Summary
Agroinfiltration of Nicotiana benthamiana is routinely used in plant science and molecular pharming to transiently express proteins of interest. Here, we discuss four phenomena that should be avoided to improve transient expression. Immune responses can be avoided by depleting immune receptors and employing pathogen‐derived effectors; transcript degradation by using silencing inhibitors or RNA interference machinery mutants; endoplasmic reticulum stress by co‐expressing chaperones; and protein degradation can be avoided with subcellular targeting, protease mutants and co‐expressing protease inhibitors. We summarise the reported increased yields for various recombinant proteins achieved with these approaches and highlight remaining challenges to further improve the efficiency of this versatile protein expression platform.
Keywords: Agrobacterium tumefaciens, agroinfiltration, ER stress, gene silencing, immunity, Nicotiana benthamiana, proteolysis, transient expression
Transient expression by infiltrating Nicotiana benthamiana leaves with Agrobacterium tumefaciens carrying genes of interest (agroinfiltration) is routinely used in plant science and molecular pharming. By transiently expressing genes of interest, one can investigate their roles in various biological processes without the need for stable transformation. Therefore, agroinfiltration facilitates the rapid assessment of gene function in a high‐throughput manner, enabling more efficient functional genomic studies. The ability of agroinfiltrated N. benthamiana to rapidly produce large amounts of foreign proteins also makes it an attractive platform for molecular pharming. This method offers several advantages over other expression systems, such as mammalian cell culture and microbial fermentation. The plant‐based transient expression typically has lower production costs and reduced risk of human pathogen contamination, is scalable, and has the potential for complex protein modifications including glycosylation. However, transient expression in N. benthamiana by agroinfiltration is still a challenge for many proteins, including antibodies and transmembrane glycoproteins, and can be further optimised. Here, we highlight four main processes that one needs to avoid to improve transient protein expression (Fig. 1).
Fig. 1.
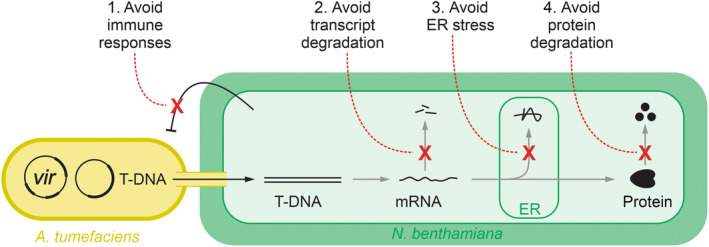
Four phenomena to avoid to improve transient protein expression. Transient expression by infiltrating leaves of Nicotiana benthamiana with Agrobacterium tumefaciens is mediated by the transfer DNA (T‐DNA) that is injected by the bacterium into the plant cell through the type‐IV secretion system. Transient expression can be improved by avoiding immune responses; avoiding transcript degradation (silencing); avoiding endoplasmic reticulum (ER) stress caused by protein accumulation in the endoplasmic reticulum; and by avoiding protein degradation by plant proteases.
Avoiding immune responses
Agroinfiltrated zones of N. benthamiana leaves normally show only weak chlorosis. However, various studies on the transcriptome, proteome, and metabolome revealed that extensive cellular reprogramming is taking place as cell homeostasis is deprioritised whilst immune responses increase (Table 1). Nearly 25% of the transcripts show differential abundance after agroinfiltration (Grosse‐Holz et al., 2018a). Upregulated genes are those involved in pathogen perception, immune signalling, protein folding, oxidative stress, and lignification (Grosse‐Holz et al., 2018a; Hamel et al., 2023b). Downregulated genes include genes for photosynthesis and housekeeping proteins, consistent with the chlorotic response. Also, SWEET family sugar efflux transporters are downregulated (Grosse‐Holz et al., 2018a; Hamel et al., 2023b), perhaps to reduce the viability of microbes in the apoplast (Chen, 2014). Within the extracellular proteome, 70% of the proteins increase in abundance upon agroinfiltration, and several without significant change in transcript abundance, suggesting post‐transcriptional regulation (Grosse‐Holz et al., 2018a,b). This includes genes encoding pathogenesis‐related proteins, cell wall remodelling proteins, molecular chaperones, and several lipases and esterases (Grosse‐Holz et al., 2018a; Hamel et al., 2023b). Metabolomic changes include increased concentrations of phytol and α‐tocopherol, consistent with chlorophyll degradation, and high levels of chlorogenic acid derivates, consistent with lignification (Drapal et al., 2021). Collectively, these transcriptome, proteome, and metabolome changes are outputs of a basal immune response, similar to pattern‐triggered immunity induced by microbe‐associated molecular patterns (Zhang & Zhou, 2010). Consequently, various strategies can be taken to avoid or suppress immune responses upon agroinfiltration of N. benthamiana to improve transformation efficiencies. Avoidance of immune responses can be achieved by depleting immune receptors that recognise Agrobacterium in N. benthamiana. NbCORE, for instance, is an immune receptor that recognises cold shock proteins of Agrobacterium but is expressed only in older N. benthamiana plants (Wang et al., 2016). Depletion of NbCORE with virus‐induced gene silencing caused an eightfold higher transient green fluorescent protein (GFP) expression in older plants (Dodds et al., 2023). Meanwhile, suppression of immune responses has been achieved with bacterial type‐III effector AvrPto, which inhibits immune‐related kinases (Xing et al., 2007). Agrobacterium expressing AvrPto and the type‐III secretion system have increased transformation efficiencies in various plants, including N. benthamiana (Raman et al., 2022). These, and other, approaches to avoid and suppress immune response can significantly increase transient expression efficiencies.
Table 1.
Differential gene expression in Nicotiana benthamiana upon agroinfiltration.
| Function | Transcriptomics | Proteomics | Metabolomics | References |
|---|---|---|---|---|
| Photosynthesis | ↓ | ↓ | ↓ | Grosse‐Holz et al. (2018a); Drapal et al. (2021); Hamel et al. (2023b) |
| Cell wall remodelling (mainly lignification) | ↑ | ↑ | ↑ | Grosse‐Holz et al. (2018a); Drapal et al. (2021); Hamel et al. (2023b) |
| Sugar depletion | ↑ | ↑ | – | Grosse‐Holz et al. (2018a);Drapal et al. (2021); Hamel et al. (2023b) |
| ROS generation | ↑ | ↑ | ↑ | Grosse‐Holz et al. (2018a); Hamel et al. (2023b) |
| Immune perception and signalling | ↑ | ↑ | n/a | Grosse‐Holz et al. (2018a); Hamel et al. (2023b) |
| Proteases and inhibitors | ↑ | ↑ | n/a | Grosse‐Holz et al. (2018a); Hamel et al. (2023b) |
| Lipases and esterases | ↑ | ↑ | n/a | Grosse‐Holz et al. (2018a); Hamel et al. (2023b) |
| Salicylic acid signalling and SAR | ↑ | ↑ | n/a | Grosse‐Holz et al. (2018a); Hamel et al. (2023b) |
| Chaperones and UPR‐related | ↑ | ↑ | n/a | Grosse‐Holz et al. (2018a); Hamel et al. (2023a,b) |
↓, significant decrease in abundance; ↑, significant increase in abundance; –, no significant change; n/a, not assessed; UPR, unfolded protein response.
Avoiding transcript degradation
Transcripts encoded by transfer DNA (T‐DNA) often become unstable through post‐transcriptional gene silencing (PTGS), which usually starts on the third day after agroinfiltration. PTGS is triggered by low levels of antisense RNA generated by random T‐DNA insertion and/or RNA‐dependent RNA polymerase, which results in double‐stranded RNA (dsRNA). dsRNA is a substrate for Dicer to generate small interfering RNAs (siRNAs) that target the degradation of homologous mRNAs. To overcome this, silencing inhibitor P19 of tomato bushy stunt virus is often co‐expressed to suppress PTGS by sequestering siRNAs (Lombardi et al., 2009). But also, various knockout lines with reduced PTGS machinery have been generated. The removal of dicer‐like proteins 2 and 4 in the dcl2dcl4 double mutant of N. benthamiana resulted in higher transient expression levels (Matsuo & Matsumura, 2017; Matsuo, 2022). Likewise, the removal of RNA‐dependent RNA polymerase 6 (rdr6) resulted in higher transient expression levels of GFP than the wild‐type (WT) plants (Matsuo & Atsumi, 2019). However, in a combinatorial study, the dcl2dcl4 plants support higher amounts of transiently expressed GFP and human fibroblast growth factor‐1 than WT and rdr6 plants (Matsuo, 2022). Likewise, other approaches to increase mRNA stability also improve transient expression. Different plant‐derived untranslated regions (UTRs) can increase mRNA stability, even in the presence of P19 (Garabagi et al., 2012). Moreover, the hypertranslatable (HT) vector system incorporates virus‐derived elements to boost transcription and translation, by increasing gene copy number and suppressing PTGS simultaneously (Sainsbury et al., 2009; Peyret & Lomonossoff, 2013).
Avoiding endoplasmic reticulum stress
Transient protein expression of proteins that are targeted to the secretory pathway can lead to endoplasmic reticulum (ER) stress due to the unfolded protein response. In plants, the ER quality control system promotes protein folding and processing of misfolded proteins (Strasser, 2018). Essential to quality control is the presence of folding factors and chaperones in the ER lumen to assist polypeptides in their correct folding. Chaperones include binding protein (BiP), HSP90 family proteins, calnexin, calreticulin, protein disulphide isomerases (PDIs), and peptidyl‐prolyl isomerases (Gupta & Tuteja, 2011). Upregulation of chaperones is an important ER‐stress response, and this often occurs in N. benthamiana upon agroinfiltration, especially when expressing large amounts of secreted proteins from other organisms (Ye et al., 2011; Margolin et al., 2020; Hamel et al., 2023a). ER stress also occurs upon the expression of high levels of membrane proteins, such as viral glycoproteins. For instance, proteomic analysis has confirmed the increased abundance of PDIs, CRT, BiP, and ER‐associated degradation components upon transient expression of a viral glycoprotein and an IgG antibody that trigger ER stress (Hamel et al., 2023a,b). Interestingly, different recombinant proteins may require specific chaperones for folding. Different IgG antibodies, for instance, either accumulate highly without triggering ER stress or accumulate poorly, associated with ER stress (Hamel et al., 2023a). To reduce ER stress upon agroinfiltration, molecular chaperones have been co‐expressed alongside the target product. Human proteins are thought to be better folded by human chaperones than by plant chaperones given the divergence of the latter. Indeed, co‐expression with human calreticulin caused a 13‐fold increase in the transient expression of HIV glycoprotein gp140, whilst avoiding the induction of ER stress marker genes (Margolin et al., 2020). Likewise, co‐expression with human calreticulin caused a threefold increase in the accumulation of the S protein ectodomain (Margolin et al., 2020; Song et al., 2022). The emerging message is that different recombinant proteins might require co‐expression with specific chaperones to alleviate ER stress and increase protein folding and accumulation.
Avoiding proteolysis
Proteolysis is a huge obstacle in transient expression. Many recombinant proteins in agroinfiltrated leaves accumulate initially and then disappear at later timepoints, and sometimes accumulate as shorter fragments, which indicates their degradation by plant proteases. Degradation has been observed for various transmembrane glycoproteins and IgG antibodies and has been studied mostly for specific IgG antibodies. The N. benthamiana genome encodes for c. 1200 putative proteases but not all of these proteases degrade recombinant proteins, as they are organelle‐specific, and many are not expressed or are inactive in agroinfiltrated leaves (Jutras et al., 2020). Most relevant for secreted recombinant proteins are probably papain‐like Cys proteases (PLCPs), subtilisins, and pepsin‐like Asp proteases that are abundant and active in the apoplast (Niemer et al., 2014; Deveuve et al., 2020; Puchol Tarazona et al., 2021). There are three main strategies taken to reduce proteolysis (Table 2). First, co‐expression with protease inhibitors has increased yields of recombinant proteins. Co‐expression with SlCYS8, for instance, caused a threefold increase in the accumulation of full‐length IgG antibody H10 (Jutras et al., 2016). Likewise, co‐expression with NbPR4, NbPot1, or HsTIMP has increased the accumulation of IgG antibody VRC01, glycohormone erythropoietin, and α‐galactosidase (Grosse‐Holz et al., 2018b). Second, potentially harmful proteases can be depleted by silencing or genome editing. Transient depletion of vacuolar processing enzymes and PLCP NbCysP6, for example reduced degradation of anti‐HIV antibody CAP256 (Singh et al., 2022). Finally, proteolysis can be prevented by targeting the protein to different subcellular locations. For instance, targeting proteins to the vacuole or retaining them in the ER has increased yields of transiently expressed 14D9 antibody by 10‐ to 15‐fold (Ocampo et al., 2016). Targeting recombinant proteins to protein bodies might be another way to avoid proteolysis (Schwestka et al., 2023). However, sensitivity to proteolysis very much depends on the recombinant protein itself, and it seems unlikely that a single strategy will avoid proteolysis for all recombinant proteins.
Table 2.
Tackling proteolysis of recombinant proteins in agroinfiltrated Nicotiana benthamiana.
| Approach | Description | Protein and accumulation 1 | Component | References |
|---|---|---|---|---|
| Protease inhibitor | Co‐expression with protease inhibitors | C5‐1 IgG antibody (LC; 70–80%) | SlCDI; SlCYS9 | Goulet et al. (2012) |
| C5‐1 IgG antibody (HC; 85%) | SlCDI | Goulet et al. (2012) | ||
| C5‐1 IgG antibody (40%) | SlCYS8 | Robert et al. (2013) | ||
| H10 IgG antibody (HC; 7.5‐fold) | SlCYS8 | Jutras et al. (2016) | ||
|
α‐Galactosidase (4–14%) Erythropoietin (16‐ to 27‐fold) VRC01 IgG antibody (2‐ to 10‐fold) |
NbPR4, NbPot1 & HsTIMP | Grosse‐Holz et al. (2018b) | ||
| Protease knockdown/out | RNAi and gene editing | CAP256 IgG antibody (−) | NbVPE‐1a, NbVPE‐1b, and NbCysP6 | Singh et al. (2022) |
| Recombinant protein compartmentation | Retention to ER or storage in protein bodies; vacuolar targeting | Fungal xylanase xyn11A (10‐fold) | HFBI | Saberianfar et al. (2015) |
| Erythropoietin (twofold) | ELP | Saberianfar et al. (2015) | ||
| Erythropoietin (twofold) | HFBI | Saberianfar et al. (2015) | ||
| Interleucin‐10 (threefold) | HFBI |
Saberianfar et al. (2015) |
||
| 14D9 IgG antibody (10‐ to 15‐fold) | ER; vacuole | Ocampo et al. (2016) | ||
| pH modulation of the plant secretory pathway | Co‐expression with proton channels | α1‐antichymotrypsin (fivefold) | Influenza M2 ion channel | Jutras et al. (2015, 2018) |
| H3 influenza A (increase; ns) | ||||
| HA influenza B (increase; ns) |
ELP, elastin‐like polypeptide; H, hemagglutinin; HFBI, hydrophobin; VPE, vacuolar processing enzymes.
Approximate accumulation according to authors' statements, − indicates no significant change, n/s indicates not specified.
Future prospects
Although agroinfiltration is already a great platform for protein expression, there are still numerous opportunities ahead of us to further improve this platform. Besides the four discussed areas, there is even more to gain from optimising co‐expression, engineering post‐translational modifications, targeting other subcellular locations, improving the protein extraction process, producing metabolites, and even optimising plant growth and agroinfiltration conditions. Much of these activities require ingenuity and the development and application of new scientific insights. There is an exciting time ahead of us.
Competing interests
None declared.
Author contributions
KB, ECW and RALH conceived the topic and wrote the manuscript together. KB and ECW contributed equally to this work.
Acknowledgements
The authors are financially supported by the BBSRC Interdisciplinary Bioscience DTP projects DDT00230 AP01.01 (KB) and AP01.15 (ECW), and ERC‐2020‐AdG project 101019324 ‘ExtraImmune’ (RALH).
References
- Chen LQ. 2014. SWEET sugar transporters for phloem transport and pathogen nutrition. New Phytologist 201: 1150–1155. [DOI] [PubMed] [Google Scholar]
- Deveuve Q, Lajoie L, Barrault B, Thibault G. 2020. The proteolytic cleavage of therapeutic monoclonal antibody hinge region: more than a matter of subclass. Frontiers in Immunology 11: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds I, Chen C, Buscaill P, van der Hoorn RAL. 2023. Depletion of the NbCORE receptor drastically improves agroinfiltration productivity in older Nicotiana benthamiana plants. Plant Biotechnology Journal 21: 1103–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapal M, Enfissi EMA, Fraser PD. 2021. Metabolic effects of agro‐infiltration on N. benthamiana accessions. Transgenic Research 30: 303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabagi F, Gilbert E, Loos A, Mclean MD, Hall JC. 2012. Utility of the P19 suppressor of gene‐silencing protein for production of therapeutic antibodies in Nicotiana expression hosts. Plant Biotechnology Journal 10: 1118–1128. [DOI] [PubMed] [Google Scholar]
- Goulet C, Khalf M, Sainsbury F, D'Aoust M‐A, Michaud D. 2012. A protease activity‐depleted environment for heterologous proteins migrating towards the leaf cell apoplast. Plant Biotechnology Journal 10: 83–94. [DOI] [PubMed] [Google Scholar]
- Grosse‐Holz F, Kelly S, Blaskowski S, Kaschani F, Kaiser M, van der Hoorn RAL. 2018a. The transcriptome, extracellular proteome and active secretome of agroinfiltrated Nicotiana benthamiana uncover a large, diverse protease repertoire. Plant Biotechnology Journal 16: 1068–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse‐Holz F, Madeira L, Zahid MA, Songer M, Kourelis J, Fesenko M, Ninck S, Kaschani F, Kaiser M, van der Hoorn RAL. 2018b. Three unrelated protease inhibitors enhance accumulation of pharmaceutical recombinant proteins in Nicotiana benthamiana . Plant Biotechnology Journal 16: 1797–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D, Tuteja N. 2011. Chaperones and foldases in endoplasmic reticulum stress signaling in plants. Plant Signaling & Behavior 6: 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel LP, Comeau MA, Tardif R, Poirier‐Gravel F, Paré MÈ, Lavoie PO, Goulet MC, Michaud D, D'Aoust MA. 2023a. Heterologous expression of influenza haemagglutinin leads to early and transient activation of the unfolded protein response in Nicotiana benthamiana . Plant Biotechnology Journal 22: 1146–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel LP, Tardif R, Poirier‐Gravel F, Rasoolizadeh A, Brosseau C, Giroux G, Lucier JF, Goulet MC, Barrada A, Paré MÈ et al. 2023b. Molecular responses of agroinfiltrated Nicotiana benthamiana leaves expressing suppressor of silencing P19 and influenza virus‐like particles. Plant Biotechnology Journal 22: 1078–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras PV, D'Aoust MA, Couture MMJ, Vézina LP, Goulet MC, Michaud D, Sainsbury F. 2015. Modulating secretory pathway pH by proton channel co‐expression can increase recombinant protein stability in plants. Biotechnology Journal 10: 1478–1486. [DOI] [PubMed] [Google Scholar]
- Jutras PV, Dodds I, van der Hoorn RAL. 2020. Proteases of Nicotiana benthamiana: an emerging battle for molecular farming. Current Opinion in Biotechnology 61: 60–65. [DOI] [PubMed] [Google Scholar]
- Jutras PV, Goulet MC, Lavoie PO, D'Aoust MA, Sainsbury F, Michaud D. 2018. Recombinant protein susceptibility to proteolysis in the plant cell secretory pathway is pH‐dependent. Plant Biotechnology Journal 16: 1928–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras PV, Marusic C, Lonoce C, Deflers C, Goulet M‐C, Benvenuto E, Michaud D, Donini M. 2016. An accessory protease inhibitor to increase the yield and quality of a tumour‐targeting mAb in Nicotiana benthamiana leaves. PLoS ONE 11: e0167086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi R, Circelli P, Villani ME, Buriani G, Nardi L, Coppola V, Bianco L, Benvenuto E, Donini M, Marusic C. 2009. High‐level HIV‐1 Nef transient expression in Nicotiana benthamiana using the P19 gene silencing suppressor protein of Artichoke Mottled Crinckle Virus . BMC Biotechnology 9: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin EA, Strasser R, Chapman R, Williamson A‐L, Rybicki EP, Meyers AE. 2020. Engineering the plant secretory pathway for the production of next‐generation pharmaceuticals. Trends in Biotechnology 38: 1034–1044. [DOI] [PubMed] [Google Scholar]
- Matsuo K. 2022. CRISPR/Cas9‐mediated knockout of the DCL2 and DCL4 genes in Nicotiana benthamiana and its productivity of recombinant proteins. Plant Cell Reports 41: 307–317. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Atsumi G. 2019. CRISPR/Cas9‐mediated knockout of the RDR6 gene in Nicotiana benthamiana for efficient transient expression of recombinant proteins. Planta 250: 463–473. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Matsumura T. 2017. Repression of the DCL2 and DCL4 genes in Nicotiana benthamiana plants for the transient expression of recombinant proteins. Journal of Bioscience and Bioengineering 124: 215–220. [DOI] [PubMed] [Google Scholar]
- Niemer M, Mehofer U, Torres Acosta JA, Verdianz M, Henkel T, Loos A, Strasser R, Maresch D, Rademacher T, Steinkellner H et al. 2014. The human anti‐HIV antibodies 2F5, 2G12, and PG9 differ in their susceptibility to proteolytic degradation: down‐regulation of endogenous serine and cysteine proteinase activities could improve antibody production in plant‐based expression platforms. Biotechnology Journal 9: 493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocampo CG, Lareu JF, Marin Viegas VS, Mangano S, Loos A, Steinkellner H, Petruccelli S. 2016. Vacuolar targeting of recombinant antibodies in Nicotiana benthamiana . Plant Biotechnology Journal 14: 2265–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyret H, Lomonossoff GP. 2013. The pEAQ vector series: the easy and quick way to produce recombinant proteins in plants. Plant Molecular Biology 83: 51–58. [DOI] [PubMed] [Google Scholar]
- Puchol Tarazona AA, Maresch D, Grill A, Bakalarz J, Torres Acosta JA, Castilho A, Steinkellner H, Mach L. 2021. Identification of two subtilisin‐like serine proteases engaged in the degradation of recombinant proteins in Nicotiana benthamiana . FEBS Letters 595: 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman V, Rojas CM, Vasudevan B, Dunning K, Kolape J, Oh S, Yun J, Yang L, Li G, Pant BD et al. 2022. Agrobacterium expressing a type III secretion system delivers Pseudomonas effectors into plant cells to enhance transformation. Nature Communications 13: 2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S, Khalf M, Goulet M‐C, D'Aoust M‐A, Sainsbury F, Michaud D. 2013. Protection of recombinant mammalian antibodies from development‐dependent proteolysis in leaves of Nicotiana benthamiana . PLoS ONE 8: e70203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberianfar R, Joensuu JJ, Conley AJ, Menassa R. 2015. Protein body formation in leaves of Nicotiana benthamiana: a concentration‐dependent mechanism influenced by the presence of fusion tags. Plant Biotechnology Journal 13: 927–937. [DOI] [PubMed] [Google Scholar]
- Sainsbury F, Thuenemann EC, Lomonossoff GP. 2009. PEAQ: versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnology Journal 7: 682–693. [DOI] [PubMed] [Google Scholar]
- Schwestka J, Zeh L, Tschofen M, Schubert F, Arcalis E, Esteve‐Gasent M, Pedrazzini E, Vitale A, Stoger E. 2023. Generation of multi‐layered protein bodies in N. benthamiana for the encapsulation of vaccine antigens. Frontiers in Plant Science 14: 1109270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AA, Pillay P, Naicker P, Alexandre K, Malatji K, Mach L, Steinkellner H, Vorster J, Chikwamba R, Tsekoa TL. 2022. Transient proteolysis reduction of Nicotiana benthamiana‐produced CAP256 broadly neutralizing antibodies using CRISPR/Cas9. Frontiers in Plant Science 13: 953654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SJ, Kim H, Jang EY, Jeon H, Diao HP, Khan MRI, Lee MS, Lee YJ, Nam JH, Kim SR et al. 2022. SARS‐CoV‐2 spike trimer vaccine expressed in Nicotiana benthamiana adjuvanted with Alum elicits protective immune responses in mice. Plant Biotechnology Journal 20: 2298–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R. 2018. Protein quality control in the endoplasmic reticulum of plants. Annual Review of Plant Biology 69: 147–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Albert M, Einig E, Fürst U, Krust D, Felix G. 2016. The pattern‐recognition receptor CORE of Solanaceae detects bacterial cold‐shock protein. Nature Plants 2: 16185. [DOI] [PubMed] [Google Scholar]
- Xing W, Zou Y, Liu Q, Liu J, Luo X, Huang Q, Chen S, Zhu L, Bi R, Hao Q et al. 2007. The structural basis for activation of plant immunity by bacterial effector protein AvrPto. Nature 449: 243–247. [DOI] [PubMed] [Google Scholar]
- Ye C, Dickman MB, Whitham SA, Payton M, Verchot J. 2011. The unfolded protein response is triggered by a plant viral movement protein. Plant Physiology 156: 741–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhou JM. 2010. Plant immunity triggered by microbial molecular signatures. Molecular Plant 3: 783–793. [DOI] [PubMed] [Google Scholar]


