Abstract
Papillomaviruses (PVs) cause disease in humans, dogs, cats, and horses. While there are some differences, many aspects of the pathogenesis, presentation and treatment of these diseases are similar between the four species. In this review, the PV-induced diseases of humans are compared to the similar diseases that develop in the companion animal species. By comparing with the human diseases, it is possible to make assumptions about some of the less common and less well-studied diseases in the veterinary species. In the first part of this review, the PV lifecycle is discussed along with the classification of PVs and the immune response to PV infection. The hyperplastic diseases caused by PVs are then discussed including PV-induced cutaneous, anogenital and oral warts within the four species.
Keywords: Papillomavirus, dogs, cats, horses, papillomas, warts, treatment, hyperplasia, oncogenesis, oncogenic viruses, canine papillomavirus, feline papillomavirus, equine papillomavirus
Introduction
Warts have been described in people since the time of the ancient Greeks (Karamanou et al., 2010). While a viral etiology of warts was proven in 1907 (Ciuffo, 1907), human papillomaviruses (HPVs) were considered of low importance until the recognition by zur Hausen (1976) that they also caused cervical cancer. Since then, there has been intense research on the mechanisms of infection, how PVs cause disease, and potential ways to prevent and treat infection. Additionally, a number of rarer syndromes in people are now recognized to be caused by PV infection.
There are also historical records of warts in animals with the Caliph of Baghdad describing equine warts in the 9th century. However, the number and variety of lesions recognized to be caused by PVs in dogs, cats and horses has expanded greatly in the last 25 years. As more has been learnt about the PV-induced diseases of the companion animal species, it has become clear that there are many similarities between the diseases caused by PVs in humans and those caused by PVs in dogs, cats, and horses. The purpose of this review is to compare the pathogenesis, clinical presentation, and clinical management of PV-induced diseases of humans to those of dogs, cats, and horses. These diseases are divided into hyperplastic diseases, which typically spontaneously resolve, and pre-neoplastic and neoplastic diseases in which spontaneous resolution is not expected. Part 1 of the review includes a discussion of the life cycle, classification, and immune response to PVs as well as the hyperplastic diseases caused by PV infection. Part 2 of this review discusses the pre-neoplastic and neoplastic diseases associated with PVs in humans, dogs, cats, and horses as well as a brief discussion on the potential use of vaccines to prevent PV-induced diseases within the companion animal species.
Papillomaviral life cycle
Papillomaviruses (PVs) are double-stranded circular DNA viruses. Their genome contains five or six early (E) genes and two late (L) genes (Fig. 1). The PV life cycle is coordinated with the division and maturation of cells within stratified epithelium (Graham, 2017; McBride, 2022). A PV infection is initiated after viral particles come into contact with the basal cells of the epithelium following microtrauma (Doorbar, 2005). Once the PV has gained entry to a basal cell, the PV E1 and E2 genes are expressed and additional viral copies are produced (Ozbun, 2002). As the basal cells replicate, the daughter cells contain PV DNA allowing the PV infection to persist (McBride, 2013). However, the production of infectious viral particles is only possible when an infected basal cell terminally differentiates and moves into the suprabasilar layer of the epithelium (Klumpp and Laimins, 1999; McBride, 2022). Here, the PV E6 and E7 proteins prevent normal loss of the nuclear machinery and stimulate replication of the infected epithelial cells (Doorbar et al., 2012). The continued replication of infected epithelial cells allows robust viral genome amplification and results in cells that contain multiple viral copies. As the infected cells approach the surface of the epithelium, expression of the PV L1 and L2 capsid proteins allows the virion to be assembled. Epithelial cells slough normally, releasing the viral particles into the environment (Graham, 2017). With the exception of the ruminant Deltapapillomaviruses, PVs are highly species-specific with most individual species infected by multiple PV types (Antonsson and Hansson, 2002; Bernard et al., 2010; Doorbar et al., 2012).
Figure 1.
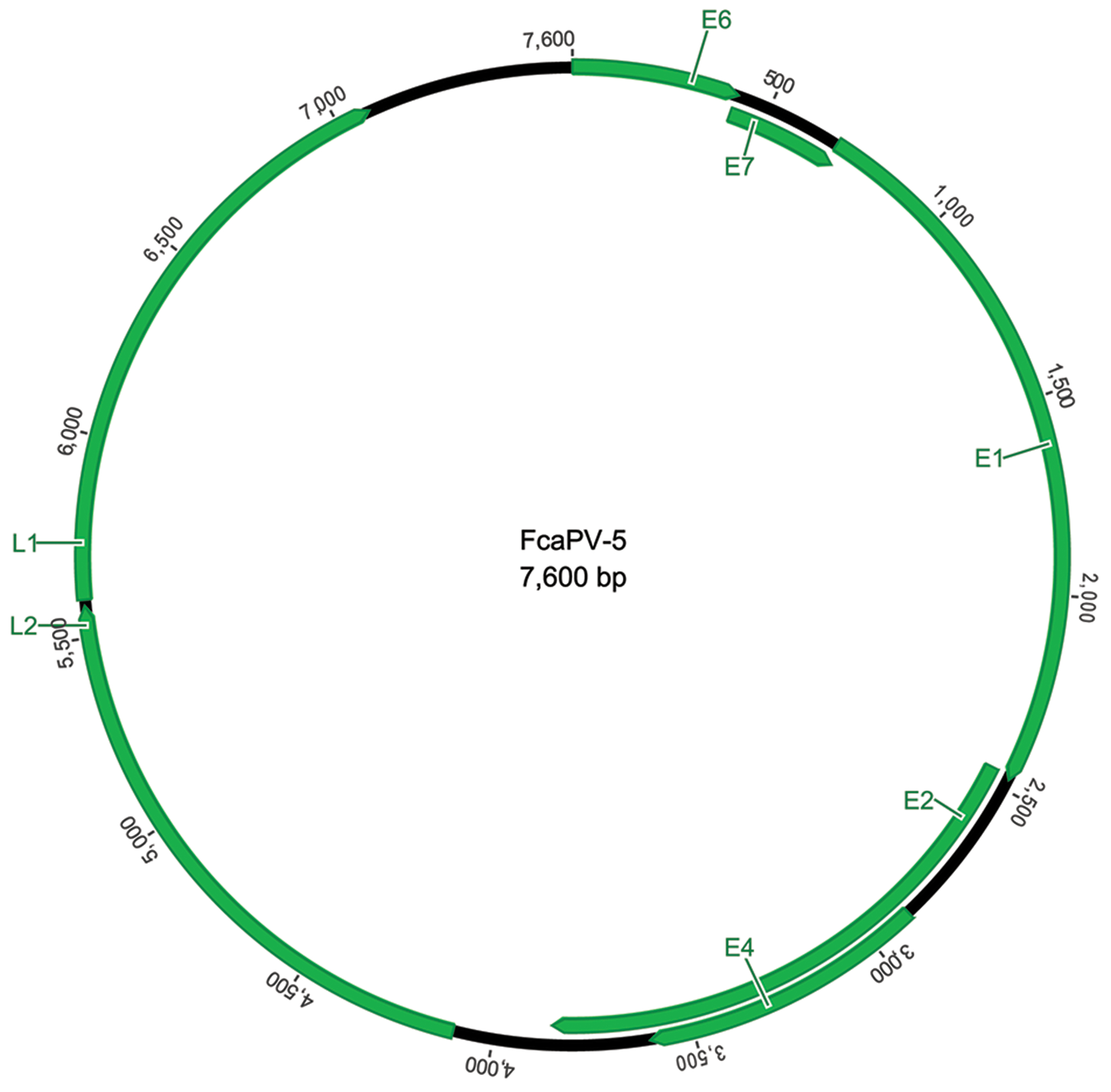
Schematic genomic organization of Felis catus papillomavirus type 5 showing the circular arrangement of the papillomaviral DNA and the organization of the 5 early (E) and 2 late (L) open reading frames.
Classification of papillomavirus types
Taxonomic classification of PVs is done using the highly conserved L1 open reading frame (ORF). Two PVs are classified as different types when they share less than 90% similarity in the L1 ORF. PVs are also classified into genera whereby PVs within the same genus share greater than 60% similarity in the L1 ORF (Bernard et al., 2010). Currently the online database within the Papillomavirus Episteme1 contains around 450 individual HPV types within the Alphapapillomavirus, Betapapillomavirus, Gammapapillomavirus, Mupapillomavirus or Nupapillomavirus genera (McBride, 2022). In dogs there are 23 Canis familiaris papillomavirus (CPV) types within the Lambdapapillomavirus, Taupapillomavirus and Chipapillomavirus genera. The episteme contains 6 Felis catus papillomavirus (FcaPV) types within the Lambdapapillomavirus, Dyothetapapillomavirus and Taupapillomavirus genera and 9 Equulis caballus papillomavirus (EcPV) types contained within the Zetapapillomavirus, Dyoiotapapillomavirus, and Dyorhopapillomavirus genera. Classification of PV types within genera is important as PV types within the same genus often infect the same, or closely related, host species and often also have a similar clinical presentation (Bernard et al., 2010). For example, the Betapapillomaviruses all infect people and typically remain asymptomatic while the Chipapillomaviruses infect dogs and cause viral plaques.
In addition to their genome-based taxonomic classification, PVs can also be broadly classified into those that cause hyperplastic papillomas (warts) and those that typically infect without causing clinical lesions (McBride, 2022). PVs that cause warts are transmitted between animals either directly or via fomites with most infections happening in young adults. When an animal is infected by one of these PV types, the PV stimulates rapid replication of epithelial cells resulting in marked epithelial hyperplasia and a visible wart (Doorbar et al., 2012; Munday et al., 2011). The ability to cause such marked epithelial hyperplasia results in the production of massive numbers of viral particles. However, warts typically also stimulate a strong immune reaction that inhibits viral replication and results in spontaneous lesion resolution (Lane et al., 2017). While warts are the most common clinical manifestation of PV infection, infection by the majority of human PV types does not typically cause visible lesions (Antonsson et al., 2003a; Antonsson et al., 2000; Doorbar, 2005). These PVs stimulate slow replication of the epithelium resulting in minimal hyperplasia and only small numbers of infective viral particles (Egawa and Doorbar, 2017; Munday et al., 2017). Infection by these types is often acquired during, or soon after birth (Antonsson et al., 2003b). Infection can be life-long and cutaneous PV infections are ubiquitous in people (Antonsson et al., 2000; Forslund et al., 1999). The development of visible lesions due to infection by these PV types is indicative of immune dysfunction (Orth et al., 1978). While there are fewer studies investigating asymptomatic PV infection of dogs, cats and horses, asymptomatic infection by PVs has been detected in all three species suggesting PV infection is likely also ubiquitous within the companion animal species (Antonsson and Hansson, 2002; Bogaert et al., 2008; Knight et al., 2013; Lange et al., 2011; Munday and Witham, 2010).
In humans, PVs are also subdivided into those that are sexually transmitted and those that are not. No PVs are established to be sexually transmitted in dogs, cats, or horses. HPVs can also be subdivided into mucosal and cutaneous types (de Villiers et al., 2004). Some PVs of companion animals similarly show a tropism for either haired skin (for example EcPV1) or mucosal surfaces (for example EcPV2). However, some canine and feline PV types have less strict tissue trophism, with CPV1, FcaPV2, FcaPV3, and FcaPV4 being detected both on haired skin and in the oral cavity (Altamura et al., 2020; Lange et al., 2019; Lange et al., 2011; Munday and Thomson, 2021). Finally, HPVs are also subdivided into low-risk types, that are either asymptomatic or cause self-resolving warts, and high-risk types, that can cause neoplasia (Doorbar et al., 2012). Similarly, some canine, feline and equine PV types have only been detected in hyperplastic lesions while other PV types are frequently detected in neoplasms.
Immune response to papillomavirus infection
There are two components of the immune response to PV infection. Firstly, a cell-mediated immune response detects and attacks infected cells with the aim of clearing the infection from the body (Nicholls et al., 2001; Stanley, 2006). The cell-mediated immune response is responsible for the resolution of PV-induced warts and, as the time of onset of the cell-mediated immune response is variable, the time taken for wart resolution also shows individual variability (Franco et al., 1999; Sancak et al., 2015). Secondly, a humoral immune response also develops after infection by a PV (Ghim et al., 2000). The development of serum antibodies does not influence resolution of the PV infection but prevents subsequent infection by the PV type (Ghim et al., 2000).
Non-genital cutaneous warts
Humans
Cutaneous warts in people are most commonly caused by members of the Alphapapillomavirus (HPV2 and 27), Mupapillomavirus (HPV1) or Gammapapillomavirus (HPV4) genera (Sterling, 2016). Cutaneous warts are most common in children and adolescents and it is estimated that up to 20% of 5 to 16 year-old children have warts at any time (Larsson and Liden, 1980; van Haalen et al., 2009). The high rates of infection in children are due a combination of factors including immune naivety, exposure to high numbers of viral particles from other children with warts, and activities that promote microtrauma such as walking around swimming pool changing rooms with bare feet.
Human cutaneous warts are subdivided into plantar warts and common warts (Bruggink et al., 2010). Plantar warts develop on the sole of the feet and can result in significant pain and difficulty walking. Common warts most frequently develop on the hands and are generally only significant for cosmetic reasons.
Sixty-five percent of cutaneous warts spontaneously resolve within 2 years and 95% resolve within 4 years (Massing and Epstein, 1963; Williams et al., 1993). Warts very rarely persist and progress to involve large areas of the body. These are referred to as ‘recalcitrant warts’ with ‘tree man syndrome’ developing if these recalcitrant warts become covered in a thick layer of keratin (Uitto et al., 2021). Most cases of recalcitrant warts are due to an inability to produce or stimulate CD4+ (T-helper) lymphocytes, preventing a normal cell-mediated immune response against PV infection (Beziat et al., 2021). The immune deficiency can result in increased susceptibility to a wide range of pathogens or be specific to cutaneous HPV infections (Beziat et al., 2021).
Histology of a human cutaneous wart reveals hyperplasia of all layers of the epidermis. The thickened epidermis can become folded resulting in filiform projections supported by a connective tissue stalk. Cells with dark irregularly shrunken nuclei surrounded by a clear halo (koilocytes) are often visible in the granular cell layer. Clumped keratohyalin granules can be visible and, depending on the causative PV type, other PV-induced cellular changes can be visible within the more superficial layers of the warts (Sterling, 2016). Warts are covered by variable degrees of hyperkeratosis.
Due to the expected spontaneous resolution and the potential pain associated with treatment, many common warts are not treated. If treatment is required, localised destruction of the infected hyperplastic cells using either cryotherapy or salicylic acid is considered most effective, although recurrence is possible (Bruggink et al., 2010; Kwok et al., 2012). As plantar warts are covered by the thick layer of keratin that covers the sole of the foot, neither cryotherapy nor topical treatments are beneficial. Surgical excision or local destruction is typically used for recalcitrant warts that interfere with normal function. However, no consistently curative treatments have been identified, presumably as the underlying immune dysfunction cannot be resolved. Progression from a cutaneous wart to a squamous cell carcinoma (SCC) is extremely rare (Egawa and Doorbar, 2017).
Dogs
Cutaneous warts are the second most common skin tumor of dogs under one year of age (Kim et al., 2022). They are most often caused by CPV2 (a Taupapillomavirus) and less commonly by CPV1 (a Lambdapapillomavirus that is also referred to as canine oral PV) with co-infections by both CPV types also detected (Chang et al., 2020; Lange et al., 2019; Orlandi et al., 2021; Sundberg et al., 1994). Canine cutaneous warts are rarely caused by CPV6 (also a Lambdapapillomavirus) (Lange et al., 2009). Warts most commonly occur on the feet due to trauma caused by walking (Fig. 2) and around the face and ears presumably secondary to self-trauma (Gould et al., 2021; Gross et al., 2005; Munday et al., 2010; Munday et al., 2017). There is rarely a history of contact with an affected dog, suggesting indirect exposure from the environment is most likely. As in humans, warts that develop on the feet can result in lameness (Gould et al., 2021) while those elsewhere on the body are generally well tolerated.
Figure 2.
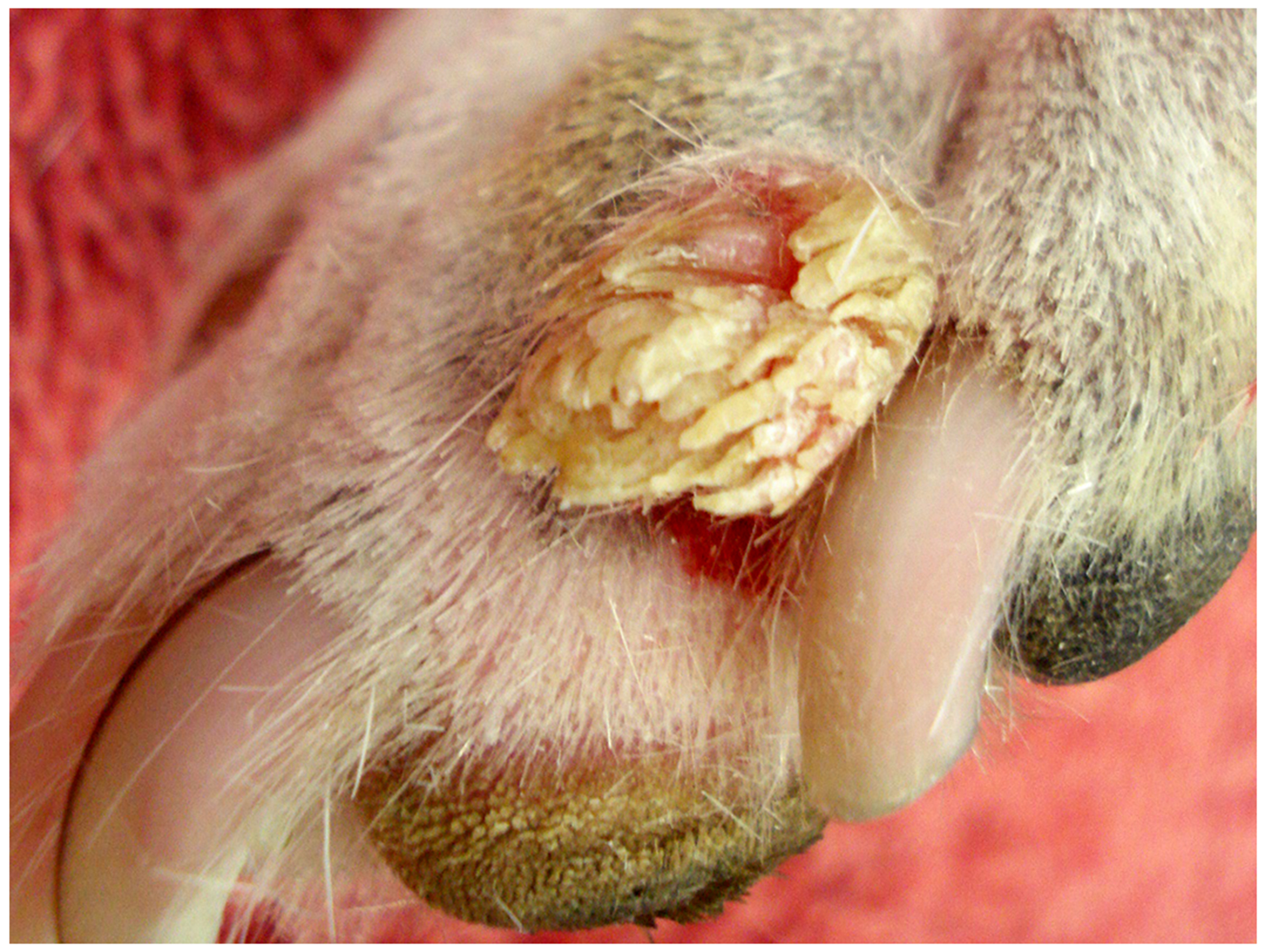
Cutaneous wart, dog. The wart appears as an exophytic vegetative mass on the toe of this dog. The frond-like appearance of the surface is due to the folding of the epidermis caused by papillomavirus-induced epidermal hyperplasia (Image courtesy of Dr Scott Martin).
The vast majority of canine cutaneous warts spontaneously regress. Most regress within 3 months, although dogs can have warts for as long as two years before resolution (Gould et al., 2021; Munday et al., 2020b). There are rare reports of CPV1-induced cutaneous warts progressing to SCC (Thaiwong et al., 2018). Additionally, multiple dogs that were being used to evaluate a treatment for severe combined immune deficiency within a research colony developed CPV2-induced warts that persisted and rapidly progressed to SCC (Goldschmidt et al., 2006). However, as in humans, most evidence suggests it is extremely rare for a canine cutaneous wart to progress to a SCC.
Histologically, lesions can be subdivided into exophytic warts in which the thickened epidermis folds out from the surface of the skin and endophytic warts in which the thickened epidermis folds inwards forming a cup-shaped structure (Goldschmidt et al., 2018). Currently there is no evidence that either subtype is more frequently associated with a particular CPV type or demonstrates a different clinical behaviour. Some exophytic warts can become covered by large quantities of keratin and these are referred to as viral cutaneous horns (Falk et al., 2017). Canine cutaneous warts generally contain large numbers of cells with expanded basophilic cytoplasm (Fig. 3). Koilocytosis and intranuclear eosinophilic inclusions are usually visible (Goldschmidt et al., 2018; Gross et al., 2005).
Figure 3.
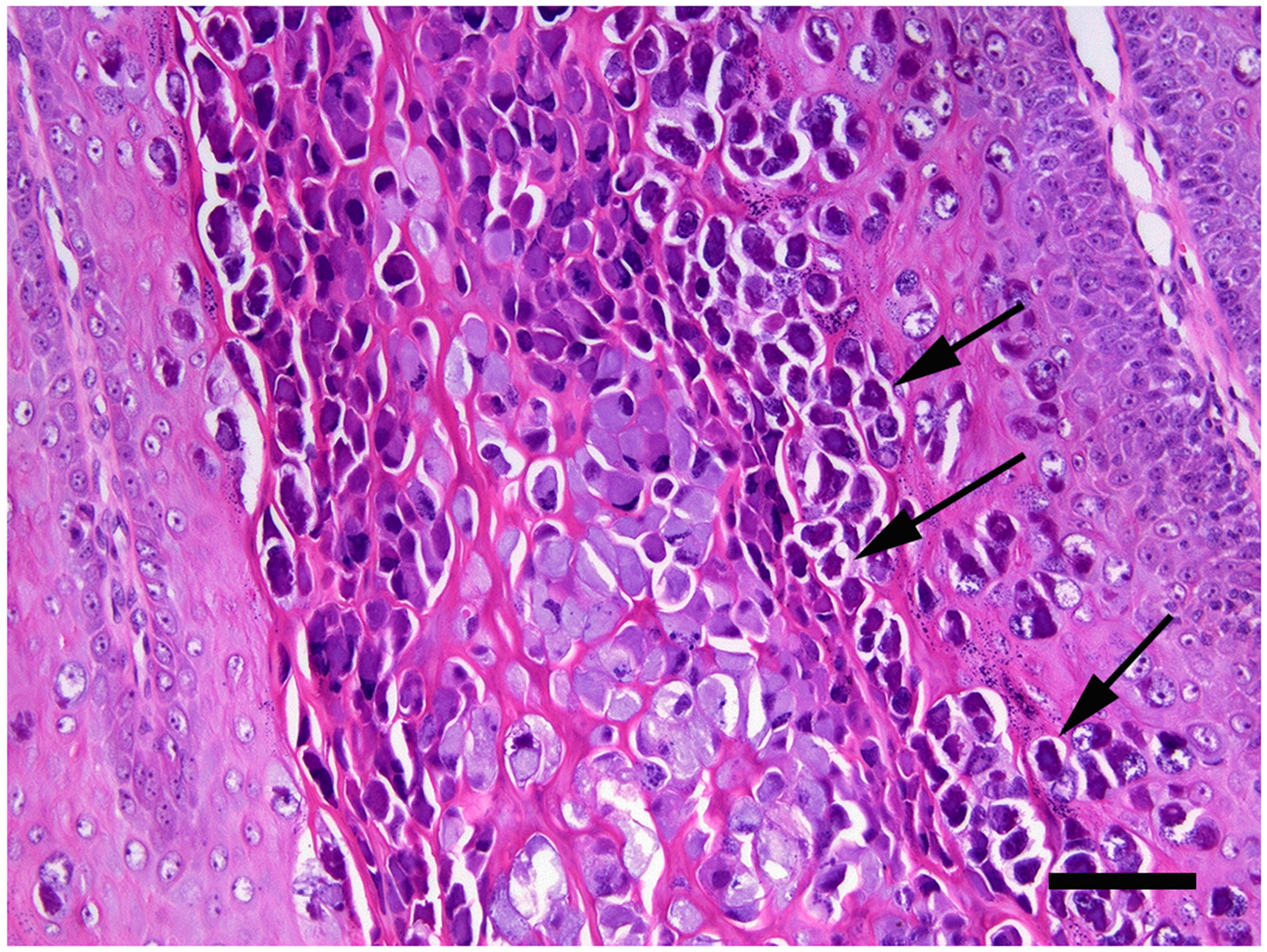
Cutaneous wart, dog. A high proportion of cells within the thickened, folded epidermis contain marked papillomaviral-induced cellular changes. These include cells with cytoplasm that is expanded by large quantities of amorphous basophilic material and cells that have a dark nucleus with an irregular nuclear membrane surrounded by a clear cytoplasmic halo (koilocytes; arrows). Scale bar = 30μm. Haematoxylin and eosin.
As in humans, most canine cutaneous warts are not treated as lesions self-resolve and do not cause discomfort. Warts were successfully treated using cryotherapy in two dogs, although proving the benefit of a particular treatment is difficult as warts spontaneously resolve after a variable length of time (Richman et al., 2017). Treatment of cutaneous warts is discussed in more detail with canine oral warts.
Cats
There are only three reports of cutaneous warts in cats. Warts were small and solitary in all cases (Carpenter et al., 1992; Munday et al., 2007). Two warts developed on the nasal planum while the other was on the eyelid. One wart contained a novel PV sequence that was most closely related to, but distinct from, the 6 currently recognized FcaPV types (Munday and Julian, 2022).
Horses
Skin warts are very common in horses with most caused by EcPV1 (Ghim et al., 2004). As in other species they are most common on the distal limbs and face of younger horses, likely due to trauma allowing infection of an immunologically naïve animal (Baker and Leyland, 1975; Junge et al., 1984; Sundberg et al., 1977; Fig. 4). Warts typically appear as multiple (up to 100), small exophytic masses covered in dark grey keratin. They do not usually cause discomfort and resolve spontaneously, usually within three months (Cook and Olson, 1951; Hamada et al., 1990). In addition to EcPV1, a novel unclassified PV type was amplified from one of two large warts that developed close to the fetlock of a horse. Both warts resolved after 14 weeks (Munday et al., 2020a).
Figure 4.
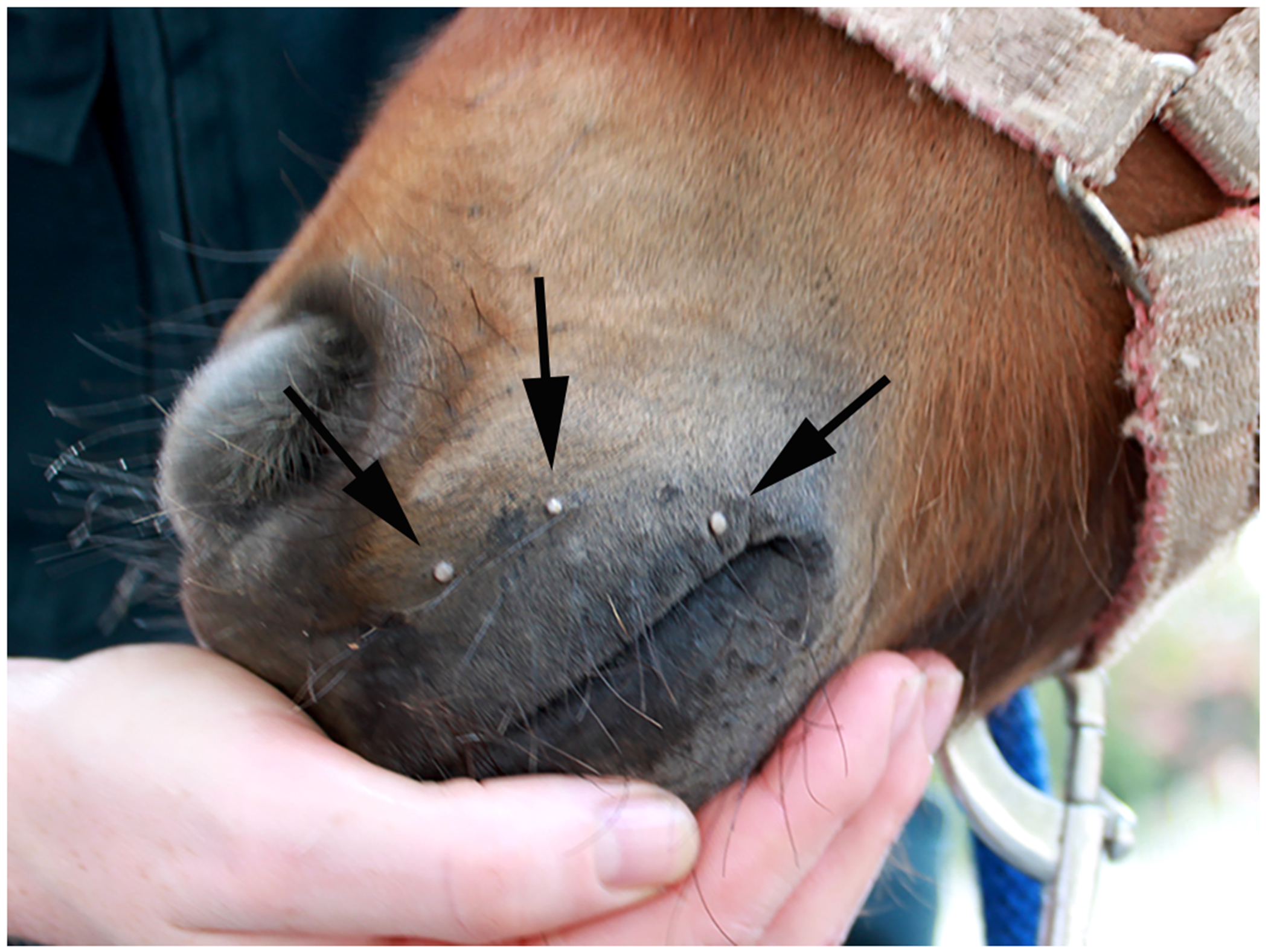
Cutaneous warts, horse. The warts appear as multiple small exophytic lesions on the muzzle of this young horse. These warts resolved spontaneously and did not appear to cause discomfort (Image courtesy of Dr Emma Gordon).
Recently, EcPV8 has been recognised as an uncommon cause of equine cutaneous warts (Linder et al., 2018; Peters-Kennedy et al., 2019). In contrast to warts caused by EcPV1, the EcPV8-associated warts were extensive and covered a significant proportion of the body, especially involving the ventrum, inguinal and axillary regions. Additionally, warts due to EcPV8 were more persistent and only resolved spontaneously in one of four horses reported with this disease. Furthermore, evidence of progression to SCC was seen in some warts. The rarity of EcPV8 as a cause of warts in horses and the subsequent persistence of these warts suggests immune deficiency could be important in disease development.
Warts due to EcPV1 show typical features of an exophytic wart. However, the EcPV8-induced lesions are more variable with some showing features of a typical exophytic wart and others showing features more suggestive of a viral plaque (Linder et al., 2018; Peters-Kennedy et al., 2019).
Anogenital cutaneous warts
Humans
Anogenital warts are most often caused by the Alphapapillomaviruses HPV6 and HPV11 (Egawa and Doorbar, 2017). Infection is mainly by sexual contact and warts are most common in 16 to 24 year-olds (Oriel, 1971). Anogenital warts are highly contagious with transmission rates of up to 88% reported (Sedlacek et al., 1986), presumably due to the likelihood of developing microtrauma in the comparatively thin skin of the anogenital regions during sex (Sterling, 2016). Infections can remain latent for up to eight months prior to wart development (Oriel, 1971). Rarely, anogenital warts can develop in younger children and these are thought to follow activation of a latent infection that was acquired during birth (Padel et al., 1990). As anogenital warts are prevented by multivalent HPV vaccines, they are becoming rare in countries with widespread vaccination programs (Flagg and Torrone, 2018).
Anogenital warts generally remain small and over 90% spontaneously resolve within two years. However, most patients want more rapid wart resolution and anogenital warts are treated more frequently than common warts. Destruction of the infected cells using cryotherapy is frequently used and is effective (Karagounis and Pomeranz, 2021). Topical application of imiquimod cream is also commonly used to treat human anogenital warts. Imiquimod is an immune-response modifier that induces the production of proinflammatory cytokines, including IFN-α, TNF-α, IL-6, and IL-8, and promotes a T-cell cytotoxic reaction against the infected epithelial cells of the wart (Hanna et al., 2016; Miller et al., 1999). While imiquimod was initially licenced for the treatment of genital warts, it does not have a specific activity against PVs and is now used to treat a variety of superficial skin lesions regardless of their cause (Hanna et al., 2016). Other, less commonly, used topical treatments include podophyllotoxin, sinecatechins, cidofovir and trichloroacetic acid. Surgical excision or laser ablation can be used for larger lesions (Karagounis and Pomeranz, 2021; Lacey et al., 2013). Multivalent HPV vaccines are currently not recommended to treat anogenital warts (Karagounis and Pomeranz, 2021). While vaccines were reported to initiate wart resolution in some individual cases (Lee et al., 2011), vaccination did not result in any significant benefit in a study of 500 patients with anogenital warts (Gilson et al., 2020). There are rare reports of persistent anogenital warts. Additionally, progression of a wart to neoplasia has been reported, although this may be because the wart contained both low-risk and high-risk HPV types (Lacey et al., 2006).
Histology of a human anogenital wart reveals marked epithelial thickening and folding, but typically little increase in the surface keratin. Koilocytosis, or other signs of viral infection, are only variably visible within the lesions (Sterling, 2016).
Dogs
Although cutaneous warts can develop in the anogenital areas of dogs, these are caused by the same PV types and probably have the same clinical behavior as warts that develop in other areas of the body (Lange et al., 2019). None of the CPV types are thought to be sexually transmitted.
Cat.
Papillomas involving the anogenital regions of cats have not been reported.
Horses
Most equine genital warts are thought to be caused by EcPV2 (Scase et al., 2010). They appear as single or numerous (up to many hundreds) raised plaques or exophytic cauliflower-like masses (Torres and Koch, 2013). Warts can be covered by a thick layer of keratin resulting in a hard grey-brown rough surface (Fig. 5). In males they may develop anywhere on the external genitalia but are more common on the free part of the penis and glans than the preputial skin or preputial fold. Warts typically involve the vulval lips, vestibular walls and clitoris of mares (Torres and Koch, 2013). Anal warts have not been reported in horses. Penile papillomatosis describes a condition in which the free part and glans of the penis are covered by innumerable coalescing warts (Knight et al., 2011). As discussed with equine genital SCCs in the second part of this review, the method of transmission of EcPV2 is currently unknown, but sexual transmission appears unlikely to be the primary mechanism of spread. How frequently horses develop warts of the penis and vulva is also uncertain. SCCs of the penis were reported to be more common than warts in a series of equine penile lesions submitted for histology (van den Top et al., 2008). However, it is probable that a genital wart is less likely to be surgically excised and submitted for histopathology than a SCC.
Figure 5.
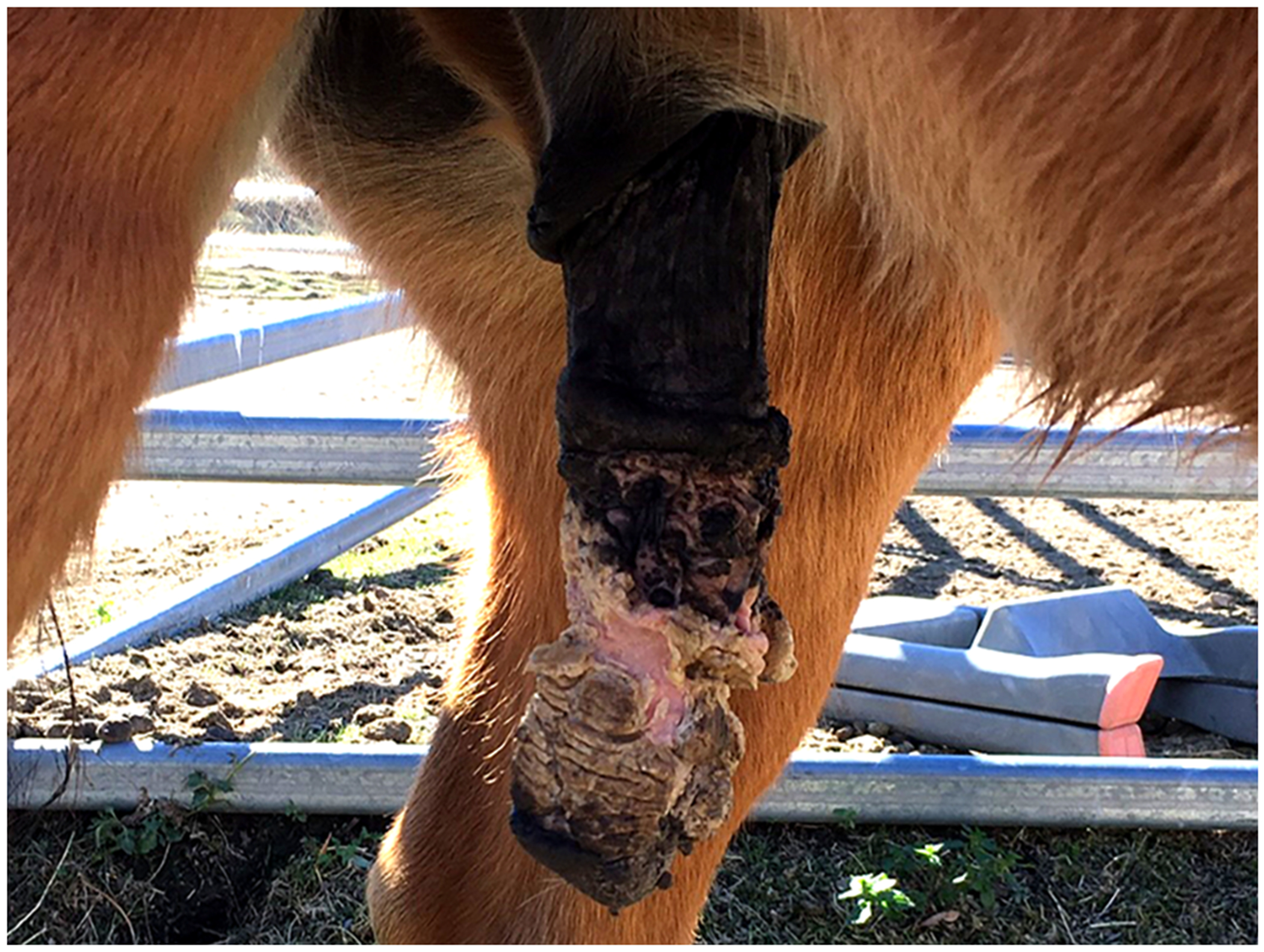
Penile papillomatosis, horse. The penis is covered by numerous warts and plaques. Some of the warts are covered in a thick layer of keratin that has resulted in a ‘horn-like’ appearance. These warts were stable for around two years. Progression to squamous cell carcinoma then occurred necessitating euthanasia of the horse (Image courtesy of Dr Richard Malik).
It is currently unknown what proportion of equine genital warts spontaneously regress or how long this takes. However, in the only published longitudinal study of equine genital warts, regression was not observed in a horse with penile papillomatosis that was observed for two years (Knight et al., 2011). Additionally, while human genital warts almost never progress to SCC, progression from a vulval wart to a SCC has been reported in a horse (Smith et al., 2009), and two of the authors of the present review have observed progression of equine penile warts to invasive SCC. Currently it appears that, compared to cutaneous and genital in humans and companion animals, EcPV2-induced equine genital warts are less likely to spontaneously regress and much more likely to progress to neoplasia. Therefore, it is probable that in the future equine genital warts will be reclassified from a hyperplastic to a pre-neoplastic lesion. There are no reports of specific treatments for equine genital warts although cryotherapy or topical treatments that cause wart destruction have been suggested (Brinsko, 1998; Torres and Koch, 2013).
Histology of an equine genital wart reveals mildly to moderately hyperplastic epithelium (Ramsauer et al., 2019). The thickened epithelium can be arranged in folds supported by a fibrous core or remain unfolded as a thickened plaque (Fig. 6). Enlarged cells with a nucleus surrounded by a clear halo (koilocytes) and depigmentation are usually visible. Cells with cytoplasm that is expanded by wispy material and clumped keratohyalin granules are variable present (Ramsauer et al., 2019).
Figure 6.
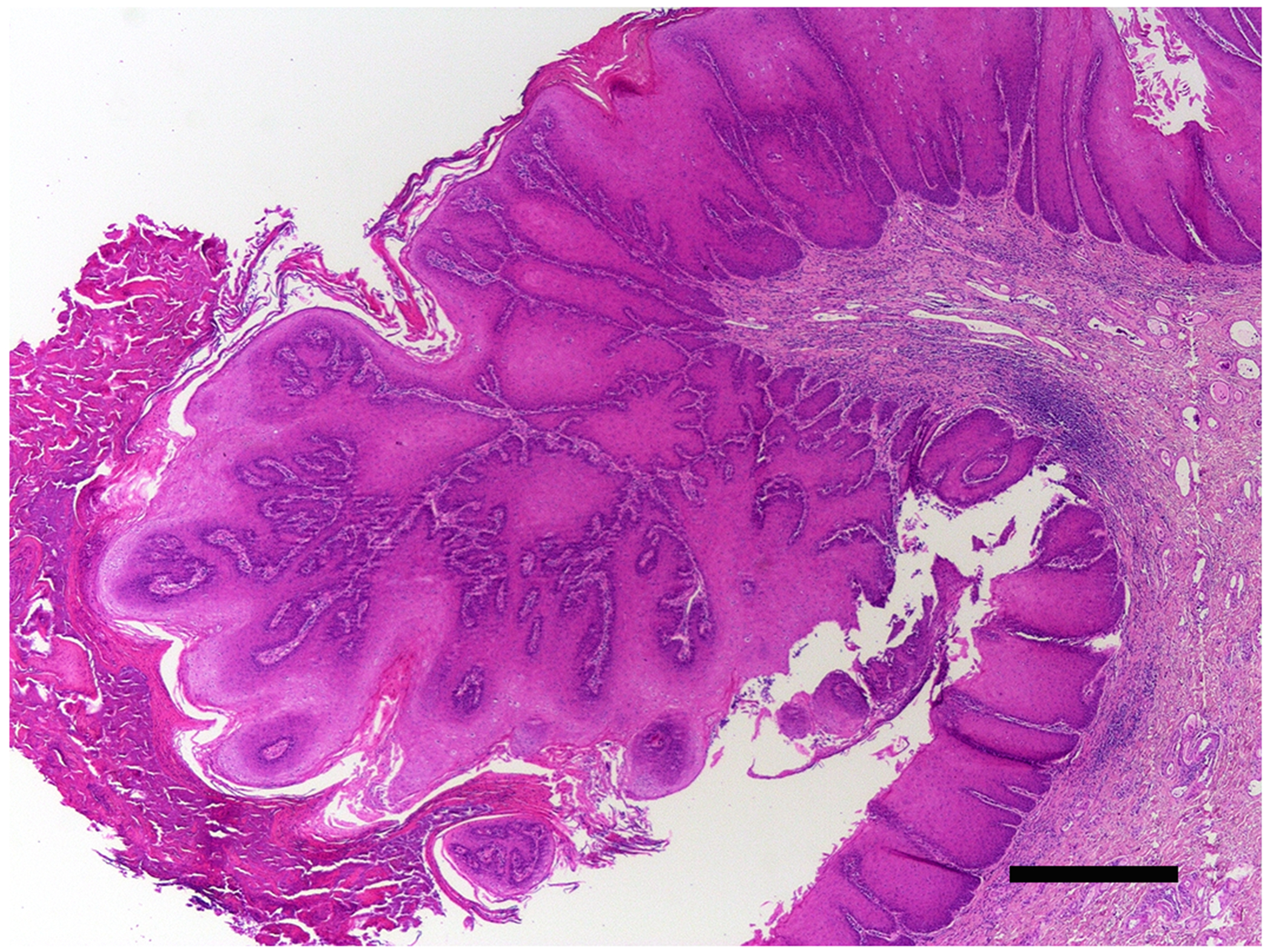
Penile wart, horse. The epithelium is markedly thickened throughout the section. However, an area of folding has resulted in an exophytic papillomatous mass that is covered by increased quantities of keratin. PCR amplified Equulis caballus papillomavirus type 2 DNA from the lesion. Scale bar = 0.5mm. Haematoxylin and eosin.
Oral warts
Humans
Oral warts are rare in people. In younger children they are caused by the same PV types that cause cutaneous warts due to autoinoculation from sucking affected fingers (Betz, 2019). Oral warts in adults are thought to be sexually transmitted and are caused by the same HPV types that cause anogenital warts (Betz, 2019). As with cutaneous and anogenital warts, spontaneous resolution of these oral warts is expected, although treatment using excisional biopsy, laser ablation or cryotherapy is often used with only rare lesion recurrence (Pringle, 2014).
Multiple non-resolving papillomas that are most numerous at the junction between respiratory columnar and stratified squamous epithelium characterise the disease recurrent respiratory papillomatosis (RRP) (Derkay and Wiatrak, 2008). RRP is subdivided into juvenile onset (around 7 years) and adult onset (around 35 years) forms of the disease (Lindeberg and Elbrønd, 1990). The papillomas are caused by the same HPVs that cause anogenital warts, but it is thought that both juvenile and adult onset RRP is caused by activation of latent HPV infections that are acquired at birth (Taliercio et al., 2015). As with recalcitrant cutaneous warts, the underlying cause of RRP is an inability to mount an appropriate immune response against the PV infection. In some patients, the papillomas become so large they significantly impact the ability of the patient to talk or even breathe (Derkay and Wiatrak, 2008). Papillomas may also undergo malignant transformation, although this appears to be rare.
Surgery is the predominant treatment for RRP with some children requiring multiple resections each year to preserve airway patency and vocal fold function (Derkay and Wiatrak, 2008). Adjunct therapy is not curative but may be used to try to extend the length of time between surgeries. Currently, treatment using monoclonal antibodies to block vascular epidermal growth factor or intralesional cidofovir is favoured (Benedict and Derkay, 2021; Hock et al. 2022). As with other non-regressing warts, little progress has been made in triggering an immune response against the papillomas.
Histologically the papillomas contain moderately hyperplastic epithelium with an increased mitotic rate and crowding of cells in the basal layer. The thickened epidermis forms multiple papillary projections supported by a fibrous core. PV-induced cellular changes are minimal within the lesions (Derkay and Wiatrak, 2008).
Dogs
Canine oral papillomatosis presents as multiple exophytic smooth or cauliflower-like warts involving the lips and mouth (Fig. 7). Similarly to canine cutaneous warts, these are particularly common in young dogs (Oğuzoğlu et al., 2017). Due to the pioneering work of M’Fadyean and Hobday (1898) these were recognized to be caused by an infectious agent over 100 years ago. These same researchers also recognized that oral warts spontaneously regressed and that these dogs were subsequently protected from the development of additional warts. The majority of canine oral warts are caused by CPV1, although there are rare reports of oral papillomas associated with other CPV types (Delius et al., 1994; Lange et al., 2012; Lange and Favrot, 2011; Nicholls et al., 1999; Oğuzoğlu et al., 2017). Few studies have investigated the epidemiology of natural infection by CPV1. There are rare reports of outbreaks of canine oral papillomatosis suggesting transmission between dogs is possible (Lane et al., 2017; Yhee et al., 2010) and there is anecdotal evidence that dogs that attend day-care facilities may be at increased risk of papilloma development. However, for many dogs there is no history of contact with an affected dog suggesting indirect transfer through the environment. It appears likely that behaviours such as stick chewing would favour transmission, especially as this behaviour can damage the oral epithelium. Dogs can be asymptomatically infected by CPV1, potentially providing a reservoir for infection (Lange et al., 2011). Most dogs do not show any systemic signs of disease although occasionally warts can interfere with normal eating or breathing (Nicholls et al., 1999).
Figure 7.
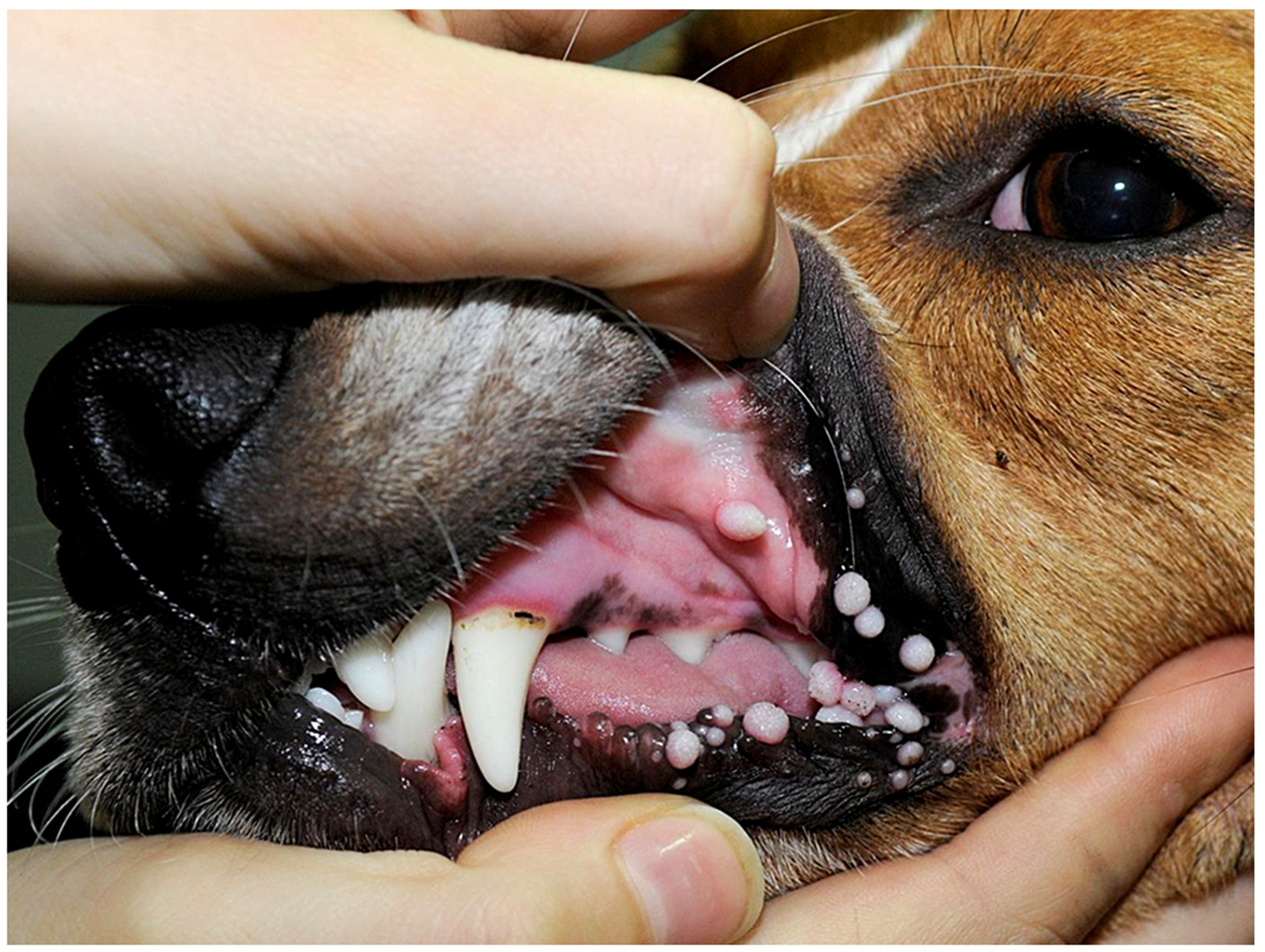
Oral warts, dog. The warts are exophytic and either appear smooth or have a more roughened surface. The warts in this dog remained small and, as expected for the vast majority of cases, resolved spontaneously (Image courtesy of Dr Anne Quain).
The overwhelming majority of canine oral warts spontaneously regress. Regression typically occurs within 4 to 8 weeks, although can take up to 12 months (Sancak et al., 2015; Yhee et al., 2010). Very rarely dogs can continue to develop additional warts that increase in size over an extended (>1 year) period (Fig. 8). These warts have been reported to spread from the oral cavity to the haired skin (Nicholls et al., 1999) or progress to SCC (Regalado Ibarra et al., 2018). No treatments have been successful and dogs that present with florid progressive warts are typically euthanatized due to the disease. Evidence from humans with recalcitrant cutaneous warts suggest that dogs probably have an underlying immune dysfunction that prevents the development of a cell-mediated immune response. In addition to the small number of dogs reported with florid persistent oral papillomatosis, evidence of progression to neoplasia was observed in around 3% of canine oral warts submitted to a diagnostic laboratory (Thaiwong et al., 2018). However, as veterinarians do not routinely excise and submit oral warts from dogs for diagnostic examination, it is likely that the oral warts included in this study had unusual clinical features. Therefore, progression of oral warts in dogs in the general population is expected to be much less common.
Figure 8.
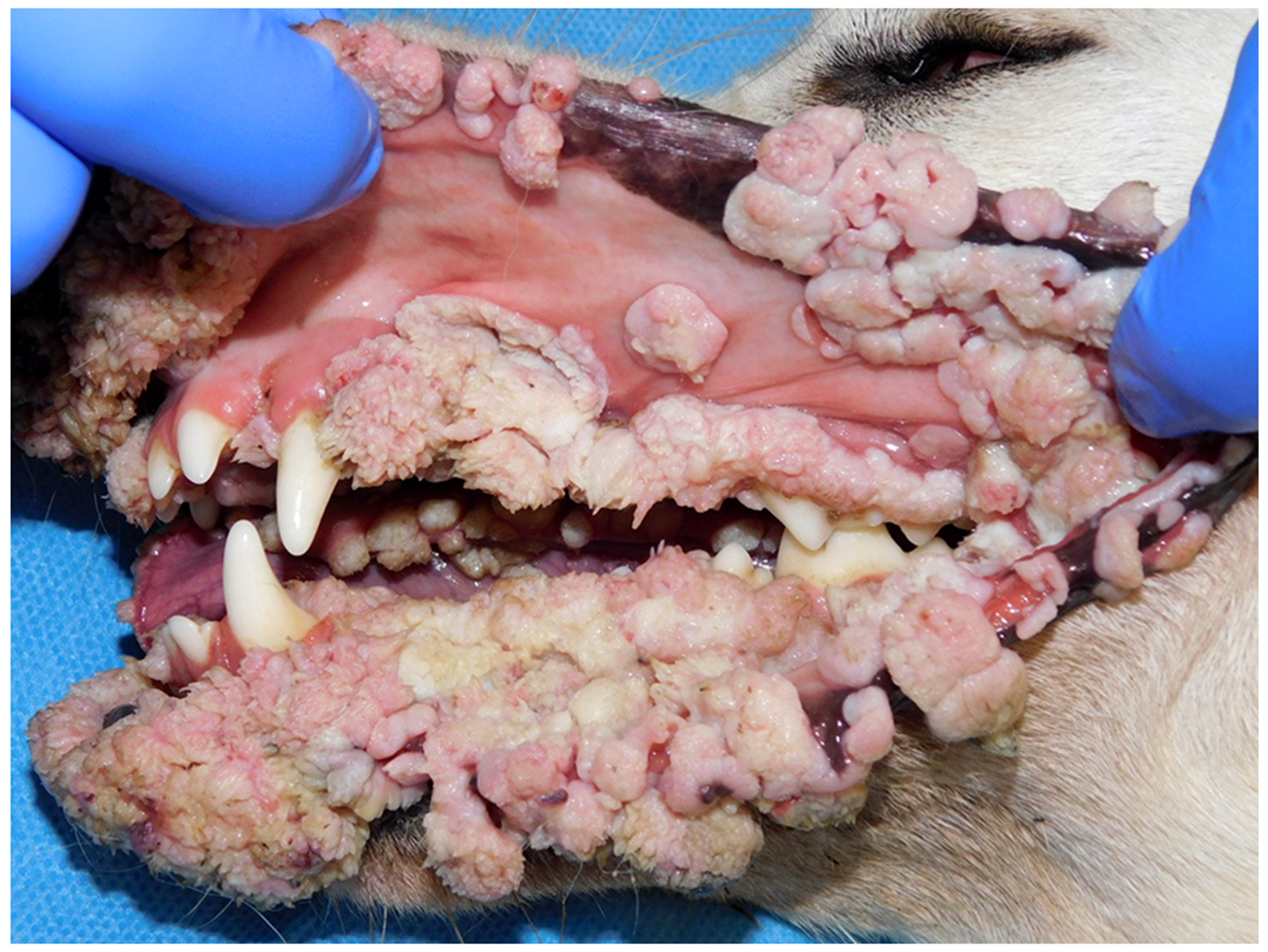
Oral warts, dog. The warts in this dog were first observed over a year ago and have continued to grow since this time. Additionally, new warts are continuing to develop in the mouth of this dog. Warts recurred after surgical excision. This dog most likely has an immune disorder that prevents the development of a normal cell-mediated response to papillomavirus infection (Image courtesy of Dr Margherita Gracis).
As oral warts typically cause little apparent discomfort and resolve spontaneously, most dogs are not treated. However, if warts become large enough to interfere with eating or breathing, they can be surgically excised or cryotherapy can be used to cause necrosis of the infected cells (Richman et al., 2017). However, recurrence is possible in the absence of an immune response against the PV. Crushing warts has been suggested as beneficial, although this is not supported by any controlled clinical studies. Numerous systemic therapies have been suggested to trigger an immune response against the oral warts and hasten spontaneous resolution. In a prospective, randomized, double-blinded placebo-controlled study of dogs with both oral and cutaneous warts, azithromycin treatment had a significant benefit with warts regressing in 10 of 10 treated, but only 1 of 7 untreated, dogs within 7 weeks (Yagci et al., 2008). While these results could suggest azithromycin triggered wart resolution, the more surprising finding was the lack of spontaneous regression of warts in untreated dogs. Indeed, in previous studies of untreated dogs, complete wart regression was reported in 7 of 7 dogs in around 3 weeks, 40 of 40 dogs within 8 weeks, and 11 of 16 dogs within 12 weeks (Sancak et al., 2015; Suzich et al., 1995; Yhee et al., 2010). Therefore, the apparent beneficial effect of azithromycin appeared mainly to be due to the chance selection of 6 dogs into the control group that had unusually long-lasting warts. Azithromycin has been used without success in multiple subsequent cases of canine warts (Regalado Ibarra et al., 2018). It is also interesting to note that the basis of using this antibiotic to treat canine warts was because it had been shown to be useful to treat a ‘papillomatous’ skin disease in humans. However, the human skin disease was not caused by PV infection (Bernstein, 2009) and azithromycin is not used to treat PV-induced diseases in humans. Cimetidine has been reported to be an effective treatment for human warts in some studies (Das et al., 2018) and there are anecdotal reports of use for canine warts. However, efficacy remains controversial in humans with several larger studies reporting no significant differences in efficacy between cimetidine and placebo (Rogers et al., 1999; Yilmaz et al., 1996). Other potential treatments for canine oral warts have generally been described in individual dogs. These include vaccination against CPV1, systemic retinoids, interferon-alpha2 as well as naturally derived treatments (Kuntsi-Vaattovaara et al., 2003; Nicholls et al., 1999). While warts resolved shortly after treatment in some dogs, oral warts are known to spontaneously resolve after a variable length of time. Therefore, without inclusion of multiple dogs and appropriate controls, it is impossible to conclude whether a treatment was beneficial or simply given coincidentally at the same time as spontaneous resolution. For a medical treatment to cause resolution of canine warts it would presumably have to trigger a cell-mediated immune response. Despite intense research, such a treatment has not been identified for warts in humans. Therefore, the evidence supporting these proposed treatments for canine warts should be interpreted with caution.
Histology of a canine oral wart reveals a typical exophytic lesion comprising thickened, folded epithelium. Prominent PV-induced cellular changes consisting of cells with cytoplasm distended by blue-grey wispy material, koilocytosis, and intranuclear viral inclusions are typically visible.
Cats
Oral warts have been rarely reported in cats. These are thought to be caused by FcaPV1 and develop in clusters on the underside of the tongue (Munday et al., 2015; Sundberg et al., 2000). They are presumed to resolve spontaneous although no cat with oral warts has been followed in a longitudinal study.
Horses
There is a single report of an EcPV2-induced laryngeal wart in a horse (Hibi et al., 2019). The wart was continuous with a SCC and the thickening of the epithelium could have been secondary to the neoplasm. Alternatively, it is possible that EcPV2 causes, as in the genital mucosa, oral warts in horses that are less likely to spontaneously resolve and more likely to progress to neoplasia than oral warts in other species.
Conclusions
Young children, dogs and horses all commonly develop cutaneous warts due to similar predisposing factors. The overwhelming majority of cutaneous non-genital warts in all species are expected to spontaneously resolve. This occurs after a variable amount of time that can be as long as 2 years in dogs and over 4 years in people. One exception is equine cutaneous warts caused by EcPV8. These appear much less likely to resolve spontaneously and these warts can also progress to neoplasia. Whether this is due to the specific PV type or due to an underlying immune dysfunction is currently unknown. While both humans and horses develop genital warts, there are significant differences between the two entities. Unlike human genital warts that are sexually transmitted, the method of transmission of the causative equine PV is currently unknown. Additionally, equine genital warts appear much less likely to spontaneously resolve and much more likely to progress to neoplasia than human genital warts. Although reported in humans, cats, and horses, oral warts are only common in dogs. The majority of canine oral warts are expected to spontaneously resolve after a variable length of time. However, a small minority persist and continue to develop over an extended time. Currently, there are no consistently effective treatments for these dogs and a poor prognosis is currently recommended when warts develop and persist over a 12-month period. While there has been intense research on ways to hasten the resolution of human warts, therapies used today are based on destruction of the wart itself with little progress made on therapies that attempt to trigger the immune system to attack cells infected by PVs. People very rarely develop progressive and persistent recalcitrant warts and these people usually have T-cell defects preventing a normal cell-mediated immune response. Due to the underlying immune defect, no consistent curative treatment has been identified for these patients. Only small numbers of dogs with a >1 year history of progressive and persistent warts have been reported. It is likely these dogs were also unable to mount an effective immune response against the PV infection and, as often in humans, no treatment was curative.
Footnotes
See: Papillomavirus Episteme. https://pave.niaid.nih.gov/#home (accessed 1 March 2022)
References
- Altamura G, Tommasino M, Borzacchiello G, 2020. Cutaneous vs. mucosal tropism: The papillomavirus paradigm comes to an “and”. Frontiers of Microbiology 11, 588663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsson A, Forslund O, Ekberg H, Sterner G, Hansson BG, 2000. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. Journal of Virology 74, 11636–11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsson A, Hansson BG, 2002. Healthy skin of many animal species harbors papillomaviruses which are closely related to their human counterparts. Journal of Virology 76, 12537–12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsson A, Erfurt C, Hazard K, Holmgren V, Simon M, Kataoka A, Hossain S, Håkangård C, Hansson BG, 2003a. Prevalence and type spectrum of human papillomaviruses in healthy skin samples collected in three continents. Journal of General Virology 84, 1881–1886. [DOI] [PubMed] [Google Scholar]
- Antonsson A, Karanfilovska S, Lindqvist PG, Hansson BG, 2003b. General acquisition of human papillomavirus infections of skin occurs in early infancy. Journal of Clinical Microbiology 41, 2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J, Leyland A, 1975. Histological survey of tumours of the horse, with particular reference to those of the skin. The Veterinary Record 96, 419–422. [DOI] [PubMed] [Google Scholar]
- Benedict JJ, Derkay CS, 2021. Recurrent respiratory papillomatosis: A 2020 perspective. Laryngoscope Investigative Otolaryngology 6, 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard HU, Burk RD, Chen Z, van Doorslaer K, Hausen H, de Villiers EM, 2010. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 401, 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein JA, 2009. Letters to the Editor. Veterinary Dermatology 20, 83–83.19320876 [Google Scholar]
- Betz SJ, 2019. HPV-related papillary lesions of the oral mucosa: A review. Head and Neck Pathology 13, 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beziat V, Casanova JL, Jouanguy E, 2021. Human genetic and immunological dissection of papillomavirus-driven diseases: new insights into their pathogenesis. Current Opinions in Virology 51, 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaert L, Martens A, Van Poucke M, Ducatelle R, De Cock H, Dewulf J, De Baere C, Peelman L, Gasthuys F, 2008. High prevalence of bovine papillomaviral DNA in the normal skin of equine sarcoid-affected and healthy horses. Veterinary Microbiology 129, 58–68. [DOI] [PubMed] [Google Scholar]
- Brinsko SP, 1998. Neoplasia of the male reproductive tract. Veterinary Clinics of North America: Equine Practice 14, 517–533. [DOI] [PubMed] [Google Scholar]
- Bruggink SC, Gussekloo J, Berger MY, Zaaijer K, Assendelft WJ, de Waal MW, Bavinck JN, Koes BW, Eekhof JA, 2010. Cryotherapy with liquid nitrogen versus topical salicylic acid application for cutaneous warts in primary care: randomized controlled trial. Canadian Medical Association Journal 182, 1624–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter JL, Kreider JW, Alroy J, Schmidt GM, 1992. Cutaneous xanthogranuloma and viral papilloma on an eyelid of a cat. Veterinary Dermatology 3, 187–190. [Google Scholar]
- Chang CY, Chen WT, Haga T, Yamashita N, Lee CF, Tsuzuki M, Chang HW, 2020. The detection and association of canine papillomavirus with benign and malignant skin lesions in dogs. Viruses 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffo G, 1907. Innesto positivo con filtrato di verruca volgare. Giornale Italiano delle Malattie Venereologia 48, 12–17. [Google Scholar]
- Cook RH, Olson C, 1951. Experimental transmission of cutaneous papilloma of the horse. American Journal of Pathology 27, 1087–1097. [PMC free article] [PubMed] [Google Scholar]
- Das BB, Anton K, Soares N, Riojas S, Mcdermott J, Knox L, Daneman S, Puente BN, 2018. Cimetidine: A safe treatment option for cutaneous warts in pediatric heart transplant recipients. Medical Sciences 6, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H, 2004. Classification of papillomaviruses. Virology 324, 17–27. [DOI] [PubMed] [Google Scholar]
- Delius H, Van Ranst MA, Jenson AB, zur Hausen H, Sundberg JP, 1994. Canine oral papillomavirus genomic sequence: a unique 1.5-kb intervening sequence between the E2 and L2 open reading frames. Virology 204, 447–452. [DOI] [PubMed] [Google Scholar]
- Derkay CS, Wiatrak B, 2008. Recurrent respiratory papillomatosis: A review. The Laryngoscope 118, 1236–1247. [DOI] [PubMed] [Google Scholar]
- Doorbar J, 2005. The papillomavirus life cycle. Journal of Clinical Virology 32, S7–15. [DOI] [PubMed] [Google Scholar]
- Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, Stanley MA, 2012. The biology and life-cycle of human papillomaviruses. Vaccine 30, F55–70. [DOI] [PubMed] [Google Scholar]
- Egawa N, Doorbar J, 2017. The low-risk papillomaviruses. Virus Research 231, 119–127. [DOI] [PubMed] [Google Scholar]
- Falk E, Lange CE, Jennings S, Ferrer L, 2017. Two cutaneous horns associated with canine papillomavirus type 1 infection in a pit bull dog. Veterinary Dermatology 28, 420–421. [DOI] [PubMed] [Google Scholar]
- Flagg EW, Torrone EA, 2018. Declines in anogenital warts among age groups most likely to be impacted by human papillomavirus vaccination, United States, 2006-2014. American Journal of Public Health 108, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forslund O, Antonsson A, Nordin P, Stenquist B, Hansson BG, 1999. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. Journal of General Virology 80, 2437–2443. [DOI] [PubMed] [Google Scholar]
- Franco EL, Villa LL, Sobrinho JP, Prado JM, Rousseau M-C, Désy M, Rohan TE, 1999. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. The Journal of Infectious Diseases 180, 1415–1423. [DOI] [PubMed] [Google Scholar]
- Ghim S, Newsome J, Bell J, Sundberg JP, Schlegel R, Jenson AB, 2000. Spontaneously regressing oral papillomas induce systemic antibodies that neutralize canine oral papillomavirus. Experimental Molecular Pathology 68, 147–151. [DOI] [PubMed] [Google Scholar]
- Ghim SJ, Rector A, Delius H, Sundberg JP, Jenson AB, Van Ranst M, 2004. Equine papillomavirus type 1: complete nucleotide sequence and characterization of recombinant virus-like particles composed of the EcPV-1 L1 major capsid protein. Biochemistry and Biophysical Research Communications 324, 1108–1115. [DOI] [PubMed] [Google Scholar]
- Gilson R, Nugent D, Bennett K, Doré CJ, Murray ML, Meadows J, Haddow LJ, Lacey C, Sandmann F, Jit M, Soldan K, Tetlow M, Caverly E, Nathan M, Copas AJ, 2020. Imiquimod versus podophyllotoxin, with and without human papillomavirus vaccine, for anogenital warts: the HIPvac factorial RCT. Health Technology Assessments 24, 1–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt MH, Kennedy JS, Kennedy DR, Yuan H, Holt DE, Casal ML, Traas AM, Mauldin EA, Moore PF, Henthorn PS, Hartnett BJ, Weinberg KI, Schlegel R, Felsburg PJ, 2006. Severe papillomavirus infection progressing to metastatic squamous cell carcinoma in bone marrow-transplanted X-linked SCID dogs. Journal of Virology 80, 6621–6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt MH, Munday JS, Scruggs JL, Klopfleisch R, Kiupel M, 2018. Epithelial Tumors of the Skin. Davis-Thomson Foundation, Gurnee, Illinois. [Google Scholar]
- Gould AP, Coyner KS, Trimmer AM, Tater K, Rishniw M, 2021. Canine pedal papilloma identification and management: a retrospective series of 44 cases. Veterinary Dermatology 32, 509–e141. [DOI] [PubMed] [Google Scholar]
- Graham SV, 2017. The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clinical Science (London) 131, 2201–2221. [DOI] [PubMed] [Google Scholar]
- Gross TL, Ihrke PJ, Walder EJ, Affolter VK, 2005. Skin diseases of the dog and cat: clinical and histopathologic diagnosis, 2nd edition. Blackwell Science, Oxford, United Kingdom. [Google Scholar]
- Hamada M, Oyamada T, Yoshikawa H, Yoshikawa T, Itakura C, 1990. Histopathological development of equine cutaneous papillomas. Journal of Compative Pathology 102, 393–403. [DOI] [PubMed] [Google Scholar]
- Hanna E, Abadi R, Abbas O, 2016. Imiquimod in dermatology: an overview. International Journal of Dermatology 55, 831–844. [DOI] [PubMed] [Google Scholar]
- Hibi H, Hatama S, Obata A, Shibahara T, Kadota K, 2019. Laryngeal squamous cell carcinoma and papilloma associated with Equus caballus papillomavirus 2 in a horse. Journal of Veterinary Medical Science 81, 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock K, Kennedy A, Howell R, Friedman A, de Alarcon A, Khosla S, 2022. Surgery and adjuvant therapy improve Derkay scores in adult and pediatric respiratory papillomatosis. The Laryngoscope DOI. 10.1002/lary.30042. [DOI] [PubMed] [Google Scholar]
- Junge R, Sundberg J, Lancaster W, 1984. Papillomas and squamous cell carcinomas of horses. Journal of the American Veterinary Medical Association 185, 656–659. [PubMed] [Google Scholar]
- Karagounis TK, Pomeranz MK, 2021. Viral venereal diseases of the skin. American Journal of Clinical Dermatology 22, 523–540. [DOI] [PubMed] [Google Scholar]
- Karamanou M, Agapitos E, Kousoulis A, Androutsos G, 2010. From the humble wart to HPV: a fascinating story throughout centuries. Oncology Reviews 4, 133–135. [Google Scholar]
- Kim D, Dobromylskyj MJ, O’Neill D, Smith KC, 2022. Skin masses in dogs under one year of age. Journal of Small Animal Practice 63, 10–15. [DOI] [PubMed] [Google Scholar]
- Klumpp DJ, Laimins LA, 1999. Differentiation-induced changes in promoter usage for transcripts encoding the human papillomavirus type 31 replication protein E1. Virology 257, 239–246. [DOI] [PubMed] [Google Scholar]
- Knight CG, Munday JS, Rosa BV, Kiupel M, 2011. Persistent, widespread papilloma formation on the penis of a horse: a novel presentation of equine papillomavirus type 2 infection. Veterinary Dermatology 22, 570–574. [DOI] [PubMed] [Google Scholar]
- Knight CG, Dunowska M, Munday JS, Peters-Kennedy J, Rosa BV, 2013. Comparison of the levels of Equus caballus papillomavirus type 2 (EcPV-2) DNA in equine squamous cell carcinomas and non-cancerous tissues using quantitative PCR. Veterinary Microbiology 166, 257–262. [DOI] [PubMed] [Google Scholar]
- Kuntsi-Vaattovaara H, Verstraete FJ, Newsome JT, Yuan H, 2003. Resolution of persistent oral papillomatosis in a dog after treatment with a recombinant canine oral papillomavirus vaccine. Veterinary Comparative Oncology 1, 57–63. [DOI] [PubMed] [Google Scholar]
- Kwok CS, Gibbs S, Bennett C, Holland R, Abbott R, 2012. Topical treatments for cutaneous warts. Cochrane Database Syst Review 2012, Cd001781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey CJN, Lowndes CM, Shah KV, 2006. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 24, S35–S41. [DOI] [PubMed] [Google Scholar]
- Lacey CJ, Woodhall SC, Wikstrom A, Ross J, 2013. 2012 European guideline for the management of anogenital warts. Journal of the European Academy of Dermatology and Venereology 27, e263–270. [DOI] [PubMed] [Google Scholar]
- Lane HE, Weese JS, Stull JW, 2017. Canine oral papillomavirus outbreak at a dog daycare facility. Canadian Veterinary Journal 58, 747–749. [PMC free article] [PubMed] [Google Scholar]
- Lange CE, Tobler K, Ackermann M, Panakova L, Thoday KL, Favrot C, 2009. Three novel canine papillomaviruses support taxonomic clade formation. Journal of General Virology 90, 2615–2621. [DOI] [PubMed] [Google Scholar]
- Lange CE, Favrot C, 2011. Canine papillomaviruses. The Veterinary Clinics of North America. Small Animal Practice 41, 1183–1195. [DOI] [PubMed] [Google Scholar]
- Lange CE, Zollinger S, Tobler K, Ackermann M, Favrot C, 2011. Clinically healthy skin of dogs is a potential reservoir for canine papillomaviruses. Journal of Clinical Microbiology 49, 707–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange CE, Ackermann M, Favrot C, Tobler K, 2012. Entire genomic sequence of novel canine papillomavirus type 13. Journal of Virology 86, 10226–10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange CE, Jennings SH, Diallo A, Lyons J, 2019. Canine papillomavirus types 1 and 2 in classical papillomas: High abundance, different morphological associations and frequent co-infections. Veterinary Journal 250, 1–5. [DOI] [PubMed] [Google Scholar]
- Larsson P, Liden S, 1980. Prevalence of skin diseases among adolescents 12-16 years of age. Acta Dermatovenereologica 60, 415–423. [PubMed] [Google Scholar]
- Lee HJ, Kim JK, Kim DH, Yoon MS, 2011. Condyloma accuminatum treated with recombinant quadrivalent human papillomavirus vaccine (types 6, 11, 16, 18). Journal of the American Academy of Dermatology 64, e130–e132. [DOI] [PubMed] [Google Scholar]
- Lindeberg H, Elbrønd O, 1990. Laryngeal papillomas: the epidemiology in a Danish subpopulation 1965-1984. Clinical Otolaryngology and Allied Sciences 15, 125–131. [DOI] [PubMed] [Google Scholar]
- Linder KE, Bizikova P, Luff J, Zhou D, Yuan H, Breuhaus B, Nelson E, Mackay R, 2018. Generalized papillomatosis in three horses associated with a novel equine papillomavirus (EcPV8). Veterinary Dermatology 29, 72–e30. [DOI] [PubMed] [Google Scholar]
- M’Fadyean J, Hobday F, 1898. Note on the experimental transmission of warts in the dog. Journal of Comparative Pathology and Therapeutics 11, 341–344. [Google Scholar]
- Massing AM, Epstein WL, 1963. Natural history of warts: A two-year study. Archives of Dermatology 87, 306–310. [DOI] [PubMed] [Google Scholar]
- McBride AA, 2013. The papillomavirus E2 proteins. Virology 445, 57–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride AA, 2022. Human papillomaviruses: diversity, infection and host interactions. National Reviews of Microbiology 20, 95–108. [DOI] [PubMed] [Google Scholar]
- Miller RL, Gerster JF, Owens ML, Slade HB, Tomai M.a., 1999. Imiquimod applied topically: a novel immune response modifier and new class of drug. International Journal of Immunopharmacology 21, 1–14. [DOI] [PubMed] [Google Scholar]
- Munday JS, Hanlon EM, Howe L, Squires RA, French AF, 2007. Feline cutaneous viral papilloma associated with human papillomavirus type 9. Veterinary Pathology 44, 924–927. [DOI] [PubMed] [Google Scholar]
- Munday JS, French AF, MacNamara AR, 2010. The development of multiple cutaneous inverted papilloma following ovariohysterectomy in a dog. New Zealand Veterinary Journal 58, 168–171. [DOI] [PubMed] [Google Scholar]
- Munday JS, Witham AI, 2010. Frequent detection of papillomavirus DNA in clinically normal skin of cats infected and noninfected with feline immunodeficiency virus. Veterinary Dermatology 21, 307–310. [DOI] [PubMed] [Google Scholar]
- Munday JS, Knight CG, French AF, 2011. Evaluation of feline oral squamous cell carcinomas for p16CDKN2A protein immunoreactivity and the presence of papillomaviral DNA. Research in Veterinary Science 90, 280–283. [DOI] [PubMed] [Google Scholar]
- Munday JS, Fairley RA, Mills H, Kiupel M, Vaatstra BL, 2015. Oral papillomas associated with Felis catus papillomavirus type 1 in 2 domestic cats. Veterinary Pathology 52, 1187–1190. [DOI] [PubMed] [Google Scholar]
- Munday JS, Thomson NA, Luff JA, 2017. Papillomaviruses in dogs and cats. Veterinary Journal 225, 23–31. [DOI] [PubMed] [Google Scholar]
- Munday JS, Hardcastle MR, Sim M, 2020a. Detection of a putative novel papillomavirus type within a large exophytic papilloma on the fetlock of a horse. Pathogens 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday JS, Piripi SA, Julian A, Martin SJ, 2020b. Long-term recurrent, yet nonprogressive, pedal viral papillomas in a dog. Veterinary Dermatology 31, 489–e128. [DOI] [PubMed] [Google Scholar]
- Munday JS, Thomson NA, 2021. Papillomaviruses in domestic cats. Viruses 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday JS, Julian A, 2022. Exophytic cutaneous papilloma associated with a novel papillomavirus sequence in a cat. Journal of Veterinary Diagnostic Investigation. doi: 10.1177/10406387221107152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls PK, Klaunberg BA, Moore RA, Santos EB, Parry NR, Gough GW, Stanley MA, 1999. Naturally occurring, nonregressing canine oral papillomavirus infection: host immunity, virus characterization, and experimental infection. Virology 265, 365–374. [DOI] [PubMed] [Google Scholar]
- Nicholls PK, Moore PF, Anderson DM, Moore RA, Parry NR, Gough GW, Stanley MA, 2001. Regression of canine oral papillomas is associated with infiltration of CD4+ and CD8+ lymphocytes. Virology 283, 31–39. [DOI] [PubMed] [Google Scholar]
- Oğuzoğlu TÇ, Timurkan MÖ, Koç BT, Alkan F, 2017. Comparison of genetic characteristics of canine papillomaviruses in Turkey. Infection, Genetics and Evolution 55, 372–376. [DOI] [PubMed] [Google Scholar]
- Oriel JD, 1971. Natural history of genital warts. British Journal of Venereal Disease 47, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi M, Mazzei M, Vascellari M, Melchiotti E, Zanardello C, Verin R, Albanese F, Necci F, Pazzini L, Lazzarini G, Abramo F, 2021. Localization and genotyping of canine papillomavirus in canine inverted papillomas. Journal of Veterinary Diagnostic Investigation 33, 1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth G, Jablonska S, Favre M, Croissant O, Jarzabek-Chorzelska M, Rzesa G, 1978. Characterization of two types of human papillomaviruses in lesions of epidermodysplasia verruciformis. Proceedings of the National Academy of Sciences 75, 1537–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbun MA, 2002. Human papillomavirus type 31b infection of human keratinocytes and the onset of early transcription. Journal of Virology 76, 11291–11300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padel AF, Venning VA, Evans MF, Quantrill AM, Fleming KA, 1990. Human papillomaviruses in anogenital warts in children: typing by in situ hybridisation. British Medical Journal 300, 1491–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters-Kennedy J, Lange CE, Rine SL, Hackett RP, 2019. Equus caballus papillomavirus 8 (EcPV8) associated with multiple viral plaques, viral papillomas, and squamous cell carcinoma in a horse. Equine Veterinary Journal 51, 470–474. [DOI] [PubMed] [Google Scholar]
- Pringle GA, 2014. The role of human papillomavirus in oral disease. Dental Clinics 58, 385–399. [DOI] [PubMed] [Google Scholar]
- Ramsauer AS, Wachoski-Dark GL, Fraefel C, Tobler K, Brandt S, Knight CG, Favrot C, Grest P, 2019. Paving the way for more precise diagnosis of EcPV2-associated equine penile lesions. BMC Veterinary Research 15, 356–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regalado Ibarra AM, Legendre L, Munday JS, 2018. Malignant transformation of a canine papillomavirus type 1-induced persistent oral papilloma in a 3-year-old dog. Journal of Veterinary Dentistry 35, 79–95. [DOI] [PubMed] [Google Scholar]
- Richman AW, Kirby AL, Rosenkrantz W, Muse R, 2017. Persistent papilloma treated with cryotherapy in three dogs. Veterinary Dermatology 28, 625–e154. [DOI] [PubMed] [Google Scholar]
- Rogers CJ, Gibney MD, Siegfried EC, Harrison BR, Glaser DA, 1999. Cimetidine therapy for recalcitrant warts in adults: Is it any better than placebo? Journal of the American Academy of Dermatology 41, 123–127. [DOI] [PubMed] [Google Scholar]
- Sancak A, Favrot C, Geisseler MD, Muller M, Lange CE, 2015. Antibody titres against canine papillomavirus 1 peak around clinical regression in naturally occurring oral papillomatosis. Veterinary Dermatology 26, 57–59, e19-20. [DOI] [PubMed] [Google Scholar]
- Scase T, Brandt S, Kainzbauer C, Sykora S, Bijmholt S, Hughes K, Sharpe S, Foote A, 2010. Equus caballus papillomavirus-2 (EcPV-2): an infectious cause for equine genital cancer? Equine Veterinary Journal 42, 738–745. [DOI] [PubMed] [Google Scholar]
- Sedlacek TV, Cunnane M, Carpiniello V, 1986. Colposcopy in the diagnosis of penile condyloma. American Journal of Obstetrics and Gynecology 154, 494–496. [DOI] [PubMed] [Google Scholar]
- Smith MA, Levine DG, Getman LM, Parente EJ, Engiles JB, 2009. Vulvar squamous cell carcinoma in situ within viral papillomas in an aged quarter horse mare. Equine Veterinary Education 21, 11–16. [Google Scholar]
- Stanley M, 2006. Immune responses to human papillomavirus. Vaccine 24, S16–22. [DOI] [PubMed] [Google Scholar]
- Sterling JC, 2016. Viral infections. Rook’s Textbook of Dermatology, Ninth Edition, 1–124. [Google Scholar]
- Sundberg J, Burnstein T, Page E, Kirkham W, Robinson F, 1977. Neoplasms of equidae. Journal of the American Veterinary Medical Association 170, 150–152. [PubMed] [Google Scholar]
- Sundberg JP, Smith EK, Herron AJ, Jenson AB, Burk RD, Van Ranst M, 1994. Involvement of canine oral papillomavirus in generalized oral and cutaneous verrucosis in a Chinese Shar Pei dog. Veterinary Pathology 31, 183–187. [DOI] [PubMed] [Google Scholar]
- Sundberg JP, Van Ranst M, Montali R, Homer BL, Miller WH, Rowland PH, Scott DW, England JJ, Dunstan RW, Mikaelian I, Jenson AB, 2000. Feline papillomas and papillomaviruses. Veterinary Pathology 37, 1–10. [DOI] [PubMed] [Google Scholar]
- Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, Newsome JA, Jenson AB, Schlegel R, 1995. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proceedings of the National Academy of Sciences of the United States of America 92, 11553–11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliercio S, Cespedes M, Born H, Ruiz R, Roof S, Amin MR, Branski RC, 2015. Adult-onset recurrent respiratory papillomatosis: A review of disease pathogenesis and implications for patient counseling. Journal of the American Medical Assocation Otolaryngology, Head & Neck Surgery 141, 78–83. [DOI] [PubMed] [Google Scholar]
- Thaiwong T, Sledge DG, Wise AG, Olstad K, Maes RK, Kiupel M, 2018. Malignant transformation of canine oral papillomavirus (CPV1)-associated papillomas in dogs: An emerging concern? Papillomavirus Research 6, 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres SM, Koch SN, 2013. Papillomavirus-associated diseases. Veterinary Clinics of North America: Equine Practice 29, 643–655. [DOI] [PubMed] [Google Scholar]
- Uitto J, Saeidian AH, Youssefian L, Saffarian Z, Casanova JL, Beziat V, Jouanguy E, Vahidnezhad H, 2021. Recalcitrant warts, epidermodysplasia verruciformis, and the tree-man syndrome: Phenotypic spectrum of cutaneous human papillomavirus infections at the intersection of genetic variability of viral and human genomes. Journal of Investigative Dermatology 142, 1265–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Top JG, de Heer N, Klein WR, Ensink JM, 2008. Penile and preputial tumours in the horse: a retrospective study of 114 affected horses. Equine Veterinary Journal 40, 528–532. [DOI] [PubMed] [Google Scholar]
- van Haalen FM, Bruggink SC, Gussekloo J, Assendelft WJ, Eekhof JA, 2009. Warts in primary schoolchildren: prevalence and relation with environmental factors. British Journal of Dermatology 161, 148–152. [DOI] [PubMed] [Google Scholar]
- Williams HC, Pottier A, Strachan D, 1993. The descriptive epidemiology of warts in British schoolchildren. British Journal of Dermatology 128, 504–511. [DOI] [PubMed] [Google Scholar]
- Yagci BB, Ural K, Ocal N, Haydardedeoglu AE, 2008. Azithromycin therapy of papillomatosis in dogs: a prospective, randomized, double-blinded, placebo-controlled clinical trial. Veterinary Dermatology 19, 194–198. [DOI] [PubMed] [Google Scholar]
- Yhee J-Y, Kwon B-J, Kim J-H, Yu C-H, Im K-S, Lee S-S, Lyoo Y-S, Chang B-J, Sur J-H, 2010. Characterization of canine oral papillomavirus by histopathological and genetic analysis in Korea. Journal of Veterinary Science 11, 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz E, Alpsoy E, Basaran E, 1996. Cimetidine therapy for warts: A placebo-controlled, double-blind study. Journal of the American Academy of Dermatology 34, 1005–1007. [DOI] [PubMed] [Google Scholar]
- zur Hausen H, 1976. Condylomata acuminata and human genital cancer. Cancer Research 36, 794. [PubMed] [Google Scholar]


