Abstract
Background
Emerging resistance to bedaquiline (BDQ) threatens to undermine advances in the treatment of drug-resistant tuberculosis (DRTB). Characterizing serial Mycobacterium tuberculosis (Mtb) isolates collected during BDQ-based treatment can provide insights into the etiologies of BDQ resistance in this important group of DRTB patients.
Methods
We measured mycobacteria growth indicator tube (MGIT)–based BDQ minimum inhibitory concentrations (MICs) of Mtb isolates collected from 195 individuals with no prior BDQ exposure who were receiving BDQ-based treatment for DRTB. We conducted whole-genome sequencing on serial Mtb isolates from all participants who had any isolate with a BDQ MIC >1 collected before or after starting treatment (95 total Mtb isolates from 24 participants).
Results
Sixteen of 24 participants had BDQ-resistant TB (MGIT MIC ≥4 µg/mL) and 8 had BDQ-intermediate infections (MGIT MIC = 2 µg/mL). Participants with pre-existing resistance outnumbered those with resistance acquired during treatment, and 8 of 24 participants had polyclonal infections. BDQ resistance was observed across multiple Mtb strain types and involved a diverse catalog of mmpR5 (Rv0678) mutations, but no mutations in atpE or pepQ. Nine pairs of participants shared genetically similar isolates separated by <5 single nucleotide polymorphisms, concerning for potential transmitted BDQ resistance.
Conclusions
BDQ-resistant TB can arise via multiple, overlapping processes, including transmission of strains with pre-existing resistance. Capturing the within-host diversity of these infections could potentially improve clinical diagnosis, population-level surveillance, and molecular diagnostic test development.
Keywords: tuberculosis, bedaquiline, drug resistance, whole-genome sequencing
Bedaquiline (BDQ) is a critical component in new regimens for drug-resistant tuberculosis. Characterizing serial Mycobacterium tuberculosis isolates collected during BDQ-based treatment identified diverse and dynamic subgroups of BDQ-resistant tuberculosis, including those with treatment-associated and pre-existing resistance.
Bedaquiline (BDQ) has markedly improved clinical outcomes for individuals with drug-resistant tuberculosis (DRTB) [1–3]. Bedaquiline-containing regimens have been standard of care for all patients with rifampin-resistant tuberculosis (TB) in South Africa since 2018 [4], where approximately 50 000 patients have received BDQ to date. Recent national-level surveillance data found baseline BDQ resistance in 3.8% of individuals with rifampin-resistant TB in South Africa, and emerging resistance to this critical new drug could jeopardize future progress in TB care and prevention [5]. Expanding population-level surveillance for BDQ-resistant TB and providing early, accurate diagnosis of BDQ-resistant infections are urgent public health priorities.
Bedaquiline resistance in clinical TB infections is almost entirely attributable to mutations in mmpR5 (Rv0678), which may also confer resistance to clofazimine. Bedaquiline resistance associated with mutations in 2 other genes, pepQ and atpE, are rare outside of in vitro experiments [6]. A diverse collection of mmpR5 mutations, involving more than 200 different loci across the gene, have been reported in clinical isolates [7]. In addition, mmpR5 mutations are associated with a wide range of BDQ-resistance phenotypes, and some mmpR5 mutations do not appear to confer BDQ resistance at all [7–9]. Continued uncertainty about how these mutations influence phenotypic BDQ susceptibility poses an important problem for the development of rapid genotypic tests for BDQ resistance.
Mycobacterium tuberculosis (Mtb) bacterial populations in individual hosts are more diverse and dynamic than previously understood [10, 11], posing challenges for the scientific and public health response to DRTB. Difficulty capturing diverse Mtb subpopulations [12, 13], including those with divergent drug-resistance phenotypes, is an important and enduring problem in the clinical management of DRTB [14]. Failure to detect these cryptic Mtb subpopulations can also result in misclassification of patients or research participants by resistance phenotype, with important implications for public health surveillance, clinical research, and molecular diagnostics for DRTB.
More comprehensive characterization of individual Mtb infections, using serial Mtb isolates collected before and during DRTB treatment, has yielded important insights into the diversity and within-host evolution of Mtb [15, 16]. Joint analysis of phenotypic drug susceptibility testing (DST) and whole-genome sequencing (WGS) data from serial Mtb isolates can differentiate infections with treatment-emergent resistance from infections involving strains with pre-existing (aka “primary”) resistance. Whole-genome sequencing also allows for the identification of polyclonal infections, which can involve different Mtb strains with divergent susceptibility phenotypes; without WGS, it is impossible to clearly differentiate this subset of polyclonal infections from monoclonal infections with treatment-emergent resistance. Last, the number of single nucleotide polymorphism (SNP) differences between isolates, derived from WGS data, provides an important quantitative measure of Mtb within-host genetic diversity and between-host genetic similarity.
This study used WGS and BDQ minimum inhibitory concentration (MIC) testing to characterize serial Mtb isolates from a sample of participants with DRTB who had evidence of decreased BDQ susceptibility while receiving BDQ-based treatment. Using this approach, we describe how infection with pre-existing resistant strains, within-host evolution, and polyclonal infection shape the dynamics of BDQ-resistant TB at the individual level.
METHODS
Study Population and Design
Pharmacokinetics, Resistance, and Outcomes of Bedaquiline in MDR and XDR-TB (PROBeX) was an observational longitudinal cohort study conducted at 3 TB referral hospitals in Gqeberha, Cape Town, and Durban, South Africa, between 2016 and 2018 [3]. PROBeX recruited 195 adult participants with culture-confirmed extensively drug-resistant (XDR) or pre-XDR TB, as defined at the time of the study [17], who received treatment with a standardized BDQ-containing regimen. The study was approved by the institutional review boards at Albert Einstein College of Medicine, the University of Cape Town, Columbia University, and Emory University. Additional details on the PROBeX parent study [3] are included in the Supplementary Methods.
Bedaquiline Minimum Inhibitory Concentration Measurements
We measured BDQ MIC values (0.125–8 µg/mL) of Mtb isolates using the BACTEC mycobacteria growth indicator tube (MGIT) 960 DST method [9] (Supplementary Methods). We performed repeat BDQ MIC measurements for any isolate with an initial MIC of 1 µg/mL or greater and, if repeat measurements were discrepant, we used the larger of the 2 MIC values. We defined BDQ-susceptible strains as those with BDQ MICs of 1 µg/mL or less (MGIT), intermediate strains as those with BDQ MICs = 2 µg/mL, and resistant strains as those with BDQ MICs of 4 µg/mL or greater [9]. (Note that both resistant and intermediate strains, as defined here, had MGIT-based MICs above the World Health Organization [WHO]–recommended critical concentration of 1 for BDQ in Mtb [18].)
Genetic Sequencing and Whole-Genome Sequencing–Based Strain Characterization
We conducted WGS for all available isolates from all participants who had at least 1 Mtb isolate with BDQ MIC greater than 1 µg/mL (n = 98). We used the Illumina NextSeq platform with read lengths of 150 bp and coverage at greater than 100× using the Nextera DNA Flex Library Preparation kit. Additional information on WGS data processing, sequence quality metrics, and WGS data availability is provided in the Supplementary Methods.
We conducted targeted Sanger sequencing to confirm mmpR5 genotypes obtained via WGS, as described in the Supplementary Methods. We accepted mmpR5 minority variants identified via WGS if they met the following criteria: (1) the proportion of all reads for a given minority variant was greater than 0.2 or (2) the minority variant was identified in another sample from the same participant. Supplementary Table 1 compares the WGS-based and Sanger-based mmpR5 genotypes for isolates in this study.
We determined lineages for each isolate using fast-lineage-caller v1.0 (https://github.com/farhat-lab/fast-lineage-caller) and the SNP-based lineage classification scheme developed by Coll et al [19]. We used Mykrobe [20] and the WHO TB drug-resistance mutations catalog [21] to generate genotypic resistance profiles to other non-BDQ TB drugs. We calculated the number of SNP differences for all between-participant and within-participant isolate pairs and constructed a phylogenetic tree using maximum-likelihood methods (Supplementary Methods). The WGS data identified 3 isolates with apparent admixture, involving multiple distinct strains within the same sample (Supplementary Table 2 and Supplementary Figure 1), which were excluded from phylogenetic analyses.
Identification of Acquired Versus Pre-existing Bedaquiline Resistance
We used clinical information on each participant, combined with BDQ MIC values and WGS-based strain characterization, to delineate whether participants had pre-existing versus treatment-emergent resistance; we also determined whether these infections were polyclonal—that is, those involving multiple, distinct Mtb strains.
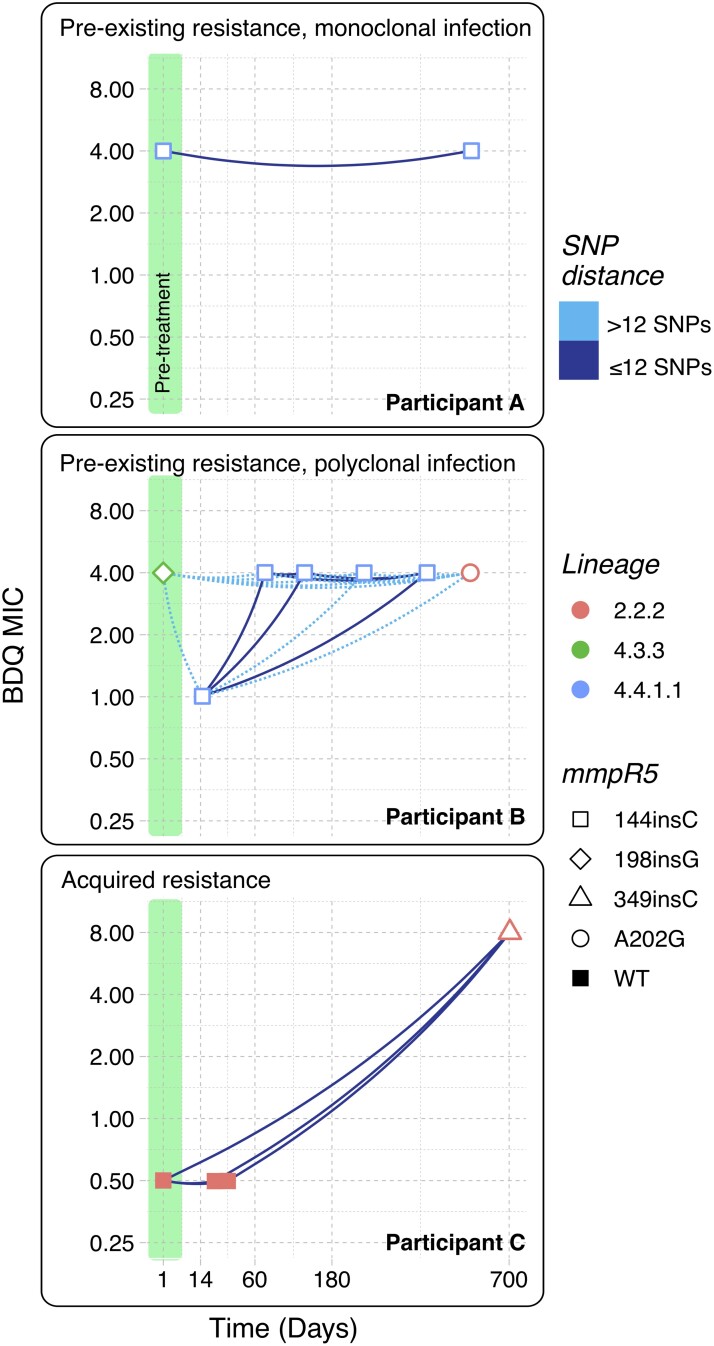
We defined participants with pre-existing BDQ resistance as those who had any pretreatment isolate with BDQ MIC greater than 1 µg/mL. We defined participants as having treatment-emergent BDQ resistance if all pretreatment isolates had MICs less than 2 and any subsequent isolate (after starting treatment) had MICs of 2 or greater. Treatment-emergent resistance, as defined, can be due to either monoclonal infections that acquire resistance de novo or polyclonal infections involving multiple strains with different BDQ MICs. To differentiate between these 2 situations, we further defined acquired resistance as the subset of cases with treatment-emergent resistance that were not polyclonal. Polyclonal infections were defined as participants who had isolates from multiple phylogeographic lineages or different isolates from the same lineage that were separated by more than 12 SNP differences. Last, we defined acquired BDQ resistance as mmpR5-mediated if pretreatment, susceptible isolates were mmpR5 wild-type and resistant isolates collected later were mmpR5 mutants. Figure 1 provides individual participant examples to illustrate these definitions.
Figure 1.
Pre-existing resistance, polyclonal infection, and acquired resistance identified using WGS characterization of serial Mtb isolates. Each panel shows BDQ MICs over time for an individual participant (participants A–C). Each timepoint represents a single isolate, colored by phylogeographic lineage. The shape of each point shows the mmpR5 genotype of each isolate. Lines between each point are colored by the SNP difference between each isolate pair. Isolates collected prior to treatment initiation are highlighted in green. The x-axis (time in days) is square root transformed to better display isolates collected early during treatment. Abbreviations: BDQ, bedaquiline; MIC, minimum inhibitory concentration; Mtb, Mycobacterium tuberculosis; SNP, single nucleotide polymorphism; WGS, whole-genome sequencing; WT, wild-type.
Genomically Linked Isolates
We used SNP differences, calculated from high-quality WGS-derived SNPs, to identify genomically linked isolate pairs. We applied 2 thresholds to classify isolates as genomically linked: fewer than 5 SNP differences [22] and a more conservative threshold of fewer than 2 SNP differences.
RESULTS
Pre-existing and Acquired Bedaquiline Resistance
We obtained Mtb BDQ MIC data for 147 of 195 participants in the PROBeX study, of whom 24 had at least 1 isolate with a BDQ MIC greater than 1. Sixteen of these participants (10.9% of 147) had at least 1 Mtb isolate with BDQ resistance (MICs ≥4) collected at any time during treatment. There were no significant differences in the demographic characteristics of these 24 participants compared with those without any BDQ-resistant isolates (Supplementary Table 3). The remaining 8 participants had at least 1 isolate with intermediate BDQ susceptibility (MIC = 2). Participants with BDQ-resistant or -intermediate strains were less likely to achieve clinical cure compared with participants with only BDQ-susceptible isolates, but this difference was not statistically significant (odds ratio: 0.87; 95% confidence interval: .36–2.11) (Supplementary Table 4).
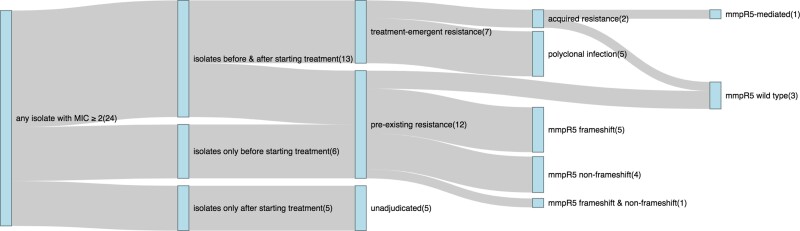
Figure 2 summarizes BDQ DST results for the 24 participants who had any isolate with a BDQ MIC greater than 1 µg/mL. Nineteen of these participants had multiple Mtb isolates collected during the study; 5 had only a single isolate. Thirteen participants had isolates collected both before and after treatment initiation.
Figure 2.
Mtb isolate collection and BDQ MIC results for 24 participants who had any isolate with a BDQ MIC ≥2. Counts for the number of participants at each node are shown in parentheses. Plot generated using the networkD3 package in R [23, 24]. Abbreviations: BDQ, bedaquiline; MIC, minimum inhibitory concentration; Mtb, Mycobacterium tuberculosis.
Taken together, 12 of 147 participants with available BDQ MIC data (8.2%) in the PROBeX study had pre-existing BDQ resistance. Only 1 participant with pre-existing resistance had prior clofazimine exposure. Seven of 147 participants (4.8%) had evidence of treatment-emergent BDQ resistance, of which 5 were polyclonal infections and 2 met criteria for acquired resistance. One case of acquired BDQ resistance was mmpR5-mediated; the second had no evidence of any mutations in mmpR5, atpE, or pepQ. Eight of 24 participants had polyclonal infections involving strains from multiple Mtb lineages or genetically distinct strains from the same lineage (ie, those separated by >12 SNP differences). One participant with polyclonal treatment-emergent resistance had isolates with a genetic admixture of multiple strains (Supplementary Table 2). Five participants did not have pretreatment isolates collected, such that we were unable to adjudicate whether these participants had pre-existing or treatment-emergent BDQ resistance. We identified 4 participants with widely different BDQ MICs (≥4-fold difference) for highly related isolates from the same lineage that were collected within relatively short time spans (<56 days apart) and had no interval changes in mmpR5 genotypes (Supplementary Figure 2).
Genomically Linked Isolate Pairs
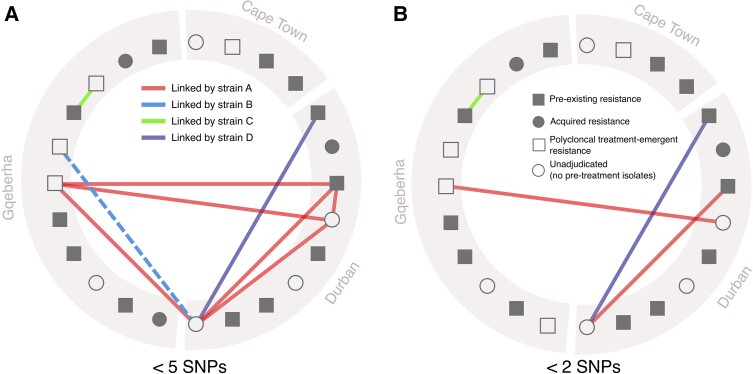
Supplementary Figure 3 shows the distributions of pairwise SNP distances for all within-participant and between-participant Mtb isolate pairs in our study sample. Using between-participant pairwise SNP distances, we identified 9 participant pairs that shared genomically linked isolates differing by less than 5 SNPs (Figure 3); we identified 4 participant pairs sharing genomically linked isolates when the less-than-2 SNP threshold was applied.
Figure 3.
Genomically linked Mtb isolate pairs shared between participants. A, Participant pairs linked by highly related Mtb isolates differing by <5 SNPs. Symbols denote whether each participant had pre-existing or treatment-emergent resistance, no categorically intermediate or resistant strains (all with MIC ≤1), or no pretreatment isolates available (such that pre-existing vs treatment-associated resistance could not be adjudicated). Lines between symbols denote participants linked by closely related isolates (differentiated by <5 SNPs or <2 SNPs in panel B) from 1 of 4 strains—strain A: BDQ-resistant, lineage 4.4.1, mmpR5 144insC (E49fs); strain B: BDQ-resistant, lineage 2.2.2, mmpR5 A202G; strain C: BDQ-susceptible, lineage 2.2.2, mmpR5 WT; strain D: BDQ-resistant, lineage 4.3.3, mmpR5 198insC. Figure generated using the igraph package in R [25]. Abbreviations: BDQ, bedaquiline; MIC, minimum inhibitory concentration; Mtb, Mycobacterium tuberculosis; SNP, single nucleotide polymorphism; WT, wild-type.
Using the less-than-5 SNP threshold, we found 4 specific strains (strains A, B, C, and D in Figure 3) involved in genetically similar between-participant isolate pairs. Strain A, a BDQ-resistant lineage 4.4.1 strain with a frameshift mmpR5 mutation (144insC [E49fs]), was involved in a genomically linked cluster of 4 participants recruited at both the Durban and Gqeberha study sites (Figure 3A). The 3 other strains (strains B, C, and D) were each involved in a single pair of linked participants. Using the most stringent threshold of less than 2 SNPs, we identified 4 participant pairs, involving 7 unique participants, that shared highly related isolates from strains A, B, or D, but not C (Figure 3B).
Tuberculosis Infections With Elevated Bedaquiline MICs Involve Diverse Mtb Populations
Phylogenetic analysis of 95 total sequenced isolates from 24 participants identified BDQ resistance across multiple lineages, including lineages 2.2.2, 4.4.1 (including strain A described above), 4.3.2, and 4.8 (Supplementary Figure 4). Isolates from certain phylogeographic lineages shared similar resistance profiles for other, non-BDQ TB drugs. For example, the majority of isolates from lineages 2.2.2 and 2.2.1 were XDR by genotyping and the majority of isolates from lineage 4.4.1 were pre-XDR (2006 definition).
Multiple mmpr5 Mutations Are Associated With Elevated Bedaquiline MICs
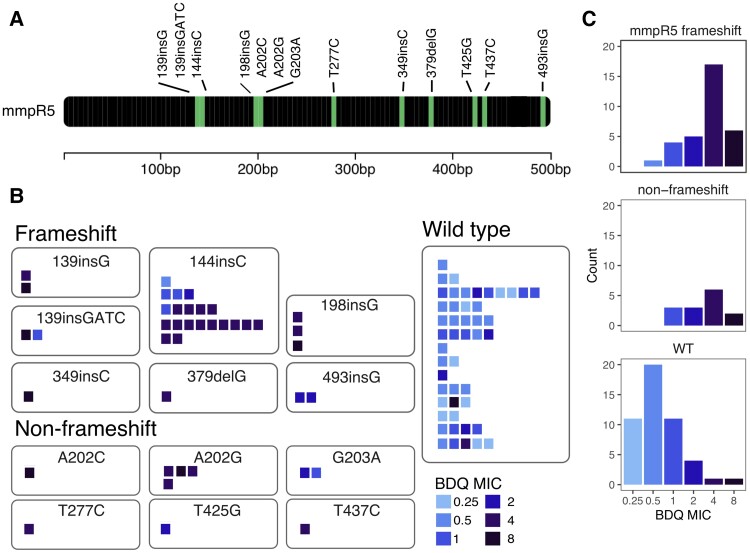
Figure 4 shows the mmpR5 mutations identified among isolates in our cohort and the BDQ MIC values of isolates carrying each mutation. Considering all non-admixed isolates from each participant (including highly similar serial isolates collected from the same patient), 23 of 33 (69.7%) isolates with mmpr5 frameshift mutations were BDQ resistant, 5 had intermediate susceptibility (15.1%), and 5 were susceptible (15.1%). Eight of 14 (57.1%) isolates with mmpR5 non-frameshift mutations were BDQ resistant, 3 (21.4%) had intermediate susceptibility, and 3 (21.4%) were susceptible. Forty-two of 48 (87.5%) mmpR5 wild-type isolates were BDQ susceptible, 4 (8.3%) were intermediate, and 2 (4.2%) isolates were BDQ resistant (Figure 4C). The mmpR5 mutations in participants with pre-existing resistance (139insG [D47fs], 139insGATC [P48fs], 144insC [E49fs], 198delG [I67fs], 198insG [I67fs], A202C [S68R], G203A [S68N], T277C [F93L], and T437C[M146T]) differed from those with acquired resistance (349insC [L117fs] and 379delG [D127fs]). There were no isolates with polymorphisms detected in atpE or pepQ.
Figure 4.
mmpR5 Mutations identified among study participants. The gene map (A) shows locations of mmpR5 mutations identified among participants in this study. Panels B and C show isolates grouped by mmpR5 mutation type (frameshift, non-frameshift, and WT). In panel B, each square represents a single isolate, each row of squares represents a single participant, and squares are colored by BDQ MIC. Abbreviations: BDQ, bedaquiline; MIC, minimum inhibitory concentration; WT, wild-type.
DISCUSSION
This study characterized serial Mtb isolates from individuals who had increased BDQ MICs during BDQ-based treatment for DRTB. This cohort included participants with pre-existing BDQ resistance, polyclonal infections with divergent BDQ resistance phenotypes, and a small number of infections with acquired BDQ resistance associated with de novo mutations in mmpR5. These infections were diverse across different participants, involving multiple Mtb strain types harboring a relatively large set of different mmpR5 mutations, and also within individuals, with diverse isolates sampled at different timepoints during TB diagnosis and treatment.
Two of our findings have implications for clinical management of BDQ-resistant TB. First, the high proportion of participants with pre-existing BDQ resistance underscores the importance of pretreatment BDQ susceptibility testing, which can have important implications for therapeutic decision making and preventing selection for resistance to other TB drugs (including pretomanid and delaminid, which have relatively lower barriers to resistance [26]). Second, our results indicate that mixed Mtb infections, involving polyclonal infections with different strain types, should be considered in patients who experience treatment failure or have unexpected changes in BDQ susceptibility during treatment. Clinical sampling often does not capture the full diversity of TB strains in a single host [10] and failure to identify Mtb populations with more extensive resistance profiles can have important consequences for TB treatment decisions and clinical outcomes [14, 27]. Emerging strategies for sequencing directly from sputum samples, without re-culturing [28], and microbiological methods for increasing the yield of differentially culturable Mtb populations [12] may be important for capturing the full genetic diversity of Mtb isolates. Repeating phenotypic DST in individuals with poor clinical or microbiological response to treatment can help identify treatment-emergent resistance and inform therapeutic decision making.
Our findings also highlight important issues relevant to the classification of TB infections by resistance phenotype. Clinical studies that rely on more limited microbiological data (eg, DST on a single sample per participant, without WGS) may fail to capture the complexity and dynamic nature of drug-resistant and BDQ-resistant TB infections, resulting in multiple kinds of potential misclassification. For example, if clinical trial participants have unrecognized polyclonal infection, their clinical outcomes (eg, treatment failure) may be incorrectly attributed to just 1 of multiple strains involved in their infection. In our study, 8 BDQ-resistant infections would have been misclassified as susceptible if only their pretreatment samples were used for classification. Misclassification of this kind can also confound genotype–phenotype correlations, obfuscating attempts to identify molecular markers of resistance to BDQ. Whole-genome sequencing is thus an important asset for clinical studies where avoiding potential misclassification, and disambiguating strains involved in polyclonal infections, are important priorities.
Four participants in our study had wide differences in BDQ MIC values on nearly clonal samples that had no identifiable changes in their mmpr5 genotypes (Supplementary Figure 2). Variability is a well-documented feature of MIC measurements in Mtb, and many of the known factors underlying this variability (including inherent laboratory measurement variance [29] and variable sampling from different disease sites and/or bacterial subpopulations [30–32]) likely apply here. Additional work is needed to understand other determinants of BDQ resistance, including genetic variants that are difficult to identify with conventional sequencing approaches (including large-scale genomic re-arrangements [33]). Last, variability in BDQ MIC measurements poses an additional, important challenge for characterizing and classifying Mtb strains by resistance phenotype.
We found genomically linked infections with a specific BDQ-resistant strain (strain A in Figure 2) involving 4 different participants recruited at 2 different study sites (Durban and Gqeberha). Strain A is a highly drug-resistant lineage 4.4.1 strain that carries the mmpR5 frameshift mutation 144insC (E49fs). This same mutation has been observed in previous studies of BDQ-resistant TB in South Africa, where a lineage 2 strain with mmpR5 144insC (E49fs) was identified in a cluster of linked infections [5]. A local genomically linked cluster of BDQ-resistant strains from lineage 4.4.1 was recently observed in eSwatini, but in this case, BDQ resistance was associated with a distinct and more diverse set of mmpR5 mutations (G121R, N98D, and M146T) that predate the clinical use of BDQ [34]. Importantly, our study was not designed to infer transmission of specific strains between individual participants, and population-level estimates of transmission-associated BDQ-resistant TB cannot be extrapolated from our data. Infections with pre-existing resistance are arguably all attributable to transmission, except for potential de novo acquisition of resistance in the interval between initial infection and treatment initiation, which is likely exceedingly rare. The evolutionary processes behind the initial emergence of currently circulating BDQ-resistant strains remain uncertain [35]. Expanded surveillance for BDQ-resistant Mtb strains, including studies specifically designed to track potential transmission of these strains, is urgently needed [36].
Our study has important limitations. First, conventional WGS can have difficulty resolving minority bacterial subpopulations without high-depth read coverage, and the standard read depths used in our WGS may fail to capture the full Mtb genetic diversity in each sample, including heteroresistant infections and polyclonal infections with very small minority strain populations. Second, SNP distance-based methods for inferring genomic linkages between isolates have several drawbacks, including ambiguity about how to choose an SNP distance threshold and bias toward either over- or under-calling linkages based on which threshold value is used [37]. The SNP distance thresholds we used are comparable to or more stringent than other studies [22] (including those in South Africa [38]) and our analysis is likely biased toward under-calling the number of genetically linked infections in our cohort. Third, potential sampling bias may have enriched our study cohort for individuals with genomically linked isolates. Participants all had highly drug-resistant TB, relatively rare infections that are caused by a restricted number of strains (compared with drug-susceptible TB), making it more likely that isolates identified in this cohort would be genetically similar. Last, the small sample size in this study and nonrandomized selection of participants for serial genotype–phenotype characterization likely limit the generalizability of our findings.
Our findings highlight the complex, dynamic nature of Mtb strains involved in BDQ-resistant TB, with important implications for patient care and scientific and clinical research. Increased investment is needed to enhance public health surveillance for BDQ-resistant TB and mitigate its potential spread.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Tyler S Brown, Section of Infectious Diseases, Boston University School of Medicine, Boston, Massachusetts, USA; Center for Communicable Disease Dynamics, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Linrui Tang, Department of Epidemiology, Columbia University Mailman School of Public Health, New York, New York, USA.
Shaheed Vally Omar, Centre for Tuberculosis, National Institute for Communicable Diseases, Johannesburg, South Africa; Department of Molecular Medicine & Hematology, School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Lavania Joseph, Centre for Tuberculosis, National Institute for Communicable Diseases, Johannesburg, South Africa.
Graeme Meintjes, Wellcome Centre for Infectious Diseases Research in Africa, Institute of Infectious Disease and Molecular Medicine, and Department of Medicine, University of Cape Town, Cape Town, South Africa.
Gary Maartens, Wellcome Centre for Infectious Diseases Research in Africa, Institute of Infectious Disease and Molecular Medicine, and Department of Medicine, University of Cape Town, Cape Town, South Africa; Division of Clinical Pharmacology, Department of Medicine, University of Cape Town, Cape Town, South Africa.
Sean Wasserman, Wellcome Centre for Infectious Diseases Research in Africa, Institute of Infectious Disease and Molecular Medicine, and Department of Medicine, University of Cape Town, Cape Town, South Africa; Division of Infectious Diseases and HIV Medicine, Department of Medicine, University of Cape Town, Cape Town, South Africa.
N Sarita Shah, Departments of Epidemiology and Global Health and Medicine, Rollins School of Public Health and Emory School of Medicine, Atlanta, Georgia, USA.
Maha R Farhat, Department of Biomedical Informatics, Harvard Medical School, Boston, Massachusetts, USA.
Neel R Gandhi, Departments of Epidemiology and Global Health and Medicine, Rollins School of Public Health and Emory School of Medicine, Atlanta, Georgia, USA.
Nazir Ismail, Centre for Tuberculosis, National Institute for Communicable Diseases, Johannesburg, South Africa.
James C M Brust, Department of Medicine, Albert Einstein College of Medicine and Montefiore Medical Center, Bronx, New York, USA.
Barun Mathema, Department of Epidemiology, Columbia University Mailman School of Public Health, New York, New York, USA.
Notes
Author Contributions. Study conception and design: B. M., J. C. M. B., T. S. B., and L. T. Data acquisition: S. V. O., L. J., G. Meintjes, G. Maartens, and S. W. Data analysis: L. T., T. S. B., S. V. O., L. J., and M. R. F. Funding and supervision: J. C. M. B., N. R. G., N. S. S., N. I., G. Meintjes, G. Maartens, and B. M. Drafting of the manuscript: T. S. B., L. T., J. C. M. B., and B. M.
Disclaimer. The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of this report. The opinions, findings and conclusions expressed in this manuscript reflect those of the authors alone and do not necessarily represent the official position of the US Department of Health and Human Services.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) (grant number R01AI114304; to J. C. M. B.). This work was also supported in part by NIH/NIAID grants R01AI151173 and R01AI169938 (to B. M.), K08AI166125 (to T. S. B.), R01AI145679 (to J. C. M. B.), K24AI155045 (to J. C. M. B.), K24AI114444 (to N. R. G.), and R01AI155765 (to M. R. .F); Einstein-Rockefeller-CUNY Center for AIDS Research P30AI124414, Emory University Center for AIDS Research P30AI050409, Einstein/Montefiore Institute for Clinical and Translational Research UL1TR001073, and the Emory/Georgia TB Research Advancement Center P30AI168386. S. W. is supported by the NIH (grant numbers K43TW011421 and U01AI170426) and the Wellcome Trust through core funding from the Wellcome Centre for Infectious Diseases Research in Africa (203135/Z/16/Z). G. Meintjes was supported by the Wellcome Trust (grant numbers 098316, 214321/Z/18/Z, and 203135/Z/16/Z) and the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation (NRF) of South Africa (grant number 64787). Additional support was provided by the South African Medical Research Council through its TB and HIV Collaborating Centres Programme, with a grant (RFA SAMRC-RFA-CC: TB/HIV/AIDS-01–2014) funded by the National Department of Health. N. S. S. reports the following support for this work: NIH/NIAID grant number K24AI165099.
This research was funded, in part, by the Wellcome Trust. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
References
- 1. Schnippel K, Ndjeka N, Maartens G, et al. Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study. Lancet Respir Med 2018; 6:699–706. [DOI] [PubMed] [Google Scholar]
- 2. Conradie F, Diacon AH, Ngubane N, et al. Treatment of highly drug-resistant pulmonary Tuberculosis. N Engl J Med 2020; 382:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brust JCM, Gandhi NR, Wasserman S, et al. Effectiveness and cardiac safety of bedaquiline-based therapy for drug-resistant tuberculosis: a prospective cohort study. Clin Infect Dis 2021; 73:2083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ndjeka N, Hughes J, Reuter A, et al. Implementing novel regimens for drug-resistant TB in South Africa: what can the world learn? Int J Tuberc Lung Dis 2020; 24:1073–80. [DOI] [PubMed] [Google Scholar]
- 5. Ismail NA, Omar SV, Moultrie H, et al. Assessment of epidemiological and genetic characteristics and clinical outcomes of resistance to bedaquiline in patients treated for rifampicin-resistant tuberculosis: a cross-sectional and longitudinal study. Lancet Infect Dis 2022; 22:496–506. [DOI] [PubMed] [Google Scholar]
- 6. Le Ray LF, Aubry A, Sougakoff W, et al. Atpe mutation in Mycobacterium tuberculosis not always predictive of bedaquiline treatment failure. Emerg Infect Dis 2022; 28:1062–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ismail N, Rivière E, Limberis J, et al. Genetic variants and their association with phenotypic resistance to bedaquiline in Mycobacterium tuberculosis: a systematic review and individual isolate data analysis. Lancet Microbe 2021; 2:e604–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nguyen TVA, Anthony RM, Bañuls AL, Nguyen TVA, Vu DH, Alffenaar JC. Bedaquiline resistance: its emergence, mechanism, and prevention. Clin Infect Dis 2018; 66:1625–30. [DOI] [PubMed] [Google Scholar]
- 9. Ismail NA, Omar SV, Joseph L, et al. Defining bedaquiline susceptibility, resistance, cross-resistance and associated genetic determinants: a retrospective cohort study. EBioMedicine 2018; 28:136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ley SD, de Vos M, Van Rie A, Warren RM. Deciphering within-host microevolution of Mycobacterium tuberculosis through whole-genome sequencing: the phenotypic impact and way forward. Microbiol Mol Biol Rev 2019; 83:e00062-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shin SS, Modongo C, Baik Y, et al. Mixed Mycobacterium tuberculosis-strain infections are associated with poor treatment outcomes among patients with newly diagnosed tuberculosis, independent of pretreatment heteroresistance. J Infect Dis 2018; 218:1974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chengalroyen MD, Beukes GM, Gordhan BG, et al. Detection and quantification of differentially culturable tubercle bacteria in sputum from patients with tuberculosis. Am J Respir Crit Care Med 2016; 194:1532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shockey AC, Dabney J, Pepperell CS. Effects of host, sample, and in vitro culture on genomic diversity of pathogenic mycobacteria. Front Genet 2019; 10:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kargarpour Kamakoli M, Sadegh HR, Farmanfarmaei G, et al. Evaluation of the impact of polyclonal infection and heteroresistance on treatment of tuberculosis patients. Sci Rep 2017; 7:41410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eldholm V, Norheim G, von der Lippe B, et al. Evolution of extensively drug-resistant Mycobacterium tuberculosis from a susceptible ancestor in a single patient. Genome Biol 2014; 15:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chesov E, Chesov D, Maurer FP, et al. Emergence of bedaquiline resistance in a high tuberculosis burden country. Eur Respir J 2022; 59:2100621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention . Notice to readers: revised definition of extensively drug-resistant tuberculosis. Morbidity Mortality Wkly Rep 2006; 55:1176. [Google Scholar]
- 18. World Health Organization . Technical report on critical concentrations for drug susceptibility testing of medicines used in the treatment of drug-resistant tuberculosis. Geneva, Switzerland: World Health Organization, 2018. [Google Scholar]
- 19. Coll F, McNerney R, Guerra-Assunção JA, et al. A robust SNP barcode for typing Mycobacterium tuberculosis complex strains. Nat Commun 2014; 5:4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hunt M, Bradley P, Lapierre SG, et al. Antibiotic resistance prediction for Mycobacterium tuberculosis from genome sequence data with Mykrobe. Wellcome Open Res 2019; 4:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walker TM, Miotto P, Köser CU, et al. The 2021 WHO catalogue of Mycobacterium tuberculosis complex mutations associated with drug resistance: a genotypic analysis. Lancet Microbe 2022; 3:e265–e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Walker TM, Ip CL, Harrell RH, et al. Whole-genome sequencing to delineate Mycobacterium tuberculosis outbreaks: a retrospective observational study. Lancet Infect Dis 2013; 13:137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allaire JJ, Gandrud C, Russell K, Yetman C. networkD3: D3 JavaScript Network Graphs from R. 0.4 ed. 2017. Available at: https://CRAN.R-project.org/package=networkD3. Accessed 23 June 2023.
- 24. R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2022. [Google Scholar]
- 25. Casardi G, Nepusz T. The igraph software package for complex network research. InterJournal Complex Systems 2006; 5:1–9. [Google Scholar]
- 26. Fujiwara M, Kawasaki M, Hariguchi N, Liu Y, Matsumoto M. Mechanisms of resistance to delamanid, a drug for Mycobacterium tuberculosis. Tuberculosis (Edinb) 2018; 108:186–94. [DOI] [PubMed] [Google Scholar]
- 27. Metcalfe JZ, Streicher E, Theron G, et al. Mycobacterium tuberculosis subculture results in loss of potentially clinically relevant heteroresistance. Antimicrob Agents Chemother 2017; 61:e00888-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown AC. Whole-genome sequencing of Mycobacterium tuberculosis directly from sputum samples. Methods Mol Biol 2021; 2314:459–80. [DOI] [PubMed] [Google Scholar]
- 29. Schon T, Jureen P, Giske CG, et al. Evaluation of wild-type MIC distributions as a tool for determination of clinical breakpoints for Mycobacterium tuberculosis. J Antimicrob Chemother 2009; 64:786–93. [DOI] [PubMed] [Google Scholar]
- 30. Kaplan G, Post FA, Moreira AL, et al. Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infect Immun 2003; 71:7099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Post FA, Willcox PA, Mathema B, et al. Genetic polymorphism in Mycobacterium tuberculosis isolates from patients with chronic multidrug-resistant tuberculosis. J Infect Dis 2004; 190:99–106. [DOI] [PubMed] [Google Scholar]
- 32. Lieberman TD, Wilson D, Misra R, et al. Genomic diversity in autopsy samples reveals within-host dissemination of HIV-associated Mycobacterium tuberculosis. Nat Med 2016; 22:1470–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sonnenkalb L, Carter JJ, Spitaleri A, et al. Bedaquiline and clofazimine resistance in Mycobacterium tuberculosis: an in-vitro and in-silico data analysis. Lancet Microbe 2023; 4:e358–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beckert P, Sanchez-Padilla E, Merker M, et al. MDR M. tuberculosis outbreak clone in Eswatini missed by Xpert has elevated bedaquiline resistance dated to the pre-treatment era. Genome Med 2020; 12:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nimmo C, Millard J, van Dorp L, et al. Population-level emergence of bedaquiline and clofazimine resistance-associated variants among patients with drug-resistant tuberculosis in Southern Africa: a phenotypic and phylogenetic analysis. Lancet Microbe 2020; 1:e165–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Rie A, Walker T, de Jong B, et al. Balancing access to BPaLM regimens and risk of resistance. Lancet Infect Dis 2022; 22:1411–2. [DOI] [PubMed] [Google Scholar]
- 37. Stimson J, Gardy J, Mathema B, Crudu V, Cohen T, Colijn C. Beyond the SNP threshold: identifying outbreak clusters using inferred transmissions. Mol Biol Evol 2019; 36:587–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nelson KN, Shah NS, Mathema B, et al. Spatial patterns of extensively drug-resistant tuberculosis (XDR-tuberculosis) transmission in KwaZulu-Natal, South Africa. J Infect Dis 2018; 218:1964–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.