Abstract
BZLF1 plays a key role in the induction of Epstein-Barr virus (EBV) replication. On the basis of limited sequence homology and mutagenesis experiments, BZLF1 has been described as a member of the bZip family of transcription factors, but this prospect has not been rigorously tested to date. Here, we present biophysical analysis of the multimerization domain of BZLF1, from three natural variants of EBV, and demonstrate for the first time that the region between amino acids 196 and 227 is sufficient to direct folding as a coiled-coil dimer in vitro.
BZLF1 (also known as Zebra, Zta, Z, and EB1) is a key component of the machinery that induces the lytic replicative cycle of Epstein-Barr virus (EBV) (3, 4, 6, 22, 27). Increased expression of BZLF1 is one of the first events that can be detected following the induction of the lytic cycle in EBV-harboring B lymphocytes, and the enforced expression of BZLF1 is sufficient to induce the lytic cycle in cells containing EBV genomes. BZLF1 functions as a transcription factor and activates its own expression and that of a subset of EBV and cellular genes through sequence-specific BZLF1 response elements within their respective promoters. Furthermore, BZLF1 also acts as a replication factor by interacting specifically with the viral lytic origin of replication (23), again through specific BZLF1 response elements (reviewed in references 21 and 24–26).
BZLF1 has been previously described as a member of the bZip family (2, 5, 7, 13–15, 20, 25, 28). In support of this conjecture, it has been shown that BZLF1 contains adjacent DNA-binding (approximately residues 175 to 195) and multimerization (approximately residues 196 to 245) regions, and the protein interacts with specific DNA sequence elements (2, 5, 8, 13, 15, 20, 28, 30) as a multimer (2, 8, 15). By analogy with other members of the bZip family, multimerization has been assumed to occur through the folding of a coiled-coil interface within this region (i.e., residues 196 to 245). The analysis of deletion mutants of BZLF1 and mutants containing one or more amino acid substitutions at residues within the proposed coiled-coil interface provided some support for this model (2, 5, 7, 13–15, 20, 25, 28), although there has been disagreement about the influence of some residues (Y200 and L225, for example) on the ability to multimerize (7, 12). Therefore, while the results of numerous studies have been consistent with the existence of a coiled-coil interface on BZLF1, the studies have fallen short of rigorously testing the model.
To date, three naturally occurring sequence variants within the multimerization domain of BZLF1 have been described. Previous analyses have focused on the BZLF1 sequence deduced from the B95-8 isolate of EBV (1); using this as a reference sequence, the two variants are A205S (19) and A206S (10). In this study, we sought to determine whether further variants of the multimerization region occur in natural isolates of EBV before exploring biophysically whether BZLF1 is indeed able to form multimers through a coiled-coil interface.
The regions surrounding exons 2 and 3 of BZLF1 were amplified by PCR using DNA from Burkitt's lymphoma cell lines (Rael, Mutu, Jijoye, and Akata) (9, 17, 27) and from nine lymphoblastoid cell lines established from patients with EBV-associated diseases. The DNA sequences were then determined. The BZLF1 coding sequence was remarkably conserved, with 48 of the 50 residues invariant (Fig. 1). However, examples of each of the known natural variants were also identified; six samples contained A205S and two contained A206S, but none of the isolates contained the double variation A205S-A206S.
FIG. 1.
Sequence variation within the BZLF1 multimerization domain. The deduced protein sequences encoded by BZLF1 exons 2 and 3 from 11 isolates of EBV are shown aligned with the B95-8 sequence. Variations are shaded.
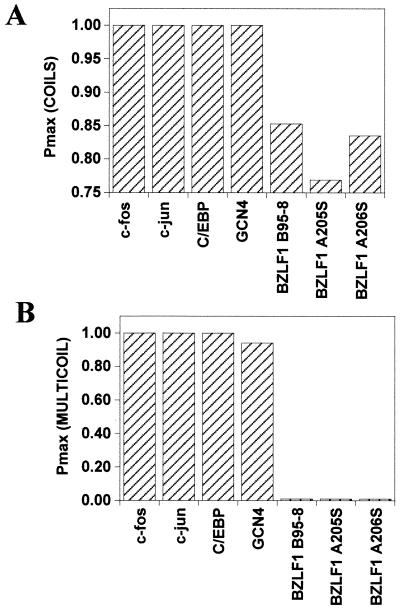
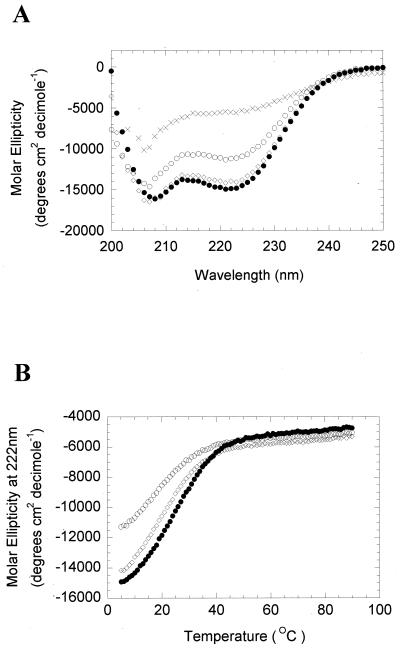
The propensity of all three natural variants of BZLF1 to form coiled coils was assessed using two structural modeling computer programs: COILS (16) and MULTICOIL (31). Surprisingly, neither program predicted the formation of a coiled-coil motif in BZLF1 approaching that of established members of the bZip family, such as GCN4 and C/EBP (Fig. 2). As a more direct test of whether the three natural variants of BZLF1 were able to fold as coiled coils, synthetic peptides encompassing the minimal multimerization domain BZLF1 (residues 196 to 227) were synthesized and their secondary structures were assessed using circular dichroism (CD) spectroscopy (Fig. 3A). The three BZLF1 peptides displayed spectra characteristic of α-helical structures, with minima around 208 and 222 nm (the more negative the signal at 222 nm, the higher the α-helical content). For comparison, a heat-denatured peptide with an unfolded, random structure displaying a negligible signal in this region is also shown. The characteristic double minima indicative of α-helical structures were clear for all three peptides, although a reduced signal intensity relative to that of the B95-8 peptide was observed for A205S and, to a lesser extent, A206S. This finding suggests that these natural variants are somewhat less helical than B95-8. Further analysis of the stability of the α-helical structures revealed that all three BZLF1 peptides exhibited sigmoidal unfolding curves typical of cooperatively folded structures, such as coiled coils (Fig. 3B). However, the midpoint temperatures of unfolding (Tm) of the peptides (25, 17, and 19°C for B95-8, A205S, and A206S, respectively, at a 100 μM concentration) were much lower than that observed for the archetypal leucine zipper peptide from GCN4, which ranges from ∼50°C at 5 μM to ∼70°C at 500 μM (18, 29).
FIG. 2.
Sequence-based coiled-coil propensity of the three natural BZLF1 variants. The maximum probability (Pmax) of coiled-coil formation was assessed for each of the three BZLF1 natural sequence variants and the indicated proteins using the programs COILS (16) and MULTICOIL (31).
FIG. 3.
The BZLF1 peptides are α-helical in solution. (A) Three BZLF1 peptides pepB95-8…LLQHYREVAAAKSSENDRLRLLLKQMCPSLDV pepA205S…LLQHYREVASAKSSENDRLRLLLKQMCPSLDV pepA206S…LLQHYREVAASKSSENDRLRLLLKQMCPSLDV were synthesized on an Applied Biosystems 432A automated, continuous-flow peptide synthesizer using solid-phase 9-fluorenylmethoxy carbonyl chemistry and purified by high-pressure liquid chromatography (as described previously [11]). The secondary structures were analyzed using CD spectroscopy using a Jasco J-715 spectropolarimeter fitted with a six-cell changer Peltier temperature controller. The buffer system contained 25 mM potassium phosphate, 100 mM sodium chloride, and 1 mM dithiothreitol. The data are shown for peptide concentrations of 100 μM measured at 5°C. pepB95-8 is shown as closed circles, pepA205S as open circles, and pepA206S as open diamonds. The spectrum for pepB95-8 at 95°C is shown as crosses. (B) The signal of each BZLF1 peptide at 222 nm was determined using CD spectroscopy across the indicated range of temperatures. pepB95-8 is shown as closed circles, pepA205S as open circles, and pepA206S as open diamonds.
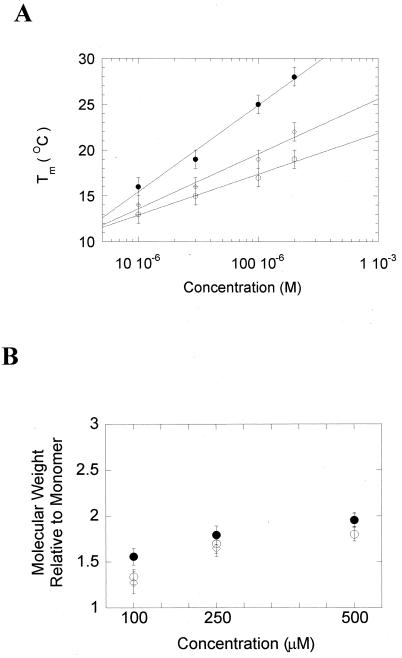
Although the data indicated that the three BZLF1 peptides are able to form α-helical structures, they did not address whether the structures are multimeric, indicative of coiled coils. The Tm of each peptide was therefore determined by CD spectroscopy at four different peptide concentrations (10, 30, 100, and 200 μM) (Fig. 4A). The increase in Tm observed for each peptide is characteristic of a cooperatively folded multimeric structure, since it reflects a shift in the equilibrium between unfolded monomers and folded multimers. We can therefore conclude that residues 196 to 227 of all three natural variants of BZLF1 are sufficient to promote the formation of α-helical, cooperatively folded, oligomeric structures. The preferred oligomeric states of the peptides were then assessed using sedimentation equilibrium ultracentrifugation. The averaged molecular weights of the three BZLF1 peptides were determined. A plot of the calculated molecular weights relative to those of the respective monomers is shown in Fig. 4B. The plots for all three peptides leveled off at a ratio of 2, suggesting that the dominant species in solution are dimers.
FIG. 4.
The three BZLF1 peptides self-associate as dimers. (A) The thermal stability of each peptide was measured by CD spectroscopy at the four concentrations indicated. pepB95-8 is shown as closed circles, pepA205S as open circles, and pepA206S as open diamonds. (B) The weight-average molecular weight of the three BZLF1 peptides was determined by sedimentation equilibrium centrifugation in a Beckman XL-I analytical ultracentrifuge fitted with a titanium An60-Ti rotor, using software supplied by Beckman Coulter UK. Three concentrations of each peptide were used (100 to 500 μM) in a buffer system containing 25 mM potassium phosphate, 100 mM sodium chloride, and 1 mM dithiothreitol. To relate this molecular weight to the extent of oligomerization, the calculated average molecular weight was divided by the monomer molecular weight for each peptide. The error bars show 95% confidence intervals.
Thus, although established coiled-coil prediction methods suggested that the BZLF1 multimerization regions either are atypical coiled coils or have a low probability of forming such structures, our subsequent biophysical analyses revealed (i) that residues 196 to 227 of BZLF1 are able to fold into helical multimers in solution and (ii) that the molecular weights of the BZLF1 peptides in solution approach those expected for dimers. In addition to providing the first biophysical data in support of a coiled-coil dimerization interface for BZLF1, our analyses revealed that the interface of all three natural variants of BZLF1 is inherently less stable than that of archetypal members of the bZip family. This suggests that other regions of BZLF1 may contribute to the formation of a robust dimer in vivo. Indeed, it has been suggested that residues carboxy terminal to position 227 may contribute to the stability of the BZLF1 multimer (20). The relevance, if any, of the small differences in Tm among the three natural variants of BZLF1 remains to be established.
Acknowledgments
This research was funded by grants from the Medical Research Council, the Wellcome Trust, and the Society of General Microbiology to A. J. Sinclair and by grants from the Wellcome Trust and BBSRC to D. N. Woolfson.
We thank K. Takada and M. Rowe for cell lines and A. Rickinson and Quin-Yun Yao for the infectious mononucleosis and nasopharyngeal carcinoma series lymphoblastoid cell lines. Peptides were synthesized by Chris Kowalczyk (University of Sussex).
REFERENCES
- 1.Baer R, Bankier A T, Biggin M D, Deninger P D, Farrell P J, Gibson T J, Hatfull G, Hudson G S, Satchwell S C, Seguin C, Tuffnell P S, Barrell B G. DNA sequence and expression of the B95–8 Epstein-Barr virus genome. Nature. 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 2.Chang Y-N, Dong D L-Y, Hayward G S, Hayward S D. The Epstein-Barr virus Zta transactivator: a member of the bZIP family with unique DNA-binding specificity and a dimerization domain that lacks the characteristic heptad leucine zipper motif. J Virol. 1990;64:3358–3369. doi: 10.1128/jvi.64.7.3358-3369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chevallier-Greco A, Manet E, Chavrier P, Mosnier J, Daillie A, Sergeant A. Both Epstein-Barr virus (EBV) encoded transcription factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986;5:3243–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Countryman J, Miller G. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc Natl Acad Sci USA. 1985;82:4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrell P J, Rowe D T, Rooney C M, Kouzarides T. Epstein-Barr virus BZLF1 trans-activator specifically binds to a consensus AP-1 site and is related to c-fos. EMBO J. 1989;8:127–132. doi: 10.1002/j.1460-2075.1989.tb03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feederle R, Kost M, Baumann M, Janz A, Drouet E, Hammerschmidt W, Delecluse H J. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J. 2000;19:3080–3089. doi: 10.1093/emboj/19.12.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flemington E, Speck S H. Evidence for coiled-coil dimer formation by an Epstein-Barr virus transactivator that lacks a heptad repeat of leucine residues. Proc Natl Acad Sci USA. 1990;87:9459–9463. doi: 10.1073/pnas.87.23.9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flemington E K, Lytle J P, Cayrol C, Borras A M, Speck S H. DNA-binding-defective mutants of the Epstein-Barr virus lytic switch activator Zta transactivate with altered specificities. Mol Cell Biol. 1994;14:3041–3052. doi: 10.1128/mcb.14.5.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregory C D, Rowe M, Rickinson A B. Different Epstein-Barr virus B-cell interactions in phenotypically distinct clones of a Burkitt's lymphoma cell line. J Gen Virol. 1990;71:1481–1495. doi: 10.1099/0022-1317-71-7-1481. [DOI] [PubMed] [Google Scholar]
- 10.Grunewald V, Bonnet M, Boutin S, Yip T, Louzir H, Levero M, Seigneurin J M, Raphael M, Touitou R, Martel-Renoir D, Cochet C, Durandy A, Andre P, Lau W, Zeng Y, Joab I. Amino acid change in the Epstein-Barr virus Zebra protein in undifferentiated nasopharyngeal carcinomas from Europe and North Africa. Int J Cancer. 1998;75:497–503. doi: 10.1002/(sici)1097-0215(19980209)75:4<497::aid-ijc2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 11.Hicks M R, Holberton D V, Kowalczyk C, Woolfson D N. Coiled-coil assembly by peptides with non-heptad sequence motifs. Folding Design. 1997;2:149–158. doi: 10.1016/S1359-0278(97)00021-7. [DOI] [PubMed] [Google Scholar]
- 12.Hong Y, Holley-Guthrie E, Kenney S. The bZip dimerization domain of the Epstein-Barr virus BZLF1 (Z) protein mediates lymphoid-specific negative regulation. Virology. 1997;229:36–48. doi: 10.1006/viro.1996.8413. [DOI] [PubMed] [Google Scholar]
- 13.Kouzarides T, Packham G, Cook A, Farrell P J. The BZLF1 protein of EBV has a coiled-coil dimerisation domain without a heptad leucine repeat but with homology to the C/EBP leucine zipper. Oncogene. 1991;6:195–204. [PubMed] [Google Scholar]
- 14.Landschultz W H, Johnson P F, McKnight S L. The leucine zipper protein: a hypothetical structure common to a new class of DNA binding proteins. Science. 1989;240:1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman P M, Berk A J. In vitro transcriptional activation, dimerization, and DNA-binding specificity of the Epstein-Barr virus Zta protein. J Virol. 1990;64:2560–2568. doi: 10.1128/jvi.64.6.2560-2568.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 17.Masucci M G, Contreras-Salazar B, Ragnar E, Falk K, Minarovits J, Ernberg I, Klein G. 5-Azacytidine up regulates the expression of Epstein-Barr virus nuclear antigen 2 (EBNA-2) through EBNA-6 and latent membrane protein in the Burkitt's lymphoma line Rael. J Virol. 1989;63:3135–3141. doi: 10.1128/jvi.63.7.3135-3141.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Shea E K, Rutkowski R, Kim P S. Evidence that the leucine zipper is a coiled coil. Science. 1989;243:538–542. doi: 10.1126/science.2911757. [DOI] [PubMed] [Google Scholar]
- 19.Packham G, Brimmell M, Cook D, Sinclair A J, Farrell P J. Strain variation in Epstein-Barr virus immediate early genes. Virology. 1993;192:541–550. doi: 10.1006/viro.1993.1070. [DOI] [PubMed] [Google Scholar]
- 20.Packham G, Economou A, Rooney C M, Rowe D T, Farrell P J. Structure and function of the Epstein-Barr virus BZLF1 protein. J Virol. 1990;64:2110–2116. doi: 10.1128/jvi.64.5.2110-2116.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Hawley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2397–2446. [Google Scholar]
- 22.Rooney C M, Rowe D T, Ragot T, Farrell P J. The spliced BZLF1 gene of Epstein-Barr virus (EBV) transactivates an early EBV promoter and induces the virus productive cycle. J Virol. 1989;63:3109–3116. doi: 10.1128/jvi.63.7.3109-3116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schepers A, Pich D, Hammerschmidt W. Activation of oriLyt, the lytic origin of DNA replication of Epstein-Barr virus, by BZLF1. Virology. 1996;220:367–376. doi: 10.1006/viro.1996.0325. [DOI] [PubMed] [Google Scholar]
- 24.Schwarzmann F, Jager M, Prang N, Wolf H. The control of lytic replication of Epstein-Barr virus in B lymphocytes. Int J Mol Med. 1998;1:137–142. doi: 10.3892/ijmm.1.1.137. [DOI] [PubMed] [Google Scholar]
- 25.Sinclair A J, Farrell P J. Epstein-Barr virus transcription factors. Cell Growth Differ. 1992;3:557–563. [PubMed] [Google Scholar]
- 26.Speck S H, Chatila T, Flemington E. Reactivation of Epstein-Barr virus: regulation and function of the BZLF1 gene. Trends Microbiol. 1997;5:399–405. doi: 10.1016/S0966-842X(97)01129-3. [DOI] [PubMed] [Google Scholar]
- 27.Takada K, Shimizu N, Sakuma S, Ono Y. trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J Virol. 1986;57:1016–1022. doi: 10.1128/jvi.57.3.1016-1022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor N, Flemington E, Kolman J L, Baumann R P, Speck S H, Miller G. ZEBRA and a Fos-GCN4 chimeric protein differ in their DNA-binding specificities for sites in the Epstein-Barr virus BZLF1 promoter. J Virol. 1991;65:4033–4041. doi: 10.1128/jvi.65.8.4033-4041.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson K S, Vinson C R, Freire E. Thermodynamic characterization of the structural stability of the coiled-coil region of the bZip transcription factor GCN4. Biochemistry. 1993;32:5491–5496. doi: 10.1021/bi00072a001. [DOI] [PubMed] [Google Scholar]
- 30.Urier G, Buisson M, Chambard P, Sergeant A. The Epstein-Barr virus early protein EB1 activates transcription from different responsive elements including AP-1 binding sites. EMBO J. 1989;8:1447–1453. doi: 10.1002/j.1460-2075.1989.tb03527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf E, Kim P S, Berger B. MultiCoil: a program for predicting two- and three-stranded coiled coils. Protein Sci. 1997;6:1179–1189. doi: 10.1002/pro.5560060606. [DOI] [PMC free article] [PubMed] [Google Scholar]