Abstract
Platelet-derived growth factor receptor (PDGFR) signaling is essential for normal embryonic development in many organisms, including frog, mouse, zebrafish, and sea urchin. The mode of action of PDGFR signaling during early development is poorly understood, however, mostly because inhibition of signaling through either the PDGFRα or PDGFRβ is embryonic lethal. In Xenopus embryos, disruption of PDGFRα signaling causes migrating anterior mesoderm cells to lose direction and undergo apoptosis through the mitochondrial pathway. To understand the mechanism of PDGFRα function in this process, we have analyzed all known effector-binding sites in vivo. By using a chemical inducer of dimerization to activate chimera PDGFRαs, we have identified a role for phospholipase Cγ (PLCγ) in protecting cells from death. PDGFRα-mediated cell survival requires PLCγ and phosphatidylinositol 3-kinase signaling, and that PDGFRα with binding sites for these two signaling factors is sufficient for this activity. Other effectors of PDGFRα signaling, Shf, SHP-2, and Crk, are not required for this process. Thus, our findings show that PDGFRα signaling through PLCγ and phosphatidylinositol 3-kinase has a protective role in preventing apoptosis in early development. Furthermore, we demonstrate that small molecule inducers of dimerization provide a powerful system to manipulate receptor function in developing embryos.
Keywords: apoptosis, gastrulation, phospholipase Cγ, Xenopus
Platelet-derived growth factor (PDGF) receptor (PDGFR) signaling is required for normal embryogenesis in a variety of organisms, including frog, mouse, zebrafish, and sea urchin (reviewed in ref. 1). The mode of action of PDGFR signaling during development, however, is poorly understood, mostly because disruption of signaling through either PDGFRα or PDGFRβ is embryonic lethal. For example, PDGFRα-null mice die during gestation and exhibit a variety of defects that arise from the failure of mesenchyme cells to migrate or differentiate (2). PDGFRα signaling is also essential for Xenopus development (3–5). In these embryos, PDGFRα and its ligand PDGF-A, come into contact for the first time during gastrulation as mesoderm cells that express the receptor migrate across ectoderm cells that express the ligand. When PDGFRα signaling is blocked with a dominant inhibitory PDGFRα (PDGFR-37) or an antisense PDGFRα morpholino oligonucleotide, this migration is disrupted, and the embryos develop with a variety of gastrulation specific defects, including an open blastopore, reduced anterior structures, and spina bifida (3, 4). These defects arise because the mesoderm cells that express PDGFR-37 are found to accumulate in the blastocoel cavity and die by apoptosis through the mitochondrial pathway (5).
The PDGFRs are receptor tyrosine kinases. Extracellular binding of PDGF stimulates the intrinsic tyrosine kinase activity in the cytoplasmic portion of each subunit of the receptor resulting in transphosphorylation of specific tyrosine residues (1). These phosphotyrosines can then serve as binding sites (pYBs) for intracellular signaling molecules by means of their Src homology 2 domains. The pYBs for PDGFRα and PDGFRβ have been identified and characterized (6). For PDGFRα, pYBs bind effectors, including Src, phosphatidylinositol 3-kinase (PI3K), phospholipase Cγ (PLCγ), the adapter proteins Crk (CrkII and CrkL) and Shf, and the phosphotyrosine phosphatase SHP-2.
Although distinct but overlapping downstream functions of the two PDGFRs have been analyzed in vitro, few studies have attempted to dissect PDGFR signaling in vivo and during embryogenesis (1). One such study in mouse embryos involved the knock-in of a mutant receptor gene back into the wild-type (wt) locus of PDGFRα-null mice. These experiments are difficult and consequently, only two effectors of PDGFRα signaling have been characterized in mouse embryos to date, PI3K and Src (7). PI3K appears to be the primary effector of PDGFRα function because PI3K-null embryos are embryonic lethal and display similar phenotypes to PDGFRα-null mice. In contrast, the role of Src family members, seems to be restricted to oligodentrocyte migration in the central nervous system. These experiments, however, do not address the importance of other PDGFRα effectors, such as PLCγ, which has been implicated to play multiple roles in conjunction with PI3K in tissue culture systems (8, 9). Analysis of PDGFRα signaling in Xenopus embryos also has proved difficult because inhibition of a specific downstream effector by a dominant-negative or antisense morpholino oligonucleotide approach may cause defects in the early embryo that mask the function of that signaling molecule later in development, given that the downstream effectors may be involved in multiple signaling pathways. For example, inhibition of PI3K with a dominant-negative p85 subunit inhibits mesoderm induction; thus, any effect of PI3K in the PDGFR pathway during gastrulation is not apparent (10).
In this study, we screened all known effector pYBs for the PDGFRα in Xenopus embryos with a chemical inducer of dimerization system to activate PDGFRα signaling. We identified a role for PLCγ in PDGFRα signaling. PDGFRα-mediated cell survival requires PLCγ and PI3K signaling, and PDGFRα with pYBs for these two signaling factors is sufficient for this activity. Other effectors of PDGFRα signaling (Shf, SHP-2 and Crk) are not required for this process. These data further show that different downstream effectors can mediate distinct responses to PDGFRα signaling in vivo.
Materials and Methods
Embryos. Xenopus embryos were fertilized in vitro, dejellied in 2% cysteine, pH 7.8, and cultured in 10% 0.1× Marc's modified Ringer's solution (11) at temperatures between 14°C and 23°C as described in ref. 3. Embryos were staged according to Nieuwkoop and Faber (12).
Plasmid Construction, Site-Directed Mutagenesis, and mRNA Synthesis. The mutant inducible PDGFRα (iPDGFRα) plasmids were constructed by PCR amplification and direct subcloning of the cytoplasmic domain of PDGFRαs (from a previously constructed vector 18F) (9) into the wt iPDGFRα-pCS2 vector or by site-directed mutagenesis, as described below. The F720, Y720, Y731/742, F988, Y988, F1018, and Y1018 mutants were made by PCR amplification of the cytoplasmic domain of each mutant PDGFRα. An XbaI restriction site was introduced for ligation into the corresponding site of the wt iPDGFRα-pCS2 plasmid. To generate the F572/4, Y572/4, F731/742, F762, F720/62, and F4 mutants, the iPDGFRα-pCS2 plasmid was subjected to site-directed mutagenesis by using the QuickChange Mutagenesis kit (Stratagene).
Synthetic mRNA transcripts were made by using the mMessage mMachine kit (Ambion, Austin, TX). Dominant negative PDGFR-37 mRNA was made from the T7 promoter of pGHE2-PDGFR-37, and iPDGFRα and β-galactosidase with a nuclear localization signal (β-gal) synthetic mRNA transcripts were synthesized from the Sp6 promoter of the pCS2-iPDGFRαs and the β-gal plasmids, respectively.
Microinjection. Embryo microinjections were carried out in a solution of 3% Ficoll in 1× Marc's modified Ringer's solution (11). Embryos were injected at the 2- to 4-cell stage into the dorsoanterior or lateral marginal zone of each blastomere with the following mRNAs. To determine the role of specific PDGFRα phosphotyrosine binding sites, embryos were injected with 100 pg of PDGFR-37 mRNA and 1 ng of mRNA encoding iPDGFRα-wt or a mutant iPDGFRα. For control experiments, 100 pg of mRNA encoding PDGFR-37 or iPDGFRα was injected. In all experiments, 100 pg to 1 ng of mRNA encoding β-gal was coinjected as a lineage tracer and to equalize the amount of total mRNA introduced into embryos. iPDGFRα receptors were activated at the beginning of gastrulation (stage 10) by microinjection of 5 nl of 10 μM AP1510 (a gift from Ariad Pharmaceuticals, Cambridge, MA) directly into the blastocoel cavity. As a negative control, embryos were similarly injected with 5 nl of DMSO, the solvent for AP1510. The embryos were cultured until the midgastrula stage (stage 11) before being fixed and stained for β-gal activity as described in ref. 3.
Histology. β-gal-stained embryos were embedded in JB-4 plastic (Polysciences) according to the manufacturer's instructions and sectioned saggitally at 5 μm. The sections were mounted and viewed on an Axiovert-35 microscope (Zeiss).
Results
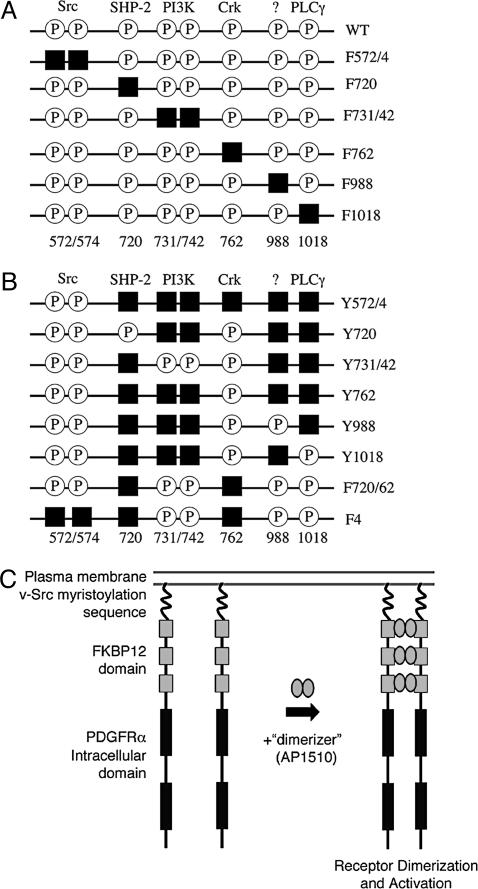
iPDGFRα Activation Restores Mesoderm Cell Survival to PDGFR-Inhibited Embryos. Substitution of specific tyrosine docking sites with phenylalanine selectively uncouples PDGFR signaling from a particular pathway (Fig. 1 A and B) (13). We have used a series of such substituted receptors in a knock-down/knock-in approach to identify which PDGFRα effectors can support mesoderm cell survival during gastrulation. In addition, these receptors were engineered so that they can be activated by chemical dimerization and not ligand binding (Fig. 1C). This technique reduces the interaction of introduced and endogenous PDGFRαs and allows the activation of PDGFRα signaling specifically at the onset of gastrulation (stage 10). Thus, the intracellular portion of a wt or a substituted PDGFRα is fused to three FKBP12 dimerization domains, which, in turn, are fused to the myristoylation signal from v-Src for targeting to the plasma membrane (Fig. 1C) (14). These iPDGFRαs are activated by addition of the synthetic ligand, AP1510 (15). Previous characterization of an iPDGFR-wt showed that it mimics wt PDGFR in Xenopus animal caps (14).
Fig. 1.
Schematic of iPDGFRα mutants. To dissect PDGFRα signaling, tyrosines that when phosphorylated (P) bind and activate specific downstream effectors were replaced by phenylalanine (black squares) by site-directed mutagenesis. (A) Subtraction mutants contain mutations that allow binding and activation of all but one downstream effector. (B) Add-back mutants contain mutations to allow binding and activation of one or more downstream effector. (C) iPDGFRα is a fusion protein of the myristoylation signal from v-Src, three tandem repeats of FKBP12 containing point mutations G89P and I90K to block calcineurin binding, and the cytoplasmic domain of the PDGFRα with or without specific Y→F mutations. The addition of the dimerizer, AP1510, activates the receptor kinase through the induced dimerization of two of the receptor fusion proteins.
Inhibition of endogenous PDGFRα signaling in Xenopus embryos causes the anterior mesoderm cells to die by apoptosis, and these dying cells accumulate in the blastocoel cavity or the vitelline space after being expelled from the embryo (for example, see Fig. 2) (5). To determine which downstream effectors of PDGFRα signaling control their survival in vivo, iPDGFRαs are introduced into Xenopus embryos in which endogenous PDGFRα function has been blocked by using dominant negative PDGFR-37 (see Materials and Methods). At the beginning of gastrulation, iPDGFRαs are activated by using AP1510, and the embryos are later analyzed for the ability of specific iPDGFRαs to restore mesoderm cell survival.
Fig. 2.
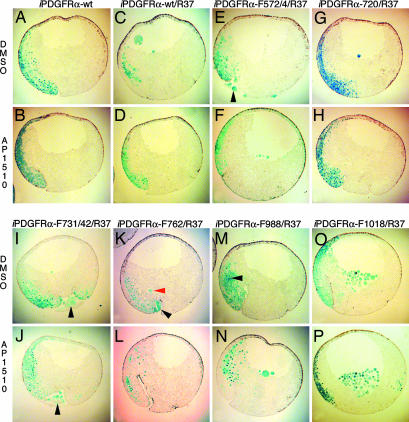
PDGFR signaling through PLCγ and PI3K, but not through SHP-2, Shf, and Crk, is required for mesoderm cell survival. (A and B) Embryos were coinjected with mRNA encoding β-gal and iPDGFRα or β-gal, PDGFR-37 (R37), and the following iPDGFRαs. (C and D) wt. (E and F) F572/74. (G and H) F720. (I and J) F731/42. (K and L) F762. (M and N) F988. (O and P) F1018. At the beginning of gastrulation (stage 10), AP1510 or DMSO was injected into the blastocoel. At the midgastrula stage (stage 11), β-gal expression was visualized (shown in blue). The stained embryos were dissected and scored for the presence or absence of nonnuclear β-gal-stained cells in the blastocoel cavity, within the vitelline membrane, or in the process of being excluded from the embryo (see red arrowhead in K), indicating the presence of apoptotic cells. The percentage of embryos containing apoptotic cells was calculated. Representative saggital sections of these embryos are shown. (G–P) Note that when signaling through PLCγ and PI3K is prevented (I, J, and M–P), activation of the receptor with AP1510 did not restore cell survival, whereas cell survival is restored when signaling through SHP-2, Shf, and Crk is prevented (G, H, K, and L). Arrowheads indicate apoptotic mesoderm cells outside the blastocoel cavity. Note that overexpression of iPDGFRα-wt mRNA alone does not cause apoptosis in mesoderm cells.
First, to validate this knock-down/knock-in approach, embryos were microinjected at the 2-cell stage in the future dorsoanterior mesoderm (16–18) with mRNA encoding PDGFR-37 and iPDGFRα-wt. In addition, mRNA encoding β-gal was included in the injection mix. β-gal mRNA was included for the later identification of apoptotic cells because it has been shown previously that as cells die by apoptosis and the nucleus breaks down, its protein product can be detected throughout the cell (5, 19). At the onset of gastrulation (stage 10), 5 nl of 10 μM AP1510 or an equivalent volume of DMSO was injected into the blastocoel cavity. After 2 h, at the mid-gastrula stage (stage 11), the embryos were fixed and stained for β-gal, and then the presence of cells with nonnuclear β-gal staining in either the blastocoel cavity or within the vitelline membrane was assessed (5).
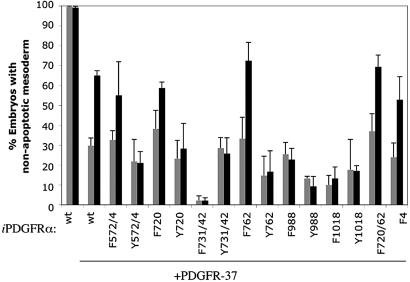
Previous work revealed that inhibition of PDGFRα signaling by injection of PDGFR-37 mRNA results in apoptosis of mesoderm cells in 68% of embryos at stage 11 and that coinjection of wt XPDGFRα mRNA rescues this phenotype to 39% of the embryos (5). A similar rescue is obtained by using the dimerizer system. In the presence of DMSO, 70% of the PDGFR-37/iPDGFRα-wt-injected embryos contained apoptotic cells, whereas addition of AP1510 rescues this to 35%, indicating that iPDGFRα-wt can restore mesoderm cell survival to these embryos to the same extent as XPDGFRα (Figs. 2, 3, 4).
Fig. 3.
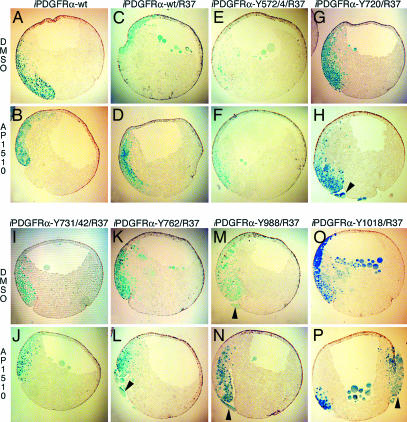
PDGFR signaling through a single downstream effector is not sufficient for mesoderm cell survival. (A and B) Embryos were coinjected with mRNA encoding β-gal and iPDGFRα or β-gal, PDGFR-37 (R37), and the following iPDGFRαs. (C and D) wt. (E and F) Y572/74. (G and H) Y720. (I and J) Y731/42. (K and L) Y762. (M and N) Y988. (O and P) Y1018. At the beginning of gastrulation (stage 10), AP1510 or DMSO was injected into the blastocoel. At the midgastrula stage (stage 11), β-gal expression was visualized (shown in blue). Representative saggital sections of these embryos are shown. (E–P) Note that single effectors do not restore cell survival. Arrowheads indicate apoptotic mesoderm cells outside the blastocoel cavity.
Fig. 4.
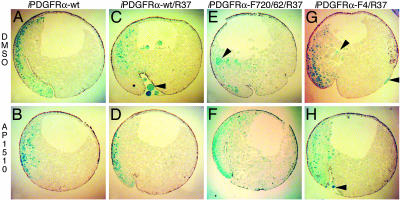
PDGFR signaling through PLCγ and PI3K is required for mesoderm cell survival. (A and B) Embryos were coinjected with mRNA encoding β-gal and iPDGFRα or β-gal, PDGFR-37 (R37), and the following iPDGFRαs. (C and D) wt. (E and F) F720/762. (G and H) F4. At the beginning of gastrulation (stage 10), AP1510 or DMSO was injected into the blastocoel. At the midgastrula stage (stage 11), β-gal expression was visualized (shown in blue). Representative saggital sections of these embryos are shown. As shown in E and F, only the presence of PLCγ, PI3K, and Src pYBs are required to restore mesoderm cell survival.
PDGFRα Signaling Requires Tyrosine Residues at Positions 572/74 for Maximum Restoration of Mesoderm Cell Survival. PDGFR signaling through Src kinase family members has been implicated in a variety of cell processes, including cell migration and cell proliferation (reviewed in ref. 20). The Src pYBs, Y572 and Y574 (21, 22), however, lie within an autoinhibition motif conserved in the PDGFR family that requires tyrosine phosphorylation for full receptor activity (23). Mutation of the equivalent Src pYBs in the PDGFRβ significantly reduces receptor activity (23, 24). PDGFRα function also appears to require Src pYBs. We find that iPDGFRα lacking the Src pYBs, with tyrosine residues 572 and 574 mutated to phenylalanine (iPDGFRα -F572/4), does not restore cell survival to the same extent as iPDGFRα-wt (Fig. 2). Thus, to ensure receptor function in the remaining mutated iPDGFRαs, the Src sites were left intact with one exception (see below).
PDGFRα Signaling Through PLCγ and PI3K but Not SHP-2, Shf, and Crk Promotes Mesoderm Cell Survival. PLCγ, a downstream effector of the PDGFRα, mediates cell migration and proliferation in a variety of cell types (6). Furthermore, PLCγ has also been shown to be required for embryogenesis, because PLCγ1-null mouse embryos die between embryonic days 10.5 and 13.5 (25). PLCγ, however, has not been linked to cell survival downstream of PDGFR signaling. PLCγ has two potential pYBs, Y988 and Y1018. Tyrosine 1018 is known to selectively bind and activate PLCγ (26). Y988 has not been fully characterized; however, there is evidence to suggest that it can also bind and activate PLCγ (26). In our assay, neither the iPDGFRα-F988 nor iPDGFRα-F1018 mutants rescue mesoderm cell death in PDGFR-37 embryos with the addition of AP1510 (Figs. 2 and 5). There is no significant difference between the AP1510-treated and DMSO-treated embryos, with ≈80% for each condition containing apoptotic cells. Taken together, these data suggest that Y1018 and Y988 are required for mesoderm cell survival and further imply that PLCγ is necessary for this process.
Fig. 5.
PDGFR signaling through PLCγ and PI3K is required for mesoderm cell survival. The data presented in Figs. 2, 3, 4 are shown in graph form. The percentage of embryos that do not contain apoptotic cells (i.e., cells with nonnuclear β-gal staining) is presented for embryos injected with mRNA as described in Figs. 2, 3, 4 and with DMSO (gray bars) or AP1510 (black bars). This percentage is low for some mutants compared with wt because there may be some basal activity of the receptor construct without the addition of dimerizer. Error bars represent standard error and were calculated from a minimum of three separate experiments. The data used to construct this graph is available in Table 1, which is published as supporting information on the PNAS web site.
PI3K has been shown to protect cells from apoptosis through the activation of Akt in cultured cells and in vivo (reviewed in ref. 27). To determine whether PI3K signaling downstream of PDGFRα is similarly required in the mesoderm, the PI3K pYB (Y731 and Y742) was mutated to phenylalanine (iPDGFRα-F731/42). Upon activation of iPDGFRα-F731/42 with AP1510, mesoderm cells still die by apoptosis, and there is no significant difference between the percentages of AP1510-treated and DMSO-treated embryos containing apoptotic mesoderm cells, 98% and 98%, respectively (Figs. 2 and 5). These data suggest that, as with PLCγ, PDGFRα signaling through PI3K is necessary for mesoderm cell survival.
In addition to Src kinases, PI3K, and PLCγ, the PDGFRα also binds the protein tyrosine phosphatase SHP-2 (Y720) (28) and the adaptor proteins Shf (Y720) (29) and Crk (Y762) (30). The downstream effects of these proteins have not been fully characterized; however, there is evidence to suggest that SHP-2 may be involved in feedback inhibition of the receptor (31), Crk may be important for cell migration (reviewed in ref. 32), and Shf may play a role in the regulation of apoptosis (29). In our assay, we found that iPDGFRα-F720 and iPDGFRα-F762 can restore mesoderm cell survival to PDGFRα blocked embryos. In all cases, a similar percentage of embryos contain apoptotic cells when iPDGFRα-F720, iPDGFRα-F762, or iPDGFRα-wt is activated (Figs. 2 and 5), suggesting that PDGFRα signaling through SHP-2, Shf, and Crk is not required for mesoderm cell survival.
PDGFRα Signaling Through PLCγ and PI3K Is Necessary and Sufficient for Mesoderm Cell Survival. These data indicate that PLCγ and PI3K play a role in PDGFRα-mediated cell survival. To determine whether these effectors act independently in this process, a series of “add-back” receptors were constructed (Fig. 1B). These receptors contain specific Y→F mutations that isolate pYBs for individual effectors. When used in our assay, none of these receptors could restore mesoderm cell survival (Figs. 3 and 5). No difference in the percentage of embryos with apoptotic cells with DMSO or AP1510 treatment was observed for any given pYB. This suggests that activation of an individual signaling pathway is not sufficient to promote cell survival of these cells and that PLCγ and PI3K are required to mediate this response to PDGFRα signaling.
To test this hypothesis, an iPDGFRα receptor was created in which all but SHP-2, Shf, and Crk pYBs are present (iPDGFRα-F720/62). This receptor restores mesoderm cell survival in our assay to a similar extent as iPDGFRα-wt receptor, supporting our contention that PDGFRα-mediated cell survival requires PLCγ and PI3K signaling and that PDGFRα with pYBs for these two signaling factors is sufficient for this activity but that pYBs for SHP-2, Shf, and Crk are not required (Fig. 4 and 5). Interestingly, iPDGFRα-F4, a receptor lacking pYBs for SHP-2, Shf, Crk, and Src restores mesoderm cell survival to a similar extent as iPDGFRα-F572/4, which only lacks the Src pYB. This finding further suggests that the region containing the Src pYB is required for receptor function, although a role for Src in cell survival cannot be ruled out.
Discussion
By using a series of iPDGFRαs that contain mutations in the intracellular domain that isolate specific effector pathways, we have identified that PDGFRα-mediated cell survival requires PLCγ and PI3K signaling and that PDGFRα with pYBs for these two signaling factors is sufficient for this activity. The other effectors of PDGFRα signaling, SHP-2, Shf, and Crk, are not important for this function of the receptor in these cells. Our data, however, cannot rule out a role for Src family kinases in this process because receptor constructs iPDGFR-F572/4 and iPDGFR-F4, which do not activate Src kinase family members, do not restore cell survival to the same extent as iPDGFRα-wt, although there is some rescue of mesoderm apoptosis in these embryos. It seems unlikely, however, that Src plays a role in PDGFRα-mediated survival of mesoderm cells because the Src family binding site (F572/74) is located in the autoinhibitory motif conserved in the PDGFR family, a region that must be tyrosine-phosphorylated for full receptor activity (23). In addition, substitution of the equivalent tyrosines in the PDGFRβ also reduces receptor function (23, 24, 33). In Xenopus, inhibition of signaling through Src family members does cause gastrulation defects; however, these embryos do not contain apoptotic mesoderm cells as seen in PDGFR-blocked embryos (34).
There is evidence in a number of cell types that PI3K plays a protective role in apoptosis downstream of growth factors (27, 35). In response to PDGFR signaling, PI3K activates the antiapoptotic kinase Akt, which in turn phosphorylates the proapoptotic Bcl-2 family member BAD. Akt phosphorylation of BAD, in conjunction with signaling from protein kinase A and mitogen-activated protein kinase, promotes the formation of an inactivation complex, protecting the cells from apoptosis. This pathway has recently been shown to function in vivo. By using a knock-in strategy, a mutant mouse was created in which BAD cannot be phosphorylated (BAD3SA) (36). Cells cultured from these transgenic mice have a decreased rate of survival even in the presence of PDGF. There is also evidence to suggest that Shf may have a protective role in apoptosis. Overexpression of Shf in mouse fibroblasts prevents serum starvation-induced death, but the role of Shf in this process is unclear (29).
Until now, PLCγ has not been implicated as a survival factor downstream of PDGFRα; however, it has recently been shown to protect developing B cells from apoptosis downstream of the B cell receptor (37, 38). PLCγ2-null mice have a reduced number of marginal zone and follicular B cells that are restored in these tissues by overexpression of Bcl-2 (38). Activation of PLCγ2 results in the up-regulation of Bcl-2 expression, suggesting that PLCγ2 promotes the survival of these cells.
These data indicate that PI3K or PLCγ can protect cells from apoptosis, through the inhibition of proapoptotic factors and the activation of antiapoptotic factors, respectively. When activated by PDGFRα signaling, however, PLCγ is not only regulated by the receptor itself but also by PI3K. PDGFRα phosphorylation of PLCγ and binding of the plekstrin homology-domain of PLCγ to phosphatidylinositol 3,4,5-triphosphate, the product of PI3K, are both required for full activation of its phospholipase activity (39).
In this study, we identified a role for PLCγ in promoting cell survival during embryonic development. We find that PDGFRα pYBs for PLCγ and PI3K are required to protect mesoderm cells from death in early Xenopus embryos. Neither pYB alone can support cell survival. Our previous work showed that the prevention of apoptosis of these cells, however, is not sufficient to restore normal cell motility, although PDGFRα signaling is essential for directed mesoderm cell migration (4, 5). Taken together, these data suggest that distinct but overlapping pathways are required to mediate a mesoderm cell's response to PDGFRα signaling during Xenopus gastrulation. The challenge now is to resolve the functions of other PDGFRα downstream effectors at this time.
Supplementary Material
Acknowledgments
We thank Paul Toselli for help with sectioning, Jian-Xin Yang and Paris Ataliotis for helpful comments on construct design, and Mark Mercola for helpful suggestions and constant encouragement. This work was supported by Massachusetts Department of Public Health Breast Cancer Research Grant DPH34088PP1033 (to K.S.); National Institutes of Health Grants CA87375 (to K.S.), AG00115 (to M.V.S.), and EY012509 (to A.K.); and by the National Institute of General Medical Sciences (S.L.S.).
Author contributions: M.V.S. and K.S. designed research; M.V.S. and K.S. performed research; A.K. and S.L.S. contributed new reagents/analytic tools; M.V.S., A.K., S.L.S., and K.S. analyzed data; and M.V.S. and K.S. wrote the paper.
Abbreviations: PDGF, platelet-derived growth factor; PDGFR, PDGF receptor; iPDGFR, inducible PDGFR; PI3K, phosphatidylinositol 3-kinase; PLCγ, phospholipase Cγ; wt, wild type; β-gal, β-galactosidase with a nuclear localization signal; pYB, phosphotyrosine serving as a binding site.
References
- 1.Hoch, R. V. & Soriano, P. (2003) Development (Cambridge, U.K.) 130, 4769-4784. [DOI] [PubMed] [Google Scholar]
- 2.Soriano, P. (1997) Development (Cambridge, U.K.) 124, 2691-2700. [DOI] [PubMed] [Google Scholar]
- 3.Ataliotis, P., Symes, K., Chou, M. M., Ho, L. & Mercola, M. (1995) Development (Cambridge, U.K.) 121, 3099-3110. [DOI] [PubMed] [Google Scholar]
- 4.Nagel, M., Tahinci, E., Symes, K. & Winklbauer, R. (2004) Development (Cambridge, U.K.) 131, 2727-2736. [DOI] [PubMed] [Google Scholar]
- 5.Van Stry, M., McLaughlin, K. A., Ataliotis, P. & Symes, K. (2004) Dev. Biol. 268, 232-242. [DOI] [PubMed] [Google Scholar]
- 6.Heldin, C. H. & Westermark, B. (1999) Physiol. Rev. 79, 1283-1316. [DOI] [PubMed] [Google Scholar]
- 7.Klinghoffer, R. A., Hamilton, T. G., Hoch, R. & Soriano, P. (2002) Dev. Cell 2, 103-113. [DOI] [PubMed] [Google Scholar]
- 8.Rameh, L. E., Rhee, S. G., Spokes, K., Kazlauskas, A., Cantley, L. C. & Cantley, L. G. (1998) J. Biol. Chem. 273, 23750-23757. [DOI] [PubMed] [Google Scholar]
- 9.Rosenkranz, S., DeMali, K. A., Gelderloos, J. A., Bazenet, C. & Kazlauskas, A. (1999) J. Biol. Chem. 274, 28335-28343. [DOI] [PubMed] [Google Scholar]
- 10.Carballada, R., Yasuo, H. & Lemaire, P. (2001) Development (Cambridge, U.K.) 128, 35-44. [DOI] [PubMed] [Google Scholar]
- 11.Peng, H. B. (1991) in Xenopus laevis: Practical Uses in Cell and Molecular Biology, eds. Kay, B. K. & Peng, H. B. (Academic, San Diego), Vol. 36, pp. 657-662. [PubMed] [Google Scholar]
- 12.Nieuwkoop, P. D. & Faber, J. (1967) Normal Table of Xenopus laevis (Daudin) (North–Holland, Amsterdam).
- 13.Heldin, C. H. & Westermark, B. (1990) Cell Regul. 1, 555-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang, J., Symes, K., Mercola, M. & Schreiber, S. L. (1998) Curr. Biol. 8, 11-18. [DOI] [PubMed] [Google Scholar]
- 15.Amara, J. F., Clackson, T., Rivera, V. M., Guo, T., Keenan, T., Natesan, S., Pollock, R., Yang, W., Courage, N. L., Holt, D. A. & Gilman, M. (1997) Proc. Natl. Acad. Sci. USA 94, 10618-10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lane, M. C. & Sheets, M. D. (2002) Dev. Dyn. 225, 434-447. [DOI] [PubMed] [Google Scholar]
- 17.Dale, L. & Slack, J. M. W. (1987) Development (Cambridge, U.K.) 100, 279-296. [DOI] [PubMed] [Google Scholar]
- 18.Moody, S. A. (1987) Dev. Biol. 119, 560-578. [DOI] [PubMed] [Google Scholar]
- 19.McCall, K. & Steller, H. (1998) Science 279, 230-234. [DOI] [PubMed] [Google Scholar]
- 20.DeMali, K. A., Godwin, S. L., Soltoff, S. P. & Kazlauskas, A. (1999) Exp. Cell Res. 253, 271-279. [DOI] [PubMed] [Google Scholar]
- 21.Gelderloos, J. A., Rosenkranz, S., Bazenet, C. & Kazlauskas, A. (1998) J. Biol. Chem. 273, 5908-5915. [DOI] [PubMed] [Google Scholar]
- 22.Hooshmand-Rad, R., Yokote, K., Heldin, C. H. & Claesson-Welsh, L. (1998) J. Cell Sci. 111, 607-614. [DOI] [PubMed] [Google Scholar]
- 23.Hubbard, S. R. (2004) Nat. Rev. 5, 464-470. [DOI] [PubMed] [Google Scholar]
- 24.Mori, S., Rönnstrand, L., Yokote, K., Engström, Å., Courtneidge, S. A., Claesson-Welsh, L. & Heldin, C.-H. (1993) EMBO J. 12, 2257-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji, Q. S., Winnier, G. E., Niswender, K. D., Horstman, D., Wisdom, R., Magnuson, M. A. & Carpenter, G. (1997) Proc. Natl. Acad. Sci. USA 94, 2999-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eriksson, A., Nanberg, E., Ronnstrand, L., Engstrom, U., Hellman, U., Rupp, E., Carpenter, G., Heldin, C. H. & Claesson-Welsh, L. (1995) J. Biol. Chem. 270, 7773-7781. [DOI] [PubMed] [Google Scholar]
- 27.Franke, T. F., Hornik, C. P., Segev, L., Shostak, G. A. & Sugimoto, C. (2003) Oncogene 22, 8983-8998. [DOI] [PubMed] [Google Scholar]
- 28.Bazenet, C. E., Gelderloos, J. A. & Kazlauskas, A. (1996) Mol. Cell. Biol. 16, 6926-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindholm, C. K., Frantz, J. D., Shoelson, S. E. & Welsh, M. (2000) Biochem. Biophys. Res. Commun. 278, 537-543. [DOI] [PubMed] [Google Scholar]
- 30.Yokote, K., Hellman, U., Ekman, S., Saito, Y., Ronnstrand, L., Heldin, C. H. & Mori, S. (1998) Oncogene 16, 1229-1239. [DOI] [PubMed] [Google Scholar]
- 31.Klinghoffer, R. A. & Kazlauskas, A. (1995) J. Biol. Chem. 270, 22208-22217. [DOI] [PubMed] [Google Scholar]
- 32.Feller, S. M. (2001) Oncogene 20, 6348-6371. [DOI] [PubMed] [Google Scholar]
- 33.Baxter, R. M., Secrist, J. P., Vaillancourt, R. R. & Kazlauskas, A. (1998) J. Biol. Chem. 273, 17050-17055. [DOI] [PubMed] [Google Scholar]
- 34.Denoyelle, M., Valles, A. M., Lentz, D., Thiery, J. P. & Boyer, B. (2001) Differentiation 69, 38-48. [DOI] [PubMed] [Google Scholar]
- 35.Yao, R. & Cooper, G. M. (1995) Science 267, 2003-2006. [DOI] [PubMed] [Google Scholar]
- 36.Datta, S. R., Ranger, A. M., Lin, M. Z., Sturgill, J. F., Ma, Y. C., Cowan, C. W., Dikkes, P., Korsmeyer, S. J. & Greenberg, M. (2002) Dev. Cell 3, 631-643. [DOI] [PubMed] [Google Scholar]
- 37.Wen, R., Chen, Y., Xue, L., Schuman, J., Yang, S., Morris, S. W. & Wang, D. (2003) J. Biol. Chem. 278, 43654-43662. [DOI] [PubMed] [Google Scholar]
- 38.Bell, S. E., Vigorito, E., McAdam, S., Reynolds, H. M., Caraux, A., Colucci, F. & Turner, M. (2004) Eur. J. Immunol. 34, 2237-2247. [DOI] [PubMed] [Google Scholar]
- 39.Katan, M., Rodriguez, R., Matsuda, M., Newbatt, Y. M. & Aherne, G. W. (2003) Adv. Enzyme Regul. 43, 77-85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.