouignan A, et al. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLoS ONE. 2019;14:e0209353. doi: 10.1371/journal.pone.0209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ryan DH, Yockey SR. Weight loss and improvement in comorbidity: differences at 5%, 10%, 15%, and over. Curr Obes Rep. 2017;6:187–194. doi: 10.1007/s13679-017-0262-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Melby CL, Paris HL, Foright RM, et al. Attenuating the biologic drive for weight regain following weight loss: must what goes down always go back up? Nutrients. 2017;9:468. doi: 10.3390/nu9050468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ugras S. Evaluating of altered hydration status on effectiveness of body composition analysis using bioelectric impedance analysis. Libyan J Med. 2020;15:1741904. doi: 10.1080/19932820.2020.1741904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020;7:22. doi: 10.3389/fcvm.2020.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang L, Yi Z. Obesity paradox and aging: visceral adiposity index and all-cause mortality in older individuals: a prospective cohort study. Front Endocrinol (Lausanne). 2022;13:975209. doi: 10.3389/fendo.2022.975209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Despres JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med. 2006;38:52–63. doi: 10.1080/07853890500383895. [DOI] [PubMed] [Google Scholar]
- 30. Chang TJ. Mechanisms and the strategy for remission of type 2 diabetes mellitus. J Diabetes Investig. 2023;14:351–353. doi: 10.1111/jdi.13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hoenig MR, Cowin G, Buckley R, et al. Low density lipoprotein cholesterol is inversely correlated with abdominal visceral fat area: a magnetic resonance imaging study. Lipids Health Dis. 2011;10:12. doi: 10.1186/1476-511X-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klopfenstein BJ, Kim MS, Krisky CM, et al. Comparison of 3 T MRI and CT for the measurement of visceral and subcutaneous adipose tissue in humans. Br J Radiol. 2012;85:e826–e830. doi: 10.1259/bjr/57987644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Janssen I, Heymsfield SB, Allison DB, et al. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr. 2002;75:683–688. doi: 10.1093/ajcn/75.4.683. [DOI] [PubMed] [Google Scholar]
- 34. Iliodromiti S, McLaren J, Ghouri N, et al. Liver, visceral and subcutaneous fat in men and women of South Asian and white European descent: a systematic review and meta-analysis of new and published data. Diabetologia. 2023;66:44–56. doi: 10.1007/s00125-022-05803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCarthy CG, Chakraborty S, Singh G, et al. Ketone body beta-hydroxybutyrate is an autophagy-dependent vasodilator. JCI Insight. 2021;6:e149037. doi: 10.1172/jci.insight.149037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCarthy CG, Waigi EW, Yeoh BS, et al. Low-dose 1,3-butanediol reverses age-associated vascular dysfunction independent of ketone body beta-hydroxybutyrate. Am J Physiol Heart Circ Physiol. 2022;322:H466–H473. doi: 10.1152/ajpheart.00486.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sanvictores T, Casale J, Huecker MR. Physiology, Fasting. Treasure Island, FL: StatPearls; 2022. [PubMed]
- 38. Badman MK, Kennedy AR, Adams AC, et al. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss. Am J Physiol Endocrinol Metab. 2009;297:E1197–204. doi: 10.1152/ajpendo.00357.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Freedland SJ, Mavropoulos J, Wang A, et al. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate. 2008;68:11–19. doi: 10.1002/pros.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nordmann AJ, Nordmann A, Briel M, et al. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:285–293. doi: 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- 41. Yuan X, Wang J, Yang S, et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr Diabetes. 2020;10:38. doi: 10.1038/s41387-020-00142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mason C, Foster-Schubert KE, Imayama I, et al. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Am J Prev Med. 2011;41:366–375. doi: 10.1016/j.amepre.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kilpatrick ES. Haemoglobin A1c in the diagnosis and monitoring of diabetes mellitus. J Clin Pathol. 2008;61:977–982. doi: 10.1136/jcp.2007.054304. [DOI] [PubMed] [Google Scholar]
- 44. Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care. 1995;18:440–447. doi: 10.2337/diacare.18.4.440. [DOI] [PubMed] [Google Scholar]
- 45. Pouwels S, Sakran N, Graham Y, et al. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord. 2022;22:63. doi: 10.1186/s12902-022-00980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Le MH, Yeo YH, Li X, et al. 2019 Global NAFLD prevalence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022;20:2809–2817.e28. doi: 10.1016/j.cgh.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 47. Targher G, Corey KE, Byrne CD, et al. The complex link between NAFLD and type 2 diabetes mellitus—mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18:599–612. doi: 10.1038/s41575-021-00448-y. [DOI] [PubMed] [Google Scholar]
- 48. Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol. 2014;5:211–218. doi: 10.1136/flgastro-2013-100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bedogni G, Bellentani S, Miglioli L, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Drinda S, Grundler F, Neumann T, et al. Effects of periodic fasting on fatty liver index—a prospective observational study. Nutrients. 2019;11:2601. doi: 10.3390/nu11112601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dhatariya KK, Glaser NS, Codner E, et al. Diabetic ketoacidosis. Nat Rev Dis Primers. 2020;6:40. doi: 10.1038/s41572-020-0165-1. [DOI] [PubMed] [Google Scholar]
- 52. Tran TTT, Pease A, Wood AJ, et al. Review of evidence for adult diabetic ketoacidosis management protocols. Front Endocrinol (Lausanne). 2017;8:106. doi: 10.3389/fendo.2017.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rozing MP, Westendorp RG, de Craen AJ, et al. ; Leiden Longevity Study (LLS) Group. Low serum free triiodothyronine levels mark familial longevity: the Leiden Longevity Study. J Gerontol A Biol Sci Med Sci. 2010;65:365–368. doi: 10.1093/gerona/glp200. [DOI] [PubMed] [Google Scholar]
- 54. Stekovic S, Hofer SJ, Tripolt N, et al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. 2019;30:462–476.e6. doi: 10.1016/j.cmet.2019.07.016. [DOI] [PubMed] [Google Scholar]