Abstract
Background
Since November 2019, the SARS-CoV-2 pandemic has created challenges for preventing and managing COVID-19 in children and adolescents. Most research to develop new therapeutic interventions or to repurpose existing ones has been undertaken in adults, and although most cases of infection in pediatric populations are mild, there have been many cases of critical and fatal infection. Understanding the risk factors for severe illness and the evidence for safety, efficacy, and effectiveness of therapies for COVID-19 in children is necessary to optimize therapy.
Methods
A panel of experts in pediatric infectious diseases, pediatric infectious diseases pharmacology, and pediatric intensive care medicine from 21 geographically diverse North American institutions was re-convened. Through a series of teleconferences and web-based surveys and a systematic review with meta-analysis of data for risk factors, a guidance statement comprising a series of recommendations for risk stratification, treatment, and prevention of COVID-19 was developed and refined based on expert consensus.
Results
There are identifiable clinical characteristics that enable risk stratification for patients at risk for severe COVID-19. These risk factors can be used to guide the treatment of hospitalized and non-hospitalized children and adolescents with COVID-19 and to guide preventative therapy where options remain available.
Keywords: pediatric, COVID-19, management, evidence-based, guidance
Background and Introduction
Following the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the coronavirus disease 2019 (COVID-19) pandemic presented an unprecedented global public health threat, with billions of infections and millions of attributable deaths [1]. Although children and adolescents are generally less vulnerable than adults to severe COVID-19 disease, with most experiencing only mild to moderate illness, they do remain at high risk for infection and sometimes develop severe or critical illness and long-term complications [2]. In this context, optimal prevention and management of COVID-19 in pediatric populations is important to reduce the use of inappropriate therapies and maximize benefits to patients.
The pandemic has been met by the rapid development of new vaccines, novel antiviral agents, and immunotherapies and by the repurposing of existing drugs for preventing and treating COVID-19 [3]. Much of the research effort has been geared towards adults, and they have been the main focus in developing these therapeutic agents, with less attention being paid to evaluating their safety and efficacy in children and adolescents. As the pandemic continues to evolve, the importance of optimizing specific strategies for managing and preventing COVID-19 in the pediatric population has become clear. Identifying pediatric populations at risk for severe COVID-19, understanding the evidence for the safety, efficacy, and effectiveness of specific preventative and therapeutic options in children and adolescents, and evaluating the applicability of data obtained from adults are all essential to guide the care of children and adolescents.
In 2022, after the publication of multiple initial and interim guidance statements for managing COVID-19 in children and adolescents, the Pediatric Infectious Diseases Society (PIDS) supported the Pediatric COVID-19 Therapies Taskforce in creating a consensus guidance document for preventing and managing COVID-19 in children and adolescents. The aim was to combine evidence and experience to create practical, evidence-based guidelines that outlined the boundaries of appropriate practice for healthcare professionals, policymakers, and caregivers.
This consensus statement has been reviewed and approved by all panelists and has been endorsed by PIDS. It replaces previous guidance on antivirals and monoclonal antibodies (mAbs) [4, 5].
To update the guidance on treatment and pre-exposure and post-exposure prophylaxis of COVID-19 in children and adolescents, we re-convened a panel of experts in pediatric infectious diseases, pediatric infectious diseases pharmacotherapy, and pediatric intensive care medicine from 21 geographically diverse US institutions. The panel evaluated the evidence regarding risk factors for severe COVID-19, as well as the available evidence regarding the safety and efficacy of preventative and therapeutic interventions in pediatric patients, and it assessed whether and how evidence obtained in adult patients should be extrapolated to pediatric patients. These data were combined with experience and expert opinion to develop recommendations. The recommendations were developed by subgroups assigned to consider (1) risk stratification, (2) early therapy for patients with COVID-19 who do not require hospitalization for COVID-19, (3) therapy for patients with COVID-19 who do require hospitalization for COVID-19, and (4) preventative therapy for patients without COVID-19, including those who have been recently exposed. After draft recommendations had been presented to and discussed with the whole panel, the feedback was incorporated by the subgroups and the panel voted on the recommendations by electronic survey. In this consensus guidance, approval by 100% of the voting panelists was required for acceptance of each recommendation.
Each statement was assigned a recommendation-strength of “recommend,” “suggest,” or “consider” [4–7]. A “recommend” statement reflects the view of the panel that the evidence base for or against a therapy is sufficiently strong that departures from these recommendations could be viewed as being outside the range of usual practice. A “suggest” statement reflects the view of the panel that there is a weighting towards risk or benefit from the therapy. A “consider” statement reflects the uncertainty of the panel concerning the risk or benefit from the therapy.
The guidance includes recommendations for risk stratification to help clinicians identify those patients who are at high risk for severe COVID-19 (Section 1), for early treatment of COVID-19 to prevent worsening disease in patients who do not require hospitalization (Section 2), and for treating COVID-19 to prevent further deterioration, critical illness, or death in patients who require hospitalization (Section 3). There are also recommendations for preventing COVID-19 in exposed and unexposed patients (Section 4).
It is important to note that some therapeutic agents discussed in the document are no longer appropriate or available for preventing or treating COVID-19 in the United States. SARS-CoV-2 has evolved rapidly, rendering ineffective some therapies that were options for treating previously circulating variants. A prerequisite for all recommendations in this guidance is the availability of at least one authorized agent that is active against the current dominant variant(s). At the time of publication, this prerequisite has not been met in the United States for pre-exposure or post-exposure prophylaxis (PrEP or PEP) or for mAbs for treating COVID-19. However, the panel has included the evidence assessment and recommendations for other areas of the world where the situation might be different, as well as for future scenarios that may arise when new products become available or existing ones again become an option.
Section 1: Risk Stratification
Recommendations:
Recommendation 1.1:
In children and adolescents with COVID-19 infection, the panel suggests using risk stratification that takes into consideration the assessment of pre-existing conditions, exacerbating factors, and prior immunity to determine appropriate management.
Recommendation 1.2:
Children and adolescents may be considered at high risk if all of the following conditions are met:
i. They have a definite or probable risk factor for severe COVID-19. Severe immunocompromise, obesity, diabetes, prematurity, and chronic cardiac, neurologic (seizures), or pulmonary disease (excluding asthma) can be considered definite risk factors. Probable risk factors include sickle cell disease, mild/moderate immunocompromise, neuro-disabilities (trisomy 21), and chronic kidney, gastrointestinal, and liver disease.
ii. They have an exacerbating condition, including multiple (≥2) comorbidities, a severe or poorly controlled comorbidity, or being <1 or ≥12 years of age.
iii. They have no prior immunity, defined as up-to-date immunization or recent infection (within the previous 4 months) in an immunocompetent host.
Recommendation 1.3:
Children and adolescents may be considered at moderate risk if they have definite or probable risk factors for severe COVID-19 but no exacerbating conditions or if they are immunocompetent and have prior immunity.
Recommendation 1.4:
Children and adolescents with no risk factors for severe COVID-19 may be considered at low risk for severe COVID-19.
Rationale for Recommendations
In developing these guidelines, the panel formulated a framework to help stratify patients who have the highest risk of their infection progressing to severe COVID-19 and who are, thus, most likely to benefit from treatment. This framework involves assessing the varying degrees of risk associated with certain pre-existing conditions, the complexity of these conditions, and prior immunity. The proposed risk stratification approach, which is illustrated in Figure 1, was developed on the basis of systematic evidence reviews and expert opinion. This approach is based on the recognition that although there may be certainty about a given risk factor (i.e., it is consistently associated with critical COVID-19 in the literature), no single risk factor will adequately capture the magnitude of risk when considered in isolation. The presence of a definite risk factor may not necessarily represent a significant risk if the condition is well controlled or if it is present in a child with prior immunity. Conversely, medical complexity and inadequately managed conditions can increase the likelihood of severe disease and should be considered even if there is uncertainty about the risk factor. Age is listed as a potential exacerbating factor because its prognostic ability is limited when no other risk factors are present. We employed age thresholds of <1 year and ≥12 years, as these were the ones most consistently used in the literature. These thresholds capture the bimodal pattern of risk, and they align with the ages specified by the United States Food and Drug Administration (FDA) in the authorization of the relevant therapeutic and prophylactic agents. However, it is important to acknowledge that these cut-offs are arbitrary in nature.
Figure 1.
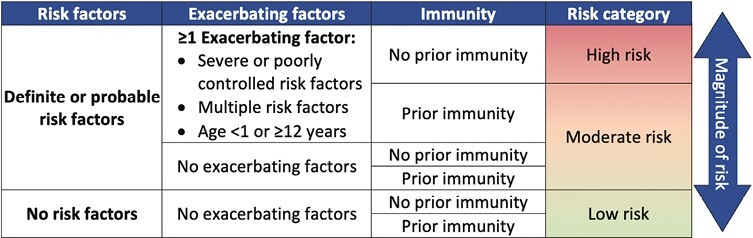
Risk stratification framework. Definite risk factors include immunocompromise, obesity, diabetes (type 1/2), prematurity, chronic cardiac, neurologic, or pulmonary disease (excluding asthma). Probable risk factors include sickle cell disease, mild/moderate immunocompromise, neuro-disabilities, chronic kidney, gastrointestinal or liver disease. Prior immunity is defined as up-to-date immunization or recent infection within the previous 4 months.
Although this conceptual framework is informed by empirical evidence, its predictive performance, as a whole, requires validation. It is presented here to help contextualize the treatment recommendations provided in this guideline. The panel also acknowledges that not all children will fit neatly into this model. Clinicians will need to exercise clinical discretion when applying this framework to individual patients. In the section below, we provide a brief overview of the risk factors that were considered, and their final classifications based on the certainty of evidence. A more comprehensive review of the evidence base for this stratification approach can be found in the accompanying systematic review and meta-analysis [8].
Evidence Acquisition and Synthesis
Systematic reviews were conducted to identify and consolidate evidence regarding factors contributing to an increased risk of progression to severe or critical COVID-19 outcomes in pediatric populations. Panel members initially focused on ascertaining evidence for the factors specifically mentioned in the Emergency Use Authorizations (EUA) for therapeutic agents and then broadened the search to provide more nuance as needed. For the purposes of these guidelines, we did not consider hospitalization alone as a severe outcome, as the decision for hospital admission frequently depends on institutional protocols and children may have been hospitalized for reasons unrelated to SARS-CoV-2. Thus, our initial systematic review focused on comparative studies that captured rates of “critical disease,” defined as disease resulting in a need for invasive mechanical ventilation, admission to an intensive care unit (ICU), and/or death. In cases where comparative studies were lacking for a particular risk factor, single-arm reports, non-pediatric studies, and studies that focused solely on hospitalization as an outcome were reviewed separately. These sources were used as indirect evidence of the potential impact of the risk factor under consideration.
Methodologists, along with a subgroup of panelists, screened the titles and abstracts of all identified papers and conducted full-text reviews of relevant articles. This evidence review included articles published from the inception of the study until August 18, 2023. Among the 655 articles screened, 136 directly explored at least one potential risk factor of interest and were, therefore, incorporated into our body of evidence. Among these 136 studies, 70 were synthesized as a meta-analysis, whereas the remaining 66 studies were reviewed qualitatively because their methods or definitions precluded direct comparisons.
Each assessed condition was classified as a Definite, Probable, or Unlikely Risk Factor based on the totality of evidence from these studies, using previously described standards [9]. Briefly, Definite Risk Factors were those for which the evidence for an increase in risk was supported by methodologically sound multicenter studies that demonstrated a large effect size, with the results being consistent across multiple studies. Large effect sizes were defined as an odds ratio (OR) or relative risk (RR) greater than 2 if adjusted for known confounders or greater than 5 if based on pooled unadjusted estimates from a meta-analysis, as previously described [10]. Risk factors were downrated to Probable if they were associated with modest effect sizes, were inconsistent, or had substantial unexplained heterogeneity across studies (I2 > 75% in meta-analysis). This group also contains factors for which substantial uncertainty exists because the best evidence came from studies with small sample sizes that could not be adjusted for confounding or for which data were available only for the adult population. Unlikely Risk Factors were those for which there was strong and consistent evidence that the characteristic was not a risk factor, supported by large multicenter studies or meta-analyses. A summary of the results from the systematic review and meta-analysis is shown in Figure 2.
Figure 2.
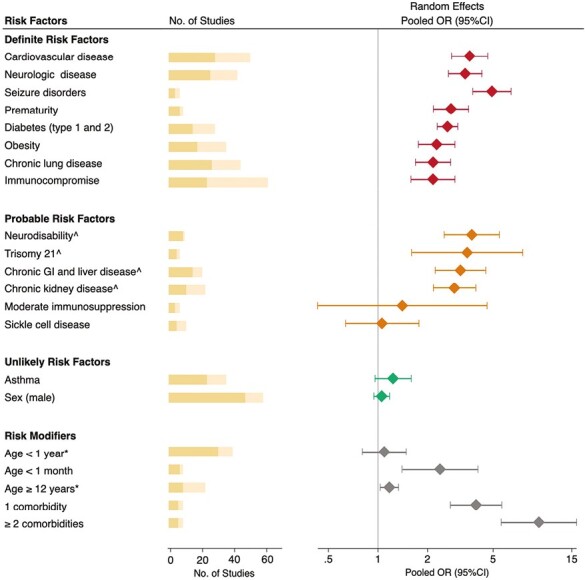
Association between comorbidities and severe COVID-19 in children. OR (95% CI) = odds ratio and 95% confidence interval, estimated using data extracted from published studies. Red = Definite risk factors; Orange = Probable risk factors; Green = Unlikely risk factors; Gray = Risk modifiers. The yellow bars represent the total number of studies evaluated, with darker shades indicating the subset of studies included in the meta-analysis. ^Certainty downgraded because of small sample sizes in studies or non-significant effects after adjusting for comorbidities. *Reference age, 1–11 years.
Summary of the Evidence for Risk Stratification
Age
The risk of critical COVID-19 is not consistent across all age groups, and it exhibits a U-shaped distribution, with the highest risk being observed in younger infants and older adolescents and the lowest risk being found in children of primary school age [11–18]. Although the results of multiple studies suggest that being younger than 1 year is a risk factor, this effect may have been confounded by prematurity, which, upon sub-analysis, we found to be a strong risk factor (pooled OR: 2.77; 95% CI: 2.17–3.54).
Medical Complexity
Medical complexity is one of the most robust and consistently observed predictors of critical COVID-19 disease. Prior meta-analyses have found an increase up to tenfold in the odds of critical disease among children with two or more underlying conditions [19]. The definition of medical complexity varies across studies, but it typically encompasses children with multiple chronic health conditions and/or a reliance on medical technology. Multiple studies have demonstrated that the degree of medical complexity increases the risk of poor outcomes in a dose-dependent manner, with each additional pre-existing disease correlating with increased odds of ICU admission or death.
Prior Immunity
Prior immunity, defined as up-to-date immunization or recent infection (within the previous 4 months), plays an important role in decreasing the level of risk. Recent findings from meta-analyses indicate that monovalent and bivalent COVID-19 vaccines were highly effective (>75%) at protecting children from severe disease during the Omicron waves [20]. Studies have shown similar protection after natural infection [21] and even higher effectiveness as a consequence of “hybrid immunity” resulting from a combination of prior infection and recent booster vaccination [22]. However, there are several important caveats regarding prior immunity that merit consideration. First, protection resulting from both previous infections and immunization wanes over time. In a recent study, the effectiveness of COVID-19 vaccines against severe Omicron infections declined from 94% to 57% after just 4 months [23]. Second, it is important to consider how closely the circulating SARS-CoV-2 variants match the strains contained in the COVID-19 vaccines. In a large multicenter study, Link-Gelles et al. showed that the effectiveness of vaccines against the recent Omicron variants differed by 10%–40%, depending on whether the monovalent or bivalent vaccine was used [24]. Another important caveat concerns immunocompromised children. As their immunological response to both vaccines and infection is variably diminished based on the timing and extent of immunosuppression [25], special consideration is needed when assessing the degree to which risk can be mitigated by prior immunity.
Immunocompromise
Evidence suggests that children with compromised immune systems are at high risk for poor outcomes after SARS-CoV-2 infections. A study by Greenan-Barrett et al. found that, after hospitalization for COVID-19, immunocompromised children and adolescents had significantly higher rates of ICU admission (12% vs. 2%), mechanical ventilation (8% vs. 1%), and death (6.5% vs. 0.2%) when compared with immunocompetent children [26]. However, the observed effect was not always consistent across studies. This variability probably stems from the diverse nature of immunocompromised conditions, which encompass both inherited and acquired forms, each conferring different degrees of immune compromise. Certain factors, such as recent chemotherapy treatment, neutropenia (absolute neutrophil count < 500/μL), lymphocytopenia (absolute lymphocyte count < 200/μL), the myeloablative conditioning regimen, graft-versus-host disease, and a recent hematopoietic cell transplant can lead to severe immunocompromise and have been associated with increased severity [27]. The evidence is more limited regarding whether mild to moderate immunocompromise is a risk factor for progression to critical COVID-19 [28–30]. The panel defined mild to moderate immunocompromise as the routine administration of non-lymphodepleting immunosuppressive or immunomodulatory medications, such as disease-modifying anti-rheumatic drugs (DMARDs) or the administration of prednisone at <20 mg/day. The largest study to evaluate this association was a multicenter single-arm study by Kearsley-Fleet et al. that involved 607 pediatric patients who received immunomodulatory therapies for rheumatologic conditions and had a hospitalization rate of 7% (43 out of 607) after COVID-19 [29]. Notably, glucocorticoid usage correlated with hospitalization on univariate analysis, although this effect lost its significance after adjustment for the effect of obesity, and no correlation was found between DMARDs and hospitalization.
Hematologic Conditions
Our review included 11 studies that assessed the outcomes in children with sickle cell disease, and five of these studies were combined in a meta-analysis. However, although the pooled OR was greater than 1, the sample sizes were small; therefore, the estimates lacked precision. Two studies included in the literature review found patients with sickle cell disease and a prior history of pain crisis, acute chest syndrome, and associated comorbidities to have an increased risk of hospitalization but not of critical disease [12, 31]. More evidence is needed to draw stronger conclusions on the risk of critical disease among children with sickle cell disease.
Obesity and Diabetes
Obesity, defined as a BMI or weight exceeding the 95th percentile for age and sex, emerged as a clear risk factor in our meta-analysis, with a pooled unadjusted OR of 2.26 (95% CI: 1.75–2.92), using data from 18 studies. Importantly, eight studies found obesity to be a predictor of critical COVID-19 outcomes even after adjustment for other known risk factors. Similarly, diabetes was a strong risk factor for severity [32–36]. One of the largest studies to examine these associations found that children and adolescents with diabetes (combined type 1 and type 2) had a higher prevalence of ICU admission (47% vs. 26%), invasive ventilation (17% vs. 10%), and death (15% vs. 8%). Notably, those with both diabetes and obesity had a hazard of death approximately four times higher than that of the general pediatric population [32].
Collectively, these findings suggest that both obesity and diabetes should be considered as definite risk factors for progression to critical COVID-19, although more data are needed to disentangle the effects of type 1 and type 2 diabetes. Kompaniyets et al. reported that both diabetes types were associated with increased risk of hospitalization, but only type 1 diabetes was associated with increased risk of critical disease (aRR: 2.38; 95% CI: 2.06–2.76) [37]. In another study, Mann et al. reviewed outcomes of 651 children with type 1 diabetes and COVID-19, and found that 12% had diabetic ketoacidosis at the time of COVID-19 diagnosis, 17% required hospitalization, and 47% of those hospitalized needed ICU care [38]. Some studies have suggested that children with type 1 diabetes who maintain good glycemic control may be at no greater risk than children without diabetes [33, 39]. However, more evidence is needed to draw this conclusion, as relatively few studies have evaluated type 1 and type 2 diabetes separately, and fewer still have taken into consideration the effect of glycemic control [37, 38, 40–42].
Cardiac and Pulmonary Disease
There is consistent evidence for the association of critical COVID-19 with cardiac and pulmonary conditions, excluding asthma. In our meta-analysis, the odds of critical disease after hospitalization were three times higher in children with cardiac disease and twice as high in those with pulmonary comorbidities. The evidence is most consistent for congenital heart disease and hypertension, which were assessed separately by more than 10 studies [11, 13, 17, 36, 43–49]. Few studies have separated out specific respiratory conditions, other than asthma, to assess their contribution to the observed risk of severe disease. Associations with severe disease have been reported for pulmonary hypertension, anatomical abnormalities, bronchopulmonary dysplasia, obstructive sleep apnea, and oxygen/ventilator dependence [11, 50–52]. In contrast, 24 studies have investigated whether asthma is a predictor of critical disease, with most (84%) finding no evidence to support this. However, it is important to acknowledge that the severity and complexity of these cardiopulmonary comorbidities may play an important role in modifying the overall effect of these conditions. For example, Ward et al. estimated that children with both cardiac and pulmonary comorbidities had 20 times greater odds of needing ICU care after being hospitalized with COVID-19 [36]. In contrast, some studies have suggested that the risks of severity may be lower in otherwise healthy children with mild, acyanotic, or corrected congenital heart disease [53–55]. There are also data suggesting that poorly controlled asthma can increase the likelihood of hospitalization, although evidence is lacking as to whether it also increases the rates of critical outcomes [12, 56, 57].
Gastrointestinal Disease
We identified 15 studies that collectively provided data on 1,015 children with diagnoses of both COVID-19 and chronic gastrointestinal conditions, including chronic liver disease, gastroesophageal disorders, short bowel syndrome, and a requirement for parenteral or enteral tube feeding for nutrition. The pooled OR derived from these studies was 3.15 (95% CI: 2.22–4.46), suggesting there is an elevated risk of severe COVID-19 in these children. A few studies suggest that certain gastrointestinal conditions have a stronger association with severe COVID-19. For example, children with liver disease, especially end-stage liver disease, experience higher rates of hepatic complications during SARS-CoV2 infection [34, 58]. Likewise, adolescents with non-alcoholic fatty liver disease may also have an increased risk of severe disease, particularly if they are obese [59]. In contrast, an international study of children with inflammatory bowel disease found that <1% (2 of 209 participants) developed critical disease [60].
Chronic Kidney Disease
Although chronic kidney disease (CKD) has been well described as a predictor of COVID-19 severity in adults, its predictive value in children is less clear. In our evidence review, the rates of CKD in children with COVID-19 were reported for eleven studies, only two of which found a statistically significant risk. However, this evidence had important limitations. For example, several studies did not differentiate between kidney injury sustained during hospitalization and pre-existing kidney disease. Additionally, most studies had small sample sizes or did not account for other comorbidities. Therefore, there is uncertainty as to whether CKD is an independent predictor of severity.
Neurodevelopmental and Psychiatric Conditions
Psychiatric conditions such as attention-deficit hyperactivity disorder (ADHD), anxiety, and depression have been associated with increased risk of hospitalization, but not necessarily for critical illness once hospitalized [12, 37, 61]. Conversely, there is good evidence that children with certain neurological conditions, such as seizure disorders, are at increased risk. In a large multicenter study that included 43,465 children and adolescents with COVID-19, epilepsy was one of the strongest risk factors for both hospitalization (aRR: 1.97; 95% CI: 1.62–2.39) and severe disease once hospitalized (aRR: 1.71; 95% CI: 1.41–2.08) [37]. Neurodevelopmental disorders that confer medical complexity (e.g., Down syndrome, cerebral palsy, and metabolic syndrome) may also place children at increased risk. In a national registry study that included 261 children with Down syndrome in Latin America, in-hospital mortality was double for children with Down Syndrome, even after adjustment for other comorbidities such as cardiac disease and obesity [62]. However, this effect has not been consistently shown in other studies [11, 37, 44, 47].
Section 2: Treatment of COVID-19 in Pediatric Patients Who Do Not Require Hospitalization for COVID-19
Recommendations (Figure 3):
Figure 3.
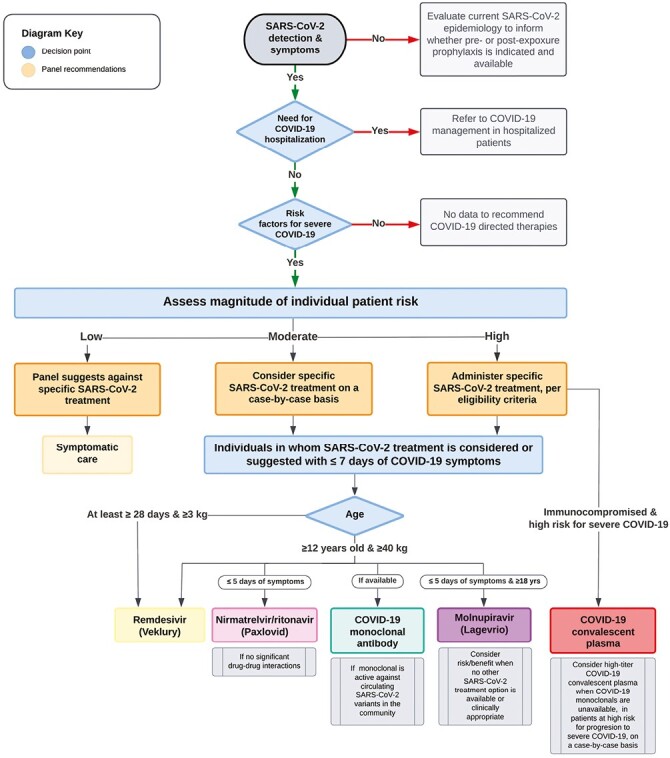
Management of COVID-19 in children and adolescents not requiring hospitalization for COVID-19.
Recommendation 2.1:
For pediatric patients with SARS-CoV-2 infection who do not require hospitalization for COVID-19 and are at low risk for progression to severe disease, the panel suggests against specific treatment for SARS-CoV-2.
Comment:
Most pediatric patients are at very low risk for severe COVID-19. The benefits of therapies for pediatric patients are not established and do not balance the potential risks of treatment.
Recommendation 2.2:
For pediatric patients with SARS-CoV-2 infection who do not require hospitalization for COVID-19 and are at moderate risk for progression to severe disease, consider specific treatment for SARS-CoV-2 on a case-by-case basis.
Recommendation 2.3:
For pediatric patients with SARS-CoV-2 infection who do not require hospitalization for COVID-19 and are at high risk for progression to severe disease, the panel suggests administering specific treatment for SARS-CoV-2.
Recommendation 2.4:
For pediatric patients with SARS-CoV-2 infection who do not require hospitalization for COVID-19 and for whom specific treatment is considered or suggested, the panel suggests treatment with one of the following: ritonavir-boosted nirmatrelvir, remdesivir, or a mAb (if expected to be effective against circulating strains). If none of these options is available or clinically appropriate, consider treatment with molnupiravir for patients ≥18 years of age.
Comments:
The efficacy and tolerability of ritonavir-boosted nirmatrelvir, remdesivir, and active mAbs for preventing severe COVID-19 appear to be very similar in published studies. The most appropriate choice of therapy should be determined by the availability, contraindications (especially drug interactions for ritonavir-boosted nirmatrelvir), ease of administration, cost, and patient/caregiver preference. Treatment should be initiated as soon as possible, ideally within 5 days of symptom onset. Consultation with a pharmacist is recommended for ritonavir-boosted nirmatrelvir because of important drug interactions that may preclude therapy or require modifying or discontinuing other medications. The use of mAb products is dependent on their expected activity against currently circulating variants of SARS-CoV-2; at the time of writing, none are available or recommended in the USA. The recommended duration of therapy for early outpatient treatment is 5 days for ritonavir-boosted nirmatrelvir, 3 days for remdesivir, and 5 days for molnupiravir.
Molnupiravir is a less-preferred option because its efficacy in preventing severe COVID-19 appears to be inferior in published studies, but its use may be considered if no other specific therapeutic options are available.
Recommendation 2.5:
For immunocompromised pediatric patients with SARS-CoV-2 infection who do not require hospitalization for COVID-19 and are at high risk for progression to severe disease, consider administering high-titer convalescent plasma to prevent disease progression.
Comments:
The benefits of convalescent plasma appear to be highest in immunocompromised patients, especially those with profound antibody deficiency. Convalescent plasma may be relatively contraindicated in patients with a history of severe allergic reactions or anaphylaxis in response to plasma transfusions. Considerations include the optimal timing of administration: early administration (as soon as possible and within 8 days of onset) is desirable, but later use may be appropriate for patients with worsening illness and profound immunocompromise, even if they do not yet require hospitalization. In settings where effective mAb therapy is available, this is preferrable to convalescent plasma.
Recommendation 2.6:
For immunocompromised pediatric patients with SARS-CoV-2 infection who do not require hospitalization for COVID-19 and are at low or moderate risk for progression to severe disease, the panel suggests against the routine administration of convalescent plasma to prevent disease progression.
Comment:
Immunocompromised pediatric patients at low to moderate risk for progression to severe disease are less likely to benefit from treatment and the panel concluded that the potential harms, such as infusion reactions and cost, may outweigh the potential benefits.
Rationale and Evidence Summaries for COVID-19 Therapies in Pediatric Patients not Requiring Hospitalization for COVID-19
Remdesivir
Remdesivir (Veklury®) is approved by the FDA for treating non-hospitalized adults and pediatric patients aged 28 days or older who weigh at least 3 kg (~7 lbs.), who have mild to moderate COVID-19, and who are at high risk for progression to severe COVID-19, including disease resulting in hospitalization or death [63].
There are no data on the benefit of remdesivir for non-hospitalized pediatric patients or for those who are fully vaccinated. The panel recommends that considerations for outpatient treatment with remdesivir should include the feasibility of outpatient administration, including the infusion center capacity, staffing sufficiency, appropriate infection prevention measures, monitoring, the ability to establish and maintain intravenous access, and patient compliance. Providers should also consider baseline renal and hepatic function, the availability of alternative agents (e.g., nirmatrelvir/ritonavir or neutralizing mAbs), and insurance approval now that remdesivir is commercially available.
Based on the study design of the randomized controlled trial used to support the FDA approval, it is recommended that remdesivir be initiated as soon as possible (within 7 days) after symptom onset in eligible patients [64].
Based on the duration recommended in the remdesivir prescribing information and outcomes in a randomized trial of non-hospitalized patients who were at high risk for COVID-19 progression, a 3-day course of remdesivir had an acceptable safety profile and resulted in an 87% lower risk of hospitalization or death when compared to placebo [64, 65].
Clinical Data
Gottlieb and colleagues conducted a randomized, double-blind, placebo-controlled trial involving 562 non-hospitalized patients at least 12 years of age with SARS-CoV-2 infection confirmed by a molecular diagnostic assay within 4 days before screening who had symptom onset within the previous 7 days and who had at least one risk factor for disease progression or were 60 years of age or older, regardless of risk factors [64]. Patients were randomly assigned to receive intravenous remdesivir (200 mg on day 1 and 100 mg on days 2 and 3) or placebo. The primary efficacy endpoint was a composite of COVID-19–related hospitalization or death from any cause by day 28. Secondary outcomes included COVID-19–related medical visits or death from any cause by day 14 and day 28, COVID-19–related hospitalization by day 14 and day 28, change in NP SARS-CoV-2 viral load from baseline to day 7, and time to alleviation of COVID-19 symptoms. There were 279 patients randomized to the remdesivir group and 283 to the placebo group. The most common comorbidities included diabetes mellitus (62% of the patients), obesity (55%), hypertension (48%), and chronic lung disease (24%). The median time from symptom onset to treatment initiation was 5 days (IQR: 3–6 days), and no patients were vaccinated against SARS-CoV-2 at baseline. By day 28, two patients (0.7%) in the remdesivir group were hospitalized because of COVID-19, compared to 15 (5.3%) in the placebo group. There was an 87% relative risk reduction (RRR) in hospitalization or death by day 28, and all hospitalizations occurred by day 14. No pediatric patients were hospitalized in either group, and there were no deaths among the study participants of any age. There was a possible improvement in the proportion of participants with symptom alleviation by day 14 (35% of the remdesivir group vs 25% of the placebo group), although there was no difference between the groups in the change in NP viral load from baseline. The incidence of adverse events (AEs) was similar in both groups (12% vs 8.8%) (rate ratio [RR]: 0.27; 95% CI: 0.1–0.7); 1.8% of the remdesivir group experienced a serious adverse event (SAE) versus 6.7% of the placebo group, and 0.7% of the remdesivir group had an event that led to discontinuation of the medication vs. 1.8% of the placebo group. There were also minimal changes in creatinine clearance from baseline and the mean change in ALT from baseline to day 14 was similar in the two study groups.
Pediatric Considerations
Although this study evaluated remdesivir in the outpatient setting, few pediatric patients aged 12–18 years were enrolled (n = 8) and few immunocompromised patients were included. Neither did the study include vaccinated patients.
Pediatric-specific data on the safety and efficacy of remdesivir is limited, and no studies, to our knowledge, have investigated its use for non-hospitalized pediatric patients. However, retrospective, mostly single-center studies of hospitalized pediatric patients treated with remdesivir have suggested that it is generally well tolerated by children [66–68]. Its use in hospitalized infants younger than 28 days and/or weighing less than 3 kg has been described, but there have been no reports of its use in non-hospitalized neonates [69].
A phase 2/3, single-arm, open-label study (NCT04431453) to evaluate the safety, tolerability, pharmacokinetics, and efficacy of remdesivir in hospitalized patients with COVID-19 ranging in age from newborn to <18 years is currently recruiting and aims to enroll 62 participants. The youngest cohort is aged 0–56 days, with a gestational age of ≤37 weeks and weighing ≥1.5 kg. The study is reported to have been completed, but no data are available at the time of writing [70]. Although this study has included only hospitalized children, it should yield important data on the safety of remdesivir in the pediatric population.
Logistically, a 3-day outpatient course of remdesivir may be challenging to administer. The feasibility of outpatient remdesivir infusions (in terms of infusion center capacity, staffing sufficiency, need for monitoring for at least 1 hour post infusion, ability to establish and maintain IV access, patient compliance, insurance approval, etc.) should be considered when prescribing remdesivir for non-hospitalized pediatric patients with mild to moderate COVID-19 who are at risk for progression to severe disease. Alternative treatments, such as ritonavir-boosted nirmatrelvir and mAbs, may be easier to administer, but remdesivir is a reasonable option to consider for children who are too young for oral antivirals or cannot swallow pills, when significant drug–drug interactions with ritonavir-boosted nirmatrelvir are to be avoided, or when the available mAbs are thought to be ineffective against the currently circulating SARS-CoV-2 variant.
Ritonavir-Boosted Nirmatrelvir (Paxlovid)
Nirmatrelvir is an inhibitor of the SARS-CoV-2 main protease (Mpro). Inhibition of Mpro prevents the processing of polyprotein precursors, resulting in the inhibition of viral replication. Ritonavir inhibits CYP3A-mediated metabolism of nirmatrelvir, resulting in increased nirmatrelvir plasma concentrations. The efficacy of ritonavir-boosted nirmatrelvir was evaluated in the phase 2/3 trial “Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients” (EPIC-HR) (ClinicalTrials.gov Identifier, NCT04960202), which included 2246 non-hospitalized, symptomatic adults with at least one risk factor for progression to severe disease. The incidence of COVID-19–related hospitalization or death through day 28 was lower in the ritonavir-boosted nirmatrelvir group than in the placebo group (RRR: 87.8%). Additional details regarding the trial are well summarized by the National Institutes of Health (NIH) COVID-19 Treatment Guidelines Panel [71]. Importantly, the EPIC-HR trial did not include patients younger than 18 years or individuals with a history of COVID-19 infection or vaccination. Ninety-eight percent of the patients enrolled in the EPIC-HR trial were infected with SARS-CoV-2 Delta variant, and there is no clinical data on the efficacy of ritonavir-boosted nirmatrelvir against the Omicron variant; however, the target for nirmatrelvir is relatively conserved, making it less susceptible to loss of efficacy against emerging variants. Concern regarding cross-resistance with other COVID-19 therapeutics is low, given the different mechanisms of activity, although there is currently a lack of data on the efficacy or safety of combination therapy.
The EPIC-SR trial (ClinicalTrials.gov Identifier, NCT05011513) included 1153 unvaccinated adults with standard risk and vaccinated adults with at least one risk factor for progression to severe disease. The data from this trial is as yet unpublished. However, the primary endpoint of self-reported, sustained alleviation of all symptoms for 4 consecutive days, as compared to placebo, was not met. There was a non-significant 51% reduction in hospitalization or death (5 of 576 patients vs. 10 of 569), with a non-significant 57% reduction in hospitalization/death among vaccinated adults with at least one risk factor. There was a 62% decrease in COVID-19–related medical visits per day across all patients (P = 0.0228) and a 72% reduction in the average number of days in hospital among ritonavir-boosted nirmatrelvir recipients versus those in the placebo arm. Observational studies have shown similar results. For example, a comparative retrospective cohort study evaluated whether ritonavir-boosted nirmatrelvir was associated with improved clinical outcomes in non-hospitalized, vaccinated (at least 1 month previously) adult patients with COVID-19 [72]. There was a 45% RRR in the primary composite outcome of all-cause ER visits, hospitalization, or death within 30 days in the ritonavir-boosted nirmatrelvir cohort (OR: 0.5; 95% CI: 0.39–0.67; P < 0.005). There was also a higher probability of event-free survival in the ritonavir-boosted nirmatrelvir group (88.15% vs. 84.16%, P = 0.002). Another propensity-matched, retrospective, observational cohort study of non-hospitalized adults with COVID-19 found that patients treated with ritonavir-boosted nirmatrelvir had reduced odds of 28-day all-cause hospitalization, 28-day all-cause mortality, and subsequent hospital visits when compared to patients who did not receive antiviral treatment [73].
Although the authorized adult dosing regimen is expected to result in serum exposures of ritonavir-boosted nirmatrelvir in patients aged 12 years or older and weighing at least 40 kg comparable to those observed in adults, the safety and effectiveness of ritonavir-boosted nirmatrelvir have not been established in pediatric patients. At the time of writing, a phase 2/3 study to investigate the efficacy and safety of ritonavir-boosted nirmatrelvir in children (ClinicalTrials.gov Identifier, NCT05261139) is not yet enrolling participants. The number needed to treat is probably much higher for pediatric patients than for adult patients because age is a protective factor for severe disease, so this aspect should be considered when individual risk vs. benefit decisions are being made.
Molnupiravir
Molnupiravir is a nucleoside analogue that inhibits SARS-CoV-2 replication by viral mutagenesis. The efficacy of molnupiravir was evaluated in the phase 3 MOVe-OUT trial, which included 1433 non-hospitalized adult patients with laboratory-confirmed mild to moderate COVID-19, at least one risk factor associated with poor disease outcomes, and symptom onset within 5 days before randomization. The incidence of all-cause hospitalization or death through day 29 was lower in the molnupiravir-treated group than in the placebo group (RRR: 30%). Additional details regarding the trial are well summarized by the NIH. Based on the bone and cartilage toxicity observed in a 3-month, repeat-dose toxicology study in rats, molnupiravir is not authorized for use in patients younger than 18 years. The safety and effectiveness of molnupiravir have not been established in pediatric patients and, at the time of writing, there are no known studies of the safety or efficacy of molnupiravir in this population. Most patients enrolled in the MOVe-OUT trial were infected with SARS-CoV-2 Delta variant, and there are no clinical data on the efficacy of molnupiravir against the Omicron variant. Concern regarding cross-resistance with other COVID-19 therapeutics is low, given the different mechanisms of activity, although there is currently a lack of data on the efficacy or safety of combination therapy. Molnupiravir has lower efficacy than ritonavir-boosted nirmatrelvir or remdesivir and, therefore, it may be considered only as an alternative.
Monoclonal Antibody (mAb) Therapy
This guidance statement is based on balancing the beneficial impact on mortality and hospitalizations seen in adult studies against the generally lower risk of progression to severe disease in children and adolescents—even among those with risk factors specifically mentioned in the EUAs—and the scarcity of pediatric evidence. Although the FDA has authorized the use of mAb therapy for adolescents at high risk for severe COVID-19, there are limited data to support such a designation among adolescents belonging to many of the risk groups mentioned in the EUA. The safety profile of these agents in adult studies has been deemed acceptable, but they have not been studied systematically in younger age groups. Furthermore, whether these agents provide protection against multisystem inflammatory syndrome in children (MIS-C) or post-COVID conditions is unknown. The intent of this guidance is not to suggest the routine use of these agents for all adolescent patients or to preclude their use for any specific group but to clarify that pediatric use should be guided by the best available evidence for the potential trajectory of SARS-CoV-2 infection in an individual patient. The guidance should supplement and not replace instructions from local health departments on using these products.
Bebtelovimab is a recombinant neutralizing human IGg1λ mAb that binds the spike protein of SARS-CoV-2 and blocks attachment to the human ACE2 receptor. In February 2022, the FDA issued an EUA for the use of bebtelovimab to treat patients aged at least 12 years and weighing at least 40 kg with mild to moderate COVID-19 who were at high risk for progression to severe disease. The mAb was to be administered within 7 days of symptom onset. Supporting evidence included in vitro data confirming the activity of the mAb against all circulating Omicron subvariants and data from a phase 2 trial, BLAZE-4 (NCT04634409), that evaluated the treatment of COVID-19 in unvaccinated, non-hospitalized adult patients with mild to moderate COVID-19. Arms 12 and 13 evaluated the clinical efficacy and safety in high-risk patients receiving bebtelovimab monotherapy or bebtelovimab in combination with bamlanivimab and etesevimab. COVID-19–related hospitalization or death from any cause by day 29 occurred in two of the patients (4%) treated with the combination therapy, as compared with three patients (3%) treated with bebtelovimab monotherapy. Two pediatric patients (aged 14 and 15 years) were enrolled in an open-label arm (14) of BLAZE-4, the main objective of which was to characterize the safety profile. In a multi-site, retrospective study of more than 3500 high-risk patients who received either IV bebtelovimab or PO nirmatrelvir–ritonavir, only 1.4% of the patients in the mAb group progressed to severe disease. This was similar to the rate of progression of 1.2% in the antiviral group. Rates of admission to the ICU were also similar at 0.4% vs 0.2%, respectively [74].
Although no study participants were infected with the Omicron (B.1.1.529) lineage or with sub-lineages of SARS-CoV-2 in the BLAZE-4 trial (Clinicaltrials.gov Identifier, NCT04634409), in vitro studies demonstrated that bebtelovimab retained activity (resulting in a <5-fold reduction) against the Omicron (B.1.1.529/BA.1), Omicron BA.2, Omicron BA.4, and Omicron BA.5 variant lineages [75]. Treatment-emergent, resistance-associated substitutions were associated with higher viral loads in the patients in whom they were detected. However, bebtelovimab is not active against the currently circulating variants of SARS-CoV-2; therefore as of November 30, 2022, it is no longer authorized for use [76, 77].
Sotrovimab is an engineered human mAb that targets a highly conserved epitope of the SARS-CoV-2 spike protein [78]. Unlike other mAbs, data suggest that sotrovimab may prevent cell–cell fusion and contribute to immune-mediated viral clearance, in addition to antiviral neutralizing activity. The COMET-ICE study was a phase 3, randomized, multi-center, double-blind, placebo-controlled trial that included 1057 non-hospitalized, unvaccinated adults. All patients included had laboratory-confirmed mild to moderate COVID-19, were at high risk for progression to severe disease, and were administered drug or placebo within 5 days of symptom onset. The primary outcome was a composite of all-cause hospitalization longer than 24 hours or death through day 29. The median age of enrolled people was 53 years. Obesity, age 55 years or older, diabetes, and moderate-to-severe asthma were the most common risk factors for progression to severe disease. More patients in the placebo group than in the sotrovimab group met the primary endpoint (30 of 529 [6%] vs. 6 of 528 [1%]; P < 0.001). Sotrovimab was associated with a 79% RRR for hospitalization or death from any cause through day 29. Adverse events were reported at similar rates for both groups (22% for the sotrovimab group vs. 23% for the placebo group). Real-world observational data from non-hospitalized adult patients also demonstrated the efficacy of sotrovimab in reducing hospitalization and 28-day all-cause mortality, as compared to the rates in matched patients who did not receive mAb [73]. The safety and efficacy of sotrovimab have not been assessed in clinical trials in pediatric patients. In limited case series of high-risk pediatric patients, sotrovimab was noted to be tolerable with no serious adverse effects [79].
Although in vitro assessments demonstrated that sotrovimab retained efficacy against the B.1.1.529/BA.1.1 Omicron variant, there was a 16-fold reduction in activity against the B.1.1.529/BA.2 spike variant of SARS-CoV-2, relative to that against the wild-type variant. As of April 2022, sotrovimab is no longer authorized for treating COVID-19 in the United States because of the increased proportion of non-susceptible circulating variants of SARS-CoV-2 [80].
Bamlanivimab plus etesevimab was the only mAb product authorized for use in children of all ages, including neonates, who were at high risk for progression to severe disease, for treating mild to moderate COVID-19 and for post-exposure prophylaxis. An open-label pediatric addition to the BLAZE-1 study included a total of 125 non-hospitalized patients treated for mild to moderate COVID-19, the youngest of whom was 10 months of age and weighed 8.6 kg. The safety profile was similar to that in adults, and no pediatric patients died or required hospitalization because of COVID-19. The phase 3 trial, BLAZE-2, supported the EUA for post-exposure prophylaxis of COVID-19, but no children were enrolled. There are no further clinical use data on using bamlanivimab plus etesevimab in pediatric patients. Dosing for children younger than 12 years was based on pharmacokinetic modeling and simulation.
Bamlanivimab plus etesevimab was associated with a >2938-fold reduction in susceptibility based on pseudotyped virus-like particle (VLP) neutralization data. In the phase 3 portion of the BLAZE-1 study, 9% of patients treated with higher-dose bamlanivimab and etesevimab and 5.3% of those who received the EUA dose were observed to have treatment-emergent variants, compared with 4% of patients who received placebo [81]. Because of the reduced activity against circulating variants of SARS-CoV-2, the FDA no longer authorizes the use of this treatment option in geographic areas where exposure or infection are most likely related to a non-susceptible viral variant [81].
Casirivimab plus imdevimab (REGEN-COV) was authorized for use in high-risk patients aged 12 years or older and weighing at least 40 kg for both treatment and post-exposure prophylaxis of COVID-19, and it could be administered via either the intravenous or subcutaneous route. Both products were discussed in prior guidance [4]. The efficacy of casirivimab plus imdevimab for treating mild to moderate COVID-19 is supported by a phase 3, placebo-controlled, adaptive trial that showed a reduced risk of COVID-19–related hospitalization or death from any cause when compared to placebo (Clinicaltrials.gov Identifier, NCT04425629). Evidence supporting the use of casirivimab plus imdevimab as post-exposure prophylaxis comes from a phase 3 trial that evaluated asymptomatic patients at least 12 years of age, but adolescents were not well represented in that trial [82]. Limited experience with casirivimab plus imdevimab in children has been published. Gupta et al. reviewed their experience with using this agent in pediatric patients after heart transplantation both to treat COVID-19 and as post-exposure prophylaxis, and they reported that casirivimab plus imdevimab was well tolerated [83].
Casirivimab plus imdevimab together were associated with a >1013-fold decrease in susceptibility to the Omicron variant, but there was no change in susceptibility to previously identified variants in VLP neutralization studies [84]. Distribution of casirivimab plus imdevimab was paused in December 2021 because of reduced in vitro activity against the current circulating strains of SARS-CoV-2 [85]. In January 2022, the FDA fact sheet was updated to reflect this; it now states that casirivimab plus imdevimab is no longer authorized for treating mild to moderate COVID-19 in geographic areas where exposure or infection is most likely related to a non-susceptible viral variant.
Pediatric-Specific Data
Several single-center case series and case reports have described the logistics, safety, and tolerability of mAb therapy in adolescent and young adult populations [86–89]. Infusion reactions have been described in a small number of patients [90]. Notably, specific pediatric efficacy and safety data for any of these products remain limited.
Convalescent Plasma
The EUA issued by the US FDA at the time of writing permits the use of high-titer convalescent plasma (CP) to treat COVID-19 in immunocompromised children and adults in both the inpatient and outpatient settings [91]. In contrast to other treatment options, there is no minimum age or weight requirement for using CP. CP is not authorized for use in immunocompetent patients because data from randomized controlled trials have not supported its use in this population and alternative therapies with better proven efficacy are available. To meet the criteria for use, CP must contain high titers of anti–SARS-CoV-2 antibodies, as defined by the EUA. There is no specified time frame in which the product must be given.
The benefits of high-titer CP in immunocompromised children are not clearly demonstrated in the current available literature, although it is reasonable to assume there may be some benefit based on the results of studies in adults. There are inherent risks in administering a blood product, as well as multiple logistical challenges. For immunocompromised pediatric patients who are at high risk for progression to severe disease, the potential benefits may outweigh the risks and administration of CP can be considered on a case-by-case basis. However, the panel considers that the risks may outweigh the potential benefits in immunocompromised pediatric patients at low to moderate risk for progression to severe disease.
Although the EUA authorizes the use of CP in pediatric patients, there is a paucity of published efficacy data on COVID-19 CP in immunocompromised children [91]. The available data is derived from small studies and case series. A recent systematic review and meta-analysis (SR & MA) of COVID-19 CP for treating immunocompromised patients has been published [92]. The SR & MA included patient-level data extracted from six publications for a total of 20 pediatric patients. The children’s immunosuppressive conditions were not specified, and none of these children died of COVID-19. Within the SR & MA, the largest pediatric population came from a pharmacokinetic (PK) study in which CP was administered to “high-risk” children at 5 mL/kg, whereupon the plasma neutralizing titers dropped quickly [93]. Therefore, the effectiveness of CP in immunocompromised children can only be inferred from adult data.
Safety and pragmatic considerations complicate decision making around the use of COVID-19 CP. It may be relatively contra-indicated in patients with a history of severe allergic reactions or anaphylaxis to plasma transfusions. Infusion reactions (including transfusion-associated circulatory overload) may occur, and transmission of transfusion-associated infections is possible. Considerations include the optimal timing of administration; this is not defined in the EUA, but early administration (as soon as possible and within 8 days of onset) is desirable; later use may be appropriate for patients with worsening illness and profound immunocompromise, even if they do not yet require hospitalization. Therefore, the logistics and lack of local availability of “high-titer” COVID-19 CP (as defined in the EUA) may preclude its use. Even if a product is available, it may not reflect current circulating variants. A pediatric dose is not defined in the EUA, but the available literature supports a dose of 5–10 mL/kg, up to the adult dose of one unit (approximately 200 mL); smaller volumes or prolonged infusions may be required for patients with impaired cardiac function/heart failure or renal disease. Repeat dosing can be considered “based on the prescribing physician’s medical judgment and the patient’s clinical response” [91]. It may be reasonable to administer CP with other treatments, such as remdesivir; however, the panel advises against administering CP in combination with mAbs or other treatments that also confer passive immunity.
Section 3: Treatment of COVID-19 in Pediatric Patients Requiring Hospitalization for COVID-19
Assessment of Hospitalized Pediatric Patients with COVID-19
Recommendation 3.1:
For patients with COVID-19 who are hospitalized for reasons other than COVID-19 respiratory disease and require no respiratory support above baseline, the panel suggests applying the approach recommended for non-hospitalized patients.
Comment:
Pediatric patients diagnosed with COVID-19 may be admitted to the hospital with no symptoms or with only mild or moderate symptoms, defined as not requiring respiratory support above baseline. For such patients, the panel suggests using the approach recommended for ambulatory patients. In pediatric patients who are admitted for another reason and are found to have COVID-19 but have no risk factors for progression to severe or critical COVID-19, antiviral therapy is not recommended. Treatment, such as a 3-day course of remdesivir, may be considered for patients who would meet the criteria for treatment described above.
Recommendation 3.2:
When planning treatment for patients who are hospitalized for COVID-19 respiratory disease, clinicians should consider the following factors: age, illness severity, vaccine status, prior infection status, duration of illness, and presence or absence of risk factors for progression to severe COVID-19.
Comment:
Clinicians caring for children hospitalized for acute COVID-19 respiratory disease should consider the following goals in therapeutic decision making: 1) preventing progression to more severe illness or death; 2) minimizing the duration of hospitalization; 3) avoiding treatment-associated adverse effects; and 4) providing cost-effective and equitable care. Age, illness severity, vaccine status, prior infection status, duration of illness, and presence or absence of risk factors for progression to severe COVID-19 are all important factors affecting which patients are most likely to benefit from therapy.
Rationale and Evidence Summary for Assessment of Hospitalized Pediatric Patients with COVID-19
Age
The FDA has approved remdesivir for use in infants and children with COVID-19 who are ≥28 days of age and weigh ≥3 kg, and it has issued EUAs for tocilizumab and baricitinib for children ≥2 years of age who are hospitalized with severe COVID-19. However, pediatric-specific efficacy data for the currently available therapies are limited. The eligibility criteria for several landmark clinical trials of COVID-19 therapeutics, including those of remdesivir, dexamethasone, and tocilizumab, included adolescents ≥12 years of age [94–97]. However, none of the reports for these trials included the specific numbers of participants younger than 18 years or provided subgroup analyses of the adolescent participants. Although there are ongoing clinical trials to determine the safety and efficacy of remdesivir, tocilizumab, and baricitinib for treating COVID-19 in the pediatric age group [70, 98–100], only small, observational datasets are currently available to support the use of these treatments in children.
In the absence of robust clinical trial data for COVID-19 therapies in children, clinicians must extrapolate their safety and efficacy from data obtained from adult studies. However, COVID-19 displays different manifestations across the age spectrum. Although several hypotheses have been proposed [101–104] to explain the age-related differences in clinical severity, the underlying mechanisms remain uncertain. Disease in adolescents is typically more severe than that in younger children, and adolescents may experience a COVID-19 syndrome more similar to that seen in adults [105–107]. In addition, older children and adolescents are more likely to have acquired comorbidities, such as hypertension, obesity, and diabetes, that are associated with more severe disease in adults [108–110]. For these reasons, results from clinical trials in adults are probably more applicable to older children and adolescents than to younger children. Conversely, younger children typically have milder disease when compared with adolescents [111, 112]. Whereas infants and young children with COVID-19 have a higher likelihood of hospital admission with COVID-19 than do older children, hospitalized infants and young children only infrequently develop severe disease requiring advanced respiratory support or intensive care [106, 113].
Acknowledging the limitations of the available literature, the current evidence base suggests that young children without other risk factors are less likely than older children and adolescents to develop severe disease, particularly illness that requires advanced respiratory support or intensive care, and, therefore, they are less likely to benefit from treatment. This pattern supports a higher threshold for initiating therapy in younger children as compared with older children and adolescents.
Prior Infection and Active and Passive Vaccination
Active and passive immunization or previous infection reduce the risk of hospitalization, despite the rising incidence of breakthrough infection due to viral evolution [114–116]. For vaccinated patients requiring hospitalization, vaccination may provide some protection against further progression [114]. However, if a vaccinated or previously infected patient requires hospitalization for acute COVID-19, this suggests they have inadequate immune protection; therefore, prior infection or immunization should not preclude antiviral therapy.
Clinicians caring for children hospitalized with acute COVID-19 should establish the patient’s history and timing of prior COVID-19 infections and COVID-19 vaccinations. Timing is important for two reasons: 1) the kinetics of the immune response, with peak neutralizing antibody titers being observed within weeks of exposure and declining over time [117]; and 2) the likelihood of the immune response against the prior antigenic exposure being active against currently circulating strains. If available and effective against circulating strains, passive immunization should also be considered. Clinicians should also consider the patient’s immune competence and the likelihood of an adequate response to infection or active vaccination.
Duration of Illness
Healthcare providers should determine the acuity of infection based on the timing of known COVID-19 exposure, symptom duration, and previous testing results. PCR tests for SARS-CoV-2 may remain positive for several weeks after acute infection and longer in the setting of immunodeficiency [118–120]. Clinical studies have consistently suggested that early initiation of antiviral therapy is likely to provide the most benefit for patients with COVID-19 [64, 121–124]. For patients who test positive for SARS-CoV-2 and present with an infectious or inflammatory syndrome inconsistent with acute COVID-19 pneumonia, an alternative diagnosis should be considered, including potential multisystem inflammatory syndrome in children (MIS-C). In such cases, treatment directed at acute COVID-19 is unlikely to be beneficial.
High-Risk Conditions
Many children hospitalized with acute COVID-19 infection have underlying medical conditions that increase their risk of hospitalization or progression to more severe disease, or both, as described above. An assessment of possible high-risk conditions, including those that may not yet have been formally diagnosed, is important for treatment decision making. In general, the threshold for initiating therapies is lower in the presence of high-risk conditions, especially if multiple such conditions coexist.
Illness Severity
COVID-19 illness severity, and by extension the likelihood of benefit from treatment, is defined primarily by respiratory support requirements above baseline, using a tiered approach similar to that used in the NIH COVID-19 Treatment Guidelines [125]. The tiers are as follows: 1) hospitalized but not requiring supplemental oxygen above baseline; 2) hospitalized and requiring supplemental oxygen above baseline via standard nasal cannula; 3) hospitalized and requiring supplemental oxygen above baseline via high-flow nasal cannula (HFNC) or non-invasive ventilation (NIV), such as bi-level positive airway pressure (BiPAP); and 4) mechanical ventilation (MV) or extracorporeal membrane oxygenation (ECMO).
For patients hospitalized because of COVID-19 respiratory disease who require supplemental oxygen or greater support, the likelihood of benefit from antiviral and anti-inflammatory therapy is higher than for less severely ill patients. Antiviral therapy has been shown to have the greatest benefit for patients who are hospitalized and require oxygen but who have not progressed to requiring advanced respiratory support such as HFNC, NIV, or MV [121]. Conversely, anti-inflammatory therapies have been shown to have the greatest benefit for patients who require or are progressing to more advanced respiratory support.
Remdesivir in Hospitalized Pediatric Patients
Recommendation 3.3:
For pediatric patients aged 12 years or older who are hospitalized for COVID-19 and require supplemental oxygen or non-invasive ventilation above baseline, the panel suggests using remdesivir.
Comments:
As described above, pediatric patients aged 12 years or older appear to be at increased risk for progression to severe or critical COVID-19. When patients in this age group require respiratory support above baseline because of COVID-19, the panel suggests administering remdesivir, based on the evidence of its benefit in adults.
Recommendation 3.4:
For pediatric patients younger than 12 years who are hospitalized for COVID-19, require supplemental oxygen or non-invasive ventilation above baseline, and are not at moderate or high risk for progression to critical COVID-19, consider remdesivir administration.
Comments:
The risk of progression to critical COVID-19 is lower in younger children than in older children. For those children younger than 12 years who have severe illness but no risk factors for progression of their illness, clinicians may consider administering remdesivir. There is no evidence for the efficacy of remdesivir in this age group. However, the safety of remdesivir in young children appears to be tolerable [126], and it may be reasonable to extrapolate from the findings of studies of adults with severe COVID-19, especially early in the course of illness.
Infants and young children may develop syndromes consistent with bronchiolitis or laryngotracheobronchitis due to SARS-CoV-2 infection [127–131]. In general, these syndromes should be managed as usual without antiviral therapy, as there is no evidence suggesting a potential benefit of COVID-19–directed therapy. In cases of particularly severe illness, it may be reasonable to consider remdesivir on a case-by-case basis in addition to usual care; however, no specific recommendation can be made.
Recommendation 3.5:
For pediatric patients younger than 12 years who are hospitalized for COVID-19, require supplemental oxygen or non-invasive ventilation above baseline, and are at moderate or high risk for progression to severe COVID-19 or are experiencing a rapid increase in their respiratory support requirements, the panel suggests administering remdesivir.
Comments:
As noted above, there is insufficient evidence by which to estimate the benefit of remdesivir for children younger than 12 years. However, for younger children with severe illness who are experiencing rapid progression of their illness or have risk factors for progression to critical COVID-19, the panel judges that the potential benefit of remdesivir probably outweighs the risk.
Recommendation 3.6:
For patients who are hospitalized for COVID-19 and require mechanical ventilation or extracorporeal membrane oxygenation (ECMO), consider remdesivir administration.
Comments:
Remdesivir has not been shown to be beneficial in clinical trials in adult patients who require mechanical ventilation or ECMO [132]. Guidelines from the World Health Organization (WHO), National Institutes of Health (NIH), and Infectious Diseases Society of America (IDSA) recommend against using remdesivir in patients with illness of this severity [125, 133, 134]. There is no high-quality evidence from pediatric patients requiring mechanical ventilation or ECMO that supports a recommendation in favor of remdesivir use. There was significant disagreement among the panel members regarding the use of remdesivir in this scenario.
Rationale and Evidence for Use of Remdesivir in Pediatric Patients with COVID-19 Requiring Hospitalization for COVID-19
Remdesivir is an adenosine nucleotide prodrug that, after intracellular triphosphorylation, acts as an analog of adenosine triphosphate (ATP) and competes with it for incorporation into RNA chains by the viral RNA-dependent RNA polymerase, resulting in premature RNA chain termination [135]. It has been approved by the US FDA for treating COVID-19 in pediatric patients aged 28 days or older who weigh at least 3 kg.
The prodrug remdesivir has a short half-life of approximately 1 hour, but several of its metabolites have longer half-lives, including the major active metabolite GS-441524, which has a half-life of 27 hours, permitting once-daily administration [135]. In adults and pediatric patients who weigh ≥40 kg, the standard regimen is 200 mg on the first day followed by 100 mg on subsequent days. Based on pharmacokinetic modeling and subsequent trials, for pediatric patients who weigh <40 kg, a dose of 5 mg/kg on day 1 followed by 2.5 mg/kg on subsequent days provides exposure similar to that with the standard adult regimen. The manufacturer recommends against administering remdesivir to patients with an estimated creatinine clearance of ≤30 mL/min because of the potential accumulation of the excipient sulfobutylether-β-cyclodextrin (SBECD) [136, 137]. ECMO and continuous renal replacement therapy appear to accelerate the clearance of remdesivir, but there are no corresponding recommended dose adjustments.
Clinical trials conducted in adults with severe COVID-19 have shown remdesivir to have a modest benefit at best; however, most of the clinical trial participants had advanced stages of COVID-19 [94, 95, 121, 132]. Although not conducted in children, the PINETREE trial demonstrated a significant reduction in the combined endpoint of hospitalization and death related to COVID-19 in adult outpatients who were at high risk for disease progression [64]. Comparable clinical trial data are not available for pediatric patients. The Clinical Administration of Remdesivir After COVID-19 Diagnosis in Children (CARAVAN) study (NCT04431453) is a phase 2/3, single-arm, open-label study evaluating the safety, pharmacokinetics, and clinical and virologic effects of remdesivir in infants and children from birth to <18 years of age who are hospitalized with COVID-19. Interim results for 53 participants indicate that remdesivir was well tolerated; constipation was the most common AE, and no SAEs were attributed to the study drug. A high proportion (85%) of the participants showed clinical improvement based on a clinical ordinal scale over the study period. The study is ongoing [126]. In addition, children receiving remdesivir have been included in several case series, but none were designed to determine the safety or efficacy of the drug [111, 138–141].
Remdesivir is recommended by IDSA and NIH guidelines for hospitalized adults with COVID-19 who require supplemental oxygen, but not for those requiring mechanical ventilation or ECMO [125, 134]. The lack of high-quality clinical trial data on the effectiveness of remdesivir in pediatric patients hospitalized with COVID-19 complicates treatment decisions for pediatric providers. Remdesivir is reported to be generally safe and well tolerated in pediatric patients, with a high proportion of treated patients showing clinical improvement, based on interim clinical trial data [126]. However, no placebo-controlled, randomized, controlled trials of remdesivir in pediatric patients have been completed, which raises the question as to whether antiviral treatment improves the clinical status of children when compared with supportive care.
Anti-Inflammatory and Immunomodulatory Therapy for Treating COVID-19 in Pediatric Patients Requiring Hospitalization for COVID-19
COVID-19 may cause significant pulmonary inflammation, resulting in hypoxemia, acute respiratory distress syndrome (ARDS), and in the most severe cases, death. Several randomized trials conducted in adults with severe and critical COVID-19 demonstrated reductions in mortality with corticosteroid administration, and corticosteroid use is recommended by the WHO, NIH, and IDSA guidelines for managing COVID-19 in selected patients with severe or critical disease [125, 133, 134].
Most studies evaluating the impact of corticosteroids on clinical outcomes in patients with COVID-19 have used dexamethasone. For this reason, the panel favors using dexamethasone over alternative corticosteroids. A randomized clinical trial evaluated the impact of high-dose dexamethasone (20 mg daily × 5 days, followed by 10 mg daily × 5 days) versus usual care (most often dexamethasone at 6 mg daily) in hospitalized adults. Among patients receiving low-flow supplemental oxygen therapy, the higher-dose dexamethasone group experienced greater mortality (19% vs 12%; RR: 1.59, 95% CI: 1.20–2.10), prompting trial enrollment to be halted for this group [142]. Based on the above evidence, the recommended dose for patients weighing ≥40 kg is 6 mg daily; for patients weighing <40 kg, the recommended daily dose is 0.15 mg/kg. Dexamethasone may be administered intravenously or orally for up to 10 days.
For patients with critical COVID-19 disease, additional anti-inflammatory therapy may be indicated. For adults with critical COVID-19 and evidence of significant inflammation, adding an interleukin (IL)-6 inhibitor, such as tocilizumab, or a Janus kinase (JAK) inhibitor, such as baricitinib, to corticosteroid therapy is associated with superior outcomes. However, published reports on the use of these therapies in pediatric patients with COVID-19 are extremely limited.
Corticosteroids for Treating COVID-19
Recommendation 3.7:
For patients who are hospitalized for COVID-19, require supplemental oxygen therapy above baseline, and are worsening, consider administering dexamethasone.
Comments:
The RECOVERY trial randomized 6424 hospitalized adults to receive dexamethasone at 6 mg/day for up to 10 days plus standard of care versus standard of care alone. Overall, there was a significant reduction in 28-day mortality in the dexamethasone group relative to the placebo group (22.9% vs 25.7%; RR: 0.83; 95% CI: 0.75–0.93; P < 0.001). However, among the approximately one-quarter of the study population who required no supplemental oxygen, there was no statistical difference in 28-day mortality, but numerically higher mortality in the dexamethasone group (17.8% versus 14%; RR: 1.19; 95% CI: 0.92–1.55) [96]. A subsequent observational study evaluated the impact of dexamethasone on 90-day mortality among adults hospitalized in the Veterans Affairs (VA) system with a positive PCR or antigen test for SARS-CoV-2. Among the 9450 hospitalized adults who did not require supplemental oxygen, 3514 received dexamethasone within 48 hours of admission. Using propensity score weighting, the hazard ratio (HR) for 90-day mortality was 1.76 (95% CI: 1.47–2.12) for patients who received dexamethasone [143]. Based on these data and on other national guidelines, the panel’s statements in support of dexamethasone use are limited to its use in hospitalized patients who require supplemental oxygen above baseline or higher levels of respiratory support [125, 134].
The RECOVERY trial demonstrated reduced mortality in hospitalized adults treated with dexamethasone relative to those treated with usual care. In the stratified analysis based on the degree of respiratory support, a modest reduction in mortality was demonstrated in the group requiring oxygen therapy (23.3% vs 26.2%; RR: 0.82; 95% CI: 0.51–0.81). However, this group was heterogeneous and included patients requiring low-flow oxygen, high-flow nasal cannula, and non-invasive mechanical ventilation, thereby precluding conclusions specific to the low-flow group [96].
Observational studies have yielded mixed results regarding the impact of corticosteroids on mortality. In a study conducted in the VA health system, using propensity score weighting, no difference in 90-day mortality was detected between those patients treated with dexamethasone and those receiving usual care (HR: 1.08; 95% CI: 0.86–1.37) among hospitalized adults requiring low-flow oxygen [143]. In contrast, a multicenter study including data from 156 hospitals demonstrated a reduction in mortality among adults receiving low-flow oxygen who were treated with dexamethasone when compared with a propensity-weighted cohort receiving usual care (adjusted OR: 0.90; 95% CI: 0.84–0.97) [144]. One multicenter pediatric study including 1163 hospitalized children with positive SARS-CoV-2 tests compared the hospital and ICU lengths of stay for children treated with corticosteroids within the first 48 hours of admission with those for untreated patients. Twenty-three percent of the patients received low-flow supplemental oxygen. There was no difference in the length of stay for patients treated with corticosteroids in the overall cohort, nor were any differences detected in the subgroup requiring supplemental oxygen or greater respiratory support [145].
Collectively, these data suggest that the benefit of corticosteroids for children requiring low-flow supplemental oxygen is uncertain, with high-dose dexamethasone being potentially harmful. This uncertainty is reflected in the panel’s statement to consider the selective use of dexamethasone in patients with escalating supplemental oxygen requirements. In an analysis stratified by duration of symptoms in the full RECOVERY study population, reductions in 28-day mortality with the use of dexamethasone were driven by those patients with symptoms for more than 7 days (RR: 0.69; 95% CI: 0.59–0.80), whereas those patients whose symptoms were present for 7 days or less experienced no benefit (RR: 1.01; 95% CI: 0.87–1.17) [96]. Therefore, for patients with an escalating oxygen requirement, clinicians may consider the duration of symptoms when making treatment decisions, as well as age and comorbidities. Finally, there are no data suggesting that the treatment of children with SARS-CoV-2–associated bronchiolitis, asthma, or croup should differ from usual care standards, which may include the use of corticosteroids in children with asthma or croup.
Recommendation 3.8:
For patients who are hospitalized for COVID-19 and require high-flow oxygen therapy, noninvasive ventilatory support, or mechanical ventilation above baseline, the panel suggests administering dexamethasone.
Comments:
Although the RECOVERY trial demonstrated a significant reduction in mortality among hospitalized adults with COVID-19 treated with dexamethasone versus usual care, the greatest benefits were seen in patients requiring mechanical ventilation (with a 28-day mortality in the stratified analysis of 29.3% versus 41.4%; RR: 0.64; 95% CI: 0.51–0.81) [96]. The association between dexamethasone use and reduced mortality in critically ill patients was further supported by a meta-analysis including the RECOVERY trial and six smaller studies [146–151]. Although a large pediatric observational study demonstrated no differences in hospital length of stay in children hospitalized with COVID-19, despite including an analysis limited to those requiring supplemental oxygen or greater respiratory support, the strength of the association between steroid use and reduced mortality in adult randomized trials supports the panel’s statement suggesting dexamethasone use for patients with critical COVID-19 [145].
The impact of corticosteroid dose has been evaluated in several trials including patients with critical COVID-19, albeit with mixed results [152–155]. The largest of these trials was the COVID STEROID 2 trial, which compared 6 mg vs 12 mg per day of dexamethasone in hospitalized adults requiring a high-flow nasal canula (>10 L/min) or greater respiratory support, approximately half of whom were on non-invasive or invasive mechanical ventilation. The median number of days alive without respiratory support within 28 days of randomization was higher for patients treated with dexamethasone at 12 mg/day, but this difference (1.3 days; 95% CI: 0–2.6 days; P = 0.07) was not statistically significant. In a prespecified Bayesian analysis, there was greater probability of benefit in the 12 mg group with low probability of harm [152]. No pediatric-specific data inform optimal steroid dose or duration in COVID-19, and the panel can make no definitive recommendation regarding dexamethasone dose. Both the NIH and IDSA guidelines recommend a dose of 6 mg daily in adults for 10 days or until hospital discharge, with the NIH acknowledging the possibility of greater benefit with a dose of 12 mg daily [125, 134]. For patients weighing less than 40 kg, a dose equivalent to 6 mg of dexamethasone is 0.15 mg/kg daily.
Use of Other Immunomodulatory Agents to Treat COVID-19
Recommendation 3.9:
For patients who are hospitalized for COVID-19; require noninvasive ventilation, mechanical ventilation, or ECMO; are worsening; and have evidence of significant inflammation, consider tocilizumab or baricitinib in addition to other therapies.
Comments:
When used in conjunction with corticosteroids, non-steroid immunomodulators have been found to improve outcomes in some hospitalized patients with COVID-19 and are recommended for adults with progressive severe or critical disease [125, 134]. Evidence supporting the use of these therapies in patients younger than 18 years is extremely limited. However, for pediatric patients with critical illness due to COVID-19, defined as requiring noninvasive ventilation, mechanical ventilation, or ECMO; worsening; and with evidence of significant inflammation, an additional immunomodulator may be considered. Such patients should also receive dexamethasone as described above.
The anti-inflammatory drugs best studied in adults include the interleukin-6 (IL-6) inhibitor tocilizumab and the Janus kinase (JAK) inhibitor baricitinib. Patients should receive only one non-steroid anti-inflammatory drug; i.e., patients should not receive a JAK inhibitor plus an IL-6 inhibitor. Tocilizumab and baricitinib both carry boxed warnings of increased risk for opportunistic infections, although no increase in opportunistic infections has been observed in patients receiving these drugs for COVID-19. However, caution should be exercised, as clinical trials did not include patients with known pre-existing infections and would have lacked adequate power to detect an increase in infrequent opportunistic infections. When initiating a non-steroid immunomodulator for COVID-19, there is no preference for one class over the other.
Baricitinib
Baricitinib is a JAK inhibitor approved for use in treating rheumatoid arthritis and alopecia areata. In a randomized, open-label clinical trial that included 8156 adults hospitalized with COVID-19, the mortality rate was 12% in baricitinib recipients vs. 14% in those who did not receive baricitinib (RR: 0.87; 95% CI: 0.77–0.99) [156]. A double-blind, placebo-controlled trial in hospitalized adults with COVID-19 demonstrated shorter time to recovery in baricitinib recipients [157].
Baricitinib carries a boxed warning of major adverse cardiovascular events and thrombosis, as observed in patients with rheumatoid arthritis. However, these AEs have not been observed when baricitinib has been used to treat COVID-19. In 2022, baricitinib was approved by the FDA for treating COVID-19 in hospitalized adults requiring supplemental oxygen, non-invasive or invasive ventilation, or ECMO. In the United States, this drug is available under an EUA, only, for patients aged 2 to <18 years [158]. The EUA-recommended dose for children aged 2 years to less than 9 years is 2 mg once daily; for children aged 9 years or older, the recommended dose is 4 mg once daily. Baricitinib is available only as tablets, which may be dispersed in water for oral or enteral administration.
Based on evidence of its efficacy in adults with severe or critical COVID-19 and safety data from pediatric patients with unrelated conditions, it is reasonable to consider using baricitinib, in addition to dexamethasone, in pediatric patients who are requiring noninvasive ventilation, mechanical ventilation, or ECMO.
Another JAK inhibitor, tofacitinib, has also shown efficacy in treating adults hospitalized with COVID-19 [159]. However, the FDA has not approved tofacitinib for treating COVID-19 and it is not labeled for use in pediatric patients.
Tocilizumab
Tocilizumab is a mAb that inhibits receptors of the pro-inflammatory cytokine IL-6. Large randomized clinical trials found that receipt of tocilizumab, as compared to placebo, was associated with a lower risk of mortality and more rapid recovery in adult patients with COVID-19 who required organ support (non-invasive or invasive mechanical ventilation and/or vasopressor or inotrope infusion) [124] and in adult patients who required supplemental oxygen with a C-reactive protein (CRP) level of ≥75 mg/L [160]. However, in hospitalized patients with less severe illness, tocilizumab did not reduce the risk of intubation [161].
Tocilizumab is approved by the FDA for treating COVID-19 in hospitalized adult patients who are receiving systemic corticosteroids and respiratory support; it is also approved for use in patients aged ≥2 years with juvenile idiopathic arthritis (JIA) or chimeric antigen receptor (CAR) T cell–induced cytokine release syndrome. For treating COVID-19 in pediatric patients in the United States, tocilizumab is available only under an EUA [162]. The EUA-recommended dose for patients weighing less than 30 kg is 12 mg/kg; for patients weighing ≥30 kg, the recommended dose is 8 mg/kg. Tocilizumab is administered as a single intravenous dose.
Serious adverse events when IL-6 inhibitors are used to treat COVID-19 appear to be rare, although gastrointestinal perforation has been reported in association with tocilizumab [134]. Reports of IL-6 inhibitor use in patients younger than 18 years are very limited [163].
Based on evidence of its efficacy in adults with severe or critical COVID-19 and safety data from pediatric patients with unrelated conditions, it is reasonable to consider using tocilizumab, in addition to dexamethasone, in pediatric patients who are requiring noninvasive ventilation, mechanical ventilation, or ECMO.
Sarilumab is another anti–IL-6 mAb with evidence of efficacy in adults with COVID-19. It is not approved by the FDA for use in pediatric patients, and the currently available evidence does not suggest that it has any benefit over tocilizumab.
Monoclonal Antibodies and COVID-19 Convalescent Plasma
Recommendation 3.10:
For patients requiring hospitalization for COVID-19 respiratory disease, the panel suggests against the routine use of mAb therapy or convalescent plasma transfusion. These therapies may be considered on a case-by-case basis for carefully selected patients, such as highly immunocompromised patients or those who are ineligible for other therapies.
Comments:
Passive immunization against COVID-19, using either COVID-19 convalescent plasma (CCP) or mAbs, emerged as a treatment strategy early in the pandemic. Commercially produced mAbs demonstrated efficacy as prophylaxis and as treatment for early, mild to moderate disease. In contrast, in large randomized controlled trials in hospitalized adult patients, outcomes in patients treated with mAb were no better than those in patients who received placebo [164, 165]. Because of their lack of clinical efficacy in hospitalized patients, mAbs, if available, are not recommended for use in hospitalized pediatric patients with COVID-19. Furthermore, as noted above, at the time of this publication, no mAbs with activity against circulating COVID-19 variants are available.
CCP was used extensively in hospitalized patients early in the pandemic. Subsequently, however, randomized controlled trials showed it to have no benefit for hospitalized adults with COVID-19 [166–168]. Furthermore, deploying CCP is resource intensive, as recoverees must be identified and screened for transfusion-transmissible pathogens, and collected plasma units must be tested to confirm that the titers of anti–COVID-19 antibodies are sufficiently high. Therefore, CCP is not recommended for use in hospitalized pediatric patients.
In immunocompromised pediatric patients with prolonged COVID-19 symptoms and evidence of persistent viral replication (i.e., persistently low cycle threshold values on COVID-19 PCR assays), passive immunization (with CCP or a mAb), usually in combination with antiviral therapy, is potentially beneficial [169]. CCP has an advantage in that it may be available shortly after the emergence of novel variants. However, evidence for the efficacy of passive immunization in this setting is limited to case reports and case series; therefore, no recommendation can be made regarding this approach.
SECTION 4: Pre-Exposure and Post-Exposure Prophylaxis
Availability of Agents for Pre-Exposure or Post-Exposure Prophylaxis
As noted above, at the time of publication, there are no authorized products available in the United States for PrEP or PEP that are active against the current dominant variants. The recommendations in this section are intended for other regions of the world, or for possible future situations in the United States, in which at least one authorized agent is available that has evidence of efficacy and safety comparable to that of the products discussed below. All recommendations pertain only to patients for whom the available products are authorized.
Criteria Used to Formulate Recommendations
Foremost among the factors weighed by the panel in formulating recommendations for PrEP or PEP were the efficacy and safety of the agents previously authorized for these indications and the resulting balance of potential benefits and harms. When assessing data on efficacy, we considered the certainty of the evidence and the effect size, the importance and meaningfulness of the outcomes studied, and the availability of pediatric evidence. Recognizing that additional factors might influence clinical decision-making and real-world use of these products [170], we also took into account their acceptability to patients, caregivers, and healthcare providers with respect to the benefits and harms and the uncertainty about these, as well as the feasibility and logistics of providing and accessing the agents. In addition, we considered how the availability of therapies for mild or moderate COVID-19 might affect the perceived value of or necessity for disease prevention.
Recommendations: Pre-Exposure Prophylaxis
Recommendation 4.1:
The panel recommends COVID-19 vaccination for all eligible patients without medical contraindications. PrEP may be used as a complementary strategy for preventing severe COVID-19, but it should not be used as a substitute for vaccination.
Recommendation 4.2:
For patients at high risk for severe COVID-19 who have (a) immunocompromising conditions associated with a poor vaccination response or (b) contraindications to vaccination, the panel suggests using PrEP.
Recommendation 4.3:
For patients at moderate risk for severe COVID-19 who have (a) immunocompromising conditions associated with a poor vaccination response or (b) contraindications to vaccination, consider using PrEP on a case-by-case basis.
Recommendation 4.4:
For patients at low risk for severe COVID-19 who have contraindications to vaccination, consider using PrEP on a case-by-case basis.
Rationale
Tixagevimab–cilgavimab (Evusheld), a combination of two long-acting neutralizing mAbs that bind to distinct epitopes of the SARS-CoV-2 spike protein receptor-binding domain [171, 172], is the sole agent to have been authorized by the US FDA for PrEP. However, on January 26, 2023, the FDA announced that the product was no longer authorized because it was likely to be inactive against >90% of current SARS-CoV-2 variants [173].
As detailed below, tixagevimab–cilgavimab was efficacious in preventing symptomatic COVID-19 due to susceptible variants, and it is also safe, with SAEs being reported only rarely in recipients and at rates comparable to those in patients receiving placebo. However, data on efficacy and safety for patients younger than 18 years are not yet available. The typically mild course of COVID-19 in children and adolescents—even in those with risk factors for severe disease—probably translates to a smaller effect size and a higher number needed to treat than are observed in trials for adults, resulting in a less favorable ratio of potential benefits to harms. In light of these factors, the panel recommends vaccinating eligible children and adolescents in accordance with the US Centers for Disease Control and Prevention guidance as the primary strategy for preventing COVID-19, with PrEP serving as a complementary strategy. We did not arrive at a recommendation of “recommend” for any patient group, and we calibrated the strength of the recommendations to the degree of risk for severe COVID-19, reasoning that the possible adverse effects of PrEP would be more justified and acceptable for patients for whom developing COVID-19 would be more likely be detrimental. Therefore, we suggest using PrEP for patients at high risk for severe COVID-19 who are either anticipated to respond poorly to vaccination because of immunocompromise or who cannot be vaccinated because of medical contraindications. For other patients for whom tixagevimab–cilgavimab was authorized, including those at low risk for severe COVID-19 who have vaccine contraindications and desire an alternate means of disease prevention, we advise considering the use of PrEP on a case-by-case basis.
Agent Previously Available for Pre-Exposure Prophylaxis
Tixagevimab–cilgavimab was first authorized for COVID-19 PrEP on December 8, 2021. It was authorized for use in patients aged 12 years or older who weighed ≥40 kg and either (1) had moderate to severe immunocompromise and, thus, might not respond adequately to COVID-19 vaccination or (2) could not receive any available COVID-19 vaccine because of a history of severe adverse reaction to a COVID-19 vaccine or vaccine components [172]. The agent was not authorized for PEP or treatment, and recipients could not be currently infected with or have had recent exposure to SARS-CoV-2. The product was administered as separate, consecutive intramuscular injections of tixagevimab and cilgavimab. The dose originally recommended was 150 mg of each component, but, as a result of decreased neutralization activity against the Omicron subvariants BA.1 and BA 1.1, the recommended dose was increased to 300 mg of each component, with repeat dosing every 6 months [174].
Evidence Summary
Efficacy
The safety and efficacy of tixagevimab–cilgavimab for PrEP was evaluated in the PROVENT study, a phase 3, randomized, double-blind, placebo-controlled trial [171, 172]. The study included a total of 5197 adults (≥18 years of age) who had an increased risk of an inadequate response to COVID-19 vaccination, an increased risk of exposure to SARS-CoV-2, or both. Participants received one dose of tixagevimab–cilgavimab or placebo (3460 in the treatment group and 1737 in the placebo group).
The primary efficacy endpoint for the study was a first episode of symptomatic COVID-19, confirmed by reverse-transcription polymerase chain reaction (RT-PCR), with an onset after the administration of tixagevimab–cilgavimab or placebo, on or before day 183. Compared with placebo recipients, recipients of the agent had a 76.7% RRR (95% CI: 46.0%–90.0%) for this outcome during primary analysis and an 82.8% RRR (95% CI: 65.8%–91.4%) at the extended 6-month follow-up. In addition, the incidence of participants who were positive for SARS-CoV-2 nucleocapsid antibodies post dose was reduced by 51.1% (95% CI: 10.6%‒73.2%) at primary analysis and by 57.7% (95% CI: 34.7%‒72.7%) at 6-month follow-up in tixagevimab-cilgavimab recipients. No COVID-19–related hospitalizations or severe or critical disease were observed in the treatment arm.
Safety
In the PROVENT trial, the incidence of any AE after intramuscular administration did not differ between recipients of placebo versus tixagevimab–cilgavimab [171]. Injection-site reaction was the most common AE reported. Only one SAE (severe mesenteric artery thrombosis) was reported as related to tixagevimab–cilgavimab in the treatment arm, but there was insufficient evidence to determine a causal relationship between the event and the trial agent. No additional AEs of special interest or unexpected longer-term safety signals were observed at the 6-month follow-up. Although a higher proportion of participants who received tixagevimab–cilgavimab versus placebo reported myocardial infarction and cardiac failure AEs, all participants with events had cardiac risk factors and/or a prior history of cardiovascular disease. A causal relationship between tixagevimab–cilgavimab and these events was not established.
In the STORM CHASER study, a phase 3, randomized, double-blind, placebo-controlled trial that evaluated tixagevimab–cilgavimab for PEP, 749 unvaccinated patients aged 18 years or greater were given 150 mg of tixagevimab and 150 mg of cilgavimab as a single intramuscular dose and compared with 372 placebo recipients [175]. Participants were enrolled within 8 days of exposure to an individual with SARS-CoV-2 infection. AEs occurred in 162 tixagevimab–cilgavimab recipients (21.6%) and 111 placebo recipients (29.8%). Most AEs were mild or moderate (95%), with headache, fatigue, and cough among the most commonly reported. SAEs, none of which were considered related to the study intervention, were reported in 5 and 3 participants (0.7% and 0.8%) in the tixagevimab–cilgavimab and placebo groups, respectively.
Safety data are also available from the TACKLE study, a phase 3, randomized, double-blind, placebo-controlled study of 300 mg of tixagevimab and 300 mg of cilgavimab administered intramuscularly to treat mild to moderate COVID-19 in non-hospitalized patients aged ≥18 years [176]. Among 452 tixagevimab–cilgavimab recipients and 451 placebo recipients, AEs were reported in 132 (29%) and 163 (36%), respectively, whereas SAEs occurred in 33 (7%) and 54 (12%), respectively. COVID-19 pneumonia was the most common AE in both arms. Cardiac SAEs in the treatment group consisted of myocardial infarction in two participants (one of whom also had cardiac failure leading to death) and sudden cardiac death in one participant, whereas one participant in the placebo group experienced arrhythmia [172]. Deaths from any cause occurred in six participants (1%) in each group and were related to COVID-19 in three tixagevimab–cilgavimab recipients (1%) and six placebo recipients (1%) [176]. No anaphylaxis or serious hypersensitivity reactions were reported [176].
Recommendations: Post-Exposure Prophylaxis
Recommendation 4.5:
For patients at high risk for severe COVID-19, the panel suggests using PEP.
Recommendation 4.6:
For patients at moderate risk for severe COVID-19, consider using PEP on a case-by-case basis. Assess risk-modifying factors such as age, vaccination-related or infection-related immunity, medical complexity, the presence of multiple risk factors, and the nature of the exposure.
Rationale
PEP is a successful strategy to prevent infections by agents such as human immunodeficiency virus, and it was one of the initial strategies to prevent symptomatic COVID-19 before antiviral therapies were available. Now, in light of the availability of FDA-approved antiviral therapy such as remdesivir and the availability of other antivirals under EUAs, PEP may be limited to patients with severe immunocompromising conditions for whom there will be a continuing need to minimize the risk of severe COVID-19.
Agents Previously Available for Post-Exposure Prophylaxis
The FDA previously updated the EUAs for casirivimab–imdevimab (on August 10, 2021) and bamlanivimab–etesevimab (on September 16, 2021) to include their use for COVID-19 PEP in certain children and adults at high risk for progression to severe COVID-19 [177, 178]. These authorizations were later withdrawn because of the high frequency of the Omicron variant (and its sublineages) of SARS-CoV-2, against which these agents have markedly decreased activity [179]. Currently, bamlanivimab–etesevimab and casirivimab–imdevimab are not authorized in any US region and may not be administered for COVID-19 PEP. As of the publication of this guidance, no such product was available in the United States for this indication.
Evidence Summary
Efficacy
Bamlanivimab
A phase III, double-blind, randomized, placebo-controlled trial was conducted to evaluate the ability of bamlanivimab, a neutralizing mAb against SARS-CoV-2, to prevent COVID-19 among residents and staff in nursing homes and assisted-living facilities [180]. Participants were enrolled from facilities with at least one index COVID-19 case. The primary outcome was SARS-CoV-2 infection with mild or worse disease within 21 days of detection and within 8 weeks of randomization. Among 966 participants who tested negative for SARS-CoV-2 by PCR and serology at baseline, bamlanivimab significantly reduced the incidence of SARS-CoV-2 infection when compared with placebo (8.5% vs 15.2%; OR: 0.43; 95% CI: 0.28–0.68), with AE rates being similar in the treatment and placebo groups. Based on these findings, bamlanivimab appeared to be moderately effective at preventing COVID-19 infection among adults without evidence of prior immunity to SARS-CoV-2. However, extrapolating these findings to children or previously vaccinated individuals is challenging.
Casirivimab–imdevimab
The mAb combination casirivimab–imdevimab was evaluated as PEP in a randomized, double-blind, placebo-controlled trial conducted in the United States, Romania, and Moldova [82]. Household contacts of an index case (positive for SARS-CoV-2 by RT-PCR) who were asymptomatic and at least 12 years of age were randomized to receive casirivimab–imdevimab or placebo by subcutaneous injection. The primary outcome was the development of symptomatic SARS-CoV-2 infection within 28 days of exposure. Participants treated with casirivimab–imdevimab had an 81.4% RRR for symptomatic infection when compared with placebo-treated participants (11 of 753 [1.5%] vs. 59 of 752 [7.8%]; OR: 0.17; 95% CI: 0.09–0.33). Secondary outcomes that also favored the treated group were a reduction in positive tests for SARS-CoV-2, a reduction in viral load for virus-positive participants, and a shorter mean symptom duration for symptomatic patients.
Safety
Overall, pediatric safety data for SARS-CoV-2 mAbs remain limited. Hypersensitivity and infusion-related reactions have been observed with all SARS-CoV-2 mAb products, although the latter may be moderated with pre-medication or by slowing the infusion rate. A 2022 Cochrane review of mAbs for preventing SARS-CoV-2 infection included two PEP studies: one randomized control trial of bamlanivimab versus placebo and one of casirivimab–imdevimab versus placebo [181]. Based on these limited data, bamlanivimab may slightly increase AEs of any severity (RR: 1.12; 95% CI: 0.86–1.46) and SAEs (RR: 1.46; 95% CI: 0.73–2.91) when compared with placebo. In contrast, casirivimab–imdevimab was associated with a slight decrease in grade 3 and higher AEs (RR: 0.5; 95% CI: 0.24–1.02) and in all-grade AEs (RR: 0.7; 95% CI: 0.61–0.8), but there was no difference in SAEs when compared with placebo.
Considerations for Decision Making about Pre-Exposure or Post-Exposure Prophylaxis
Degree and Type of Immunocompromise
As with many infectious diseases, the risk of severe COVID-19 is affected by several host (i.e., patient) factors. Based largely on experience with other respiratory viral infections, certain immunocompromising conditions are believed to predispose children to more severe manifestations of COVID-19 (detailed in Section 1, above). Several conditions and therapies lead to moderate or severe immunocompromise in children and adults, making them eligible for alternative vaccination schedules, as well as for various treatments and preventative agents in the setting of SARS-CoV-2 infection and exposure [182]. Fortunately, relatively few pediatric groups have been clearly found to be at increased risk for severe COVID-19 (e.g., children with severe T-cell immunodeficiencies or severe combined immunodeficiencies or those who have received significant T-cell immunosuppression), making quantification of the benefits of PrEP and PEP difficult (Figure 1).
Presence of multiple risk factors
Non-immunologic risk factors (e.g., diabetes, underlying pulmonary disease, obesity, and severe heart disease) also influence the severity COVID-19 infections in children. When more than one risk factor is present, this risk is compounded. Eligible children who have multiple conditions associated with severe COVID-19 should be deemed at highest risk and be strongly considered for preventative measures and timely treatment (Figure 1).
Vaccination history
Vaccine-mediated protection against severe COVID-19 requires completion of a COVID-19 vaccine primary series (including a third dose for immunocompromised individuals) and receipt of the most recent booster dose recommended based on age [183]. Individuals who are not up to date with their COVID-19 vaccination should be vaccinated as expediently as possible and, if eligible, be considered for PrEP, as well as for PEP in the event of a qualifying SARS-CoV-2 exposure. There is insufficient information to guide PrEP based on the time from last vaccine dose. Individuals who have immunocompromising conditions associated with a poor vaccination response and are at moderate or high risk for severe disease may benefit from PrEP or PEP regardless of having received all recommended COVID-19 vaccine doses.
Efficacy of vaccines against currently circulating variants
Although vaccine efficacy against the Omicron variant and its sublineages is decreased when compared to the efficacy against prior SARS-CoV-2 variants, mRNA COVID-19 vaccines, at the time of publication, continued to offer protection against severe outcomes and complications in children and adolescents [184, 185]. Booster doses are effective at increasing vaccine efficacy in adolescents, as measured by the incidence of symptomatic COVID-19 infection [186].
Infection-related immunity
SARS-CoV-2 reinfection within 90 days of the initial infection can occur with the same virus variant as in the initial infection or with a different variant, with variable disease severity. Previous infection has been shown to provide some protection. However, moderately to severely immunocompromised individuals may not mount an appropriate response to the infection. Individuals who have immunocompromising conditions and are at moderate or high risk for severe disease may benefit from PrEP or PEP regardless of their history of COVID-19.
Prior receipt of pre-exposure prophylaxis
Currently, individuals who meet the criteria for PrEP with tixagevimab–cilgavimab, including living in a region with susceptible circulating SARS-CoV-2 variants, can receive it every 6 months [174]. Individuals who have a known COVID-19 exposure more than 6 months from their last dose of tixagevimab–cilgavimab and are at moderate or high risk for severe disease may benefit from PEP.
Patient/family preferences and weighing of risks/benefits
There are several additional considerations surrounding clinical decision-making for PrEP/PEP from the perspective of the patient and their caregivers. These largely relate to the process of therapy administration; previously authorized agents required intramuscular or intravenous administration in a healthcare setting. In partnership with the treating clinician, patients and their caregivers should evaluate the potential benefits and risks of therapy as discussed above and consider their personal weighting of these benefits and risks in light of the patient’s clinical circumstances, in particular their risk for progression to severe disease. The requirement for intravenous infusion or injection in some cases will preclude the use of PrEP/PEP because of patient or caregiver concerns about this mode of administration or a need for sedation for peripheral intravenous catheter placement. When the patient is considered an appropriate candidate for PrEP/PEP, subsequent considerations include the logistics of access to a facility, the time cost (including missed work, school, etc.), and out-of-pocket expenses (co-pays, transportation, etc.). Other potential barriers include access to a facility that is equipped to administer PrEP/PEP for pediatric patients, including the necessary staffing support. Although home administration is not, to our knowledge, commonly offered, extensive favorable experience with pediatric home infusions of other therapies via homecare nursing services may provide an opportunity to address some of these barriers in the future.
Acknowledgements
The authors gratefully acknowledge the review and endorsement of this guideline by the Pediatric Infectious Diseases Society.
The authors would like to acknowledge the following former committee members who made significant contributions to the project:
Candace L. Johnson, MD, Department of Pediatrics, Columbia University, New York, NY
Gabriella S. Lamb, MD, MPH Division of Infectious Diseases, Boston Children’s Hospital, Boston, MA; Adam J. Ratner, MD, Department of Pediatrics, NYU Grossman School of Medicine, New York, NY; Rebecca Same, MD, Department of Pediatrics, Perelman School of Medicine of the University of Pennsylvania; Division of Infectious Diseases, Children’s Hospital of Philadelphia, Philadelphia, PA; Alpana Waghmare, MD, Department of Pediatrics, University of Washington School of Medicine, and Division of Infectious Diseases, Seattle Children’s Hospital, Seattle WA
Rachel L. Wattier, MD, MHS Department of Pediatrics, University of California, San Francisco, San Francisco, CA; Jennifer L. Young, PharmD, St. Louis Children’s Hospital, St. Louis, MO; Philip Zachariah, Department of Pediatrics, Columbia University, New York, NY; and Vaccine Research and Development, Pfizer, Inc., Pearl River, NY.
The authors are grateful for systematic evidence review provided by Camila Aparicio, MD, Department of Pediatrics, Yale University School of Medicine, New Haven, CT. This work was funded by the PIDS SUMMERS program.
The authors are grateful for editorial assistance provided by:
Keith A. Laycock, PhD, ELS, Department of Scientific Editing, St. Jude Children’s Research Hospital, Memphis, TN
Funders:
This work was supported, in part, by the National Institutes of Health grant number K23AI159518 (C.R.O). Contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Conflicts of Interest:
J.B. has received support to his institution from AstraZeneca for participation in sponsored research. W.C.H. has served as a consultant to Pfizer, Inc. and ADMA Biologics, and owns stock in Pfizer, Bristol Meyers Squibb, and Zimmer Biomet. S.H.J has been a consultant for Bayer, outside the scope of this work. G.M.M. receives research support from Astellas and SymBio Pharma. P.K.S. has received support to his institution from Gilead Sciences Inc., Merck Inc. and Allovir Inc. for participation in sponsored research. R.O has received support to her institution from Gilead Sciences for participation in sponsored research. Z.I.W. has received support to his institution from Pfizer and Merck for participation in sponsored research. J.W. has received research support to his institution from Merck Inc., Scynexis Inc. and Karius Inc.
Contributor Information
Zachary I Willis, Department of Pediatrics, University of North Carolina School of Medicine, Chapel Hill, NC, USA.
Carlos R Oliveira, Department of Pediatrics, Yale University School of Medicine, New Haven, CT, USA.
Mark J Abzug, Department of Pediatrics, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora, CO, USA.
Brenda I Anosike, Department of Pediatrics, The Children’s Hospital at Montefiore and Albert Einstein College of Medicine, Bronx, NY, USA.
Monica I Ardura, Department of Pediatrics, ID Host Defense Program, Nationwide Children’s Hospital & The Ohio State University, Columbus, OH, USA.
Laura L Bio, Department of Pharmacy, Lucile Packard Children’s Hospital, Stanford, CA, USA.
Juri Boguniewicz, Department of Pediatrics, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora, CO, USA.
Kathleen Chiotos, Departments of Anesthesiology, Critical Care Medicine, and Pediatrics, Perelman School of Medicine at the University of Pennsylvania, Divisions of Critical Care Medicine and Infectious Diseases, Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
Kevin Downes, Department of Pediatrics, Perelman School of Medicine of the University of Pennsylvania, Division of Infectious Diseases, Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
Steven P Grapentine, Department of Pharmacy, University of California San Francisco Benioff Children’s Hospital, San Francisco, CA, USA.
Adam L Hersh, Department of Pediatrics, Division of Infectious Diseases, University of Utah, Salt Lake City, UT, USA.
Sarah M Heston, Department of Pediatrics, Duke University School of Medicine, Durham, NC, USA.
Diego R Hijano, Department of Infectious Diseases, St. Jude Children’s Research Hospital and Department of Pediatrics, University of Tennessee Health Science Center, Memphis, TN, USA.
W Charles Huskins, Department of Pediatric and Adolescent Medicine, Mayo Clinic, Rochester, MN, USA.
Scott H James, Department of Pediatrics, Division of Pediatric Infectious Diseases, University of Alabama at Birmingham, Birmingham, AL, USA.
Sarah Jones, Department of Pharmacy, Boston Children’s Hospital, Boston, MA, USA.
Christine R Lockowitz, Department of Pharmacy, St. Louis Children’s Hospital, St. Louis, Missouri, USA.
Elizabeth C Lloyd, Department of Pediatrics, University of Michigan, Ann Arbor, MI, USA.
Christine MacBrayne, Department of Pharmacy, Children’s Hospital Colorado, Aurora, CO, USA.
Gabriela M Maron, Department of Infectious Diseases, St. Jude Children’s Research Hospital and Department of Pediatrics, University of Tennessee Health Science Center, Memphis, TN, USA.
Molly Hayes McDonough, Center for Healthcare Quality & Analytics, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
Christine M Miller, Department of Pediatrics, Yale University School of Medicine, New Haven, CT, USA.
Theodore H Morton, Department of Pharmacy, St Jude’s Children’s Research Hospital, Memphis, Tennessee, USA.
Rosemary M Olivero, Department of Pediatrics and Human Development, Michigan State College of Human Medicine and Helen DeVos Children’s Hospital of Corewell Health, Grand Rapids, MI, USA.
Rachel C Orscheln, Department of Pediatrics, Washington University, St. Louis, MO, USA.
Hayden T Schwenk, Department of Pediatrics, Stanford School of Medicine, Stanford, CA, USA.
Prachi Singh, Department of Pediatrics, University of California San Francisco, San Francisco, CA, USA.
Vijaya L Soma, Department of Pediatrics, NYU Grossman School of Medicine, New York, NY, USA.
Paul K Sue, Department of Pediatrics, Columbia University, New York, NY, USA.
Surabhi B Vora, Department of Pediatrics, University of Washington School of Medicine, and Division of Infectious Diseases, Seattle Children’s Hospital, Seattle, WA, USA.
Mari M Nakamura, Antimicrobial Stewardship Program and Division of Infectious Diseases, Boston Children’s Hospital, Boston, MA, USA.
Joshua Wolf, Department of Infectious Diseases, St. Jude Children’s Research Hospital and Department of Pediatrics, University of Tennessee Health Science Center, Memphis, TN, USA.
References
- 1. World Health Organization. COVID-19 Epidemiological Update - 29 September 2023. Available at: https://www.who.int/publications/m/item/covid-19-epidemiological-update---29-september-2023. Accessed October 15, 2023.
- 2. Zimmermann P, Curtis N. Why does the severity of COVID-19 differ with age?: understanding the mechanisms underlying the age gradient in outcome following SARS-CoV-2 infection. Pediatr Infect Dis J 2022; 41:e36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Toussi SS, Hammond JL, Gerstenberger BS, Anderson AS. Therapeutics for COVID-19. Nat Microbiol 2023; 8:771–86. [DOI] [PubMed] [Google Scholar]
- 4. Wolf J, Abzug MJ, Anosike BI, et al. Updated guidance on use and prioritization of monoclonal antibody therapy for treatment of COVID-19 in adolescents. J Pediatric Infect Dis Soc 2022; 11:177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiotos K, Hayes M, Kimberlin DW, et al. Multicenter interim guidance on use of antivirals for children With Coronavirus Disease 2019/Severe Acute Respiratory Syndrome Coronavirus 2. J Pediatric Infect Dis Soc 2021; 10:34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolf J, Abzug MJ, Wattier RL, et al. Initial guidance on use of monoclonal antibody therapy for treatment of Coronavirus Disease 2019 in children and adolescents. J Pediatric Infect Dis Soc 2021; 10:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chiotos K, Hayes M, Kimberlin DW, et al. Multicenter initial guidance on use of antivirals for children with Coronavirus Disease 2019/Severe Acute Respiratory Syndrome Coronavirus 2. J Pediatric Infect Dis Soc 2020; 9:701–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aparicio A, Willis ZI, Nakamura MM, Wolf J, et al. PIDS Pediatric COVID-19 Therapies Task Force. Risk Factors for Pediatric Critical COVID-19: A Systematic Review and Meta-Analysis. medRxiv 2024; doi: 10.1101/2024.01.17.24301452. [DOI] [Google Scholar]
- 9. CDC. Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals. 2023. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. Accessed May 1, 2023.
- 10. Guyatt GH, Oxman AD, Sultan S, et al. ; GRADE Working Group. GRADE guidelines: 9 Rating up the quality of evidence. J Clin Epidemiol 2011; 64:1311–6. [DOI] [PubMed] [Google Scholar]
- 11. Farrar DS, Drouin O, Moore Hepburn C, et al. Risk factors for severe COVID-19 in hospitalized children in Canada: a national prospective study from March 2020-May 2021. Lancet Reg Health Am 2022; 15:100337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Forrest CB, Burrows EK, Mejias A, et al. Severity of acute COVID-19 in children <18 years old march 2020 to december 2021. Pediatrics 2022; 149:e2021055765. Available at: https://www.ncbi.nlm.nih.gov/pubmed/35322270 [DOI] [PubMed] [Google Scholar]
- 13. Martinez-Valdez L, Richardson Lopez Collada V, Castro-Ceronio LE, Rodriguez Gutierrez AM, Bautista-Marquez A, Hernandez-Avila M. Risk factors for COVID-19 hospitalisations and deaths in Mexican children and adolescents: retrospective cross-sectional study. BMJ Open 2022; 12:e055074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kufa T, Jassat W, Cohen C, et al. Epidemiology of SARS-CoV-2 infection and SARS-CoV-2 positive hospital admissions among children in South Africa. Influenza Other Respir Viruses 2022; 16:34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hillesheim D, Tomasi YT, Figueiro TH, Paiva KM. Severe acute respiratory syndrome due to COVID-19 among children and adolescents in Brazil: profile of deaths and hospital lethality as at Epidemiological Week 38, 2020. Epidemiol Serv Saude 2020; 29:e2020644. [DOI] [PubMed] [Google Scholar]
- 16. Sedighi I, Fahimzad A, Pak N, et al. A multicenter retrospective study of clinical features, laboratory characteristics, and outcomes of 166 hospitalized children with coronavirus disease 2019 (COVID-19): a preliminary report from Iranian Network for Research in Viral Diseases (INRVD). Pediatr Pulmonol 2022; 57:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Solorzano-Santos F, Miranda-Lora AL, Marquez-Gonzalez H, Klunder-Klunder M. Survival analysis and mortality predictors of COVID-19 in a pediatric cohort in Mexico. Front Public Health 2022; 10:969251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parri N, Magista AM, Marchetti F, et al. ; CONFIDENCE and COVID-19 Italian Pediatric Study Networks. Characteristic of COVID-19 infection in pediatric patients: early findings from two Italian Pediatric Research Networks. Eur J Pediatr 2020; 179:1315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harwood R, Yan H, Talawila Da Camara N, et al. Which children and young people are at higher risk of severe disease and death after hospitalisation with SARS-CoV-2 infection in children and young people: a systematic review and individual patient meta-analysis. EClinicalMedicine 2022; 44:101287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Piechotta V, Siemens W, Thielemann I, et al. Safety and effectiveness of vaccines against COVID-19 in children aged 5-11 years: a systematic review and meta-analysis. Lancet Child Adolesc Health 2023; 7:379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin DY, Gu Y, Xu Y, et al. Effects of vaccination and previous infection on omicron infections in children. N Engl J Med 2022; 387:1141–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Powell AA, Kirsebom F, Stowe J, et al. Protection against symptomatic infection with delta (B.1.617.2) and omicron (B.1.1.529) BA.1 and BA.2 SARS-CoV-2 variants after previous infection and vaccination in adolescents in England, August, 2021-March, 2022: a national, observational, test-negative, case-control study. Lancet Infect Dis 2023; 23:435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piche-Renaud PP, Swayze S, Buchan SA, et al. ; CANADIAN IMMUNIZATION RESEARCH NETOWRK (CIRN) PROVINICAL COLLABORATIVE INVESTIGATORS. COVID-19 Vaccine effectiveness against omicron infection and hospitalization. Pediatrics 2023; 151:e2022059513. Available at: https://www.ncbi.nlm.nih.gov/pubmed/36866446 [DOI] [PubMed] [Google Scholar]
- 24. Link-Gelles R, Ciesla AA, Rowley EAK, et al. Effectiveness of monovalent and bivalent mRNA vaccines in preventing COVID-19-associated emergency department and urgent care encounters among children aged 6 months-5 years - VISION Network, United States, July 2022-June 2023. MMWR Morb Mortal Wkly Rep 2023; 72:886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morgans HA, Bradley T, Flebbe-Rehwaldt L, et al. Humoral and cellular response to the COVID-19 vaccine in immunocompromised children. Pediatr Res 2023; 94:200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greenan-Barrett J, Aston S, Deakin CT, Ciurtin C. The impact of immunocompromise on outcomes of COVID-19 in children and young people-a systematic review and meta-analysis. Front Immunol 2023; 14:1159269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Averbuch D, de la Camara R, Tridello G, et al. Risk factors for a severe disease course in children with SARS-COV-2 infection following hematopoietic cell transplantation in the pre-Omicron period: a prospective multinational Infectious Disease Working Party from the European Society for Blood and Marrow Transplantation group (EBMT) and the Spanish Group of Hematopoietic Stem Cell Transplantation (GETH) study. Bone Marrow Transplant 2023; 58:558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alqanatish J, Almojali A, Alfadhel A, et al. COVID-19 and pediatric rheumatology: a comprehensive study from a leading tertiary center in Saudi Arabia. J Epidemiol Glob Health 2023; 13:676–84. Available at: https://www.ncbi.nlm.nih.gov/pubmed/37594620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kearsley-Fleet L, Chang ML, Lawson-Tovey S, et al. ; CARRA Registry Investigators. Outcomes of SARS-CoV-2 infection among children and young people with pre-existing rheumatic and musculoskeletal diseases. Ann Rheum Dis 2022; 81:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sengler C, Eulert S, Minden K, et al. Clinical manifestations and outcome of SARS-CoV-2 infections in children and adolescents with rheumatic musculoskeletal diseases: data from the National Paediatric Rheumatology Database in Germany. RMD Open 2021; 7:e001687. Available at: https://www.ncbi.nlm.nih.gov/pubmed/34312307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mucalo L, Brandow AM, Dasgupta M, et al. Comorbidities are risk factors for hospitalization and serious COVID-19 illness in children and adults with sickle cell disease. Blood Adv 2021; 5:2717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oliveira EA, Mak RH, Colosimo EA, et al. Risk factors for COVID-19-related mortality in hospitalized children and adolescents with diabetes mellitus: An observational retrospective cohort study. Pediatr Diabetes 2022; 23:763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Banull NR, Reich PJ, Anka C, et al. Association between endocrine disorders and severe COVID-19 disease in pediatric patients. Horm Res Paediatr 2022; 95:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Madani S, Shahin S, Yoosefi M, et al. Red flags of poor prognosis in pediatric cases of COVID-19: the first 6610 hospitalized children in Iran. BMC Pediatr 2021; 21:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanchez-Piedra C, Gamino-Arroyo AE, Cruz-Cruz C, Prado-Galbarro FJ. Impact of environmental and individual factors on COVID-19 mortality in children and adolescents in Mexico: An observational study. Lancet Reg Health Am 2022; 8:100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ward JL, Harwood R, Smith C, et al. Risk factors for PICU admission and death among children and young people hospitalized with COVID-19 and PIMS-TS in England during the first pandemic year. Nat Med 2022; 28:193–200. [DOI] [PubMed] [Google Scholar]
- 37. Kompaniyets L, Agathis NT, Nelson JM, et al. Underlying medical conditions associated with severe COVID-19 illness among children. JAMA Netw Open 2021; 4:e2111182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mann EA, Rompicherla S, Gallagher MP, et al. Comorbidities increase COVID-19 hospitalization in young people with type 1 diabetes. Pediatr Diabetes 2022; 23:968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karavanaki K, Rodolaki K, Soldatou A, Karanasios S, Kakleas K. Covid-19 infection in children and adolescents and its association with type 1 diabetes mellitus (T1d) presentation and management. Endocrine 2023; 80:237–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes 2021; 22:202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murillo-Zamora E, Trujillo X, Huerta M, Rios-Silva M, Lugo-Radillo A, Mendoza-Cano O. Decreased survival in children inpatients with COVID-19 and antibiotic prescription. BMC Infect Dis 2022; 22:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: data from the T1D exchange surveillance study. J Clin Endocrinol Metab 2022; 107:410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fisler G, Izard SM, Shah S, et al. ; Northwell COVID-19 Research Consortium. Characteristics and risk factors associated with critical illness in pediatric COVID-19. Ann Intensive Care 2020; 10:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gotzinger F, Santiago-Garcia B, Noguera-Julian A, et al. ; ptbnet COVID-19 Study Group. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health 2020; 4:653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nguyen PNT, Thuc TT, Hung NT, et al. Risk factors for disease severity and mortality of children with Covid-19: A study at a Vietnamese Children’s hospital. J Infect Chemother 2022; 28:1380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saatci D, Ranger TA, Garriga C, et al. Association between race and COVID-19 outcomes among 2.6 million children in England. JAMA Pediatr 2021; 175:928–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ungar SP, Solomon S, Stachel A, et al. Hospital and ICU admission risk associated with comorbidities among children with COVID-19 ancestral strains. Clin Pediatr (Phila) 2023; 62:1048–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Verma S, Lumba R, Dapul HM, et al. Characteristics of hospitalized children with SARS-CoV-2 in the New York City metropolitan area. Hosp Pediatr 2021; 11:71–8. [DOI] [PubMed] [Google Scholar]
- 49. Mania A, Faltin K, Mazur-Melewska K, et al. Clinical picture and risk factors of severe respiratory symptoms in COVID-19 in children. Viruses 2021; 13:2366. Available at: https://www.ncbi.nlm.nih.gov/pubmed/34960635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Martin B, DeWitt PE, Russell S, et al. Characteristics, outcomes, and severity risk factors associated with SARS-CoV-2 infection among children in the US National COVID cohort collaborative. JAMA Netw Open 2022; 5:e2143151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moeller A, Thanikkel L, Duijts L, et al. COVID-19 in children with underlying chronic respiratory diseases: survey results from 174 centres. ERJ Open Res 2020; 6:00409–2020. Available at: https://www.ncbi.nlm.nih.gov/pubmed/33263054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Woodruff RC, Campbell AP, Taylor CA, et al. Risk factors for severe COVID-19 in children. Pediatrics 2022; 149:e2021–053418. Available at: https://www.ncbi.nlm.nih.gov/pubmed/34935038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ammar LA, Nassar JE, Bitar F, Arabi M. COVID-19 in cyanotic congenital heart disease. Can J Infect Dis Med Microbiol 2023; 2023:5561159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zareef R, Salameh E, Hammoud R, Tannouri T, Bitar F, Arabi M. COVID-19 in congenital heart disease patients: what did we learn?! Front Cardiovasc Med 2023; 10:1235165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Strah DD, Kowalek KA, Weinberger K, et al. Worse hospital outcomes for children and adults with COVID-19 and congenital heart disease. Pediatr Cardiol 2022; 43:541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dolby T, Nafilyan V, Morgan A, Kallis C, Sheikh A, Quint JK. Relationship between asthma and severe COVID-19: a national cohort study. Thorax 2023; 78:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shi T, Pan J, Katikireddi SV, et al. ; Public Health Scotland and the EAVE II Collaborators. Risk of COVID-19 hospital admission among children aged 5-17 years with asthma in Scotland: a national incident cohort study. Lancet Respir Med 2022; 10:191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sousa BLA, Brentani A, Costa Ribeiro CC, et al. Non-communicable diseases, sociodemographic vulnerability and the risk of mortality in hospitalised children and adolescents with COVID-19 in Brazil: a cross-sectional observational study. BMJ Open 2021; 11:e050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nicastro E, Ebel NH, Kehar M, et al. The impact of severe acute respiratory syndrome coronavirus type 2 on children with liver diseases: a joint European society for pediatric gastroenterology, hepatology and nutrition and society of pediatric liver transplantation position paper. J Pediatr Gastroenterol Nutr 2022; 74:159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brenner EJ, Pigneur B, Focht G, et al. Benign evolution of SARS-Cov2 infections in children with inflammatory bowel disease: results from two international databases. Clin Gastroenterol Hepatol 2021; 19:394–396.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Catalan A, Aymerich C, Bilbao A, et al. Psychosis and substance abuse increase the COVID-19 mortality risk. Psychol Med 2022; 53:4236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Leung C, Su L, Simoes ESAC, Arocha LS, de Paiva KM, Haas P. Risk for severe illness and death among pediatric patients with down syndrome hospitalized for COVID-19, Brazil. Emerg Infect Dis 2023; 29:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Food and Drug Administration. Highlights of prescribing information: VEKLURY® (remdesivir) (package insert). 2023; Available at: https://www.gilead.com/-/media/files/pdfs/medicines/covid-19/veklury/veklury_pi.pdf. Accessed October 28, 2023.
- 64. Gottlieb RL, Vaca CE, Paredes R, et al. ; GS-US-540-9012 (PINETREE) Investigators. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med 2022; 386:305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Approves First COVID-19 Treatment for Young Children. FDA, 2022. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-approves-first-covid-19-treatment-young-children. Accessed March 20, 2023. [Google Scholar]
- 66. Samuel AM, Hacker LL, Zebracki J, et al. Remdesivir use in pediatric patients for SARS-CoV-2 treatment: single academic center study. Pediatr Infect Dis J 2023; 42:310–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Goldman DL, Aldrich ML, Hagmann SHF, et al. Compassionate use of remdesivir in children with severe COVID-19. Pediatrics 2021; 147:e2020047803. [DOI] [PubMed] [Google Scholar]
- 68. Méndez-Echevarría A, Pérez-Martínez A, Gonzalez Del Valle L, et al. Compassionate use of remdesivir in children with COVID-19. Eur J Pediatr 2021; 180:1317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sarhan MA, Casalino M, Paopongsawan P, et al. SARS-CoV-2 associated respiratory failure in a preterm infant and the outcome after remdesivir treatment. Pediatr Infect Dis J 2022; 41:e233–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gilead Sciences. A Phase 2/3 Single-Arm, Open-Label Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of Remdesivir (GS-5734TM) in Participants From Birth to < 18 Years of Age With COVID-19. 2023. Available at: https://clinicaltrials.gov/study/NCT04431453. Accessed December 31, 2022.
- 71. National Institutes of Health. COVID-19 treatment guidelines panel. coronavirus disease 2019 (COVID-19) treatment guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/. A. Accessed December 29, 2021. [PubMed]
- 72. Hammond J, Leister-Tebbe H, Gardner A, et al. ; EPIC-HR Investigators. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med 2022; 386:1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Aggarwal NR, Molina KC, Beaty LE, et al. Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA4 and BA5 in Colorado, USA: a retrospective cohort study. Lancet Infect Dis 2023; 23:696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Razonable RR, O’Horo JC, Hanson SN, et al. Comparable outcomes for bebtelovimab and ritonavir-boosted nirmatrelvir treatment in high-risk patients with coronavirus disease-2019 during severe acute respiratory syndrome coronavirus 2 BA2 omicron epoch. J Infect Dis 2022; 226:1683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Food and Drug Administration. Bebtelovimab EUA Letter of Authorization. Available at: https://www.fda.gov/media/156152/download. Accessed December 3, 2022. [Google Scholar]
- 76. Food and Drug Administration. FDA Announces Bebtelovimab is Not Currently Authorized in Any US Region. FDA; 2022; Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-bebtelovimab-not-currently-authorized-any-us-region. Accessed December 3, 2022. [Google Scholar]
- 77. Imai M, Ito M, Kiso M, et al. Efficacy of antiviral agents against omicron subvariants BQ11 and XBB. N Engl J Med 2023; 388:89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. ; COMET-ICE Investigators. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate Covid-19: A randomized clinical trial. JAMA 2022; 327:1236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Butzer SK, Habbig S, Mehler K, et al. Use of Sotrovimab in 14 Children with COVID-19: A Single-center Experience. Pediatr Infect Dis J 2023; 42:e61–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Takashita E, Yamayoshi S, Simon V, et al. Efficacy of antibodies and antiviral drugs against omicron BA2121, BA4, and BA5 subvariants. N Engl J Med 2022; 387:468–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Food and Drug Administration. Fact sheet for health care providers: Emergency Use Authorization (EUA) of bamlanivimab and etesevimab. Available at: https://www.fda.gov/media/145802/download. Accessed December 31, 2021.
- 82. O’Brien MP, Forleo-Neto E, Musser BJ, et al. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N Engl J Med 2021; 385:1184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gupta D, Clifford SA, Fricker FJ. Use of REGEN-COV in children after heart transplantation for treatment and post-exposure prophylaxis of COVID-19. Cardiol Young 2023; 33:496–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Food and Drug Administration. Fact sheet for health care providers: Emergency Use Authorization (EUA) of casirivimab and imdevimab. Available at: https://www.fda.gov/media/145611/download. Accessed December 31, 2021.
- 85. Anti-SARS-CoV-2 Monoclonal Antibodies. Available at: https://www.covid19treatmentguidelines.nih.gov/tables/variants-and-susceptibility-to-mabs/. Accessed October 9, 2023.
- 86. Vora SB, Englund JA, Trehan I, et al. Monoclonal antibody and antiviral therapy for mild-to-moderate COVID-19 in pediatric patients. Pediatr Infect Dis J 2023; 42:32–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bahakel H, Murphy C, Frenck RW, et al. Single site experience of the use of monoclonal antibodies for the treatment of COVID-19 in high-risk pediatric and young adult patients. Pediatr Infect Dis J 2022; 41:985–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Romani L, Calò Carducci FI, Chiurchiù S, et al. Safety of monoclonal antibodies in children affected by SARS-CoV-2 infection. Children (Basel) 2022; 9:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Santos JDL, Bhisitkul D, Carman M, et al. The use of monoclonal antibody therapy in pediatric patients with COVID-19: a retrospective case series. Int J Emerg Med 2022; 15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Peters ME, Strayer J, Wald ER. Serious infusion reactions in two adolescents receiving bebtelovimab. Pediatr Infect Dis J 2023; 42:e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Food and Drug Administration. Fact sheet for health care providers: Emergency Use Authorization (EUA) of Covid-19 convalescent plasma for treatment of hospitalized patients with Covid-19. 2021; Available at: https://www.fda.gov/media/141478/download
- 92. Senefeld JW, Franchini M, Mengoli C, et al. COVID-19 convalescent plasma for the treatment of immunocompromised patients: a systematic review and meta-analysis. JAMA Network Open 2023; 6:e2250647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gordon O, Brosnan MK, Yoon S, et al. Pharmacokinetics of high-titer anti–SARS-CoV-2 human convalescent plasma in high-risk children. JCI Insight 2022; 7:e151518. Available at: https://insight.jci.org/articles/view/151518. Accessed October 9, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Goldman JD, Lye DCB, Hui DS, et al. ; GS-US-540-5773 Investigators. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med 2020; 383:1827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate covid-19: a randomized clinical trial. JAMA 2020; 324:1048–57. Available at: https://jamanetwork.com/journals/jama/fullarticle/2769871. Accessed September 8, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 2021; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe COVID-19 pneumonia. N Engl J Med 2021; 384:1503–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pfizer. A phase 2/3, interventional safety, pharmacokinetics, and efficacy, open-label, multi-center, single-arm study to investigate orally administered PF-07321332 (nirmatrelvir)/ritonavir in nonhospitalized symptomatic pediatric participants with Covid-19 who are at risk of progression to severe disease. 2022. Available at: https://clinicaltrials.gov/ct2/show/NCT05261139. Accessed August 25, 2022.
- 99. Hoffmann-La Roche. A Phase Ib, Single-Arm, Open-Label Study Evaluating the Pharmacokinetics, Pharmacodynamics, and Safety of Tocilizumab in Pediatric Patients Hospitalized With COVID-19. clinicaltrials.gov, 2022. Available at: https://clinicaltrials.gov/ct2/show/NCT05164133. Accessed September 26, 2022.
- 100. Eli Lilly and Company. A Multicenter, Open-Label, Pharmacokinetic and Safety Study of Baricitinib in Pediatric Patients From 1 Year to Less Than 18 Years Old Hospitalized With COVID-19. clinicaltrials.gov, 2022. Available at: https://clinicaltrials.gov/ct2/show/NCT05074420. Accessed September 26, 2022.
- 101. Singh T, Heston SM, Langel SN, et al. Lessons From COVID-19 in children: key hypotheses to guide preventative and therapeutic strategies. Clin Infect Dis 2020; 71:2006–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Do LAH, Anderson J, Mulholland EK, Licciardi PV. Can data from paediatric cohorts solve the COVID-19 puzzle? PLoS Pathog 2020; 16:e1008798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wong LSY, Loo EXL, Kang AYH, Lau HX, Tambyah PA, Tham EH. Age-related differences in immunological responses to SARS-CoV-2. J Allergy Clin Immunol Pract 2020; 8:3251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hurst JH, McCumber AW, Aquino JN, et al. Age-related changes in the nasopharyngeal microbiome are associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and symptoms among children, adolescents, and young adults. Clin Infect Dis 2022; 75:e928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Antoon JW, Grijalva CG, Thurm C, et al. Factors associated with COVID-19 disease severity in US children and adolescents. J Hosp Med 2021; 16:603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ouldali N, Yang DD, Madhi F, et al. ; investigator group of the PANDOR study. Factors associated with severe SARS-CoV-2 infection. Pediatrics 2021; 147:e2020023432. [DOI] [PubMed] [Google Scholar]
- 107. Wanga V, Gerdes ME, Shi DS, et al. ; BMBS1. Characteristics and clinical outcomes of children and adolescents aged <18 years hospitalized with COVID-19 - six hospitals, United States, July-August 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Stefan N, Birkenfeld AL, Schulze MB. Global pandemics interconnected - obesity, impaired metabolic health and COVID-19. Nat Rev Endocrinol 2021; 17:135–49. [DOI] [PubMed] [Google Scholar]
- 109. May AL, Freedman D, Sherry B, Blanck HM; Centers for Disease Control and Prevention (CDC). Obesity - United States, 1999-2010. MMWR Suppl 2013; 62:120–8. [PubMed] [Google Scholar]
- 110. Jackson SL, Zhang Z, Wiltz JL, et al. Hypertension among youths - United States, 2001-2016. MMWR Morb Mortal Wkly Rep 2018; 67:758–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Götzinger F, Santiago-García B, Noguera-Julián A, et al. ; ptbnet COVID-19 Study Group. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health 2020; 4:653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Graff K, Smith C, Silveira L, et al. Risk factors for severe COVID-19 in children. Pediatr Infect Dis J 2021; 40:e137–45. [DOI] [PubMed] [Google Scholar]
- 113. Bhuiyan MU, Stiboy E, Hassan MZ, et al. Epidemiology of COVID-19 infection in young children under five years: a systematic review and meta-analysis. Vaccine 2021; 39:667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tenforde MW, Self WH, Adams K, et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA 2021; 326:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Frenck RW, Klein NP, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med 2021; 385:239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet 2021; 398:1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021; 385:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Owusu D, Pomeroy MA, Lewis NM, et al. ; Household Transmission Study Team. Persistent SARS-CoV-2 RNA shedding without evidence of infectiousness: a cohort study of individuals with COVID-19. J Infect Dis 2021; 224:1362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wang K, Zhang X, Sun J, et al. Differences of severe acute respiratory syndrome coronavirus 2 shedding duration in sputum and nasopharyngeal swab specimens among adult inpatients with coronavirus disease 2019. Chest 2020; 158:1876–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wang B, Van Oekelen O, Mouhieddine TH, et al. A tertiary center experience of multiple myeloma patients with COVID-19: lessons learned and the path forward. J Hematol Oncol 2020; 13:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Beigel JH, Tomashek KM, Dodd LE, et al. ; ACTT-1 Study Group Members. Remdesivir for the treatment of COVID-19 — final report. N Engl J Med 2020; 383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Al Sulaiman K, Korayem GB, Eljaaly K, et al. Early dexamethasone use as a protective measure in non-mechanically ventilated critically ill patients with COVID-19: a multicenter, cohort study. Sci Rep 2022; 12:9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hussain Alsayed HA, Saheb Sharif-Askari F, Saheb Sharif-Askari N, Hussain AAS, Hamid Q, Halwani R. Early administration of remdesivir to COVID-19 patients associates with higher recovery rate and lower need for ICU admission: A retrospective cohort study. PLoS One 2021; 16:e0258643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gordon AC, Mouncey PR, Al-Beidh F, et al. ; REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med 2021; 384:1491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/. Accessed October 2, 2023.
- 126. Ahmed A, Rojo P, Agwu A, et al. P168 Remdesivir in the treatment of children 28 days to < 18 years of age hospitalised with COVID-19 in the CARAVAN study. Thorax 2022; 77:A172–3. [Google Scholar]
- 127. Duarte-Salles T, Vizcaya D, Pistillo A, et al. Thirty-day outcomes of children and adolescents with COVID-19: an international experience. Pediatrics 2021; 148:e2020042929. [DOI] [PubMed] [Google Scholar]
- 128. Andina-Martinez D, Alonso-Cadenas JA, Cobos-Carrascosa E, et al. ; EPICO-AEP Working Group. SARS-CoV-2 acute bronchiolitis in hospitalized children: neither frequent nor more severe. Pediatr Pulmonol 2022; 57:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Brewster RC, Parsons C, Laird-Gion J, et al. COVID-19-associated croup in children. Pediatrics 2022; 149:e2022056492. [DOI] [PubMed] [Google Scholar]
- 130. Lefchak B, Nickel A, Lammers S, Watson D, Hester GZ, Bergmann KR. Analysis of COVID-19-related croup and SARS-CoV-2 variant predominance in the US. JAMA Netw Open 2022; 5:e2220060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Tunҫ EM, Koid Jia Shin C, Usoro E, et al. Croup during the coronavirus disease 2019 omicron variant surge. J Pediatr 2022; 247:147–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. WHO Solidarity Trial Consortium. Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet 2022; 399:1941–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. World Health Organization. Therapeutics and COVID-19: Living guideline, 13 January 2023. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2023.1 [PubMed] [Google Scholar]
- 134. Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Version 11.0.0. Infectious Diseases Society of America Available at: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Accessed August 3, 2023. [DOI] [PMC free article] [PubMed]
- 135. Lamb YN. Remdesivir: first approval. Drugs 2020; 80:1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Pettit NN, Pisano J, Nguyen CT, et al. Remdesivir use in the setting of severe renal impairment: a theoretical concern or real risk? Clin Infect Dis 2021; 73:e3990–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ackley TW, McManus D, Topal JE, Cicali B, Shah S. A valid warning or clinical lore: an evaluation of safety outcomes of remdesivir in patients with impaired renal function from a multicenter matched cohort. Antimicrob Agents Chemother 2021; 65:e02290–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Chao JY, Derespina KR, Herold BC, et al. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 at a tertiary care medical center in New York City. J Pediatr 2020; 223:14–19.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Shekerdemian LS, Mahmood NR, Wolfe KK, et al. ; International COVID-19 PICU Collaborative. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr 2020; 174:868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Zachariah P, Johnson CL, Halabi KC, et al. ; Columbia Pediatric COVID-19 Management Group. Epidemiology, clinical features, and disease severity in patients with Coronavirus Disease 2019 (COVID-19) in a children’s hospital in New York City, New York. JAMA Pediatr 2020; 174:e202430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kim L, Whitaker M, O’Halloran A, et al. ; COVID-NET Surveillance Team. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19 - COVID-NET, 14 States, March 1-July 25, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1081–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. RECOVERY Collaborative Group. Electronic address: recoverytrial@ndphoxacuk, RECOVERY Collaborative Group Higher dose corticosteroids in patients admitted to hospital with COVID-19 who are hypoxic but not requiring ventilatory support (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2023; 401:1499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Crothers K, DeFaccio R, Tate J, et al. ; Veterans Aging Cohort Study Clinical COVID-19 Working Group. Dexamethasone in hospitalised COVID-19 patients not on intensive respiratory support. Eur Respir J 2022; 60:2102532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Mourad A, Thibault D, Holland TL, et al. Dexamethasone for Inpatients With COVID-19 in a National Cohort. JAMA Netw Open 2023; 6:e238516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Tripathi S, Nadiger M, McGarvey JS, et al. ; Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 Registry Investigator Group. Association of early steroid administration with outcomes of children hospitalized for COVID-19 without multisystem inflammatory syndrome in children. JAMA Pediatr 2022; 176:1208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Sterne JAC, Murthy S, Diaz JV, et al. ; WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19: a meta-analysis. JAMA 2020; 324:1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Tomazini BM, Maia IS, Cavalcanti AB, et al. ; COALITION COVID-19 Brazil III Investigators. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA 2020; 324:1307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Angus DC, Derde L, Al-Beidh F, et al. ; Writing Committee for the REMAP-CAP Investigators. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA 2020; 324:1317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Dequin P-F, Heming N, Meziani F, et al. ; CAPE COVID Trial Group and the CRICS-TriGGERSep Network. Effect of hydrocortisone on 21-day mortality or respiratory support among critically Ill patients with COVID-19: a randomized clinical trial. JAMA 2020; 324:1298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Jeronimo CMP, Farias MEL, Val FFA, et al. ; Metcovid Team. Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID-19; Metcovid): a randomized, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis 2021; 72:e373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Munch MW, Meyhoff TS, Helleberg M, et al. Low-dose hydrocortisone in patients with COVID-19 and severe hypoxia: The COVID STEROID randomised, placebo-controlled trial. Acta Anaesthesiol Scand 2021; 65:1421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Granholm A, Munch MW, Myatra SN, et al. Dexamethasone 12 mg versus 6 mg for patients with COVID-19 and severe hypoxaemia: a pre-planned, secondary Bayesian analysis of the COVID STEROID 2 trial. Intensive Care Med 2022; 48:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Toroghi N, Abbasian L, Nourian A, et al. Comparing efficacy and safety of different doses of dexamethasone in the treatment of COVID-19: a three-arm randomized clinical trial. Pharmacol Rep 2022; 74:229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Maskin LP, Bonelli I, Olarte GL, et al. High- versus low-dose dexamethasone for the treatment of COVID-19-related acute respiratory distress syndrome: a multicenter, randomized open-label clinical trial. J Intensive Care Med 2022; 37:491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Bouadma L, Mekontso-Dessap A, Burdet C, et al. ; COVIDICUS Study Group. High-dose dexamethasone and oxygen support strategies in intensive care unit patients with severe COVID-19 acute hypoxemic respiratory failure: the COVIDICUS randomized clinical trial. JAMA Intern Med 2022; 182:906–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. RECOVERY Collaborative Group. Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis. Lancet 2022; 400:359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kalil AC, Patterson TF, Mehta AK, et al. ; ACTT-2 Study Group Members. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med 2021; 384:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. U.S. Food and Drug Administration. Fact Sheet for Healthcare Providers: Emergency Use Authorization (EUA) of Baricitinib. 2022; Available at: https://www.fda.gov/media/143823/download. Accessed October 5, 2023.
- 159. Guimarães PO, Quirk D, Furtado RH, et al. ; STOP-COVID Trial Investigators. Tofacitinib in patients hospitalized with covid-19 pneumonia. N Engl J Med 2021; 385:406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021; 397:1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Stone JH, Frigault MJ, Serling-Boyd NJ, et al. ; BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with covid-19. N Engl J Med 2020; 383:2333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. U.S. Food and Drug Administration. Fact Sheet for Healthcare Providers: Emergency Use Authorization for Actemra(R). 2021; Available at: https://www.fda.gov/media/150321/download. Accessed October 5, 2023.
- 163. Teoh Z, Danziger-Isakov L, Courter JD, et al. Tocilizumab for treatment of children and young adults with severe acute COVID-19: experience at a quaternary-care children’s hospital. Pediatr Infect Dis J 2023; 42:119–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Lundgren JD, Grund B, Barkauskas CE, et al. ; ACTIV-3/TICO LY-CoV555 Study Group. A neutralizing monoclonal antibody for hospitalized patients with covid-19. N Engl J Med 2021; 384:905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group. Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis 2022; 22:622–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Estcourt LJ, Turgeon AF, McQuilten ZK, et al. ; Writing Committee for the REMAP-CAP Investigators. Effect of convalescent plasma on organ support-free days in critically Ill patients with COVID-19: a randomized clinical trial. JAMA 2021; 326:1690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Bégin P, Callum J, Jamula E, et al. ; CONCOR-1 Study Group. Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial. Nat Med 2021; 27:2012–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet 2021; 397:2049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Mikulska M, Sepulcri C, Dentone C, et al. Triple combination therapy with 2 antivirals and monoclonal antibodies for persistent or relapsed severe acute respiratory syndrome coronavirus 2 infection in immunocompromised patients. Clin Infect Dis 2023; 77:280–6. [DOI] [PubMed] [Google Scholar]
- 170. Alonso-Coello P, Schünemann HJ, Moberg J, et al. ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices 1: Introduction. BMJ 2016; 353:i2016. [DOI] [PubMed] [Google Scholar]
- 171. Levin MJ, Ustianowski A, De Wit S, et al. Intramuscular AZD7442 (tixagevimab–cilgavimab) for prevention of Covid-19. N Engl J Med 2022;386(23):2188–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Food and Drug Administration. Fact sheet for healthcare providers: Emergency Use Authorization for EvusheldTM (tixagevimab co-packaged with cilgavimab). Available at: https://www.fda.gov/media/154701/download. Accessed April 4, 2022.
- 173. Center for Drug Evaluation and Research. FDA announces Evusheld is not currently authorized for emergency use in the U.S. FDA, 2023. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-evusheld-not-currently-authorized-emergency-use-us. Accessed April 5, 2023. [Google Scholar]
- 174. Center for Drug Evaluation and Research, Food and Drug Administration. FDA authorizes revisions to Evusheld dosing. FDA; 2022; Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-evusheld-not-currently-authorized-emergency-use-us. Accessed April 24, 2023. [Google Scholar]
- 175. Levin MJ, Ustianowski A, Thomas S, et al. ; COVID-19 Study to Optimally Reduce Morbidity in CareHomes and Sites with Enhanced Risk (STORMCHASER) Study Group. AZD7442 (tixagevimab/cilgavimab) for post-exposure prophylaxis of symptomatic coronavirus disease 2019. Clin Infect Dis: Off Publ Infect Dis Soc Am 2023; 76:1247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Montgomery H, Hobbs FDR, Padilla F, et al. Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2022; 10(10):985–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Center for Drug Evaluation and Research, Food and Drug Administration. FDA authorizes bamlanivimab and etesevimab monoclonal antibody therapy for post-exposure prophylaxis (prevention) for COVID-19. FDA; 2021; Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-authorizes-bamlanivimab-and-etesevimab-monoclonal-antibody-therapy-post-exposure-prophylaxis. Accessed April 24, 2023. [Google Scholar]
- 178. Center for Drug Evaluation and Research, Food and Drug Administration. FDA authorizes REGEN-COV monoclonal antibody therapy for post-exposure prophylaxis (prevention) for COVID-19. FDA; 2021; Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-authorizes-regen-cov-monoclonal-antibody-therapy-post-exposure-prophylaxis-prevention-covid-19. Accessed April 24, 2023. [Google Scholar]
- 179. Office of the Commissioner, Food and Drug Administration. Coronavirus (COVID-19) Update: December 23, 2021. FDA, 2021. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-december-23-2021. Accessed April 24, 2023. [Google Scholar]
- 180. Cohen MS, Nirula A, Mulligan MJ, et al. ; BLAZE-2 Investigators. Effect of bamlanivimab vs placebo on incidence of Covid-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial. JAMA 2021; 326:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Hirsch C, Park YS, Piechotta V, et al. SARS-CoV-2-neutralising monoclonal antibodies to prevent COVID-19. Cochrane Database Syst Rev 2022; 6:CD014945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Centers for Disease Control and Prevention. People Who Are Immunocompromised. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-who-are-immunocompromised.html. Accessed April 24, 2023.
- 183. Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention. ACIP COVID-19 Vaccine Recommendations | CDC. 2022. Available at: https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html. Accessed April 24, 2023. [Google Scholar]
- 184. Klein NP, Stockwell MS, Demarco M, et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA vaccination in preventing COVID-19-associated emergency department and urgent care encounters and hospitalizations among nonimmunocompromised children and adolescents aged 5-17 Years - VISION Network, 10 states, April 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022; 71:352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Price AM, Olson SM, Newhams MM, et al. ; Overcoming Covid-19 Investigators. BNT162b2 protection against the omicron variant in children and adolescents. N Engl J Med 2022; 386:1899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Fleming-Dutra KE, Britton A, Shang N, et al. Association of prior BNT162b2 COVID-19 vaccination with symptomatic SARS-CoV-2 infection in children and adolescents during omicron predominance. JAMA 2022; 327:2210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


