Abstract
We report that both primary and laboratory-adapted infectious human immunodeficiency virus type 1 (HIV-1) isolates in a cell-free form are capable of transcytosis through a tight and polarized monolayer of human endometrial cells. Trancytosis of cell-free HIV occurs in a strain-selective fashion and appears to be dependent on interactions between HIV envelope glycoproteins and lectins on the apical membrane of the epithelial cells. These findings provide new insights into the initial events occurring during heterosexual transmission of the virus.
Transmission of human immunodeficiency virus type 1 (HIV-1) occurs through monostratified mucosal surfaces (27, 29). Genital secretions of HIV-1-seropositive individuals contain both cell-free HIV-1 particles and virus that is cell associated in the form of infected monocytes/macrophages and CD4+ T lymphocytes (23, 25, 27). Whereas HIV-1 recovered from individuals undergoing primary infection is largely R5-tropic and of the non-syncytium-inducing (NSI) phenotype (28, 31), both X4-tropic syncytium-inducing variants and R5-tropic NSI variants are found in blood and genital secretions of HIV-1-seropositive individuals at a later stage of disease (7, 32). Thus, a selection process favoring R5-tropic NSI phenotypes occurs during or soon after transmucosal penetration of the virus.
Transcytosis of HIV-1 through a tight monolayer of epithelial cells has been proposed as an in vitro model mimicking the penetration of HIV-1 through unistratified epithelia (21, 22). Although transcytosis of cell-associated virus has been consistently demonstrated in this model (2, 22), transcytosis of cell-free HIV-1 particles remains controversial (2, 4, 17).
Transcytosis of free and cell-associated HIV-1 across a monolayer of epithelial cells.
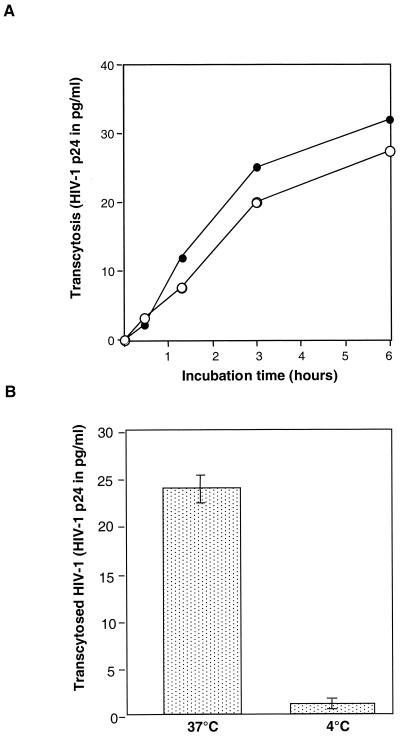
We first investigated whether cell-associated R5- and X4-tropic viruses, as well as the corresponding free viral particles, were capable of transcytosis through the HEC-1 monolayer. A significant amount of transcytosis was consistently observed in the case of both cell-associated virus and free virus following contact with the apical membrane of HEC-1 cells at 37°C (Fig. 1A). When performing the experiment at 4°C, we observed that transcytosis of free HIV-1NDK was inhibited by 90% (Fig. 1B). Virus that was recovered from the basal chamber, whether it originated from transcytosis of cell-associated HIV-1 or of free HIV-1, was infectious in vitro, as assessed by its ability to infect phytohemagglutinin (PHA)- and interleukin-2 (IL-2)-stimulated peripheral blood lymphocytes (PBL) from healthy individuals.
FIG. 1.
Transcytosis of cell-free and cell-associated HIV-1 through a tight monolayer of HEC-1 cells. (A) Kinetics of transcytosis of cell-free (full circles) and PBL-associated (open circles) HIV-1NDK. Twenty nanograms of p24 (free virus) and 2 × 106 infected PBL were deposited in the apical chamber of the transwell system. The results are expressed as the amount of p24 antigen recovered in the basolateral chamber as a function of time. (B) Temperature dependency of transcytosis. Transcytosis of free HIV-1NDK through the HEC-1 cells monolayer was assessed at 37 and at 4°C by measuring the amount of p24 antigen in the basal chamber after 3 h of contact of cell-free virus (20 ng) with the apical membrane of HEC-1 cells. Results are expressed as means and standard deviations of three separate experiments.
Detection of intracellular HIV-1 gp160 in transcytosed HEC-1 cells.
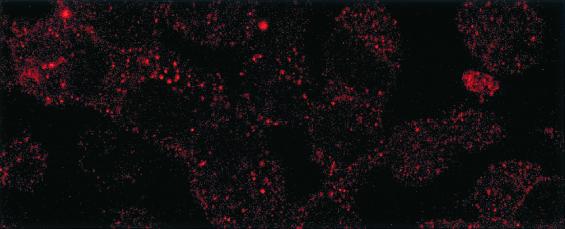
Indirect immunofluorescence allowed detection of HIV gp160 antigen by confocal microscopy within the cytosol of HEC-1 cells, after exposure of the apical side of the monolayer to free HIV-1NDK during 3 h (Fig. 2).
FIG. 2.
Detection of intracellular HIV-1 gp160 antigen (red) in transcytosed HEC-1 cells by immunoflorescence. The HEC-1 cells used in the transcytosis assays were washed, fixed with paraformaldehyde (4% in phosphate-buffered saline [PBS]) for 15 min, quenched of free aldehydes with 200 mM NH4Cl in PBS, and permeabilized for 10 min with 0.5% of Triton X-100 in PBS. After being washed with PBS, cells were incubated for 1 h with human anti-gp160 IgG diluted in PBS buffer with 1% bovine serum albumin. Phycoerythrin-labeled F(ab′)2 goat anti-human IgG (Jackson Immunoresearch, West Grove, Pa.) was further added at a dilution of 1/10. The coverslips were mounted in Mowiol (Sigma, St. Louis, Mo.) and observed by confocal microscopy using a Leica microscope (Leica, Wetzlar, Germany). Magnification, ×630.
Selectivity of transcytosis of free HIV-1 through a monolayer of endometrial cells.
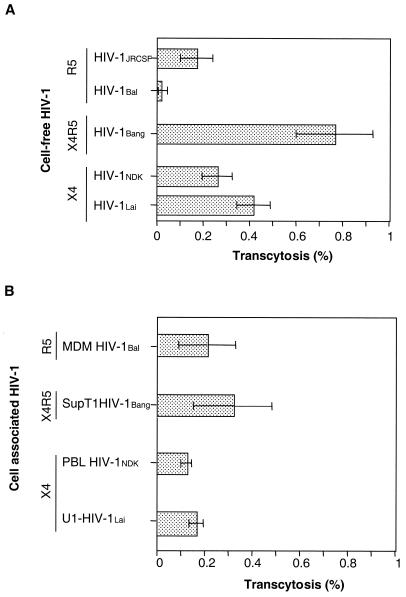
When HIV-1 was delivered as free viral particles to the apical chamber of the transwells, the recovery in the basal compartment, as measured by quantitating p24 antigen, was 0.41% ± 0.07% of deposited HIV-1Lai (mean ± the standard error of the mean), 0.26% ± 0.06% of HIV-1NDK, 0.77% ± 0.16% of HIV-1Bang, 0.17% ± 0.07% of deposited HIV-1JRCSF, and 0.01% ± 0.005% of HIV-1Bal, respectively (Fig. 3A). The amount of HIV-1Bal recovered in the basal chamber in an experiment performed at 37°C did not exceed that of HIV-1NDK recovered at 4°C (i.e., 0.01% of deposited virus, used as a cutoff in the assay), despite the fact that significant transcytosis of the HIV-1NDK, HIV-1Bang, and HIV-1Lai isolates occurs under the same experimental conditions.
FIG. 3.
Transcytosis of various isolates of HIV-1 through HEC-1 cells. (A) Transcytosis of cell-free HIV. (B) Transcytosis of cell-associated HIV. The viral strains that were used included the primary R5-tropic HIV-1JRCSF (clade B) grown on PBL following stimulation with PHA and IL-2, the R5-tropic HIV-1Bal (clade B) which was amplified in monocyte-derived macrophages of healthy donors, the laboratory-adapted R5X4-tropic HIV-1Bang originating from a patient infected with clade A virus and further amplified in the Sup T1 T-cell line, the primary X4-tropic HIV-1NDK (clade D) grown in PBL of healthy donors following stimulation with PHA and IL-2, and the laboratory-adapted X4-tropic HIV-1Lai (clade B) amplified in U1 monocytic cells. Transcytosis was assessed as previously described (14). Filters were used when the resistivity of the monolayer had reached 200 Ω/cm2 after 6 days. Free virus (20 ng/well) or HIV-1-infected cells (2 × 106 cells) were added to the apical chamber of transwells. After 180 min at 37°C, transcytosis was quantified by measuring the p24 antigen in samples taken from the basolateral chamber by means of a capture enzyme-linked immunosorbent assay (DuPont de Nemours, Wilmington, Del.) (threshold of detection, 3 pg/ml). Results were expressed as a percentage of virus recovered in the basal chamber, calculated from the amount of HIV-1 applied in the apical chamber that represented 100%. The data are expressed as means and standard deviations for three independent experiments.
No significative difference was observed between strains with regard to transcytosis of cell-associated viruses. The mean percentages of deposited Sup T1-associated HIV-1Bang, peripheral blood lymphocyte (PBL)-associated HIV-1NDK, U1-associated HIV-1Lai, and monocyte-derived macrophage-associated HIV-1Bal that were recovered in the basal chamber of the transwell systems in three independent experiments were 0.32% ± 0.17%, 0.12% ± 0.02%, 0.17% ± 0.03%, and 0.21% ± 0.12%, respectively (Fig. 3B).
Involvement of gp160 in transcytosis of free HIV-1.
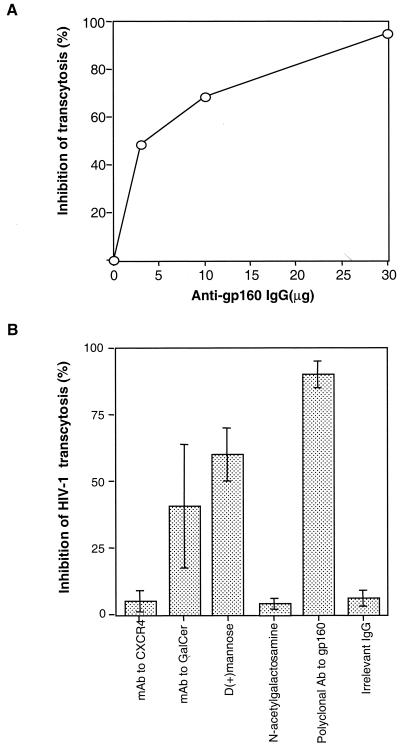
HEC-1 cells were found to express CXCR4 and Galcer antigens (approximately 70% of HEC-1 cells) by indirect immunofluorescence staining, but they failed to express the CD4 and CCR5 antigens (not shown). Transcytosis of free HIV-1NDK was not inhibited by anti-CXCR4 monoclonal antibody (MAb), nor by a mixture of four anti-CCR5 MAbs to the apical chamber of the transwells. In contrast, human polyclonal antibodies to gp160 blocked, in a dose-dependent manner, up to 95% ± 3% of the transcytosis of free HIV-1NDK (Fig. 4A). Transcytosis of free HIV-1NDK was partially inhibited by the anti-Galcer antibodies (40.5% ± 23%) and by d-(+)-mannose (60% ± 4%). Irrelevant IgG and N-acetylgalactosamine did not inhibit cell-free HIV-1 transcytosis (Fig. 4B).
FIG. 4.
Inhibition of transcytosis of free HIV-1NDK through a tight HEC-1 epithelial barrier by anti-env and antireceptor antibodies. (A) Virus was incubated with serial amounts of purified polyclonal human antibodies to gp160 for 15 min at 37°C prior to being deposited in the apical chamber of the transwell system. (B) Cells were preincubated with 12G5 MAbs to CXCR4 (R & D Systems, Minneapolis, Minn.), and virus was incubated with rabbit polyclonal anti-Galcer antibodies (Sigma), d-(+)-mannose (Sigma), and N-acetylgalactosamine (Sigma). A positive control in the experiment was polyclonal IgG against gp160 purified from serum of HIV-1-seropositive individuals. The negative control was an irrelevant IgG purified from pooled serum of HIV-1-seronegative blood donors. The results are expressed as the percent inhibition of transcytosis and expressed as means ± standard deviations for four separate experiments.
Lack of infection of HEC-1 endometrial cells upon transcytosis of cell-free and cell-associated HIV-1.
The epithelial cells used in the transcytosis assays were recovered from filter by trypsinization and further cultured for 30 days in order to eliminate contaminating cells. The presence of HIV-1 provirus was then investigated using 106 epithelial cells by means of a nested PCR in the pol gene region, as described previously (20). No viral DNA was detected in HEC-1 cells that had been exposed to all free and cell-associated HIV isolates.
Although transcytosis of cell-associated virus has been observed consistently (2, 21), transcytosis of cell-free HIV-1 particles through monolayers of epithelial cells remains controversial. Thus, evidence of transcytosis of cell-free virus through HEC1 endometrial cells could not be shown by Bomsel (2). In contrast, Kage and colleagues reported that cell-free HIV-1 can be taken up and released by a monolayer of primary human gingival cells and that recovered virus remained infectious for CD4+ T cells in vitro (17). In this study, we reevaluated the transcytosis of cell-free HIV-1 using a tight polarized monolayer of HEC-1 endometrial cells. The integrity of the monolayer was carefully assessed by measuring the resistivity between the apical and basal chambers prior to transcytosis and after transcytosis had taken place. We observed that cell-free as well as cell-associated HIV-1 was transported through the monolayer of HEC-1 cells. Immunofluorescence staining of HIV-1 gp160 in HEC-1 cells exposed to cell-free HIV gave direct evidence that the virus passed through the cell. We chose to detect the envelope gp160 rather than nucleoprotein antigens to differentiate between transcytosis and infection. Virus that was recovered in the basal chamber following transcytosis through HEC-1 cells remained infectious, as demonstrated by its ability to infect IL-2- and PHA-activated PBL of healthy donors in vitro. Transcytosis was an active process, since it was suppressed by more than 90% at 4°C. Whereas transcytosis of virus occurred with all strains that we tested when virus was in a cell-associated form, transcytosis appeared to be dependent on the viral strain in the case of cell-free viral particles. Thus, the cell-free R5-tropic HIV-1Bal strain did not cross the epithelial monolayer, whereas under the same experimental conditions using a twofold-lower amount of HIV-1, transcytosis occurred with other R5-tropic and X4-tropic cell-free HIV-1 strains. Transcytosis occurred with an efficiency that was dependent on the viral strain. Differences in efficiency were not related to the R5 or X4 tropism of the virus.
In order to investigate the nature of the molecules involved in initiating transcytosis at the apical membrane of endometrial cells, we analyzed the expression of receptors for HIV-1 and the ability of antibodies directed against these molecules to inhibit transcytosis when added to the apical chamber of the transwell system. HEC-1 cells were found not to express either CD4 or CCR5, whereas the cells strongly stained for CXCR4 and Galcer, a glycolipid that has been suggested to function as a receptor for the penetration of HIV-1 in CD4-negative cells (6, 8, 9) and in transcytosis of cell-associated HIV-1 (2). Transcytosis of cell-free and cell-associated HIV-1 was not inhibited by a large excess of either anti-CD4, anti-CXCR4, or anti-CCR5 antibodies. Antibodies to Galcer resulted in a limited (35 to 40%), although significant, inhibition of the transcytosis of cell-free virus. Affinity-purified human polyclonal immunoglobulin G (IgG) against gp160 consistently inhibited transcytosis by 95%. These observations suggest that transcytosis occurs through a receptor-mediated mechanism utilizing the HIV-1 envelope and partially involving Galcer residues. Inhibition experiments using mannose showed that it inhibited 60% of transcytosis of free HIV-1, further suggesting that this lectin is also involved in the interaction between gp160 and the apical membrane of HEC-1 cells. Taken together, the data suggest that cell-free infectious HIV-1 particles can penetrate epithelia following the interaction between gp160 and lectin residues, such as mannose, and to a lesser extent the Galcer molecule expressed on the mucosal surface. Thus, although the Galcer molecule can bind to HIV-1 envelope glycoprotein, leading to the infection of some epithelial cells (5, 8), the glycolipid does not appear as a major attachment receptor used at initial events of free HIV-1 transcytosis through a monolayer of epithelial cells.
Whether the transmucosal passage of HIV-1 involves infection of the epithelial cells during sexual transmission remains unknown. The issue remains controversial, since some authors have reported infection (3, 15, 26) whereas others have not (2, 11). We have been unable to detect viral DNA by means of a nested PCR in HEC-1 cells that had been exposed to free or to cell-associated virus, washed, and further subcultured for 30 days prior to PCR testing. These results indicate that HEC-1 cells allow viral transcytosis in the absence of infection. However, transcytosis in association with concomitant infection of epithelial cells may occur, as previously reported in the case of HeLa cervical epithelial cells (19).
The demonstration that infectious cell-free HIV-1 may cross epithelial cells is consistent with observations of infection of adult macaques challenged intravaginally with cell-free SIV and chimeric simian/human immunodeficiency viruses (12, 13, 16, 18, 30). Furthermore, the risk of transmission of HIV-1 from mother to infant by breast feeding has been shown to be directly correlated to the amount of cell-free virus in milk (24). The amounts of virus that were recovered in the basal chamber of the transwell system did not exceed 0.7% in the case of cell-free virus and 0.3% in the case of cell-associated HIV-1. Thus, it is likely that sexual transmission by this mechanism will require a high inoculum and may not occur at a high frequency, which is consistent with epidemiological data (23). The risk of transmission would also depend, at least in the case of cell-free virus, on the relative fitness of the variants within the inoculum and local factors, including the presence of mucus and the glycocalyx on the apical surface of epithelial cells (10).
The observation that transcytosis occurs with both X4- and R5-tropic viruses contrasts with the prevailing perception that only R5-tropic NSI HIV-1 is transmitted during sexual infection and rather suggests that a selection process favoring R5-tropic NSI strains occurs in the submucosa following crossing of epithelial cells by the virus. The expansion of R5-tropic, NSI HIV-1 variants observed in vivo during primary infection could be the result of a favorable cytokine environment at submucosal sites during the transamplification of virus in the submucosa (1).
Acknowledgments
We thank Françoise Barré-Sinoussi and Elisabeth Menu from Institut Pasteur, Paris, who kindly provided the primary HIV-1NDK and HIV-1JRCSF strains. We also thank Theano Eirinopoulou for confocal microscopy processing.
This work was supported by the Agence Nationale de Recherches sur le SIDA (ANRS), Paris, France.
REFERENCES
- 1.Agace W W, Amara A, Roberts A I, Pablos J L, Thelen S, Uguccioni M, Li X Y, Marsal J, Arenzana-Seisdedos F, Delaunay T, Ebert E C, Moser B, Parker C M. Constitutive expression of stromal derived factor-1 by mucosal epithelia and its role in HIV transmission and propagation. Curr Biol. 2000;10:325–328. doi: 10.1016/s0960-9822(00)00380-8. [DOI] [PubMed] [Google Scholar]
- 2.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med. 1997;3:42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- 3.Chenine A L, Matouskova E, Sanchez G, Reischig J, Pavlikova L, LeContel C, Chermann J C, Hirsch I. Primary intestinal epithelial cells can be infected with laboratory-adapted strain HIV type 1 NDK but not with clinical primary isolates. AIDS Res Hum Retrovir. 1998;14:1235–1238. doi: 10.1089/aid.1998.14.1235. [DOI] [PubMed] [Google Scholar]
- 4.Collins K B, Patterson B K, Naus G J, Landers D V, Gupta P. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat Med. 2000;6:475–479. doi: 10.1038/74743. [DOI] [PubMed] [Google Scholar]
- 5.Cook D G, Fantini J, Spitalnik S L, Gonzalez S F. Binding of human immunodeficiency virus type I (HIV-1) gp120 to galactosylceramide (GalCer): relationship to the V3 loop. Virology. 1994;201:206–214. doi: 10.1006/viro.1994.1287. [DOI] [PubMed] [Google Scholar]
- 6.Delezay O, Koch N, Yahi N, Hammache D, Tourres C, Tamalet C, Fantini J. Co-expression of CXCR4/fusin and galactosylceramide in the human intestinal epithelial cell line HT-29. AIDS. 1997;11:1311–1308. doi: 10.1097/00002030-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Delwart E L, Mullins J I, Gupta P, Learn G H, Jr, Holodniy M, Katzenstein D, Walker B D, Singh M K. Human immunodeficiency virus type 1 populations in blood and semen. J Virol. 1998;72:617–623. doi: 10.1128/jvi.72.1.617-623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fantini J, Cook D G, Nathanson N, Spitalnik S L, Gonzalez S F. Infection of colonic epithelial cell lines by type 1 human immunodeficiency virus is associated with cell surface expression of galactosylceramide, a potential alternative gp120 receptor. Proc Natl Acad Sci USA. 1993;90:2700–2704. doi: 10.1073/pnas.90.7.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fantini J, Hammache D, Delezay O, Pieroni G, Tamalet C, Yahi N. Sulfatide inhibits HIV-1 entry into CD4− CXCR4+ cells. Virology. 1998;246:211–220. doi: 10.1006/viro.1998.9216. [DOI] [PubMed] [Google Scholar]
- 10.Frey A, Giannasca K T, Weltzin R, Giannasca P J, Reggio H, Lencer W I, Neutra M R. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting. J Exp Med. 1997;184:1045–1059. doi: 10.1084/jem.184.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenhead P, Hayes P, Watts P S, Laing K G, Griffin G E, Shattock R J. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol. 2000;74:5577–5586. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harouse J M, Gettie A, Eshetu T, Tan R C, Bohm R, Blanchard J, Baskin G, Cheng-Mayer C. Mucosal transmission and induction of simian AIDS by CCR5-specific simian human immunodeficiency virus SHIV (SF162P3) J Virol. 2001;75:1990–1995. doi: 10.1128/JVI.75.4.1990-1995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harouse J M, Tan R C, Gettie A, Dailey P, Marx P A, Luciw P A, Cheng-Mayer C. Mucosal transmission of pathogenic CXCR4-utilizing SHIVSF33A variants in rhesus macaques. Virology. 1998;248:95–107. doi: 10.1006/viro.1998.9236. [DOI] [PubMed] [Google Scholar]
- 14.Hocini H, Belec L, Iscaki S, Garin B, Pillot J, Becquart P, Bomsel M. High-level ability of secretory IgA to block HIV type 1 transcytosis: contrasting secretory IgA and IgG responses to glycoprotein 160. AIDS Res Hum Retrovir. 1997;13:1179–1185. doi: 10.1089/aid.1997.13.1179. [DOI] [PubMed] [Google Scholar]
- 15.Howell A L, Edkins R D, Rier S E, Yeaman G R, Stern J E, Fanger M W, Wira C R. Human immunodeficiency virus type 1 infection of cells and tissues from the upper and lower human female reproductive tract. J Virol. 1997;71:3498–3506. doi: 10.1128/jvi.71.5.3498-3506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu J, Gardner M B, Miller C J. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kage A, Shoolian E, Rokos K, Ozel M, Nuck R, Reutter W, Kottgen E, Pauli G. Epithelial uptake and transport of cell-free human immunodeficiency virus type 1 and gp120-coated microparticles. J Virol. 1998;72:4231–4236. doi: 10.1128/jvi.72.5.4231-4236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mascola J R, Stiegler G, VanCott T C, Katinger H, Carpenter C B, Hanson C E, Beary H, Hayes D, Frankel S S, Birx D L, Lewis M G. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 19.Morizono K, Harada S. Human immunodeficiency virus type 1 (HIV-1) infection and transcytosis activity of a HIV-1 susceptible clone from HeLa cell. Microbiol Immunol. 1998;42:313–320. doi: 10.1111/j.1348-0421.1998.tb02288.x. [DOI] [PubMed] [Google Scholar]
- 20.Nijhuis M, Boucher C A, Schipper P, Leitner T, Schuurman R, Albert J. Stochastic processes strongly influence HIV-1 evolution during suboptimal protease-inhibitor therapy. Proc Natl Acad Sci USA. 1998;95:14441–14446. doi: 10.1073/pnas.95.24.14441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips D M. The role of cell-to-cell transmission in HIV infection. AIDS. 1994;8:719–731. doi: 10.1097/00002030-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Phillips D M, Bourinbaiar A S. Mechanism of HIV spread from lymphocytes to epithelia. Virology. 1992;186:261–273. doi: 10.1016/0042-6822(92)90080-9. [DOI] [PubMed] [Google Scholar]
- 23.Royce R A, Sena A, Cates W, Jr, Cohen M S. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. . (Erratum, 337:799.) [DOI] [PubMed] [Google Scholar]
- 24.Semba R D, Kumwenda N, Hoover D R, Taha T E, Quinn T C, Mtimavalye L, Biggar R J, Broadhead R, Miotti P G, Sokoll L J, van der Hoeven L, Chiphangwi J D. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 1999;180:93–98. doi: 10.1086/314854. [DOI] [PubMed] [Google Scholar]
- 25.Shepard R N, Schock J, Robertson K, Shugars D C, Dyer J, Vernazza P, Hall C, Cohen M S, Fiscus S A. Quantitation of human immunodeficiency virus type 1 RNA in different biological compartments. J Clin Microbiol. 2000;38:1414–1418. doi: 10.1128/jcm.38.4.1414-1418.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan X, Pearce-Pratt R, Phillips D M. Productive infection of a cervical epithelial cell line with human immunodeficiency virus: implications for sexual transmission. J Virol. 1993;67:6447–6452. doi: 10.1128/jvi.67.11.6447-6452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van de Perre P. Transmission of human immunodeficiency virus type 1 through breast-feeding: how can it be prevented? J Infect Dis. 1999;179(Suppl. 3):S405–S407. doi: 10.1086/314793. [DOI] [PubMed] [Google Scholar]
- 28.van't Wout A B, Kootstra N A, Mulder-Kampinga G A, Albrecht-van Lent N, Scherpbier H J, Veenstra J, Boer K, Coutinho R A, Miedema F, Schuitemaker H. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission. J Clin Investig. 1994;94:2060–2067. doi: 10.1172/JCI117560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeaman G R, White H D, Howell A, Prabhala R, Wira C R. The mucosal immune system in the human female reproductive tract: potential insights into the heterosexual transmission of HIV. AIDS Res Hum Retrovir. 1998;14(Suppl. 1):S57–S62. [PubMed] [Google Scholar]
- 30.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus K A, Reimann K A, Reinhart T A, Rogan M, Cavert W, Miller C J, Veazey R S, Notermans D, Little S, Danner S A, Richman D D, Havlir D, Wong J, Jordan H L, Schacker T W, Racz P, Tenner-Racz K, Letvin N L, Wolinsky S, Haase A T. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. . (Erratum, 286:2273.) [DOI] [PubMed] [Google Scholar]
- 31.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 32.Zhu T, Wang N, Carr A, Nam D S, Moor-Jankowski R, Cooper D A, Ho D D. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J Virol. 1996;70:3098–3107. doi: 10.1128/jvi.70.5.3098-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]