Abstract
Objective
Determine the effectiveness of intradiscal corticosteroid injection (IDCI) for the treatment of discovertebral low back pain.
Design
Systematic review.
Population
Adults with chronic low back pain attributed to disc or vertebral end plate pain, as evidenced by positive provocation discography or Modic 1 or 2 changes on magnetic resonance imaging.
Intervention
Fluoroscopically guided or computed tomography–guided IDCI.
Comparison
Sham/placebo procedure including intradiscal saline, anesthetic, discography alone, or other active treatment.
Outcomes
Reduction in chronic low back pain reported on a visual analog scale or numeric rating scale and reduction in disability reported by a validated scale such as the Oswestry Disability Index.
Methods
Four reviewers independently assessed articles published before January 31, 2023, in Medline, Embase, CENTRAL, and CINAHL. The quality of evidence was evaluated with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework. The risk of bias in randomized trials was evaluated with the Cochrane Risk of Bias tool (version 2).
Results
Of the 7806 unique records screened, 6 randomized controlled trials featuring 603 total participants ultimately met the inclusion criteria. In multiple randomized controlled trials, IDCI was found to reduce pain and disability for 1–6 months in those with Modic 1 and 2 changes but not in those selected by provocation discography.
Conclusion
According to GRADE, there is low-quality evidence that IDCI reduces pain and disability for up to 6 months in individuals with chronic discovertebral low back pain as evidenced by Modic 1 and 2 changes but not in individuals selected by provocation discography.
Study registration
PROSPERO (CRD42021287421).
Keywords: end plate, vertebrogenic, Modic, spine, steroid
Introduction
Low back pain (LBP) is among the most common musculoskeletal conditions and affects almost all individuals at some point in their lives. Chronic low back pain (CLBP) is the leading cause of disability and work nonattendance,1–4 and costs associated with treatment have been estimated to be in excess of 100 billion dollars per year as recently as 2016.5 CLBP is a complex multidimensional condition, and it has been a pursuit of clinicians and researchers to categorize its multiple etiologies better to develop more targeted and effective treatments. Although CLBP can be multifactorial, in a proportion of individuals, it can be attributed to nociception arising from the intervertebral disc.6,7 Historically, discogenic pain was thought to be related to degenerative intervertebral disc changes leading to structural and biomechanical instability, inflammation, and nerve ingrowth.8,9 However, clinical and anatomic evidence indicates that the vertebral end plates contribute to CLBP in patients classically diagnosed with “discogenic” pain.10,11 Nociceptive signals are transmitted from the vertebral end plates by the basivertebral nerve, a branch of the sinuvertebral nerve.12–14
An updated paradigm of anterior element spinal pain describes the intervertebral disc and vertebral end plate as a structural and functional unit known as the discovertebral complex; injuries to either one of these structures can affect the other.15 The vertebral end plate is a porous structure with capillaries that aid in nutrient transport to the avascular intervertebral disc.16,17 Injuries or chronic stresses to the vertebral end plate can lead to an inflammatory response mediated by cytokines and other inflammatory factors when nucleus pulposus migrates through the end plate and into the vertebral body. This inflammatory response gives rise to neovascularization, increased basivertebral nerve termini density, and increased density of nociceptive receptors at these termini, leading to pain sensitization.18 These changes might be observed radiographically as Modic type 1 changes (fibrovascular replacement) or Modic type 2 changes (fatty marrow replacement).19
The current treatment options for discovertebral CLBP are limited. Guidelines for the treatment of axial LBP generally recommend a 6- to 12-week course of conservative treatment, including physical therapy, analgesic medications, acupuncture, and chiropractic treatment, before an interventional paradigm of injections or surgical treatment is considered. For those who do not respond to first-line treatments, interventional treatments that historically have been used include chymopapain injection, intradiscal electrothermal annuloplasty, nucleoplasty, methylene blue injection, ozone injection, and fibrin sealant injections.20–24 Ablation of the basivertebral nerve via a transpedicular approach, though effective for specific patients, requires specialized training and is often performed with the patient under deep sedation or general anesthesia.25–30 Intradiscal injections of disc allograft and allogeneic mesenchymal cells have also shown promising early results.31,32 These more recently developed treatments are characteristically expensive and not yet covered by most payers. Some patients with recalcitrant discovertebral pain might ultimately undergo costly and potentially ineffective spinal fusion surgery. Intradiscal glucocorticoid injection is purported to target local inflammation within the discovertebral complex, thereby reducing pain and associated disability.33–35 Intradiscal corticosteroid injection (IDCI) could provide a relatively affordable and effective treatment strategy in carefully selected patients with discovertebral pain, but a systematic review has not been performed to determine whether adequate evidence supports this practice.
Objectives and rationale
The objective of this systematic review was to identify and evaluate the quality of studies on the efficacy of IDCI for the treatment of chronic discovertebral LBP.
Methods
Protocol and registration
This review was registered on PROSPERO (CRD42021287421) on November 24, 2021. For transparency and reproducibility, this review adheres to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines for reporting of the protocol, searches, and review.36–38 The review was conducted with methodological guidance from the Cochrane Handbook for Systematic Reviews of Interventions (version 6.2).39
Eligibility criteria
Population
The eligible population consisted of adults 18 years of age or older with CLBP attributed to disc or vertebral end plate pain as evidenced by positive provocation discography or by Modic 1 or 2 changes on MRI, respectively. Studies including patients with radicular pain were excluded.
Intervention
Eligible interventions were (1) fluoroscopically–guided or computed tomography (CT)–guided IDCI or (2) adjunctive treatment in addition to IDCI. Peridiscal or epidural administrations of steroids were excluded. In cases in which an adjunctive treatment was provided in addition to IDCI, a random-effects meta-analysis was planned to assess heterogeneity related to the adjunct.
Comparison
Eligible comparisons were sham/placebo procedures including intradiscal saline, anesthetic, provocation discography alone, or other active treatment. The protocol was amended to include active treatments as eligible for inclusion during the literature search, as few sham-controlled trials were identified.
Outcome
Outcomes were reduction in CLBP reported on a visual analog scale (VAS) or numeric rating scale (NRS) and reduction in disability reported by validated scales such as the Oswestry Disability Index (ODI). When the data were available, the percentages of patients reporting at least 50% reduction in index pain and greater than 15-point ODI reduction at short-term (up to 6 months) and medium-term (6 to 12 months) time points were calculated, along with 95% confidence intervals, with a primary endpoint of 3 months.
Studies
This review was designed to evaluate randomized controlled trials (RCTs). Nonrandomized studies with or without an internal control were captured and reported narratively but were not eligible for meta-analysis. Case reports and expert opinion pieces were excluded. Unpublished and non–English-language studies were excluded.
Information sources and search
An information specialist (M.M.M.) developed the search for our primary database, Medline, from exemplar articles and team feedback, and then translated the search to the other databases. A library colleague peer-reviewed search strategies using the Peer Review of Electronic Search Strategies (PRESS) guidelines.40
Clinical outcome studies on the effectiveness of IDCI for the treatment of chronic discovertebral LBP were obtained by searching Medline (Ovid) 1946–2023, Embase (embase.com) 1974–2023, CENTRAL—Cochrane Central Register of Controlled Trials (Wiley) 1898–2023, and CINAHL Complete (Ebscohost) 1937–2023 with a combination of keywords and subject terms. No date limits or methodological filters were applied. Searches were first conducted on December 9–12, 2021, and were updated January 31, 2023. See Supplementary File S1 for search strategies. Literature was also identified from the cited references of included publications. No additional studies or data were sought by contacting authors or experts.
Study selection
Covidence, a Web-based systematic review platform, was used for screening study selection.41 Per Cochrane Handbook and PRISMA guidelines,39,42 the selection of studies for inclusion was performed in tandem by multiple authors (D.C., S.M., M.C., and A.C.) independently and in a blinded fashion through all phases of the review. Disagreements were resolved by consensus or by involvement of an additional reviewer.
Data items and collection
Data extraction was performed in Microsoft Excel by 3 authors (M.C., D.C., S.M.) and checked for accuracy by a fourth author (A.C.). Disagreements were resolved by consensus or by involvement of a fourth reviewer (A.C.). Information related to the population of interest was extracted, including the method used to determine the presence of discovertebral pain. This included clinical evaluation plus either provocation discography or presence of Modic 1 or 2 changes on MRI. Demographic information related to age, gender, and baseline pain and disability was also collected. In terms of the intervention, items collected included the type of imaging guidance, the location of the injection (ie, the level), and the specific injectate. Outcome data collected included VAS score, NRS score, ODI score, and scores of other validated scales assessing back pain–related disability. Study type and details relating to methodology were also recorded.
Risk of bias and methodological assessment
Risk of bias was assessed with the Cochrane Risk of Bias Tool (version 2.0).43 This tool was designed to assess risk of bias within 5 domains related to randomization, deviations from the intended intervention, missing outcome data, measurement of the outcome, and selection of the reported results. Within each domain, a judgment of “low risk of bias,” “some concerns,” or “high risk of bias” was ascertained, which then informed the overall risk-of-bias judgment for the result assessed. Judgments were performed independently in a blinded fashion by 2 reviewers (M.C. and D.C.), with disagreements being resolved by consensus or by involvement of a third reviewer (A.C.).
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system of appraisal was used to evaluate the body of evidence to determine the quality of the evidence for the effectiveness of IDCI.44 The GRADE system evaluates the body of evidence across multiple domains, which include imprecision, inconsistency, indirectness, risk of bias, and publication bias. GRADE allows for an initial rating of quality based on the best available evidence and allows for upgrading (eg, large magnitude of effect, dose–response gradient) or downgrading (eg, risk of bias, indirectness) of the evidence quality. Judgements were performed independently among 3 reviewers (M.C., S.M., and A.C.), with disagreements being resolved by consensus.
Summary measures and synthesis of results
The primary outcome of interest was reduction in CLBP reported on the VAS or NRS and reduction in disability reported by a validated scale such as the ODI. For categorical variables, we considered a greater than 50% reduction in NRS/VAS scores and a greater than 15-point ODI reduction at 3 months to represent robust, clinically significant improvements.45,46 Planned summary measures for binary outcomes included risk difference and relative risk. Planned summary measures for continuous data included calculations of standardized mean differences between groups, along with standard deviations and 95% confidence intervals. If an adequate number of studies was discovered, a GRADE evidence profile was planned and was to be based on GRADE assessment and calculation of treatment effect.44,47,48
Results
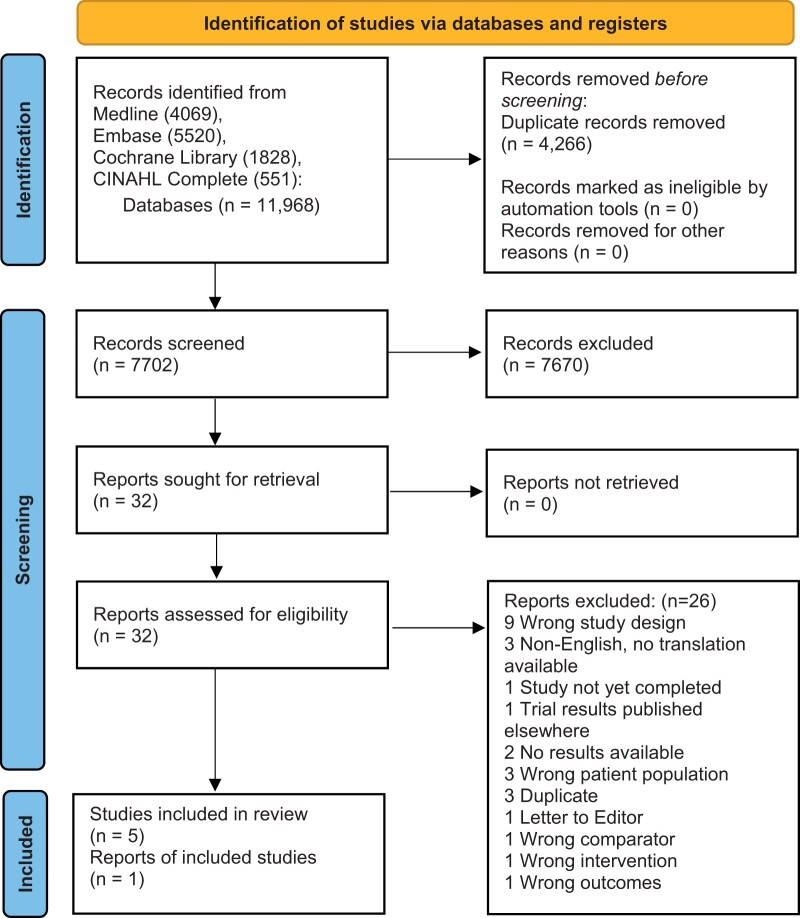
Search results yielded 11 968 records. After removal of duplicates, 7806 were screened by title and abstract, resulting in 32 full-text articles assessed for eligibility. Ultimately, 6 RCTs (total n = 603; n = 319 steroid, n = 284 placebo [saline = 100, contrast alone = 153, contrast+ lidocaine = 22, platelet-rich plasma releasate = 9]) met the inclusion criteria.49–54 See Figure 1 for an overview of the results. Included studies were organized by the study design and characteristics of individual studies and are summarized in Table 1. Given the paucity and heterogeneity of studies, a meta-analysis was not performed.
Figure 1.
PRISMA 2020 flow diagram for new systematic reviews, which included searches of databases and registers only.
From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71
For more information, visit: https://www.prisma-statement.org/.
Table 1.
Study characteristics.
| Reference | Inclusion criteria | Injectate details | Injection imaging and location(s) | Outcome measures | Follow-up | Adverse events | Funding / author disclosures |
|---|---|---|---|---|---|---|---|
| Double-blinded RCTs | |||||||
| Cao et al., 2011 | Age 20–60 years; Modic 1 or 2 on MRI at 1 level; positive discography |
|
CT guided at level of positive discography | 3 and 6 months | Not reported | None | |
| Nguyen et al., 2017 | Age 18–70 years; failure of conservative treatment (analgesics, NSAIDs) or other spinal steroid injections (epidural or facet joint); intradiscal injection ≥6 months before inclusion; Social Security coverage |
|
Fluoroscopically guided at lumbar disc level correlating with Modic 1 changes (no provocative discography used) |
|
1, 3, 6, and 12 months | SAEsd at 12 months: n = 29 (GC IDI group), n = 27 (control group) | Funded by French Ministry of Health |
| Akeda et al., 2022 | Age >18 years; LBP >3 months, VAS >40 mm; ODI score >20% at baseline; painful DDD ≥1 lumbar level from L3–L4 to L5–S1 confirmed by radiographic findings and provocation discography; disc degeneration on MRI (Pfirrmann grade >II); <50% disc height loss; positive provocation discography |
|
Fluoroscopically guided at levels L3–L4 (n = 1 PRPr group; n = 3 CS group), L4–L5 (n = 5 PRPr group; n = 6 CS group), and L5–S1 (n = 5 PRPr group; n = 1 CS group) |
|
4, 8, 12, 16, 20, 34, and 60 weeks after initial injection | Two separate instances of post-injection pain: n = 1 (PRPr group) | Funded by Okasan-Kato Foundation |
| Single-blinded RCTs | |||||||
| Khot et al., 2004 | No age requirements reported; discogenic LBP with DDD on MRI; failure of conservative treatment >6 weeks; concordant pain with positive discography |
|
Fluoroscopically guided at level of positive discography | 12 months | Not reported | None | |
| Tavares et al., 2021 | Age 18–80 years; LBP >6 weeks; Modic 1 changes; conservative treatment failure |
|
Fluoroscopically guided at levels L2–L3 (n = 5), L3–L4 (n = 2), L4–L5 (n = 21), and L5–S1 (n = 22) | 1, 3, and 6 months | Hospitalization for usual care of CLBP: n = 3 (GC group), n = 4 (control group) | Funded by CHU Montpellier and CHU Nimes | |
| Nonblinded RCT | |||||||
| Buttermann, 2004 | Age 18–65 years; conservative treatment failure; LBP >1 year with DDD diagnosis based on combination of clinical examination, medical history, and MRI |
|
Fluoroscopically guided at level(s) with positive discography | 1–3 months, 4–6 months, 7–12 months, 1–2 years | Not reported | None | |
| Retrospective case series | |||||||
| Fayad et al., 2007 | Modic changes on MRI; conservative treatment failure ≥3 months |
|
Fluoroscopically guided at levels L2–L3 (n = 6), L3–L4 (n = 5), L4–L5 (n = 30), and L5–S1 (n = 33) | 1, 3, and 6 months | None | None | |
| Beaudreuil et al., 2012 | Conservative treatment failure; underwent lumbar MRI |
|
Fluoroscopically guided at level(s) with Modic changes or DDD |
|
24 hours, latest follow-up (mean 14 ± 2 months) | Not reported | None |
| Prospective, observational study | |||||||
| Yavuz et al., 2012 | Conservative treatment failure ≥3 months; DDD on MRI; positive provocation discography |
|
Fluoroscopically guided at levels T12–L1 (n = 1), L1–L2 (n = 1), L3–L4 (n = 2), L4–L5 (n = 13), and L5–S1 (n = 3) | 2 weeks, 3 months | None | None | |
Abbreviations: CHU= Centre Hospitalier Universitaire; CLBP= chronic low back pain; CS = corticosteroid; CT= computed tomography; DDD = degenerative disc disease; DPQ = Dallas Pain Questionnaire; GC = glucocorticoid; GC IDI = glucocorticoid intradiscal injection; HADS = Hospital Anxiety and Depression Scale; ISI = intradiscal steroid injection; JOABPEQ = Japanese Orthopaedic Association Back Pain Evaluation Questionnaire; LBP = low back pain; MOS = medical outcomes study; NRS = numeric rating scale; NSAID = nonsteroidal anti-inflammatory drug; ODI = Oswestry Disability Index; PGA = Patient Global Assessment; PRPr = platelet-rich plasma releasate; QBPDS = Quebec Back Pain Disability Scale; RCT= randomized controlled trial; RDQ= Roland-Morris Disability Questionnaire; SAE = serious adverse event; SF-12 = Short Form-12; SF-36 = Short Form-36; VAS = visual analog scale.
None of the included studies reported the prespecified categorical outcome of interest for disability reduction (>15-point reduction on ODI).
Prespecified continuous outcome of interest for pain reduction.
Prespecified continuous outcome of interest for disability reduction.
Prespecified categorical outcome of interest for pain reduction.
SAEs were defined as “any untoward medical occurrence that resulted in death, were life-threatening, required inpatient hospitalization or prolongation of existing hospitalization, or resulted in persistent or clinically significant disability,” excluding infections.50
In addition to the 6 RCTs, we also captured data from 3 observational studies.55–57 Of the 9 total studies, 5 studies (4 RCTs and 1 observational study) evaluated the effectiveness of IDCI for the treatment of chronic discovertebral LBP as evidenced by Modic changes.49,50,52,54,55 The presence of discovertebral pain was determined in the remaining 4 studies by the identification of “disc degeneration” or “degenerative disc disease” (DDD) on MRI with or without provocation discography.51,53,56,57 Injections were performed under fluoroscopic guidance in all but 1 study, which used CT guidance.49 Across all studies, intradiscal steroid injections consisted of glucocorticoids (methylprednisolone51,57; prednisolone50,52,55; betamethasone49,53,54,56), with injectate volumes ranging from 1 mL to 3 mL.
The 6 RCTs compared the effectiveness of intradiscal injection with steroid vs that of a placebo injectate (saline49,51; contrast alone50,54; contrast+ 2% lidocaine52) or an active treatment (platelet-rich plasma releasate [PRPr]53). Among the 3 observational studies, 2 studies had no control group,55,56 and 1 study compared response to IDCI in patients with Modic I changes to that of a control group of patients diagnosed with DDD in the absence of Modic I changes.57 Pain outcomes were assessed with VAS scores in all but 1 study, which used NRS scores.50 Disability was measured with the ODI in 5 studies49,51–54 and with the Quebec Back Pain Disability Scale (QBPDS) in 3 studies.50,54–56 Postprocedural follow-up time points ranged from 24 hours to 2 years.54,57 For the remaining studies, final follow-up visits occurred at 3 months,56 6 months,49,52,55 12 months,50,51,53 and an average of 14 ± 2 months.57
Randomized controlled trials
In 2011, Cao et al. published results of a double-blinded RCT evaluating CT-guided IDCI with betamethasone or IDCI with betamethasone plus Chinese herbal songmeile compared with CT-guided intradiscal saline injection in 120 adults with CLBP.49 Participants were adults 21–60 years of age with CLBP without radicular pain and failure of more than 6 weeks of conservative treatment. Radiographic inclusion criteria were MRI showing DDD, Modic type 1 or 2 changes, and subsequent positive provocation discography at the level of interest. Technical details of discography, such as pressure threshold, were not reported. Participants were initially categorized as having either Modic type 1 (Group A, n = 60) or type 2 (Group B, n = 60) changes. Further subgroup analysis was conducted as follows: Subgroup A1 (saline, n = 20); Subgroup A2 (betamethasone, n = 20); Subgroup A3 (betamethasone+ songmeile, n = 20); Subgroup B1 (saline, n = 20); Subgroup B2 (betamethasone, n = 20); and Subgroup B3 (betamethasone+ songmeile, n = 20). The volume of injectate was 3 mL, but the concentration of betamethasone was not reported. Outcomes of VAS and ODI were collected in each subgroup at 3 and 6 months after the CT-guided injections, with a reported 100% follow-up rate. No outcome data were collected beyond 6 months. Comparisons between the betamethasone and betamethasone+ songmeile treatment protocols revealed no significant differences in average VAS or ODI score improvement at either follow-up time point for patients with Modic type 1 (A2 vs A3) or type 2 (B2 vs B3) changes. As such, we have reported outcomes for the Modic type 1 and type 2 betamethasone subgroups (A2 and B2) only. At both 3 and 6 months, significant improvements in both VAS and ODI scores were noted in the betamethasone subgroups when compared with the saline subgroups. In the saline control group with type 1 Modic changes, average VAS scores did not improve significantly from baseline (7.1 ± 1.6) at either 3 months or 6 months (7.0 ± 1.3 and 7.5 ± 1.1, respectively; P > .05); this pattern was also observed in the saline control group with type 2 Modic changes (6.5 ± 1.2 at baseline vs 6.8 ± 1.0 and 6.4 ± 1.1 at 3 and 6 months, respectively; P > .05). Significant reductions in average VAS scores from baseline occurred in the betamethasone subgroups with type 1 Modic changes (6.5 ± 1.2 at baseline vs 1.8 ± 1.0 and 2.3 ± 1.0 at 3 and 6 months; P < .05) and with type 2 Modic changes (6.8 ± 1.3 at baseline vs 1.6 ± 0.8 and 2.1 ± 1.0 at 3 and 6 months; P < .05). Results for change in disability as reported on ODI mirrored those for VAS pain scores. No improvements from baseline were observed in average ODI scores for the saline control subgroups with Modic type 1 (37.9 ± 14.7 at baseline vs 42.0 ± 13.9 and 44.4 ± 14.0 at 3 and 6 months, respectively; P > .05) or Modic type 2 changes (32.4 ± 9.7 at baseline vs 33.3 ± 10.6 and 33.8 ± 12.0 at 3 and 6 months, respectively; P > .05). Average ODI score improvements from baseline were significant at both follow-up time points for the betamethasone subgroups with type 1 (35.7 ± 11.1 at baseline vs 13.1 ± 2.2 and 14.7 ± 3.2 at 3 and 6 months; P < .05) and type 2 Modic changes (31.5 ± 5.9 at baseline vs 12.7 ± 2.1 and 13.8 ± 2.3 at 3 and 6 months; P < .05). There were no significant differences in these outcomes between those with Modic 1 vs Modic 2 changes at any time point for each injection protocol. Adverse events were not reported. No funding disclosure was provided.
In 2017, Nguyen et al. published results of a multicenter double-blinded RCT comparing fluoroscopically guided IDCI (25 mg prednisolone acetate) with intradiscal iodixanol contrast (control) in 135 adults with CLBP.50 Participants were adults 18–70 years of age referred for management of CLBP with last-48-hour average NRS greater than 40/100 and Modic 1 changes on MRI for less than 6 months. Exclusion criteria were previous disc surgery, current oral steroid treatment, ankylosing spondylitis, sciatica, previous or going infectious spondylodiscitis, fever, and multilevel Modic type 1 changes on MRI. Outcome measures, including NRS and QBPDS, were obtained at 1, 3, 6, and 12 months after the index injection. At 1 month, the mean reduction in LBP intensity from baseline as reported on the NRS (range 0–100) was significantly greater for the IDCI group than for the control group (–32.5 [CI –38.2 to –26.8] vs –17.5 [CI –23.3 to –11.7]; P < .001). At 12 months, however, this between-group difference was no longer significant (–18.2 [95% CI –24.2 to –12.2] for IDCI vs –24.8 [95% CI –30.9 to –18.7] for control; P = .122). Between-group comparisons of mean reduction in disability from baseline as reported on the QBPDS (range 0–100) were not statistically significant at 1-month (–11.9 [95% CI –16.0 to –7.8] for IDCI vs –6.7 [95% CI –10.8 to –2.7] for control; P = .069) or 12-month (–6.9 [95% CI –11.6 to –2.2] for IDCI vs –7.6 [95% CI –12.4 to –2.8] for control; P = .83) follow-up time points. These and additional study outcomes are reported in Table 2. No cases of infection or discitis were reported in either group. One case of sciatica, which was deemed possibly related to the intervention, was reported in the control group. Funding was provided by a research grant from the French Ministry of Health.
Table 2.
Pain and disability improvement outcomes data as reported on NRS/VAS and ODI/QBPDS for individual studies.
| Reference | Outcome measures | Follow-up | Pain reduction outcomes | Disability reduction outcomes |
|---|---|---|---|---|
| Double-blinded RCTs | ||||
| Cao et al., 2011 | VAS, ODI |
|
|
|
| Nguyen et al., 2017 | NRS, QBPDS |
|
|
• No difference in mean QBPDS score change from baseline between GC IDI group and control group at 1 month (–11.9 [95% CI –16.0 to –7.8] vs –6.7 [95% CI –10.8 to –2.7]) or at 12 months (–6.9 [95% CI –11.6 to –2.2] vs –7.6 [95% CI –12.4 to –2.8]). |
| Akeda et al., 2022 | VAS, ODI |
|
|
• No significant differences in ODI score change or % change from baseline between PRPr and CS groups for any follow-up time point. |
| Single-blinded RCTs | ||||
| Khot et al., 2004 | VAS, ODI | • 12 months | • No significant difference between steroid group and control group in median VAS pain score change from baseline at 12-month follow-up (0 [IQR –0.25 to 1] vs 0 [IQR –1 to1]). | • Primary study outcome: No significant difference in mean percentage disability change on ODI from baseline between steroid group and control group at 12-month follow-up (2.28 [SE 2.5] vs 3.4 [SE 1.8]). |
| Tavares et al., 2021 | VAS, ODI |
|
|
• No significant between-group differences in ODI score changes from baseline at 1, 3, or 6 months. |
| Nonblinded RCT | ||||
| Buttermann, 2004 | VAS, ODI |
|
|
|
| Retrospective case series | ||||
| Fayad et al., 2007 | VAS, QBPDS |
|
|
• No significant differences between groups in QBPDS score changes at any follow-up time point. |
| Beaudreuil et al., 2012 | VAS |
|
|
|
| Prospective, observational study | ||||
| Yavuz et al., 2012 | VAS, QBPDS |
|
• Mean VAS spinal pain score decreased significantly from baseline (66.4 ± 13.7) at both 2-week (37.5 ± 17.1) and 3-month (39.2 ± 19.6) follow-up time points. | • Mean QBPDS score improved significantly from baseline (35.1 ± 15.9) at both 2-week (23.7 ± 14.5) and 3-month (24.4 ± 13.8) follow-up time points. |
Abbreviations: CI = confidence interval; CS = corticosteroid; GC IDI = glucocorticoid intradiscal injection; IQR = interquartile range; ISI = intradiscal steroid injection; LBP = low back pain; NRS = numeric rating scale; ODI = Oswestry Disability Index; PRPr = platelet-rich plasma releasate; QBPDS = Quebec Back Pain Disability Scale; RCT= randomized controlled trial; SE = standard error; VAS = visual analog scale.
The primary study endpoint occurred at 8 weeks after the initial injection of either CS or PRPr. At this time point, all study participants were offered an optional PRPr injection. Subsequent time point (12–60 weeks) analyses of outcome data were conducted only for participants who received the optional injection.
In 2004, Khot et al. published results of a single-blinded RCT comparing fluoroscopically guided IDCI (methylprednisolone acetate) with intradiscal saline injection in 120 adults with CLBP.51 Participants were adults with “discogenic” LBP without radicular pain and failure of at least 6 weeks of conservative treatment without previous surgery. Radiographic inclusion criteria were MRI showing DDD and concordant pain on provocation discography of a “degenerative disc.” The presence or absence of Modic changes was not reported. All fluoroscopically guided injections were performed at levels associated with positive provocation discography and were comprised of 1 mL of 40 mg/mL methylprednisolone or 1 mL saline. Outcomes including VAS and ODI were reported but only at the 12-month time point. At 12 months, no significant differences were noted between the 2 groups for either median change in LBP intensity on VAS (0 [IQR –0.25 to 1] for the steroid group vs 0 [IQR –1 to 1] for the saline control group; P = .72) or mean change in disability on the ODI (2.3 [SE 2.5] for the steroid group vs 3.4 [SE 1.8] for the saline control group; P = .71). Adverse events were not reported. The authors reported no funding for support of the study.
In 2021, Tavares et al. published results of a multicenter, single-blinded RCT comparing fluoroscopically guided IDCI (prednisolone acetate) with intradiscal lidocaine injection in 50 adults with CLBP.52 Participants were adults 18–80 years of age with at least 6 weeks of LBP associated with single-level type 1 Modic changes that had not improved with conservative treatment. Those with multilevel Modic changes were excluded. All fluoroscopically guided injections were performed at levels with type 1 Modic changes. Outcomes included VAS, ODI, Dallas Pain Questionnaire, analgesic treatment, and work status at weeks 1–4 and at 3 and 6 months after injection. Collection of patient outcome data was concluded at the 6-month follow-up time point. With regard to the primary outcome, mean change in LBP intensity as reported on VAS was significantly greater at 1 month for the IDCI group than for the lidocaine control group (–2.7 ± 2.3 vs 0.1 ± 2.0; P < .001), but no significant differences were found between the 2 groups at 3 and 6 months (P = .31 and .45, respectively). No between-group differences in disability reduction as reported on the ODI were significant at 1, 3, or 6 months (P = .3, .2, and .4, respectively). There were significant differences in the daily activities of the Dallas Pain Questionnaire in favor of the active group, with the mean “daily activity” subscore improving in the active group by 21.2 and 30.6 points at 1 and 3 months, respectively, compared with 3.3- and 9.4-point improvements in the control group. Adverse events were defined as “hospitalization for usual care of chronic LBP” and reported as 3 in the study group and 4 in the control group. The study was supported by a research grant from Centre Hospitalier Universitaire Montpellier and Centre Hospitalier Universitaire Nimes. It was stated that funders had no role in study design, conduct, or reporting.
In 2022, Akeda et al. published results of a double-blinded RCT comparing fluoroscopically guided IDCI (methylprednisolone [CS]) with autologous PRPr in 16 adults with CLBP, with 15 patients receiving an optional PRPr injection 8 weeks after the first injection.53 Participants were adults 18 years of age or older with CLBP lasting at least 3 months, a VAS >40 mm, ≥1 lumbar disc (L3/4 to L5/S1) with evidence of degeneration on MRI, and ≥1 symptomatic disc, with these levels confirmed by “standard provocative discography.” The primary outcome was changes in VAS from baseline at 8 weeks, with secondary outcomes being pain, disability, quality of life, and safety for up to 60 weeks. At 8 weeks, both the PRPr (–30.9 ± 22.7) and CS (–26.3 ± 29.8) groups’ VAS scores had decreased significantly from baseline (P < .01), with no significant differences between groups. Reductions in disability at 8 weeks as captured by ODI did not differ significantly between the PRPr (–14.5 ± 11.6) and CS groups (–7.7 ± 8.9); however, the study authors did not indicate whether these changes in ODI scores represented significant decreases from baseline values. Additional secondary outcomes, after optional PRPr injection in 15/16 participants at 8 weeks, are shown in Table 2. Post-injection pain was the only adverse event reported during this study. This study was supported by a grant from the Okasan-Kato foundation.
In 2004, Buttermann published results of a nonblinded RCT comparing fluoroscopically guided discography with or without concurrent administration of IDCI (betamethasone) in 171 adults categorized according to the presence (n = 78) or absence (n = 93) of inflammatory end plate (Modic type 1) changes on MRI.54 Participants, who were 18–65 years of age, were part of a larger study population receiving epidural steroid injections to treat CLBP lasting at least 1 year that was refractory to conservative care. All were diagnosed with DDD on the basis of a combination of clinical examination, medical history, and MRI. Patients whose CLBP did not improve with epidural steroid injections were deemed potential spinal fusion surgery candidates and subsequently randomized to provocative discography, either as a standalone procedure or accompanied by simultaneous injection of intradiscal steroid. All injections were performed at levels with concordant pain upon provocation discography. Pain and disability outcomes were assessed with VAS and ODI, respectively, at follow-up time periods of 1–3 months, 4–6 months, 7–12 months, and 1–2 years. Additional outcomes included reductions in total area covered on pain diagrams, use of analgesic medications, and patients’ assessment of whether the injection was successful (yes vs no) at treating their LBP symptoms. No prespecified primary or secondary outcomes were defined. Reporting of study outcomes and measures of statistical significance was limited to figures, with no supporting information provided in tables or the main text with regard to the magnitude or significance levels of changes to patient pain and functioning. Reductions from baseline in both LBP intensity on VAS and disability on ODI were significantly greater at 1–3 months for participants who received IDCI at the time of discography than for those who received discography alone (P < .005), regardless of whether they were identified as having Modic 1 changes at study initiation. However, precipitous study dropout rates by participants in the discography control group precluded statistical comparisons for time periods beyond 3 months. Among participants in the discography+ IDCI treatment group, individuals with Modic 1 changes had significantly greater reductions from baseline in VAS LBP scores at 1–3 months and 4–6 months (P < .05) and ODI disability scores at all follow-up time periods (P < .04) than did participants without Modic 1 changes. Mean VAS LBP and ODI scores were significantly decreased from baseline at all follow-up time periods for participants with Modic 1 changes in the discography+ IDCI group (P < .05), while mean VAS LBP scores for participants without Modic 1 changes showed significant improvements from baseline at 1–3 months and 4–6 months after discography+ IDCI. Adverse events were reported for 6 patients (all were dural punctures during epidural steroid injection), but it is unclear whether these individuals were among the subset of surgical candidates randomized to either of the provocative discography study groups. No study funding was reported.
Observational studies
In 2007, Fayad et al. published results of a single-center, retrospective, observational study of fluoroscopically guided IDCI (prednisolone) in 74 adults with CLBP.55 Participants were adults 32–70 years of age with disabling CLBP with Modic changes on MRI of the lumbar spine, not responding to at least 3 months of conservative treatment. All fluoroscopically guided injections were performed at levels with Modic changes (type I = pure end plate edema, I-2 = mixture of type 1 and type 2 but predominantly edema changes, and II-1 = predominantly fatty changes). The primary outcome was change in LBP intensity, measured by VAS, from baseline to 1 month after injection. Secondary outcomes included change in LBP intensity recorded at 3 and 6 months and change in disability score at 1, 3, and 6 months, as well as the proportion of responders with at least 50% reduction in pain intensity at 1 month and the patient’s global assessment of treatment efficacy at 1 month. Of note, data were available for 93.2% of patients (n = 69) at 1 month and for 81.1% and 75.7% of patients at 3 and 6 months, respectively. The primary outcome of pain intensity decreased from baseline to 1 month by a mean of 30.2 ± 26.6 in the Modic I group, 29.4 ± 21.5 in the Modic I-2 group, and 5.3 ± 25.5 in the Modic II-1 group, with reductions significantly higher in the Modic I and I-2 groups than in the Modic II group (P = .009 and .017). At 1 month, 54.5% of Modic I, 52% of Modic I-2, and 8.3% of Modic II-1 participants had at least 50% reduction in pain. The patient’s global assessment of treatment efficacy was rated as excellent or good in 54.4% of patients (n = 18) in the Modic I group, 32% of patients in the Modic I-2 group, and 10.2% of patients in the Modic II-1 group. The reduction in disability was greater in the Modic I and Modic I-2 groups than in the Modic II-1 group but not with statistical significance. Of note, at 3 and 6 months, both the Modic I and I-2 groups tended to have better results for all outcome measures than did the Modic II-1 group, but the result was not statistically significant. No adverse events of infection or hematoma were reported. Sources of funding were not reported.
In 2012, Yavuz et al. published results of a prospective, single-arm observational study of fluoroscopically guided IDCI (betamethasone) in 18 adults with CLBP and positive provocation discography who had failed to improve after at least 3 months of conservative treatment and who were noted to have DDD findings on MRI.56 Clinical parameters were recorded at baseline, 2 weeks, and subsequently 3 months after treatment and included LBP intensity on VAS, QBPDS, fingertip-to-floor distance, and duration of sitting without pain. Mean VAS scores were found to have decreased significantly from baseline (66.4 ± 13.7) at 2 weeks and 3 months after treatment with IDCI (37.5 ± 17.1 and 39.2 ± 19.6, respectively; P = .001 and .002). Similar decreases in mean patient QBPDS scores from baseline (35.1 ± 15.9) were observed at 2 weeks and 3 months (23.7 ± 14.5 and 24.4 ± 13.8, respectively), again showing statistical significance at both time points (P = .001 and .002). Other secondary outcomes, including the mean fingertip-to-floor distance and mean duration of sitting without pain, showed statistical significance at both the 2-week and 3-month follow-up time points. No adverse events of infection or hematoma were reported. The authors reported no conflicts of interest and reported no funding for the study.
In 2012, Beaudreuil et al. published results of a retrospective study of fluoroscopically guided IDCI (methylprednisolone) in 97 adults with CLBP.57 Participants were adults with severe, disabling CLBP whose disease had not responded to usual conservative treatments and who lacked evidence of systemic inflammatory disorder, metabolic bone disease, local infection, or malignancy, and all had undergone lumbar spine MRI with T1- and T2- weighted sequences. Participants were divided into 3 groups on the basis of MRI evaluations of Modic changes. Individuals with type I Modic changes were categorized by whether they had no history of disc surgery or nucleolysis treatment (Modic I-a) or had undergone one or both of these interventions ≥6 months before IDCI treatment (Modic I-b). A final control group consisted of patients with DDD but no Modic type I changes. Outcome measures including self-assessed improvement (yes vs no) and VAS scores (range 0–100 mm) for back and radiating pain were obtained at 24 hours after the index injection and then subsequently at the latest mean follow-up of 14 ± 2 months. Although both Modic I groups’ VAS scores showed significant decreases in LBP intensity from baseline at 24 hours (Modic I-a, 52.0 ± 5.0 vs 28.0 ± 5.0; Modic I-b, 62.0 ± 4.0 vs 37.0 ± 5.0; P < .05), these improvements were not maintained through long-term follow-up. At a final mean follow-up of 14 ± 2 months, VAS LBP scores did not significantly differ from baseline for any of the 3 groups (P > .05). No discussion of adverse events was reported. The authors reported no conflicts of interest.
GRADE quality assessment
According to GRADE, there is low-quality evidence that IDCI provides short-term reduction in pain and disability in patients with discovertebral CLBP as evidenced by type 1 or type 2 Modic changes at involved segments. Although multiple RCTs have evaluated IDCI in patients with Modic changes, the body of evidence is limited by small study sizes (imprecision), risk of bias (only 1 RCT without concern for risk of bias49), and inconsistency of results. There is low-quality evidence that IDCI is ineffective at reducing pain and disability at 1 year in those with discovertebral pain as evidenced by positive provocation discography in the absence of Modic changes on MRI. There was insufficient evidence to provide a GRADE evidence quality rating for IDCI in those with discovertebral pain as evidenced by positive discography (without Modic changes) at short and intermediate time points; the sole RCT reported outcomes only at 1 year.51 On the basis of a single RCT with high risk of bias and small sample size,53 there is very-low-quality evidence to suggest that PRPr and intradiscal steroid produce similar reductions in pain and disability in those with discovertebral pain selected by positive provocation discography for up to 1 year. Given the paucity of RCTs, a GRADE evidence profile was not constructed. See Supplemental File S2 for results of the risk of bias assessment.
Discussion
Clinicians and researchers have made efforts to comprehend and classify the diverse causes of LBP, recognizing it as an often multifaceted and intricate condition. As the understanding of the underlying causes of CLBP has advanced, there has been a shift in focus toward the development of target-specific treatments. Increasing knowledge of the inflammatory nature of pathological discovertebral degeneration has prompted a number of new studies testing biological agents and a resurgence of interest in IDCI for those with chronic discovertebral pain.16,58,59
The aim of this systematic review was to identify and evaluate the quality of studies examining the effectiveness of IDCI for the treatment of chronic discovertebral LBP as evidenced by provocation discography or Modic type 1 or 2 changes. The review ultimately yielded 6 RCTs (total n = 603; n = 319 steroid, n = 284 placebo [saline = 100, contrast alone = 153, contrast+ lidocaine = 22, platelet-rich plasma releasate = 9]) that met the inclusion/exclusion criteria. The quality of the evidence supporting the use of IDCI for discovertebral LBP was considered “low.” Short-term effectiveness of IDCI based on outcomes reported up to 6 months was found in all included studies other than Khot et al.; however, that study assessed outcomes only at 12-month follow-up and not before that time point.51 Evaluations of effect duration with regard to improvements in both pain and disability ranged from 1 to 6 months, but not thereafter, in all studies. It is possible that the short-term effectiveness of IDCI is secondary to a systemic corticosteroid effect, but intramuscular injection of corticosteroid has been shown to produce clinically significant reductions in pain in only a minority of patients (21%) at 1 month.60
To provide precise and effective treatment, it is imperative to establish an accurate diagnosis of the underlying cause of CLBP. All studies in the present systematic review met the minimum eligibility requirement of selecting patients for IDCI treatment on the basis of Modic changes (type 1 or 2) or positive provocation discography at concordant levels. These diagnostic criteria were often combined with clinical findings, as well as radiographic evidence of DDD, to confirm that a patient’s CLBP was indeed discovertebral in origin. Previous studies have shown DDD in 37% to 96% of asymptomatic individuals, with prevalence increasing with age.61 Further research investigating the complex innervation and signaling pathways in both healthy and injured discovertebral segments could enhance our ability to appropriately provide sustained treatment of DDD by targeting the intervertebral disc only. In the present review, along with clinical suspicion, different imaging parameters were used with mixed prognostic results: IDCI was restricted to patients with Modic changes in 3 of 6 RCTs and 1 of 3 observational studies,49,50,52,55 whereas IDCI administration in the remaining studies was based on “disc degeneration” findings with or without provocation discography.51,53,54,56,57 Four studies (2 RCTs and 2 observational studies) investigated whether Modic changes had predictive value for patient outcomes. Buttermann observed that LBP and disability were significantly reduced in patients with inflammatory end plate changes (Modic type 1) compared with patients without Modic 1 changes.54 Between-group disparities with regard to LBP improvement persisted through 6 months, whereas significant differences in disability reduction were still present at the final study follow-up period of 1–2 years. However, these findings were likely influenced by patients dropping out from the study: Attrition rates at 1- to 2-year follow-up were 68% and 76% among patients with and without Modic 1 changes, respectively. In a retrospective analysis comparing IDCI treatment outcomes in patients with and without Modic 1 changes, Beaudreuil et al. found no significant differences in LBP improvement at the average latest follow-up time of 14 months.57 Cao et al. also observed no statistically significant difference in outcomes between participants with Modic I and II changes49; however, Fayad et al. did show statistically significant improvements in patients with Modic type I changes compared with those with Modic type II changes at all time points assessed.53 These observations warrant further clinical investigation.
As is evidenced in this systematic review, our current understanding of imaging and pain sources in CLBP relies largely on clinical suspicion combined with imaging and potentially provocation discography. Clinical suspicion based on patient history and physical examination is inadequate for diagnosing discogenic pain.62,63 The high prevalence of disc degeneration and disruption in asymptomatic individuals makes advanced imaging a similarly insufficient diagnostic tool.64,65 When performed and interpreted according to current clinical guidelines from the Spine Intervention Society (SIS) / International Association for the Study of Pain (IASP), provocation discography provides superior diagnostic value for discogenic pain with low false positive rates.7 Because no included studies provided necessary technical details (eg, pressure threshold, pain response, etc.) for determining whether discography procedures and subsequent interpretation of results met these standards, we cannot make inferences about the utility of provocation discography for predicting IDCI treatment outcomes. Evidence suggests that surgical outcomes for discectomy and spinal fusion are improved when patient selection criteria include a guideline-concordant positive discography response compared with clinical and imaging findings alone.7 However, the prognostic value of discography for assessing the likelihood that a patient will benefit from IDCI remains undetermined. The limitations of these diagnostic approaches underscore the need for consistent implementation and reporting of protocols when discography is used to confirm suspected discogenic sources of CLBP.
Although IDCI does appear to provide short- to medium-term pain relief, effective and durable treatments are needed in this difficult-to-treat population. A previous systematic review for “regenerative” therapies, including intradiscal platelet-rich plasma and stem cells, suggested mixed results and overall very low-quality evidence.66 Results from the Akeda et al. study were included in the present review.53 This RCT investigated outcomes at 8 weeks for patients who had received either IDCI or PRPr injections. Both the IDCI and PRPr groups showed statistically significant improvement in VAS pain scores from baseline, with no differences between groups. However, all subjects were offered an optional PRPr injection at 8 weeks regardless of their original injection type; 15 out of 16 patients elected to receive the optional injection, making further outcomes analysis of IDCI difficult past that time point.
As discussed, the present systematic review provides evidence of potential short-term pain relief after IDCI. In addition to intradiscal treatments, targeting the intraosseous portion of the basivertebral nerve has emerged as a safe and effective treatment for pain arising from the discovertebral complex.12,13,59,67 In contrast to the short-term relief observed in multiple studies discussed previously, research has shown that an updated technique for the basivertebral nerve ablation procedure might provide long-term effectiveness for vertebrogenic pain, as studied up to 5 years.25,68,69
With IDCI exhibiting short-term effectiveness, consideration and reporting of adverse events are important, and reporting was absent in 2 of 6 included RCTs and 1 of 3 cohort studies. One study suggested adverse events rates of 39% to 43% between groups50; although the authors explicitly stated that none of the adverse events were infections, they provided no further explanation for the study’s unexpectedly high adverse event rate compared with those reported in the literature.70,71
Limitations of the present review and its findings must be acknowledged. Despite the exhaustive search strategy, this review yielded only 6 RCTs and 3 cohort studies of IDCI for the treatment of discovertebral pain. This small number inherently limits our ability to draw firm conclusions. It also is worth mentioning that the steroid injectate differed among RCTs (n = 1 methylprednisolone; n = 2 prednisolone; n = 3 betamethasone), which might have influenced the observed results in these studies.
Conclusion
According to GRADE, there is low-quality evidence that IDCI provides a short-term reduction in pain and disability in those with chronic discovertebral LBP as evidenced by Modic 1 and 2 changes. There is low-quality evidence that IDCI does not provide reduction in pain and disability in those with chronic discovertebral LBP when selected by positive provocation discography alone. IDCI does not appear to be effective beyond 6 months, regardless of selection method.
Supplementary Material
Contributor Information
Scott Miller, Department of Physical Medicine and Rehabilitation, University of Utah, Salt Lake City, UT 84108, United States.
Marc Caragea, Department of Physical Medicine and Rehabilitation, University of Utah, Salt Lake City, UT 84108, United States.
Dan Carson, Department of Physical Medicine and Rehabilitation, University of Utah, Salt Lake City, UT 84108, United States.
Mary M McFarland, Eccles Health Sciences Library, University of Utah, Salt Lake City, UT 84112, United States.
Masaru Teramoto, Department of Physical Medicine and Rehabilitation, University of Utah, Salt Lake City, UT 84108, United States.
Daniel M Cushman, Department of Physical Medicine and Rehabilitation, University of Utah, Salt Lake City, UT 84108, United States.
Amanda N Cooper, Department of Physical Medicine and Rehabilitation, University of Utah, Salt Lake City, UT 84108, United States.
Taylor Burnham, Department of Physical Medicine and Rehabilitation, University of Utah, Salt Lake City, UT 84108, United States.
Zachary L McCormick, Department of Physical Medicine and Rehabilitation, University of Utah, Salt Lake City, UT 84108, United States.
Aaron Conger, Department of Physical Medicine and Rehabilitation, University of Utah, Salt Lake City, UT 84108, United States.
Supplementary material
Supplementary material is available at Pain Medicine online.
Funding
This review was supported by an investigator-initiated research grant from the Skaggs Research Foundation (paid directly to the University of Utah). The sponsor had no role in the design or conduct of the review or in approving the final manuscript. The protocol, search, data extraction, and statistical analysis were developed and performed independently.
Conflict of interest: Z.L.M. serves on the board of directors of the Spine Intervention Society. None of the other authors have any relevant financial conflicts of interest to disclose.
References
- 1. Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64(6):2028-2037. 10.1002/art.34347 [DOI] [PubMed] [Google Scholar]
- 2. Murray CJL, Barber RM, Foreman KJ, et al. ; GBD 2013 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet. 2015;386(10009):2145-2191. 10.1016/S0140-6736(15)61340-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shmagel A, Foley R, Ibrahim H. Epidemiology of chronic low back pain in US adults: data from the 2009-2010 National Health and Nutrition Examination Survey. Arthritis Care Res (Hoboken). 2016;68(11):1688-1694. 10.1002/acr.22890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hartvigsen J, Hancock MJ, Kongsted A, et al. ; Lancet Low Back Pain Series Working Group. What low back pain is and why we need to pay attention. Lancet. 2018;391(10137):2356-2367. 10.1016/S0140-6736(18)30480-X [DOI] [PubMed] [Google Scholar]
- 5. Dieleman JL, Cao J, Chapin A, et al. US health care spending by payer and health condition, 1996-2016. JAMA. 2020;323(9):863-884. 10.1001/jama.2020.0734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DePalma MJ, Ketchum JM, Saullo T. What is the source of chronic low back pain and does age play a role? Pain Med. 2011;12(2):224-233. 10.1111/j.1526-4637.2010.01045.x [DOI] [PubMed] [Google Scholar]
- 7. McCormick ZL, DeFrancesch F, Loomba V, Moradian M, Bathina R, Rappard G. Diagnostic value, prognostic value, and safety of provocation discography. Pain Med. 2018;19(1):3-8. 10.1093/pm/pnx034 [DOI] [PubMed] [Google Scholar]
- 8. Yang G, Liao W, Shen M, Mei H. Insight into neural mechanisms underlying discogenic back pain. J Int Med Res. 2018;46(11):4427-4436. 10.1177/0300060518799902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohtori S, Inoue G, Miyagi M, Takahashi K. Pathomechanisms of discogenic low back pain in humans and animal models. Spine J. 2015;15(6):1347-1355. 10.1016/j.spinee.2013.07.490 [DOI] [PubMed] [Google Scholar]
- 10. Conger A, Smuck M, Truumees E, Lotz JC, DePalma MJ, McCormick ZL. Vertebrogenic pain: a paradigm shift in diagnosis and treatment of axial low back pain. Pain Med. 2022;23(Suppl 2):S63-S71. 10.1093/pm/pnac081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Michalik A, Conger A, Smuck M, Maus TP, McCormick ZL. Intraosseous basivertebral nerve radiofrequency ablation for the treatment of vertebral body endplate low back pain: current evidence and future directions. Pain Med. 2021;22(Suppl 1):S24-S30. 10.1093/pm/pnab117 [DOI] [PubMed] [Google Scholar]
- 12. Fields AJ, Liebenberg EC, Lotz JC. Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. Spine J. 2014;14(3):513-521. 10.1016/j.spinee.2013.06.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Antonacci MD, Mody DR, Heggeness MH. Innervation of the human vertebral body. J Spinal Disord. 1998;11(6):526-531. 10.1097/00002517-199812000-00013 [DOI] [PubMed] [Google Scholar]
- 14. Fras C, Kravetz P, Mody DR, Heggeness MH. Substance P-containing nerves within the human vertebral body. an immunohistochemical study of the basivertebral nerve. Spine J. 2003;3(1):63-67. [DOI] [PubMed] [Google Scholar]
- 15. Dudli S, Sing DC, Hu SS, et al. ISSLS prize in basic science 2017: intervertebral disc/bone marrow cross-talk with Modic changes. Eur Spine J. 2017;26(5):1362-1373. 10.1007/s00586-017-4955-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferguson SJ, Ito K, Nolte LP. Fluid flow and convective transport of solutes within the intervertebral disc. J Biomech. 2004;37(2):213-221. 10.1016/s0021-9290(03)00250-1 [DOI] [PubMed] [Google Scholar]
- 17. Sampson SL, Sylvia M, Fields AJ. Effects of dynamic loading on solute transport through the human cartilage endplate. J Biomech. 2019;83:273-279. 10.1016/j.jbiomech.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dudli S, Fields AJ, Samartzis D, Karppinen J, Lotz JC. Pathobiology of Modic changes. Eur Spine J. 2016;25(11):3723-3734. 10.1007/s00586-016-4459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1 Pt 1):193-199. 10.1148/radiology.166.1.3336678 [DOI] [PubMed] [Google Scholar]
- 20. Kallewaard JW, Wintraecken VM, Geurts JW, et al. A multicenter randomized controlled trial on the efficacy of intradiscal methylene blue injection for chronic discogenic low back pain: the IMBI study. Pain. 2019;160(4):945-953. 10.1097/j.pain.0000000000001475 [DOI] [PubMed] [Google Scholar]
- 21. Levi DS, Horn S, Walko E. Intradiskal methylene blue treatment for diskogenic low back pain. PM R. 2014;6(11):1030-1037. 10.1016/j.pmrj.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 22. Hashemi M, Poorfarokh M, Mohajerani SA, et al. Injection of intradiscal O2-O3 to reduce pain and disability of patients with low back pain due to prolapsed lumbar disk. Anesthesiol Pain Med. 2014;4(5):e19206. 10.5812/aapm.19206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Helm Ii S, Deer TR, Manchikanti L, et al. Effectiveness of thermal annular procedures in treating discogenic low back pain. Pain Phys. 2012;3;15(3;5):E279-E304. [PubMed] [Google Scholar]
- 24. Yin W, Pauza K, Olan WJ, Doerzbacher JF, Thorne KJ. Intradiscal injection of fibrin sealant for the treatment of symptomatic lumbar internal disc disruption: results of a prospective multicenter pilot study with 24-month follow-up. Pain Med. 2014;15(1):16-31. 10.1111/pme.12249 [DOI] [PubMed] [Google Scholar]
- 25. Fischgrund JS, Rhyne A, Franke J, et al. Intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 2-year results from a prospective randomized double-blind sham-controlled multicenter study. Int J Spine Surg. 2019;13(2):110-119. 10.14444/6015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khalil JG, Smuck M, Koreckij T, et al. ; INTRACEPT Trial Investigators. A prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain. Spine J. 2019;19(10):1620-1632. 10.1016/j.spinee.2019.05.598 [DOI] [PubMed] [Google Scholar]
- 27. Conger A, Schuster NM, Cheng DS, et al. The effectiveness of intraosseous basivertebral nerve radiofrequency neurotomy for the treatment of chronic low back pain in patients with Modic changes: a systematic review. Pain Med. 2021;22(5):1039-1054. 10.1093/pm/pnab040 [DOI] [PubMed] [Google Scholar]
- 28. McCormick ZL, Sperry BP, Boody BS, et al. Pain location and exacerbating activities associated with treatment success following basivertebral nerve ablation: an aggregated cohort study of multicenter prospective clinical trial data. Pain Med. 2022;23(Suppl 2):S14-S33. 10.1093/pm/pnac069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boody BS, Sperry BP, Harper K, Macadaeg K, McCormick ZL. The relationship between patient demographic and clinical characteristics and successful treatment outcomes after basivertebral nerve radiofrequency ablation: a pooled cohort study of three prospective clinical trials. Pain Med. 2022;23(Suppl 2):S2-S13. 10.1093/pm/pnac050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCormick ZL, Conger A, Smuck M, et al. Magnetic resonance imaging characteristics associated with treatment success from basivertebral nerve ablation: an aggregated cohort study of multicenter prospective clinical trials data. Pain Med. 2022;23(Suppl 2):S34-S49. 10.1093/pm/pnac093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amirdelfan K, Bae H, McJunkin T, et al. Allogeneic mesenchymal precursor cells treatment for chronic low back pain associated with degenerative disc disease: a prospective randomized, placebo-controlled 36-month study of safety and efficacy. Spine J. 2021;21(2):212-230. 10.1016/j.spinee.2020.10.004 [DOI] [PubMed] [Google Scholar]
- 32. Beall DP, Wilson GL, Bishop R, Tally W. VAST clinical trial: safely supplementing tissue lost to degenerative disc disease. Int J Spine Surg. 2020;14(2):239-253. 10.14444/7033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li JY, Xie W, Strong JA, Guo QL, Zhang JM. Mechanical hypersensitivity, sympathetic sprouting and glial activation are attenuated by local injection of corticosteroid near the lumbar ganglion in a rat model of neuropathic pain. Reg Anesth Pain Med. 2011;36(1):56-62. 10.1097/AAP.0b013e318203087f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ramesh G, Meisner OC, Philipp MT. Anti-inflammatory effects of dexamethasone and meloxicam on Borrelia burgdorferi-induced inflammation in neuronal cultures of dorsal root ganglia and myelinating cells of the peripheral nervous system. J Neuroinflammation. 2015;12(1):240. 10.1186/s12974-015-0461-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johansson A, Hao J, Sjölund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anaesthesiol Scand. 1990;34(5):335-338. 10.1111/j.1399-6576.1990.tb03097.x [DOI] [PubMed] [Google Scholar]
- 36. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moher D, Shamseer L, Clarke M, et al. ; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rethlefsen ML, Kirtley S, Waffenschmidt S, et al. ; PRISMA-S Group. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10(1):39. 10.1186/s13643-020-01542-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2. Cochrane. Updated February 2021. Accessed November 2021. www.training.cochrane.org/handbook.
- 40. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40-46. 10.1016/j.jclinepi.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 41. Innovation VH. Covidence Systematic Review Software. Melbourne, Australia. Published 2017. Accessed December 2021. www.covidence.org.
- 42. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269, W64. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 43. Higgins P, Savovic H, Page M, Sterne J. Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2) short version (CRIBSHEET). RoB 2.o Development Group. Updated August 2019. Accessed August 2023. https://drive.google.com/file/d/1Q4Fk3HCuBRwIDWTGZa5oH11OdR4Gbhdo/view.
- 44. Guyatt GH, Oxman AD, Santesso N, et al. GRADE guidelines: 12. Preparing summary of findings tables—binary outcomes. J Clin Epidemiol. 2013;66(2):158-172. 10.1016/j.jclinepi.2012.01.012 [DOI] [PubMed] [Google Scholar]
- 45. Hung M, Saltzman CL, Kendall R, et al. What are the MCIDs for PROMIS, NDI, and ODI instruments among patients with spinal conditions? Clin Orthop Relat Res. 2018;476(10):2027-2036. 10.1097/CORR.0000000000000419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ostelo RWJG, de Vet HCW. Clinically important outcomes in low back pain. Best Pract Res Clin Rheumatol. 2005;19(4):593-607. 10.1016/j.berh.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 47. Guyatt GH, Thorlund K, Oxman AD, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles—continuous outcomes. J Clin Epidemiol. 2013;66(2):173-183. 10.1016/j.jclinepi.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 48. Smith SM, Dworkin RH, Turk DC, et al. Interpretation of chronic pain clinical trial outcomes: IMMPACT recommended considerations. Pain. 2020;161(11):2446-2461. 10.1097/j.pain.0000000000001952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cao P, Jiang L, Zhuang C, et al. Intradiscal injection therapy for degenerative chronic discogenic low back pain with end plate Modic changes. Spine J. 2011;11(2):100-106. 10.1016/j.spinee.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 50. Nguyen C, Boutron I, Baron G, et al. Intradiscal glucocorticoid injection for patients with chronic low back pain associated with active discopathy: a randomized trial. Ann Intern Med. 2017;166(8):547-556. [DOI] [PubMed] [Google Scholar]
- 51. Khot A, Bowditch M, Powell J, Sharp D. The use of intradiscal steroid therapy for lumbar spinal discogenic pain: a randomized controlled trial. Spine (Phila Pa 1976). 2004;29(8):833-836; discussion 837. 10.1097/00007632-200404150-00002 [DOI] [PubMed] [Google Scholar]
- 52. Tavares I, Thomas E, Cyteval C, et al. Intradiscal glucocorticoids injection in chronic low back pain with active discopathy: a randomized controlled study. Ann Phys Rehabil Med. 2021;64(2):101396. 10.1016/j.rehab.2020.05.003 [DOI] [PubMed] [Google Scholar]
- 53. Akeda K, Ohishi K, Takegami N, et al. Platelet-rich plasma releasate versus corticosteroid for the treatment of discogenic low back pain: a double-blind randomized controlled trial. J Clin Med. 2022;11(2):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Buttermann GR. The effect of spinal steroid injections for degenerative disc disease. Spine J. 2004;4(5):495-505. [DOI] [PubMed] [Google Scholar]
- 55. Fayad F, Lefevre-Colau MM, Rannou F, et al. Relation of inflammatory Modic changes to intradiscal steroid injection outcome in chronic low back pain. Eur Spine J. 2007;16(7):925-931. 10.1007/s00586-006-0301-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yavuz F, Taskaynatan M, Aydemir K, et al. The efficacy of intradiscal steroid injections in degenerative lumbar disc disease. Turk J Phys Med Rehab. 2012;58(2):88-92. [Google Scholar]
- 57. Beaudreuil J, Dieude P, Poiraudeau S, Revel M. Disabling chronic low back pain with Modic type 1 MRI signal: acute reduction in pain with intradiscal corticotherapy. Ann Phys Rehabil Med. 2012;55(3):139-147. [DOI] [PubMed] [Google Scholar]
- 58. Berg-Johansen B, Fields AJ, Liebenberg EC, Li A, Lotz JC. Structure-function relationships at the human spinal disc-vertebra interface. J Orthop Res. 2018;36(1):192-201. 10.1002/jor.23627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brown MF, Hukkanen MVJ, McCarthy ID, et al. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg Br. 1997;79(1):147-153. 10.1302/0301-620X.79B1.6814 [DOI] [PubMed] [Google Scholar]
- 60. Ghahreman A, Ferch R, Bogduk N. The efficacy of transforaminal injection of steroids for the treatment of lumbar radicular pain. Pain Med. 2010;11(8):1149-1168. 10.1111/j.1526-4637.2010.00908.x [DOI] [PubMed] [Google Scholar]
- 61. Brinjikji W, Luetmer P H, Comstock B, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradio. 2015;36(4):811–816. 10.3174/ajnr.A4173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schwarzer AC, Aprill CN, Derby R, et al. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine (Phila Pa 1976). 1995;20(17):1878-1883. [DOI] [PubMed] [Google Scholar]
- 63. Young S, Aprill C, Laslett M. Correlation of clinical examination characteristics with three sources of chronic low back pain. Spine J. 2003;3(6):460-465. [DOI] [PubMed] [Google Scholar]
- 64. Jensen MC, Brant-Zawadzki MN, Obuchowski N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331(2):69-73. [DOI] [PubMed] [Google Scholar]
- 65. Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(3):403-408. [PubMed] [Google Scholar]
- 66. Schneider BJ, Hunt C, Conger A, et al. The effectiveness of intradiscal biologic treatments for discogenic low back pain: a systematic review. Spine J. 2022;22(2):226-237. 10.1016/j.spinee.2021.07.015 [DOI] [PubMed] [Google Scholar]
- 67. Ohtori S, Inoue G, Ito T, et al. Tumor necrosis factor-immunoreactive cells and PGP 9.5-immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back pain and Modic type 1 or type 2 changes on MRI. Spine (Phila Pa 1976). 2006;31(9):1026-1031. 10.1097/01.brs.0000215027.87102.7c [DOI] [PubMed] [Google Scholar]
- 68. Fischgrund JS, Rhyne A, Macadaeg K, et al. Long-term outcomes following intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 5-year treatment arm results from a prospective randomized double-blind sham-controlled multi-center study. Eur Spine J. 2020;29(8):1925-1934. 10.1007/s00586-020-06448-x [DOI] [PubMed] [Google Scholar]
- 69. Koreckij T, Kreiner S, Khalil JG, et al. ; INTRACEPT Trial Investigators. Prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 24-month treatment arm results. N Am Spine Soc J. 2021;8:100089. 10.1016/j.xnsj.2021.100089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Willems PC, Jacobs W, Duinkerke ES, De Kleuver M. Lumbar discography: should we use prophylactic antibiotics? A study of 435 consecutive discograms and a systematic review of the literature. J Spinal Disord Tech. 2004;17(3):243-247. 10.1097/00024720-200406000-00013 [DOI] [PubMed] [Google Scholar]
- 71. Mattie R, Schneider BJ, Miller DC, Popescu A, Smith CC, McCormick ZL. Factfinders for patient safety: antibiotics for disc access and spinal cord stimulation trials. Intervent Pain Med. 2022;1(4):100150. 10.1016/j.inpm.2022.100150 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.