Abstract
Benzyl alcohol (BA) is a major biomass derivative and can be further converted into deoxybenzoin (DOB) and benzoin (BZ) as high-value products for industrial applications through photocatalytic C–C coupling reaction. The photocatalytic process contains two reaction steps, which are (1) the C–C coupling of BA to hydrobenzoin (HB) intermediates and (2) either dehydration of HB to DOB or dehydrogenation of HB to BZ. We found that generation of DOB or BZ is mainly determined by the activation of Cα–H or O–H bonds in HB. In this study, phase junction CdS photocatalysts and Ni/CdS photocatalysts were elaborately designed to precisely control the activation of Cα–H or O–H bonds in HB by adjusting the adsorption orientation of HB on the photocatalyst surfaces. After orienting the Cα–H groups in HB on the CdS surfaces, the Cα–H bond dissociation energy (BDE) at 1.39 eV is lower than the BDE of the O–H bond at 2.69 eV, therefore improving the selectivity of the DOB. Conversely, on Ni/CdS photocatalysts, the O–H groups in HB orient toward the photocatalyst surfaces. The BDE of the O–H bonds is 1.11 eV to form BZ, which is lower than the BDE of the Cα–H bonds to the DOB (1.33 eV), thereby enhancing the selectivity of BZ. As a result, CdS photocatalysts can achieve complete conversion of BA to 80.4% of the DOB after 9 h of visible light irradiation, while 0.3% Ni/CdS photocatalysts promote complete conversion of BA to 81.5% of BZ after only 5 h. This work provides a promising strategy in selective conversion of BA to either DOB or BZ through delicate design of photocatalysts.
Keywords: C−C coupling of benzyl alcohol, Cα−H bond activation, O−H bond activation, photocatalysis, cadmium sulfide
1. Introduction
Benzyl alcohol (BA) is a promising biomass derivative and can be further converted into high-value products for industrial applications, such as deoxybenzoin (DOB) as the flame retardant in the polymer industry,1,2 and benzoin (BZ) for surface coatings and photosensitive polymer materials.3−5 However, traditional methods for generating DOB and BZ, such as N-heterocyclic carbene (NHC)-catalyzed BZ condensation3,6−8 and Friedel–Crafts acylation,9,10 respectively, show several significant drawbacks. First, these methods require the use of activators, such as diazabicycloundecane (DBU), HCl, NaOH, or TEA for the NHC-catalyzed process6,8 and K2CO3, KF, K3PO4, or KOAc in Friedel–Crafts acylation,10 which increase the production costs. Second, the recycling and reusability of these catalysts are limited, with significant decreases in the yield of products after several cycles.6−8 Third, the preparation of NHC catalysts is particularly complex and costly, requiring expensive palladium or copper catalysts along with BINAP and X-Phos ligands for the introduction of alkyl or aryl groups at specific N positions in the NHC precursors.11,12 These drawbacks limit the applications of these traditional strategies for generation of DOB and BZ. Photocatalytic C–C coupling of BA to DOB and BZ is an efficient and cost-effective alternative, overcoming the need for additional activators, simplifying catalyst preparation, and enhancing photocatalyst reusability.
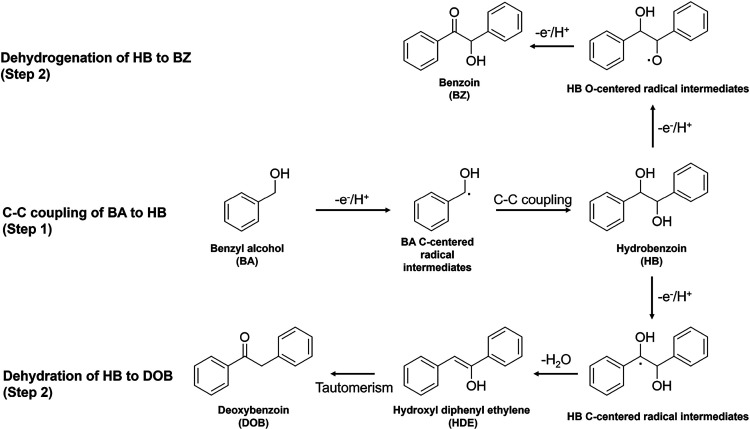
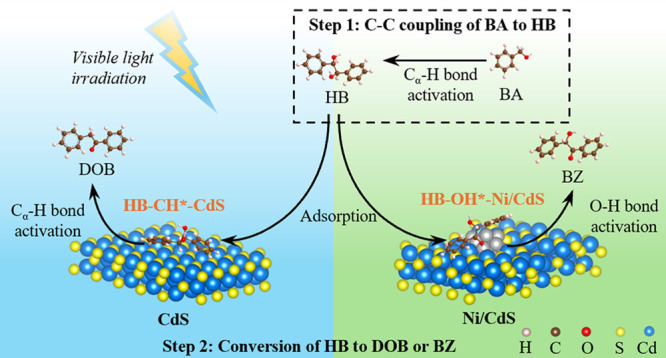
Previous works have demonstrated that the generation of DOB and BZ through photocatalytic C–C coupling of BA is a two-step reaction process.13−15 As shown in Scheme 1, the Cα–H bonds in BA are first activated to form BA C-centered radical intermediates and then convert to hydrobenzoin (HB) as an intermediate product through self-C–C coupling reaction (Step 1).13−15 In Step 2, HB intermediates are further converted to either DOB through dehydration of HB or BZ through dehydrogenation of HB. In these reactions, the selectivity of DOB and BZ is mainly controlled by the competition between the dehydration and dehydrogenation of HB intermediates.13−15 Previous works have demonstrated that the dehydration of HB intermediates to DOB is mainly determined by the Cα–H bond activation.14 Wang’s group has demonstrated that the activation of Cα–H bonds in HB to generate HB C-centered radical intermediates by ZnIn sulfide photocatalysts is an important step, as the intermediates can be readily reduced to hydroxyl diphenyl ethylene (HDE) by photoexcited electrons and then generate DOB through HDE tautomerism.14 However, the mechanism in the dehydrogenation of HB intermediates to BZ is still unclear. Generally, the dehydrogenation of alcoholic compounds to corresponding products, such as dehydrogenation of BA to BH and methanol to formaldehyde, is mainly determined by the O–H bond activation.16−19 For example, in the dehydrogenation of methanol into formaldehyde, the activation of O–H bonds in methanol to generate CH3O• radical intermediates can significantly reduce the bond dissociation energy (BDE) of C–H bonds from 1.57 eV in methanol to 0.21 eV, thereby enhancing formaldehyde production.20−22 Inspired by these works, the selectivity in the generation of DOB or BZ from C–C coupling of BA should be precisely controlled by the activation of either Cα–H bonds or O–H bonds in HB intermediates, respectively.
Scheme 1. Photocatalytic C–C Coupling of BA to DOB and BZ.
Reproduced from refs (13) and (14). Copyright [2020] American Chemical Society.
Based on the above discussions, it is important to design photocatalysts that can precisely control the activation of either the Cα–H bonds or the O–H bonds in HB intermediates to improve the selectivity of either DOB or BZ as desirable products, respectively. Metal sulfide photocatalysts show promising potential in the activation of the Cα–H bonds in alcoholic compounds to generate corresponding products, such as C–C coupling of methane to ethylene glycol and C–C coupling of furfuryl alcohol (FA) to hydrofuroin (HF). The sulfur moieties on the surfaces of the metal sulfide photocatalysts exhibit high activity for the Cα–H bond activation in these alcoholic compounds.22−24 For example, Wang’s group demonstrated that the sulfur moieties on the CdS surfaces can improve the activation of Cα–H bonds in methanol to form •CH2OH instead of activation of O–H bonds to form CH3O•, as the sulfur moieties on CdS surfaces act as proton acceptors, significantly reducing the reaction energy of Cα–H bond activation to 0.8 eV compared to 1.6 eV for O–H bond activation, therefore improving the generation of ethylene glycol.22 Amal’s group has demonstrated that the sulfur moieties on the ZnxIn2S3+x photocatalysts can accumulate the photogenerated holes to form a sulfur anion radical (S–•). S–• can promote the concerted proton–electron transfer (CPET) pathway and improve the Cα–H bond activation in FA, therefore improving the generation of HF.24 Herein, the elaborately designed CdS photocatalysts with exposed sulfur moieties might have a high potential to improve the Cα–H bond activation in HB intermediates and enhance the selectivity of DOB as a desirable product.
Activation of the O–H bonds in HB intermediates can enhance the generation of BZ as a desirable product from C–C coupling of BA. Generally, metal clusters, including Pt, Au, Ru, and Ni, have been proved as cocatalysts to improve the activation of the O–H bonds in alcoholic compounds in the generation of dehydrogenated products, such as dehydrogenation of BA to BH and dehydrogenation of methanol to formaldehyde. These metal clusters can improve the adsorption of O atoms in O–H groups on the photocatalyst surfaces, therefore enhancing the activation of O–H bonds in alcoholic compounds.16,25−27 For example, Li’s group demonstrated that the introduction of Ru clusters on the surfaces of g-C3N4–x photocatalysts can decrease the adsorption energy of BA from −0.024 eV without Ru clusters to −1.542 eV and elongate the O–H bonds in BA from 0.994 to 1.011 Å, therefore enhancing the selectivity of BH as the dehydrogenated product.27 Xu’s work showed that Ni clusters on CdS nanoparticles act as an electron acceptor, improving the activation of O–H bonds in absorbed alcohols to form O-centered radical intermediates, which then oxidize into corresponding dehydrogenated ketone compounds.16 Inspired by the above research, the introduction of metal clusters as cocatalysts on the photocatalysts might have potential in improving the activation of the O–H bonds in HB intermediates to enhance the selectivity of BZ as a desirable product.
In summary, the generation of DOB and BZ from C–C coupling of BA contains two reaction steps: (1) the C–C coupling of BA to HB intermediates and (2) the conversion of HB intermediates into either DOB through a dehydration process or into BZ through a dehydrogenation process. Activations of Cα–H bonds and O–H bonds in HB intermediates are the determined steps to control the selectivity of DOB and BZ as desirable products, respectively. In this study, phase junction CdS photocatalysts (CdS) and decoration of Ni clusters on the surfaces of phase junction CdS photocatalysts (Ni/CdS) were designed to improve the Cα–H bond activation and the activation of the O–H bond in HB, respectively. In detail, CdS photocatalysts adsorb the HB intermediates with an orientation of the Cα–H groups toward the CdS surfaces (R-CH*). This orientation significantly reduces the energy for Cα–H bond activation in HB in comparison to that of the activation of the O–H bond, therefore enhancing the selectivity of the DOB. Conversely, the Ni/CdS photocatalysts preferentially adsorb the HB intermediates with orientation of the O–H groups toward its surfaces (R-OH*), which dramatically improves the activation of the O–H bond in HB, therefore improving the selectivity of BZ. Our experimental results demonstrated that phase junction CdS photocatalysts can achieve complete conversion of BA to 80.4% of DOB after 9 h of visible light irradiation, while 0.3% Ni/CdS photocatalysts show the complete conversion of BA to 81.5% of BZ after only 5 h of visible light irradiation, which exhibit the best photocatalytic performance compared to previous works.
2. Materials and Methods
2.1. Materials
All reagents were of analytical grade and were used without further purification. Trisodium citrate dihydrate (99%+), thioacetamide (99%+), CH3CN, BZ, and NiCl2·4H2O (98%) were purchased from Fisher Scientific International, Inc. Methanol (MEOH), 5,5-dimethyl-1-pyrroline N-oxide (DMPO), Cd(NO3)2·4H2O (98%), BA, BH, DOB, HB, 1,1-diphenylethylene (DPE), furfuryl alcohol (FA), methylbenzyl alcohol (Me-BA), methoxybenzyl alcohol (MeO-BA), Na2S·9H2O (98%+), Na2S2O8 (99%), and Na2SO3 (98.5%) were purchased from Sigma-Aldrich Co., Ltd. Ethylene glycol (EG) was purchased from VWR International, LLC. Deionized (DI) water was produced by CENTRA R200 Centralized Purification and Distribution Systems.
2.2. Preparation of Phase Junction CdS Nanoparticles
The phase junction CdS photocatalysts (CdS) were prepared by a one-pot hydrothermal method. Typically, 308 mg of Cd(NO3)2·4H2O and 150 mg of trisodium citrate were dissolved into a 15 mL water and EG mixed solution (vwater/vEG = 1/5). After ultrasonic dispersion for 10 min and then vigorous stirring for 30 min, 375 mg of thioacetamide was added into the solution. After being stirred for another 30 min, the mixture was transferred into a 25 mL stainless Teflon-lined autoclave reactor. The autoclave reactor was subsequently heated to 160 °C with a 3 °C min–1 heating rate in an oven, and the temperature was maintained for 4 h. After being naturally cooled down, the sample was collected by centrifugation (9000 rpm) and rinsed several times with ethanol and water. The solid samples were then dried under vacuum at 60 °C for 4 h.
2.3. Preparation of Ni/CdS
The Ni/CdS photocatalysts were formed from CdS and NiCl2 through in situ photocatalytic synthesis. Typically, 10 mg of CdS nanoparticles was dispersed in a 5 mL CH3CN solution containing 10 mg of BA and x% of NiCl2 (x% = molar of NiCl2/molar of 10 mg CdS; 0.1, 0.3, and 0.5%). The mixed solution was stirred, and argon was purged in the sealed reactor for 30 min. Then, the sealed reactor was illuminated with a xenon arc lamp with a 420 nm UV filter. During the irradiation stage, the temperature of the solution was kept at 20 °C with cooling water. After the irradiation, the sample was collected by centrifugation (9000 rpm) and rinsed several times with CH3CN. The solid samples were dried under vacuum at 60 °C for 4 h and stored under an inert atmosphere condition.
2.4. Photocatalytic Activity Evaluation
Photocatalytic reactions were performed in a customized quartz reactor with a cooling water jacket. As shown in Figure S1, the visible light source was provided by a xenon arc lamp (Perfect Light Company, PE300BF) equipped with a 420 nm UV filter. The position of the lamp was fixed during the entire experiment to maintain a constant light intensity of 0.35 W cm–2. A 10 mg portion of reactants (BA, MeO-BA, Me-BA, and FA) and 10 mg of CdS photocatalysts were dispersed in 5 mL of CH3CN in the reactor with a certain amount of NiCl2 (x% = molar of NiCl2/molar of 10 mg of CdS; 0, 0.1, 0.3, and 0.5%). Cooling water was employed to keep the temperature of reaction solution at 20 °C. The photocatalysts were dispersed by magnetic stirring, and the reactor was firmly sealed after 30 min of argon purge (10 mL min–1). The sealed reactor with 200 rpm of magnetic stirring was illuminated under visible light irradiation. After the reaction, the solution was collected by centrifugation and methylparaben was added as the internal standard. The obtained solution was qualitatively analyzed by gas chromatography–mass spectrometry (GC-MS, QP2010 SE, Shimadzu, Rxi-5 ms column) and quantitatively analyzed by gas chromatography (GC, GC-2010 plus, Shimadzu, Stabilwax-MS column). The following equations were used to calculate the conversion rate of reactants, the selectivity of products, and the yield of products:
2.5. Characterization
The morphologies of as-prepared CdS and 0.3% Ni/CdS were imaged by high-resolution transmission electron microscopy (TEM/HRTEM, JEOL JEM-2100F). The composition of x% Ni/CdS photocatalysts was determined by inductively coupled plasma optical emission spectroscopy (ICP-OES, Varian Vista Pro). The chemical states of each element in x% Ni/CdS photocatalysts and X-ray photoelectron valence band spectra (XPS-VB) were characterized by X-ray photoelectron spectroscopy (XPS, ThermoFisher K-Alpha) equipped with an Al Kα X-ray source. All peaks have been calibrated with the C 1s peak, where the standard binding energy (B.E.) is 284.8 eV for the adventitious carbon source. X-ray diffraction (XRD) patterns were obtained using a Bruker Phaser-D2 diffractometer with a Cu Kα X-ray source at the voltage of 40 kV and the current of 40 mA. The light absorption properties and bandgap results of prepared x% Ni/CdS were obtained using UV/vis diffuse reflectance spectroscopy (DRS) on a JASCO V-670 spectrophotometer equipped with an integration sphere in the spectral range of 200–1000 nm and BaSO4 was used as the reflectance standard. The photoelectrochemical (PEC) measurements were performed in a standard three-electrode system by using an electrochemical workstation (CHI660E, Chenhua, shanghai) under visible light illumination in 50 mL of CH3CN with 30 mg of BA and 0.2 M Na2ClO4. Photoluminescence (PL) analyses were conducted on a Shimadzu RF-6000. The time-resolved photoluminescence (TRPL) experiments were conducted on an Edinburgh FLS1000. More detailed characterization information is provided in the Supporting Information.
3. Results and Discussion
3.1. Characterization of Phase Junction CdS and Ni/CdS Photocatalysts
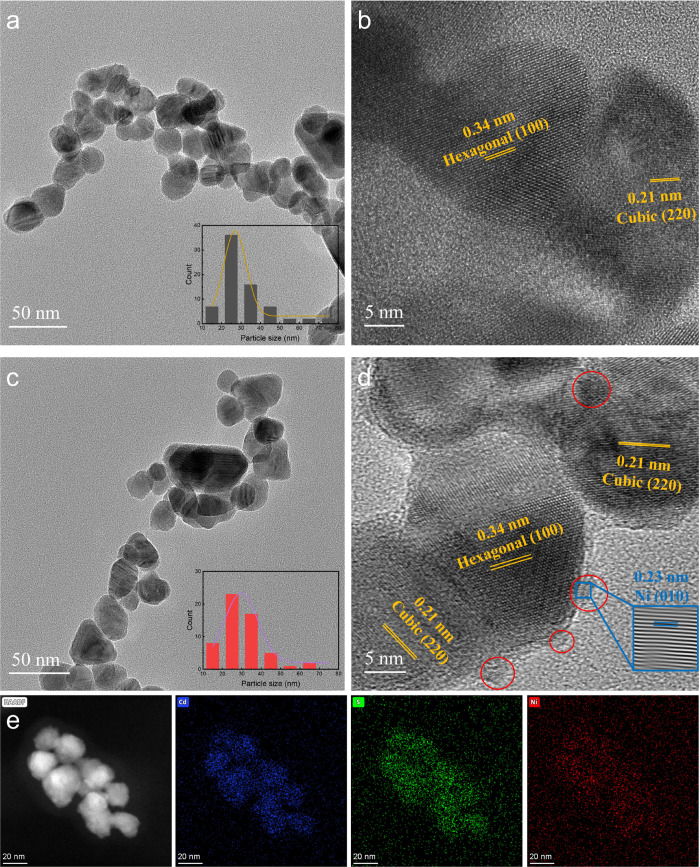
TEM/HRTEM, XRD, ICP-OES, and XPS were conducted to verify the successful synthesis of phase junction CdS nanoparticles (CdS) and the loading of Ni clusters onto phase junction CdS nanoparticles (Ni/CdS). The phase junction CdS photocatalysts were prepared through the hydrothermal process reported in our previous work,28 while the Ni/CdS photocatalysts were prepared through an in situ photodeposition process using NiCl2 as the precursor under visible light irradiation. As shown in Figure 1a,c, TEM images display comparable irregular nanoparticles with an average particle size of around 30 nm for both CdS and 0.3% Ni/CdS photocatalysts. The TEM element mappings (Figure 1e and Figure S2) show Ni uniformly dispersed on the surfaces of the CdS nanoparticles. HRTEM images (Figure 1b,d) confirm the presence of hexagonal CdS (100) and cubic CdS (220) facets in both CdS and 0.3% Ni/CdS photocatalysts. Additionally, the 0.3% Ni/CdS photocatalysts exhibit a lattice spacing of 0.23 nm (red circle), corresponding to the (010) plane of metallic Ni.29
Figure 1.
TEM and HRTEM images of phase junction CdS (a, b) and 0.3% Ni/CdS (c, d). (e) HAADF-STEM images and corresponding elemental mappings of 0.3% Ni/CdS photocatalysts. The inset graphs in panels (a) and (c) are corresponding particle size distributions.
Figure S3 presents the XRD patterns of CdS and x% Ni/CdS and the standard patterns of CdS (hexagonal phase of CdS: JCPDS no. 41-1049; cubic phase of CdS: JCPDS no. 10-0454). These results exhibit the presence of both hexagonal CdS phase and cubic CdS phase in CdS and x% Ni/CdS. Upon decorating CdS with Ni clusters, there are no obvious changes in the XRD patterns of CdS, indicating that the photodeposition of Ni onto CdS does not alter the structure of CdS.
XPS was conducted to further investigate the chemical states of CdS and 0.3% Ni/CdS photocatalysts. The full XPS spectra are presented in Figure S4a and demonstrate that both CdS and 0.3% Ni/CdS photocatalysts exhibit peaks of Cd and S elements, and 0.3% Ni/CdS additionally displays peaks of the Ni element. Figure S4b,c demonstrates the high-resolution XPS spectra of Cd and S elements in CdS and 0.3% Ni/CdS photocatalysts, respectively. In CdS, the XPS S 2p peaks are observed at 161.5 eV (S 2p3/2) and 162.8 eV (S 2p1/2), whereas in 0.3% Ni/CdS, the bonding energy of these peaks shows the positive shift to 161.8 eV (S 2p3/2) and 163.1 eV (S 2p1/2), respectively. Similarly, the Cd 3d peaks in CdS are located at 405.1 eV (Cd 3d5/2) and 411.9 eV (Cd 3d3/2), whereas in 0.3% Ni/CdS, the binding energy of these peaks exhibits the positive shift to 405.5 eV (Cd 3d5/2) and 412.2 eV (Cd 3d3/2), respectively. Generally, the binding energy of an element originates from the Coulomb attraction between the electrons of atom and the atomic nucleus.15,30 A higher binding energy of an element indicates a decreased electron density, as the reduction in electron density enhances the Coulomb attraction between the remaining electrons and the atomic nucleus.15,30 The positive shifts of both S 2p and Cd 3d from CdS to 0.3% Ni/CdS exhibit the decreased electron density of S 2p and Cd 3d in 0.3% Ni/CdS, as the electrons transfer from CdS to Ni. As shown in Figure S4d, the high-resolution XPS spectrum of the Ni element in 0.3% Ni/CdS reveals the presence of metallic Ni, evidenced by a peak at 852.4 eV. In addition, three fitted peaks are also observed at 855.9 eV (Ni 2p3/2), 860.7 eV (Ni 2p3/2), and 873 eV (Ni 2p1/2), corresponding to the oxidation of Ni to Ni2+.31 The oxidation of Ni to Ni2+ is attributed to the inevitable oxidation occurring during the transportation stage of the samples for XPS characterization.
ICP-OES was conducted to measure the actual content of the Ni element in x% Ni/CdS. As shown in Table S1, the ratio of Ni/Cd in x% Ni/CdS increases from 0 in CdS to 0.43% in 0.5% Ni/CdS. The results indicate that the concentration of Ni clusters on the surfaces of CdS proportionally increases with the amount of NiCl2 added in the in situ photodeposition process, closely matching the theoretical amount of Ni in x% Ni/CdS photocatalysts. In summary, all characterizations prove that phase junction CdS photocatalysts and Ni/CdS photocatalysts were successfully prepared.
3.2. C–C Coupling of BA to DOB and BZ Using CdS and Ni/CdS Photocatalysts
After the structural characteristics of CdS and Ni/CdS photocatalysts were understood, both photocatalysts were employed to facilitate the C–C coupling of BA to DOB and BZ as desirable products. The mass spectrometry data from GC-MS, GC spectra, and 1H and 13C NMR spectra were collected to investigate the generated products in the photocatalytic C–C coupling of BA. As shown in Figure S5, the mass spectra of generated products along with the corresponding standard mass spectra from the GC-MS library confirm that the generated products were BH, HB, DOB, and BZ. As shown in Figure S6, the GC spectra demonstrate that BZ is the main product when using 0.3% Ni/CdS photocatalysts after 5 h of visible light irradiation, whereas DOB is the major product when using CdS photocatalysts after 9 h of reaction. In addition, 1H and 13C NMR analyses have been applied to BA, all products, and the reaction solutions after visible light irradiation. The results indicate that the main product is BZ when using 0.3% Ni/CdS and DOB when using CdS (the detailed discussions are presented in Figure S7).
This reaction contains two reaction steps, as shown in Scheme 1. In the first reaction step, BA as a reactant can convert to HB as an intermediate product through self-C–C coupling reaction, alongside with production of BH as a byproduct. In the second reaction step, HB intermediates can either dehydrogenate to BZ or dehydrate to DOB. Generally, both conversion of BA to BH and dehydrogenation of HB to BZ are net oxidation reactions that primarily rely on photogenerated holes in the photocatalytic system.14 In contrast, the C–C coupling of BA to HB and the dehydration of HB to DOB require both reductive and oxidative capabilities,14 needing both photogenerated holes and electrons. In this reaction step, DOB and BZ might have the potential to interconvert. To verify this possibility, DOB and BZ are separately introduced as a reactant into the reaction system, revealing their nonconvertibility (entries 1 and 2 in Table 1). Previous works demonstrated that BH can be converted to HB intermediates through Cα=O bond activation.32 To exclude this possibility, BH was added as the reactant in our reaction system, and the results demonstrate that BH is an ultimate byproduct and cannot be converted to HB intermediates (entry 3 in Table 1).
Table 1. Controlled Reaction Conditions for C–C Coupling of BA to DOB and BZa.
| conversion/selectivity |
||||||||
|---|---|---|---|---|---|---|---|---|
| entry | photocatalyst | atmosphere | reactant | BA | BH | DOB | BZ | HB |
| 1 | 0.3% Ni/CdS | Ar | DOB | |||||
| 2 | 0.3% Ni/CdS | Ar | BZ | |||||
| 3 | 0.3% Ni/CdS | Ar | BH | |||||
| 4 | 0.3% Ni/CdS | air | BA | 74% | 96.5% | 1.04% | 0.6% | 0.2% |
| 5 | 0.3% Ni/CdS | O2 | BA | 46.8% | 83.1% | 2.6% | 12.9% | 2.5% |
| 6 | 0.3% Ni/CdS | Ar | BA | 100% | 15% | 1.2% | 81.5% | 3.2% |
| 7 | 0.3% Ni/CdS | N2 | BA | 100% | 16.7% | 3.8% | 79.8% | 1.1% |
| 8 | 0.3% Ni/CdS | He | BA | 100% | 16.8% | 2.6% | 81% | 1.5% |
| 9 | 0.3% NiS2/CdS | Ar | BA | 100% | 13.6% | 34.5% | 34.6% | 14.9% |
| 10 | 0.3% Ni/CdS(E) | Ar | BA | 100% | 12.3% | 5.5% | 72.4% | 7.8% |
Typical reaction conditions: the reactant is 10 mg; the photocatalyst is 10 mg; CH3CN is 5 mL; Ar is at 1 atm; the visible light power is 0.35 W cm–2; 5 h.
Before evaluating the photocatalytic performance of CdS and Ni/CdS photocatalysts developed in this study, it is essential to optimize the atmosphere in the reaction system to achieve the high selectivity of desirable products. As shown in entries 4 and 5 in Table 1, 74 and 46.8% of BA are converted to 96.5 and 83.1% of BH as an oxidized byproduct under air and oxygen atmospheres, respectively. The results indicate a negative effect of oxygen on the generation of DOB and BZ. The photocatalytic results in the inert atmospheres (N2, He, and Ar) are presented in entries 6–8 in Table 1, which show a similar conversion rate of BA and selectivity to desirable products. In detail, BA completely converts to around 81% BZ and around 3% DOB using 0.3% Ni/CdS photocatalysts under different inert atmospheres after 5 h of visible light irradiation. Hence, the following experiments are performed in an Ar inert atmosphere.
The preparation of Ni/CdS photocatalysts through an in situ photodeposition process is an effective method to improve the conversion rate of BA to BZ with high selectivity. Under visible light irradiation, CdS generates electrons to reduce NiCl2 precursors to metallic Ni and then Ni is adsorbed on the CdS surfaces, forming Ni cluster-decorated CdS photocatalysts.33 Ni clusters have been considered as the active sites to improve the photocatalytic performances in various applications, such as hydrogen evolution31,33 and coupling of amines to imines.29,34 To further validate the importance of Ni clusters on CdS surfaces for conversion of BA to BZ, NiS2/CdS photocatalysts prepared by using an in situ hydrothermal method and Ni/CdS(E) photocatalysts prepared by using a traditional photodeposition method were compared (both synthesizing details are provided in the Supporting Information). As shown in entry 9 in Table 1, only 34.6% of BZ is generated after 5 h of visible light irradiation, indicating that NiS2 on the CdS surfaces cannot significantly improve the conversion rate of BA to BZ. In addition, 0.3% Ni/CdS(E) photocatalysts generate 72.4% of BZ after 5 h of irradiation (entry 10 in Table 1), and 0.3% Ni/CdS provides 81.5% selectivity of BZ. The results demonstrate that Ni clusters on the CdS surfaces significantly improve the yield of BZ. Furthermore, the better photocatalytic performance using 0.3% Ni/CdS than 0.3% Ni/CdS(E) might be attributed to the optimization of the distribution and size of Ni clusters on the CdS surfaces in the presence of BA during the in situ photodeposition process, which is more conducive to the conversion of BA to BZ.
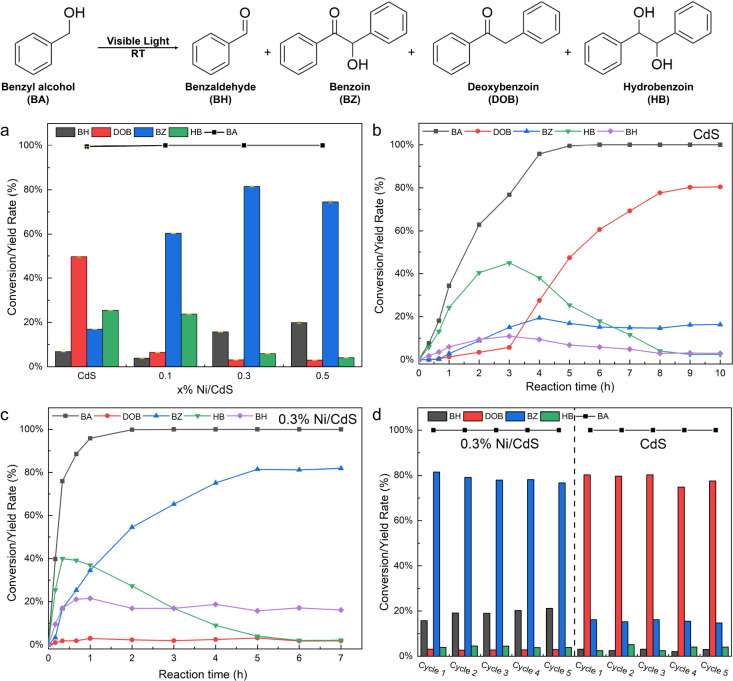
The CdS and x% Ni/CdS photocatalysts show promising potential in C–C coupling of BA with high selectivities of DOB and BZ, respectively. As shown in Figure 2a, CdS photocatalysts achieve complete conversion of BA to 50% of DOB and 17% of BZ as desirable products after 5 h of visible light irradiation. In this reaction, 25.4% of HB intermediates remain, indicating that the reaction time needs to be extended to achieve their complete conversion. The results demonstrate that CdS nanoparticles have a high potential in conversion of BA to high productivity of DOB. All x% Ni/CdS photocatalysts also show complete conversion of BA to HB intermediates in the first reaction step after 5 h of visible light irradiation. In the second reaction step, x% Ni/CdS shows significantly improved capability in the conversion of HB intermediates. The residual HB intermediates reduce from 23.8% in 0.1% Ni/CdS to only 4.1% in 0.5% Ni/CdS, indicating that the conversion rate of HB intermediates increases with Ni content on CdS photocatalysts. The selectivity of BZ as the desirable product increases from 60.4% in 0.1% Ni/CdS to 81.5% in 0.3% Ni/CdS but decreases to 74.5% in 0.5% Ni/CdS. The 0.3% Ni/CdS photocatalysts show the highest selectivity of BZ compared to other photocatalysts in this work.
Figure 2.
(a) Photocatalytic performance in C–C coupling of BA to corresponding products with CdS and x% Ni/CdS after 5 h of visible light irradiation. Reaction kinetics of photocatalytic C–C coupling of BA was studied using (b) CdS and (c) 0.3% Ni/CdS. (d) Stability test of the CdS and Ni/CdS photocatalysts. Reaction conditions: BA is 10 mg; the photocatalyst is 10 mg; CH3CN is 5 mL; Ar is at 1 atm; the visible light power is 0.35 W cm–2.
3.3. C–C Coupling of BA to DOB and BZ through a Two-Step Reaction
The C–C coupling of BA to DOB and BZ contains two reaction steps, which are (1) C–C coupling of BA to HB intermediates and (2) either dehydration of HB to DOB or dehydrogenation of HB to BZ.13−15,35,36 The reaction kinetics of CdS and 0.3% Ni/CdS photocatalysts were determined to investigate the two-step reaction in the C–C coupling of BA to DOB and BZ, respectively. As shown in Figure 2b, using CdS photocatalysts, BA can achieve complete conversion to corresponding products after 5 h of visible light irradiation. In the first 3 h, the primary product is HB intermediates, with a yield of 45%. Afterward, the yield of HB gradually decreases to 2.4%, and the generation of DOB significantly increases from only 5.7% after 3 h to 80.4% after 9 h. Similarly, as shown in Figure 2c, 0.3% Ni/CdS photocatalysts achieve complete conversion of BA to corresponding products after 2 h of visible light irradiation. The yield of HB intermediates significantly increases to 40% after the first 20 min and then gradually decreases to 1.1% after 5 h. Meanwhile, the yield of BZ as the desirable product significantly increases to 81.5% after 5 h, while the generation of DOB retains a minimal selectivity at 1.2%. Both reaction kinetics confirm that DOB and BZ are generated through a two-step reaction pathway. As a result, CdS photocatalysts can achieve complete conversion of BA to 80.4% of DOB after 9 h of visible light irradiation, while 0.3% Ni/CdS photocatalysts promote a complete conversion of BA to 81.5% of BZ after only 5 h. For better comparison with previous works, the apparent quantum yield (AQY) for generation of desirable products was calculated based on the method provided in previous studies,35,36 and the calculated details are presented in the Supporting Information. In our reaction system, the calculated AQY for BZ generation using 0.3% Ni/CdS photocatalysts is 4.42%, while the calculated AQY for DOB generation using CdS photocatalysts is 3.38%. Compared with previous studies, our results demonstrate the highest AQY for DOB generation using CdS and for BZ generation using 0.3% Ni/CdS (Table S2).13,14,36,37
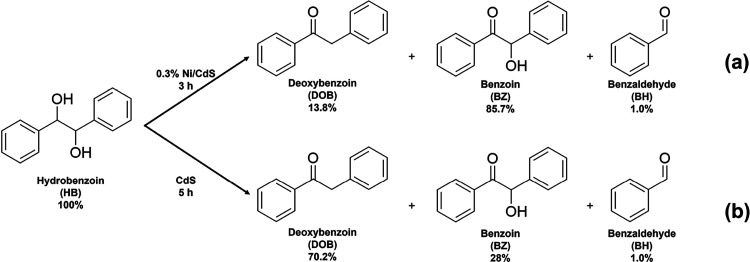
To further investigate the two-step reaction in C–C coupling of BA into DOB and BZ, HB intermediates as the reactant were used to generate DOB and BZ by using CdS and Ni/CdS photocatalysts, respectively. As shown in Figure S8, in C–C coupling of the BA reaction, GC-MS spectra reveal two different peaks of HB intermediates, corresponding to different chiral forms of HB, which might be (R,R)-HB, (S,S)-HB and meso-HB. To evaluate the potential impact of these chiral forms on the conversion rate to DOB and BZ, (R,R)-HB, (S,S)-HB, and meso-HB were separately used as reactants to generate BZ and DOB. As shown in Table S3, the selectivity of BZ from three chiral forms of HB shows similar results that are around 86% when using 0.3% Ni/CdS after 3 h of visible light irradiation. The results indicate that the chiral forms of HB do not influence its conversion rate to DOB or BZ. Hence, meso-HB was used as the reactant for the following experiments. As shown in Scheme 2, 0.3% Ni/CdS photocatalysts can achieve complete conversion of HB to generate 85.7% BZ and 13.8% DOB after 3 h of visible light irradiation. Also, CdS photocatalysts can completely convert HB to 70.2% of DOB and 28% of BZ after 5 h. The selectivity of DOB from the conversion of HB using CdS is similar to that from C–C coupling of BA using the same photocatalyst, and the selectivity of BZ from the conversion of HB using 0.3% Ni/CdS is also similar to that from the C–C coupling of BA using the same Ni/CdS catalyst. These results confirm that the selectivity of DOB and BZ is primarily determined by the conversion of HB intermediates in the second reaction step.
Scheme 2. Photocatalytic Conversion of HB Intermediates to DOB, BZ, and BH Using (a) 0.3% Ni/CdS and (b) CdS.
Reaction conditions: HB is 10 mg; the photocatalyst is 10 mg; CH3CN is 5 mL; Ar is at 1 atm; the visible light power is 0.35 W cm–2.
3.4. Photostability and Feasibility of CdS and Ni/CdS Photocatalysts
The feasibility and photostability of CdS and Ni/CdS photocatalysts are important factors in evaluating the long-term application of photocatalysts in the C–C coupling of alcoholic compounds to desirable products. In the fragmentation of biomass to alcohol-type derivatives, the typical derivatives from lignin conversion are BA with substitutes of −Me and −OMe at the para-position (Me-BA and MeO-BA), as both substitutes are major groups in the native lignin structures.14 Additionally, FA is a basic alcohol-type unit in cellulose and hemicellulose.38−43 To evaluate the feasibility of CdS and Ni/CdS photocatalysts, Me-BA, MeO-BA, and FA were used to investigate their conversion efficiency to the corresponding C–C coupled products. As shown in Scheme 3b,d, 0.3% Ni/CdS photocatalysts can achieve complete conversion of Me-BA and MeO-BA compounds to 70.4% of Me-BZ and 63% of MeO-BZ, respectively, as desirable products after 6 h of visible light irradiation. In addition, using CdS photocatalysts, the conversion rate of both Me-BA and MeO-BA achieves 100%, with the selectivity of the corresponding DOB products at 74.7% of Me-DOB and 70% of MeO–DOB, respectively, after 12 h of visible light irradiation (Scheme 3a,c). The generated products are important compounds in chemical industrial applications. For example, the generated Me-BZ and MeO-BZ are valuable precursors in the synthesis of 5H-1,4-benzodiazepine derivatives, which are important building blocks in various fields, such as organic chemistry, natural product synthesis, biochemistry, and pharmaceutical synthesis.44,45 Similarly, the produced Me-DOB and MeO-DOB are also important materials in generation of the diepoxide of bishydroxydeoxybenzoin (BEDB) that is a cross-linker with low flammability for epoxy adhesive chemistry46 and in generation of benzothieno[2,3-b]quinolones that shows practical applications in synthesis of anticancer agents, antibacterial and antiviral agents, and fluorescent probes and dyes.47
Scheme 3. Photocatalytic Conversion of Different Alcoholic Compounds to Corresponding C–C Coupled Products Using CdS and 0.3% Ni/CdS Photocatalysts.
(a, b) Me-BA; (c, d) MeO-BA; (e, f) FA. Reaction conditions: the reactant is 10 mg; the photocatalyst is 10 mg; CH3CN is 5 mL; Ar is at 1 atm; the visible light power is 0.35 W cm–2.
We also investigated the C–C coupling of FA to generate the corresponding products. As shown in Scheme 3e,f, FA converts to HF as the sole C–C coupled product, with selectivities of 86.7% when using CdS photocatalysts after 12 h of visible light irradiation and 54.6% using 0.3% Ni/CdS photocatalysts after 6 h. However, HF cannot further convert to dehydrated or dehydrogenated products, which is consistent with many studies that have demonstrated HF as the sole C–C coupled product.24,48−52 To investigate the possibility of conversion of HF to dehydrated or dehydrogenated products in our reaction system, the reaction time for FA conversion using both photocatalysts was prolonged to 24 h of visible light irradiation. As shown in Table S4, 100% of FA was converted to 88.6% of HF using CdS photocatalysts, and 100% of FA was converted to 63.4% of HF using 0.3% Ni/CdS. Both results confirm that HF is the sole C–C coupled product and cannot be further converted to dehydrated or dehydrogenated products. Two possible reasons could explain the results. First, the furan groups in HF show a weaker conjugation effect compared to phenyl groups in HB, Me-HB, or MeO-HB,53,54 which reduce the efficiency of photogenerated electron migration from photocatalysts to HF.55−57 Second, the O atom in furan groups is the electron-withdrawing group (EWG),58 decreasing the electron density of O–H or Cα–H groups in HF.59−61 Despite this, HF is a high-value product, as it can be used as the jet fuel precursor to generate C10 alkanes for aviation fuel.24,48−52 These experiments demonstrate that both CdS and Ni/CdS photocatalysts have promising potential in the conversion of alcoholic compounds into their corresponding C–C coupled products, affirming their high potential feasibility in practical applications.
Photocorrosion and photostability are important factors to investigate the applicability of CdS and Ni/CdS photocatalysts in the photocatalytic C–C coupling of BA into DOB and BZ, respectively. The photocorrosion and photostability of both photocatalysts were evaluated through recycling experiments. As shown in Figure 2d, after 5 recycling tests, the recycled 0.3% Ni/CdS photocatalysts can completely convert BA to around 78% of BZ after 5 h of visible light irradiation. Similarly, the recycled CdS photocatalysts also keep good stability through 5 recycling tests, achieving complete conversion of BA to around 77% of DOB after 9 h. XRD analysis was conducted to evaluate the structural integrity of both CdS and 0.3% Ni/CdS photocatalysts after 5 recycling tests. As shown in Figure S9, both recycled photocatalysts show similar XRD patterns with fresh samples. These results indicate that CdS and 0.3% Ni/CdS photocatalysts have low photocorrosion and high photostability in C–C coupling of BA into desirable products.
3.5. Improved Interaction between BA and Photogenerated Charges on 0.3% Ni/CdS to Enhance the Conversion Rate of BA
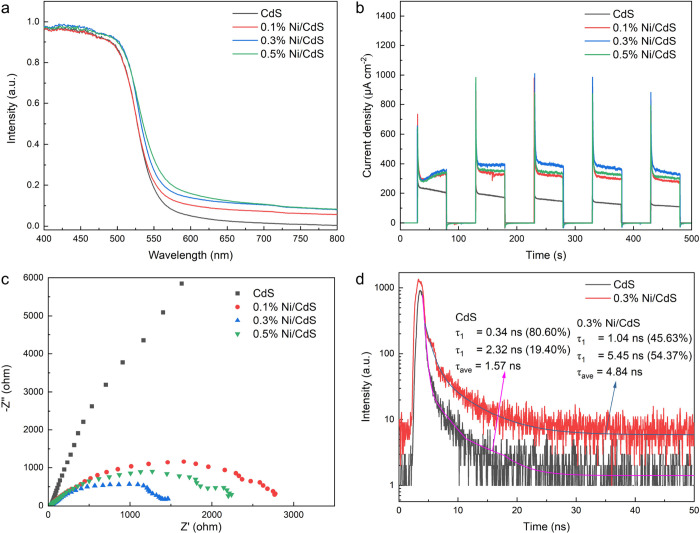
The reaction kinetics in Figure 2b,c show that the conversion rate of BA to BZ using 0.3% Ni/CdS photocatalysts is faster than the conversion rate of BA to DOB using CdS photocatalysts. This improvement is mainly attributed to the fact that the 0.3% Ni/CdS photocatalysts can enhance the visible light absorption capability and improve the photogenerated charge carrier migration efficiency to effectively enhance the interaction between BA and photogenerated charge carriers, therefore increasing the conversion rate in the BA C–C coupling reaction.15 Diffuse reflectance spectroscopy (DRS), photoelectrochemical (PEC) measurements, photoluminescence (PL), and time-resolved photoluminescence (TRPL) were employed to examine photophysical properties of CdS and x% Ni/CdS photocatalysts. DRS was employed to investigate the visible light absorption capabilities of photocatalysts. As shown in Figure 3a, the red shifts are observed from CdS to 0.5% Ni/CdS photocatalysts, indicating that the visible light absorption capability is increased with the amount of Ni on CdS photocatalysts. The bandgap energy (Eg) of CdS can be determined based on the DRS results, with details provided in the Supporting Information. As shown in Figure S10, the Eg of CdS is determined to be 2.30 eV. In addition, the Eg of x% Ni/CdS is equal to the Eg of CdS, as the introduction of Ni clusters on the surfaces of CdS cannot alter the crystal structure of CdS (XRD patterns in Figure S3).29,31 Although the introduction of Ni clusters cannot change the Eg of CdS, it does improve the visible light absorption capability, therefore enhancing the photocatalytic conversion rate of BA to BZ using 0.3% Ni/CdS photocatalysts.
Figure 3.
(a) UV–vis diffuse reflectance spectra of CdS and x% Ni/CdS photocatalysts. (b) Transient photocurrent of CdS and x% Ni/CdS photocatalysts upon turning on and off visible light. (c) EIS results of CdS and x% Ni/CdS photocatalysts under visible light irradiation. (d) TRPL spectra of CdS and 0.3% Ni/CdS photocatalysts.
The PEC measurements, which include transient photocurrent responses and electrochemical impedance spectroscopy (EIS), were conducted to investigate the photogenerated charge carrier separation efficiency of CdS and x% Ni/CdS photocatalysts. As shown in Figure 3b,c, 0.3% Ni/CdS photocatalysts show the highest photocurrent density and the smallest arc radius compared to other photocatalysts, indicating that 0.3% Ni/CdS photocatalysts significantly enhance the separation efficiency of photoexcited charge carriers and decrease the transfer resistance of charge carriers, therefore enhancing the photocatalytic performance.
PL and TRPL spectra were measured to investigate photogenerated charge carriers’ recombination and migration dynamics. PL emission spectra of CdS and 0.3% Ni/CdS photocatalysts were measured at 400 nm excitation wavelength to investigate the charge carriers’ quenched efficiency. As shown in Figure S11, both photocatalysts exhibit two emission peaks, which are a band-edge emission peak at 559 nm and a defect-mediated emission peak at 595 nm. Generally, the band-edge emission represents the direct recombination of excitons in the photocatalysts, while the defect-mediated emission arises from radiative recombination through defect states.62,63 The PL spectrum of phase junction CdS (Figure S11) exhibits that the intensity of defect-mediated emission is significantly higher than that of band-edge emission, indicating that the radiative recombination through defect states is the major pathway for photogenerated charge carriers’ recombination. For the phase junction CdS, the defect-mediated emission might attribute to the formation of defect states by lattice mismatch or interfacial inhomogeneity in the phase junction region.64−66 The introduction of Ni clusters on the surfaces of CdS can reduce the intensities of both emissions, as photogenerated electrons transfer from CdS to Ni cocatalysts, which not only facilitates electron separation but also passivates defect states in the phase junction region of CdS photocatalysts.29,31 Ni/CdS (0.3%) with lower emission intensities shows enhanced separation efficiency of photogenerated charge carriers and prolonged recombination time, thereby improving the conversion rate of BA to the corresponding products. TRPL was further conducted to investigate their charge carrier decay lifetime. As shown in Figure 3d, the decay curves of two photocatalysts are fitted by two-exponential functions, indicating the presence of two main carrier recombination pathways, corresponding to band-edge recombination (fast decay, τ1) and defect-mediated recombination (slow decay, τ2).67−69 In detail, CdS exhibits τ1 and τ2 of 0.34 and 2.32 ns, respectively, while 0.3% Ni/CdS shows τ1 and τ2 of 1.04 and 5.45 ns, respectively. Both longer τ1 and τ2 in 0.3% Ni/CdS indicate that the introduction of Ni clusters in CdS not only can increase the band-edge recombination time but also prolong the defect-mediated recombination time, which is consistent with the PL results observed for CdS and 0.3% Ni/CdS. The average emission lifetime (τave) was further calculated based on τave =(A1τ12 + A2τ22)/(A1τ1 + A2τ2), where τ1 and τ2 are the fluorescence lifetime and A1 and A2 are pre-exponential factors.70,71 The τave increases from 1.57 ns in CdS to 4.84 ns in 0.3% Ni/CdS. Both PL spectra and TRPL results demonstrate that the 0.3% Ni/CdS photocatalysts can prolong the residence time of photogenerated charge carriers, as the introduction of Ni clusters on the surfaces of CdS photocatalysts can mainly facilitate the migration of photogenerated electrons from CdS to Ni clusters and passivates defect states in the phase junction region of CdS photocatalysts. In summary, 0.3% Ni/CdS photocatalysts with the highest visible light absorption capability, the highest photoexcited charge carriers’ separation efficiency, the lowest charge carriers’ transfer resistance, and the longest recombination time of charge carriers compared to other photocatalysts can significantly improve the generation of charge carriers and promote the contact between charge carriers and the reactant, therefore improving the conversion rate of BA to BZ.
3.6. Mechanistic Study
As discussed in Section 3.3, the C–C coupling of BA into DOB and BZ contains two reaction steps, which are (1) C–C coupling of BA to HB as an intermediate product and (2) dehydration or dehydrogenation of HB to DOB or BZ, respectively. Herein, we delve into the distinct roles played by photogenerated charge carriers and generated radical intermediates in these two reaction steps, aiming to understand the mechanisms in the high selectivity of DOB and BZ using CdS and Ni/CdS photocatalysts, respectively.
3.6.1. C–C Coupling of BA to HB Intermediates through Cα–H Bond Activation
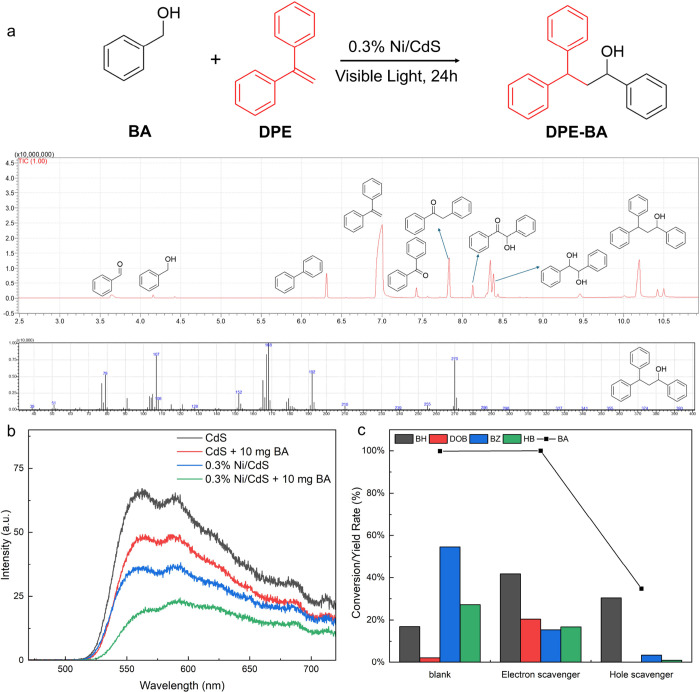
The C–C coupling of BA to HB intermediates is the first step in the generation of DOB and BZ as desirable products. Previous studies demonstrated that the Cα–H bond activation in BA is a crucial step for C–C coupling of BA to HB.13−15,72 In this study, DPE as the radical trapping agent was used to capture the BA C-centered radical intermediates after Cα–H bond activation. As shown in Figure 4a, DPE is added into the reaction system, which can significantly suppress the conversion rate of BA and the selectivity of C–C coupled products. In detail, 90% of BA converts to only 2.2% of DOB, 34.3% of BZ, and 15.8% of HB intermediates as the C–C coupled products after 24 h of visible light irradiation. The inability to achieve stoichiometric balance in this result is attributed to the fact that the BA C-centered radical intermediates are captured by DPE to generate DPE-BA (Figure 4a), instead of generation of C–C coupled products. These results demonstrate that C–C coupling of BA involves the radical intermediate mechanism, and the Cα–H bond activation in BA can determine the generation of HB intermediates.
Figure 4.
(a) GC-MS spectra of DPE capturing the BA C-radical intermediate and the MS spectrum of DPE-BA. Reaction conditions: BA is 20 mg; DPE is 30 mg; 0.3% Ni/CdS is 20 mg; CH3CN is 10 mL; Ar is at 1 atm; the visible light power is 0.35 W cm–2; 24 h. (b) PL emission spectra of CdS and 0.3% Ni/CdS with and without 10 mg of BA under visible light irradiation (excitation wavelength at 420 nm). (c) Conversion of BA to corresponding products using different scavengers. Reaction conditions: BA is 10 mg; the photocatalyst is 10 mg; CH3CN is 5 mL; Ar is at 1 atm; the visible light power is 0.35 W cm–2; 2 h. Hole scavengers are 20 mg of Na2S and 10 mg of Na2SO3; electron scavengers are 30 mg of Na2S2O8.
In the first reaction step, both photogenerated holes and electrons play important roles in the C–C coupling of the BA into HB intermediates. PL analysis was conducted to confirm the charge transfer event between photogenerated charge carriers and BA, as the fast transfer efficiency facilitates the generation of HB intermediates. As shown in Figure 4b, the emission intensities of excited states of both CdS and 0.3% Ni/CdS photocatalysts are relatively low in the presence of 10 mg of BA compared to that in the absence of BA under an excitation wavelength of 420 nm, indicating that the photogenerated charges are quenched with BA. The results demonstrate that the photogenerated holes and electrons transfer from photocatalysts to BA, therefore prolonging the recombination time of charge carriers and improving the efficiency of charge carriers’ utilization. In addition, the emission intensities of 0.3% Ni/CdS in both the presence and absence of 10 mg of BA are lower than those of CdS. The results further confirm that the efficiency of photogenerated charge carriers’ utilization in 0.3% Ni/CdS is higher than in CdS, resulting in a faster conversion rate of BA using 0.3% Ni/CdS compared to using CdS (Figure 2b,c). To investigate their individual roles, hole scavengers and electron scavengers were separately employed to hinder the photogenerated holes and electrons on photocatalysts, respectively. As shown in Figure 4c, the addition of electron scavengers (30 mg of Na2S2O8) in the reaction system shows that BA can still achieve complete conversion, but the yield of the BH byproduct significantly increases from 16 to 41.7% using 0.3% Ni/CdS after 2 h of visible light irradiation, demonstrating that the photoexcited electrons (e–) mainly improve the C–C coupling of BA to HB intermediates and decrease the selectivity of the BH byproduct. The addition of hole scavengers (20 mg of Na2S and 10 mg of Na2SO3) significantly decreases the conversion rate of BA from 100 to 34.7% and dramatically increases the yield of the BH byproduct from 16 to 88% after 2 h of visible light irradiation, indicating that the Cα–H bond activation in BA is mainly determined by the photogenerated holes (h+). These trapping experiments show that both h+ and e– can collaboratively improve the Cα–H bond activation in BA and enhance the conversion rate of BA into HB intermediates.
3.6.2. Conversion of HB Intermediates into DOB through Cα–H Bond Activation or into BZ through O–H Bond Activation
As discussed in Section 3.2, the selectivity of DOB or BZ from C–C coupling of BA is mainly determined by the second reaction step. In detail, HB intermediates can be dehydrated by CdS photocatalysts to improve the selectivity of DOB or dehydrogenated by 0.3% Ni/CdS photocatalysts to enhance the selectivity of BZ. Herein, the core question about the photocatalytic C–C coupled mechanism is how do CdS and 0.3% Ni/CdS photocatalysts exhibit high selectivity for the conversion of HB intermediates to DOB and BZ, respectively.
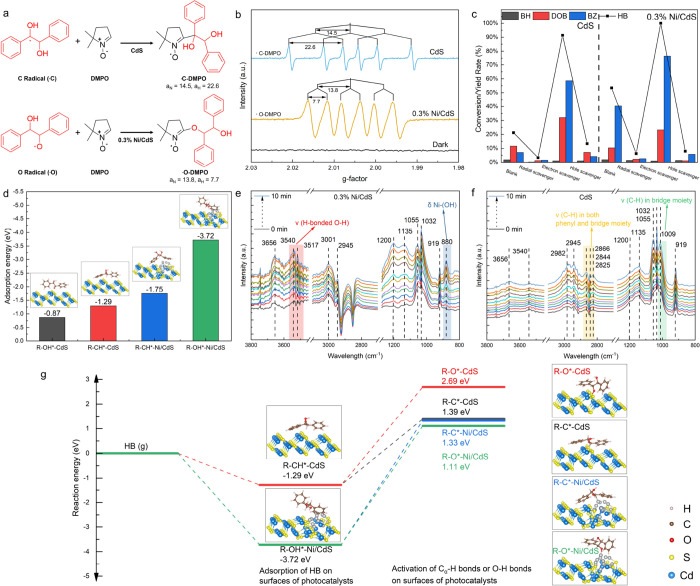
To understand the mechanism in the generation of DOB and BZ from the conversion of HB intermediates, in situ electron spin resonance (ESR) spectroscopy with DMPO as a spin-trapping agent was conducted to identify the generated radical intermediates from HB using CdS and 0.3% Ni/CdS photocatalysts, respectively. As shown in Figure 5a,b, using CdS photocatalysts, sextet signals with a ratio of 1:1:1:1:1:1 (αN = 14.5; αH = 22.6) are observed after 30 min of visible light irradiation, corresponding to the formation of HB C-centered radical intermediates (•C), whereas using 0.3% Ni/CdS photocatalysts, sextet signals with a ratio of 1:1:1:1:1:1 (αN = 13.8; αH = 7.7) are identified, representing the formation of HB O-centered radical intermediates (•O).22,24,29,73 Generally, the HB C-centered radical intermediates are generated through the Cα–H bond activation in HB, whereas the HB O-centered radical intermediates are generated through the O–H bond activation in HB.22 These results indicate that CdS photocatalysts have a high potential for the activation of Cα–H bonds in HB intermediates to generate DOB, and 0.3% Ni/CdS photocatalysts show a high potential for the activation of the O–H bonds in HB intermediates to generate BZ.
Figure 5.
(a, b) In situ ESR spectra in conversion of HB intermediates to DOB and BZ using CdS and 0.3% Ni/CdS photocatalysts after 30 min of visible light irradiation in the presence of DMPO as a spin-trapping agent. The details are provided in the Supporting Information. (c) Conversion of HB intermediates to corresponding products with different scavengers using CdS and 0.3% Ni/CdS photocatalysts. Reaction conditions: HB is 10 mg; the photocatalyst is 10 mg; CH3CN is 5 mL; Ar is at 1 atm; the visible light power is 0.35 W cm–2; 20 min. Hole scavengers are 20 mg of Na2S and 10 mg of Na2SO3; electron scavengers are 30 mg of Na2S2O8, and free radical scavengers are 30 mg of DMPO. (d) Calculated adsorption energy of HB on surfaces of CdS and Ni/CdS photocatalysts. (e, f) Time-resolved ATR spectra for the adsorption of HB on 0.3% Ni/CdS and CdS. (g) Calculated reaction energy of Cα–H bond activation and O–H bond activation in HB intermediates on surfaces of CdS and Ni/CdS photocatalysts.
The adsorption orientations of HB intermediates on both photocatalysts are the key factors to promote Cα–H bond activation and O–H bond activation. Generally, the adsorption of HB on both photocatalyst surfaces contains two distinct orientations, which are (1) the orientation of O–H groups in HB toward the photocatalyst surfaces (R-OH*) and (2) the orientation of Cα–H groups in HB toward the photocatalyst surfaces (R-CH*).73 Hence, DFT calculations and time-resolved ATR analyses were conducted to investigate the adsorption orientations of the HB intermediates on both photocatalysts. DFT simulations were first used to calculate the adsorption energies of HB intermediates with two adsorption orientations (R-OH* and R-CH*) on CdS and Ni/CdS photocatalysts. We constructed the cubic CdS (220) surfaces as the CdS photocatalyst reaction platform, as our previous work has demonstrated that the cubic (220) facets on phase junction CdS photocatalysts play a crucial role in the Cα–H bond activation in lignin to generate desirable aromatic monomers.28 Ni/CdS was built by loading Ni13 clusters onto the created cubic CdS (220) surfaces for HB adsorption and both bond activations (Figure S12). As shown in Figure 5d, on the CdS surfaces, the adsorption energy of R-OH* at −0.87 eV is higher than that of R-CH* at −1.29 eV, indicating a preference for HB adsorption with the R-CH* orientation. Conversely, on the Ni/CdS surfaces, the adsorption energy of R-OH* at −3.72 eV is significantly lower than that of R-CH* at −1.75 eV, suggesting a predilection for HB adsorption with an R-OH* orientation. To further investigate the adsorption orientations of HB intermediates on both photocatalysts, time-resolved ATR analyses were conducted. As shown in Figure 5e,f, several peaks are observed from 3800 to 2800 cm–1 and from 1200 to 800 cm–1, and their relevant detected bands and assignment are listed in Table S5. For 0.3% Ni/CdS photocatalysts, two peaks of 3540 and 3517 cm–1 (red area) are observed and gradually increase with the adsorption time, corresponding to H-bonded −OH groups on photocatalyst surfaces (ν (H-bonded O–H)), and another peak of 880 cm–1 (blue area) is also observed, representing the bending vibration of Ni–OH bonds (δ Ni–(OH)).74 The results indicate that the adsorption orientation of HB on Ni/CdS photocatalysts is mainly R-OH*. For CdS photocatalysts, four peaks occur in comparison with the ATR spectra of 0.3% Ni/CdS, which are 2866, 2844, 2825, and 1009 cm–1. The intensity of these peaks gradually increases with the adsorption time. The peaks between 2866 and 2825 cm–1 (orange area) represent the stretching vibration of C–H bonds (ν (C–H)) in both phenyl (C6H5) and the bridge moiety (CH–CH) of HB intermediates, and a peak of 1009 cm–1 (green area) corresponds to ν (C–H) in the bridge moiety (CH–CH) of HB intermediates.75 The results confirm that the adsorption orientation of HB on CdS photocatalysts is R-CH*. Based on DFT and in situ ATR results, the Cα–H groups in HB intermediates tend to orient toward the CdS surfaces (R-CH*) and the O–H groups in HB tend to orient toward the Ni/CdS surfaces (R-OH*).
DFT calculations were further conducted to investigate the reaction energies of Cα–H bond activation and O–H bond activation in HB intermediates based on adsorption of HB on CdS surfaces with an R-CH* orientation and on Ni/CdS surfaces with an R-OH* orientation. As shown in Figure 5g, on CdS surfaces, the bond dissociation energy (BDE) of O–H bond activation to form R-O* is 2.69 eV, which is significantly higher than the BDE of Cα–H bond activation at 1.39 eV to generate R-C*. Conversely, on the Ni/CdS photocatalyst surfaces, the BDE of the activation of the O–H bond to form R-O* is 1.11 eV, which is lower than that of the activation of the Cα–H bond at 1.33 eV to generate R-C*. In summary, CdS photocatalysts show the adsorption of HB with an R-CH* orientation, which mainly activates the Cα–H bonds in HB intermediates and improves the generation of DOB, while Ni/CdS photocatalysts show the adsorption of HB with an R-OH* orientation, which predominantly activates the O–H bonds in HB and enhances the selectivity of BZ.
The in situ ESR and DFT results indicate that the generation of HB C-centered radical intermediates through the Cα–H bond activation and the generation of HB O-centered radical intermediates through the activation of the O–H bond are important factors in determining the yields of DOB and BZ, respectively. Therefore, several radical scavengers were applied to further investigate the generation of both radical intermediates in two reaction systems. As shown in Figure 5c, DMPO was added into the photocatalytic system to quench both generated radical intermediates to investigate their effect on the photocatalytic performance in conversion of HB to DOB and BZ. The results show that the conversion rate of HB is significantly suppressed to 3.3% by addition of DMPO in the CdS reaction system and to 6.2% in the 0.3% Ni/CdS reaction system after 20 min of visible light irradiation, indicating that the formation of both radical intermediates is essential to improve the conversion rate of HB to DOB and BZ. DMPO is a general free radical scavenger that can capture both radicals in our reaction systems. To specifically evaluate the generation of HB C-centered radicals or HB O-centered radicals using both photocatalysts, MEOH and DPE were further conducted to hinder the specific radicals and investigate the photocatalytic performances. MEOH is a common scavenger to quench hydroxyl radicals (•OH) and R-O• radicals, as reported in previous studies.76−78 In our reaction systems, the main radicals are HB C-centered or HB O-centered (R-O•) radicals. Therefore, the addition of MEOH can capture the generated HB O-centered radicals. As shown in Figure S13, the addition of 30 mg of MEOH improves the conversion rate of HB from 21.2 to 30.5% using CdS and from 53.4 to 90.6% using 0.3% Ni/CdS photocatalysts. Also, the yields of DOB are significantly improved from 11.6 to 22.9% using CdS and from 10.3 to 64.1% using 0.3% Ni/CdS. The results further demonstrate that the generation of BZ is mainly determined by the formation of HB O-centered radicals. Previous studies demonstrated that DPE scavengers could preferentially quench C-centered radicals.14,79 In our reaction system, the additions of DPE have the potential to quench the generation of HB C-centered radicals. As shown in Figure S13, the addition of 30 mg of DPE reduces the conversion rate of HB to 8.4% using CdS and 14.4% using 0.3% Ni/CdS. The yields of BZ are higher than that of DOB using both photocatalysts. For CdS, the yields of BZ and DOB are 5.3 and 2.1%, respectively, while for 0.3% Ni/CdS, the yields of BZ and DOB are 10.1 and 3.3%, respectively. The results demonstrate that the generation of BZ in our reaction system is mainly determined by the HB O-centered radicals.
In addition, previous works demonstrated that the selectivity of the DOB and BZ can be determined by the band structure of the photocatalysts. For example, photocatalysts with high reductive capability can enhance the selectivity of DOB by promoting the reductive cleavage of the Cα–OH bonds in the formed HB C-centered radical intermediates.14 Inspired by this study,14 the cleavage of Cα–H bonds in the formed HB O-centered radical intermediates can improve the generation of BZ, and the cleavage of Cα–H bonds is mainly determined by the photogenerated holes (photo-oxidative capability).13,14,25,73,80 Hence, it is important to investigate the band structure of CdS and Ni/CdS photocatalysts, as these photocatalysts exhibit different selectivities in the generation of DOB and BZ. Generally, a lower ECB represents a stronger reductive capability, while a higher EVB corresponds to a stronger oxidative capability.81,82EVB was obtained from the XPS-VB results, and ECB can be calculated using the equation ECB = EVB – Eg (the details are presented in Table S6). As a result, CdS shows EVB at 1.17 eV and ECB at −1.13 eV, while 0.3% Ni/CdS shows EVB at 1.73 eV and ECB at −0.57 eV. Therefore, the photo-oxidative capability of 0.3% Ni/CdS is stronger than that of CdS, while the photoreductive capability of CdS is stronger than that of 0.3% Ni/CdS. As a result, 0.3% Ni/CdS with a stronger photo-oxidative capability can improve the cleavage of Cα–H bonds in the formed HB O-centered radical intermediates to enhance the generation of BZ, while CdS with a stronger photoreductive capability can improve the cleavage of Cα–OH bonds in the formed HB C-centered radical intermediates to enhance the generation of DOB.
The hole and electron scavengers were further used to hinder the photogenerated holes or electrons in the reaction system and investigate their respective impacts on the conversion of HB intermediates to DOB and BZ. As shown in Figure 5c, the addition of hole scavengers (20 mg of Na2S and 10 mg of Na2SO3) can significantly suppress the conversion rate of HB intermediates on both photocatalysts after 20 min of visible light irradiation, as the first activation step (Cα–H bond activation or O–H bond activation in HB) requires the photogenerated holes. The addition of electron scavengers (30 mg of Na2S2O8) can significantly improve the reaction rate of HB from 53.4 to 100% using 0.3% Ni/CdS and from 21.2 to 91.3% using CdS photocatalysts after 20 min of visible light irradiation. Also, the yield of BZ dramatically enhances from 40.4 to 76.4% using 0.3% Ni/CdS photocatalysts and from 7 to 58.6% using CdS photocatalysts. The improved conversion rate of HB is mainly attributed to the fact that the addition of electron scavengers can facilitate the consumption of photogenerated electrons and improve the generation of holes, therefore improving the first activation step by holes. The improved yields of BZ on both photocatalysts mainly due to the extra photogenerated holes can facilitate the cleavage of Cα–H bonds after the first activation step. Based on these analyses, we can conclude that both activations of Cα–H bonds or O–H bonds in HB require the photo-oxidative capability provided by holes, the cleavage of Cα–OH bonds in the formed HB C-centered radicals requires stronger photoreductive capability provided by electrons, and the cleavage of Cα–H bonds in the formed HB O-centered radicals requires stronger photo-oxidative capability.
3.6.3. Photocatalytic Mechanism for C–C Coupling of BA to DOB and BZ
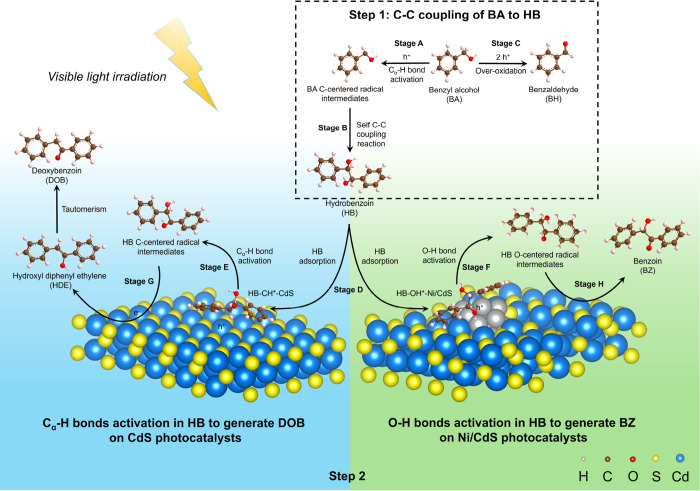
Based on the results discussed above, we proposed a plausible mechanism for photocatalytic C–C coupling of BA to DOB or BZ with high selectivity as desirable products using CdS or Ni/CdS photocatalysts, respectively. As shown in Scheme 4, the conversion of BA into DOB and BZ requires two reaction steps, which are (1) C–C coupling of BA to HB intermediates and (2) dehydration or dehydrogenation of HB intermediates to DOB or BZ, respectively. In the first reaction step, h+ activates the Cα–H bonds in BA to form BA C-centered radical intermediates and then desorbs on the surfaces of photocatalysts to generate HB as an intermediate product through the self-C–C coupling reaction (Stages A and B). In this step, BH as a byproduct is converted through overoxidation of BA by h+ (Stage C). In the second reaction step, HB intermediates adsorb on the photocatalyst surfaces and then activate the Cα–H bonds to generate HB C-centered radical intermediates on CdS surfaces or activate the O–H bonds to form HB O-centered radical intermediates on Ni/CdS surfaces (Stages D–F). Using the CdS photocatalysts, the Cα–H groups in HB intermediates preferentially orient toward the photocatalyst surfaces, as the adsorption energy of R-OH* at −0.87 eV is higher than that of R-CH* at −1.29 eV (Figure 5d), therefore improving the preference of Cα–H bond activation to form HB C-centered radical intermediates (Stage E). In Stage G, the HB C-centered radical intermediates can be reduced by e– to form HDE, which subsequently leads to the generation of DOB through the tautomerization of HDE.14 In addition, using Ni/CdS photocatalysts, O–H groups in HB are preferred to orient toward the Ni/CdS surfaces, attributed to the fact that the adsorption energy of R-OH* at −3.72 eV is lower than that of R-CH* at −1.75 eV, therefore improving the activation of the HB O–H bonds to generate HB O-centered radical intermediates (Stage F). In Stage H, the formed O-centered radical intermediates can be further oxidized by h+ to generate BZ as a desirable product (Stage H). In summary, the prepared phase junction CdS nanoparticles can significantly improve the generation of DOB by enhancing the Cα–H bond activation, and the prepared Ni/CdS photocatalysts can enhance the yield of BZ by improving the activation of the O–H bond.
Scheme 4. Proposed Mechanism in C–C Coupling of BA to DOB and BZ as Desirable Products through Two-Step Reactions.
4. Conclusions
In this study, we found that the photocatalytic C–C coupling of BA to generate DOB and BZ as desirable products contains two reaction steps, which are (1) C–C coupling of BA to generate HB intermediates and (2) dehydration of HB to DOB or dehydrogenation of HB to BZ. The generated radical intermediates in both reaction steps are important in determining the selectivity of the corresponding products. In detail, in the first reaction step, the activation of Cα–H bonds in BA through h+ forms BA C-centered radical intermediates, and the formed radicals determine the formation of HB intermediates through the self-C–C coupling reaction. In the second reaction step, the selectivity of DOB and BZ is mainly controlled by the activation of Cα–H bonds to form HB C-centered radical intermediates and by the activation of O–H bonds to form HB O-centered radical intermediates, respectively. The elaborately designed phase junction CdS photocatalysts and Ni/CdS photocatalysts can precisely control the Cα–H bond activation and the O–H bond activation in HB intermediates by adjusting the adsorption orientation of HB on the photocatalysts surfaces. Using phase junction CdS photocatalysts, the Cα–H groups in HB tend to orient toward the surfaces (R-CH*), as the adsorption energy of R-CH* at −1.29 eV is lower than that of R-OH* at −0.87 eV. The R-CH* orientation improves the activation of Cα–H bonds in HB, thereby enhancing the selectivity of DOB. Conversely, using Ni/CdS photocatalysts, the O–H groups in HB prefer to orient toward the surfaces (R-OH*), as the adsorption energy of R-OH* at −3.72 eV is lower than that of R-CH* at −1.75 eV. This orientation enhances the activation of O–H bonds in HB, resulting in an increased selectivity of BZ as the desirable product. As a result, phase junction CdS photocatalysts can achieve complete conversion of BA to 80.4% of DOB after 9 h of visible light irradiation, 0.3% Ni/CdS photocatalysts show the complete conversion of BA to 81.5% of BZ after only 5 h of visible light irradiation, and both elaborately developed photocatalysts exhibit the best photocatalytic performance compared to previous works.
Acknowledgments
We acknowledge the support received from the Engineering and Physical Sciences Research Council (EPSRC) (tender numbers: EP/W027593/1 and EP/V041665/1). Special thanks go to Mr. Mark Lauchlan and Mr. Fergus Dingwall from the Engineering School of the University of Edinburgh for their critical help in this study.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.4c03426.
Experimental details; photocatalytic reaction setup system; HAADF-STEM image; ICP-OES results; XRD patterns; XPS spectra; GC-MS results; GC spectra; 1H and 13C NMR spectra; photocatalytic performance; band structure; PL spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Hu X.; Wang Y.; Yu J.; Zhu J.; Hu Z. Synthesis of a Deoxybenzoin Derivative and Its Use as a Flame Retardant in Poly(Trimethylene Terephthalate). J. Appl. Polym. Sci. 2018, 135 (8), 1–8. 10.1002/app.45904. [DOI] [Google Scholar]
- Li H. Q.; Luo Y.; Song R.; Li Z. L.; Yan T.; Zhu H. L. Design, Synthesis, and Immunosuppressive Activity of New Deoxybenzoin Derivatives. ChemMedChem. 2010, 5 (7), 1117–1122. 10.1002/cmdc.201000107. [DOI] [PubMed] [Google Scholar]
- Menon R. S.; Biju A. T.; Nair V. Recent Advances in N-Heterocyclic Carbene (NHC)-Catalysed Benzoin Reactions. Beilstein J. Org. Chem. 2016, 12, 444–461. 10.3762/bjoc.12.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.; Yang S.; Zhang X.; Lin Q.; Das D. K.; Liu J.; Fang X. Dynamic Kinetic Resolution Enabled by Intramolecular Benzoin Reaction: Synthetic Applications and Mechanistic Insights. J. Am. Chem. Soc. 2016, 138 (25), 7932–7938. 10.1021/jacs.6b02929. [DOI] [PubMed] [Google Scholar]
- Lei W. L.; Yang B.; Zhang Q. B.; Yuan P. F.; Wu L. Z.; Liu Q. Visible-Light-Enabled Aerobic Synthesis of Benzoin Bis-Ethers from Alkynes and Alcohols. Green Chem. 2018, 20 (24), 5479–5483. 10.1039/C8GC02766H. [DOI] [Google Scholar]
- Iwamoto K. I.; Kimura H.; Oike M.; Sato M. Methylene-Bridged Bis(Benzimidazolium) Salt as a Highly Efficient Catalyst for the Benzoin Reaction in Aqueous Media. Org. Biomol. Chem. 2008, 6 (5), 912–915. 10.1039/b719430g. [DOI] [PubMed] [Google Scholar]
- Iwamoto K. I.; Hamaya M.; Hashimoto N.; Kimura H.; Suzuki Y.; Sato M. Benzoin Reaction in Water as an Aqueous Medium Catalyzed by Benzimidazolium Salt. Tetrahedron Lett. 2006, 47 (40), 7175–7177. 10.1016/j.tetlet.2006.07.153. [DOI] [Google Scholar]
- Banu S. F.; Indira M.; Sreeshitha M.; Reddy P. V. G. Highly Efficient N-Heterocyclic Carbene Precursor from Sterically Hindered Benzimidazolium Salt for Benzoin Condensation. ChemistrySelect 2024, 9 (17), e202401297 10.1002/slct.202401297. [DOI] [Google Scholar]
- Cao H.; Yang H.; Fang Y.; Zeng Y.; Cai X.; Ma J. Study on Trifluoromethanesulfonic Acid-Promoted Synthesis of Daidzein: Process Optimization and Reaction Mechanism. Chin. J. Chem. Eng. 2024, 71, 132–139. 10.1016/j.cjche.2024.03.026. [DOI] [Google Scholar]
- Chen X.; Chen Z.; So C. M. Exploration of Aryl Phosphates in Palladium-Catalyzed Mono-α-Arylation of Aryl and Heteroaryl Ketones. J. Org. Chem. 2019, 84 (10), 6337–6346. 10.1021/acs.joc.9b00669. [DOI] [PubMed] [Google Scholar]
- Morgan J. P.; Torres E. E.; Averill R.; Brody A. M. Updating the Benzoin Condensation of Benzaldehyde Using Microwave-Assisted Organic Synthesis and N-Heterocyclic Carbene Catalysis. J. Chem. Educ. 2023, 100 (2), 986–990. 10.1021/acs.jchemed.2c00982. [DOI] [Google Scholar]
- Hsieh M. H.; Huang G. T.; Yu J. S. K. Can the Radical Channel Contribute to the Catalytic Cycle of N-Heterocyclic Carbene in Benzoin Condensation?. J. Org. Chem. 2018, 83 (24), 15202–15209. 10.1021/acs.joc.8b02483. [DOI] [PubMed] [Google Scholar]
- Han G.; Liu X.; Cao Z.; Sun Y. Photocatalytic Pinacol C-C Coupling and Jet Fuel Precursor Production on ZnIn2S4 nanosheets. ACS Catal. 2020, 10 (16), 9346–9355. 10.1021/acscatal.0c01715. [DOI] [Google Scholar]
- Luo N.; Hou T.; Liu S.; Zeng B.; Lu J.; Zhang J.; Li H.; Wang F. Photocatalytic Coproduction of Deoxybenzoin and H2 through Tandem Redox Reactions. ACS Catal. 2020, 10 (1), 762–769. 10.1021/acscatal.9b03651. [DOI] [Google Scholar]
- Lee S. G.; Kang M. J.; Park M.; Kim K. J.; Lee H.; Kim H. S. Selective Photocatalytic Conversion of Benzyl Alcohol to Benzaldehyde or Deoxybenzoin over Ion-Exchanged CdS. Appl. Catal. B 2022, 304, 120967 10.1016/j.apcatb.2021.120967. [DOI] [Google Scholar]
- Chai Z.; Zeng T. T.; Li Q.; Lu L. Q.; Xiao W. J.; Xu D. Efficient Visible Light-Driven Splitting of Alcohols into Hydrogen and Corresponding Carbonyl Compounds over a Ni-Modified CdS Photocatalyst. J. Am. Chem. Soc. 2016, 138 (32), 10128–10131. 10.1021/jacs.6b06860. [DOI] [PubMed] [Google Scholar]
- Li H.; Qin F.; Yang Z.; Cui X.; Wang J.; Zhang L. New Reaction Pathway Induced by Plasmon for Selective Benzyl Alcohol Oxidation on BiOCl Possessing Oxygen Vacancies. J. Am. Chem. Soc. 2017, 139 (9), 3513–3521. 10.1021/jacs.6b12850. [DOI] [PubMed] [Google Scholar]
- Dai Y.; Li C.; Shen Y.; Zhu S.; Hvid M. S.; Wu L. C.; Skibsted J.; Li Y.; Niemantsverdriet J. W. H.; Besenbacher F.; Lock N.; Su R. Efficient Solar-Driven Hydrogen Transfer by Bismuth-Based Photocatalyst with Engineered Basic Sites. J. Am. Chem. Soc. 2018, 140 (48), 16711–16719. 10.1021/jacs.8b09796. [DOI] [PubMed] [Google Scholar]
- Jiao L.; Zhang D.; Hao Z.; Yu F.; Lv X. J. Modulating the Energy Band to Inhibit the Over-Oxidation for Highly Selective Anisaldehyde Production Coupled with Robust H2 Evolution from Water Splitting. ACS Catal. 2021, 11 (14), 8727–8735. 10.1021/acscatal.1c01520. [DOI] [Google Scholar]
- Zhang J.; Peng C.; Wang H.; Hu P. Identifying the Role of Photogenerated Holes in Photocatalytic Methanol Dissociation on Rutile TiO2(110). ACS Catal. 2017, 7 (4), 2374–2380. 10.1021/acscatal.6b03348. [DOI] [Google Scholar]
- Guo Q.; Xu C.; Ren Z.; Yang W.; Ma Z.; Dai D.; Fan H.; Minton T. K.; Yang X. Stepwise Photocatalytic Dissociation of Methanol and Water on TiO2(110). J. Am. Chem. Soc. 2012, 134 (32), 13366–13373. 10.1021/ja304049x. [DOI] [PubMed] [Google Scholar]
- Xie S.; Shen Z.; Deng J.; Guo P.; Zhang Q.; Zhang H.; Ma C.; Jiang Z.; Cheng J.; Deng D.; Wang Y. Visible Light-Driven C-H Activation and C-C Coupling of Methanol into Ethylene Glycol. Nat. Commun. 2018, 9 (1), 1–7. 10.1038/s41467-018-03543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Sun Y.; Zhang F.; Hu J.; Hu W.; Xie S.; Wang Y.; Feng J.; Li Y.; Wang G.; Zhang B.; Wang H.; Zhang Q.; Wang Y. Precisely Constructed Metal Sulfides with Localized Single-Atom Rhodium for Photocatalytic C–H Activation and Direct Methanol Coupling to Ethylene Glycol. Adv. Mater. 2023, 35 (5), 1–9. 10.1002/adma.202205782. [DOI] [PubMed] [Google Scholar]
- Gunawan D.; Yuwono J. A.; Kumar P. V.; Kaleem A.; Nielsen M. P.; Tayebjee M. J. Y.; Oppong-Antwi L.; Wen H.; Kuschnerus I.; Chang S. L. Y.; Wang Y.; Hocking R. K.; Chan T. S.; Toe C. Y.; Scott J.; Amal R. Unraveling the Structure-Activity-Selectivity Relationships in Furfuryl Alcohol Photoreforming to H2 and Hydrofuroin over ZnxIn2S3+x Photocatalysts. Appl. Catal. B Environ. 2023, 335, 122880 10.1016/j.apcatb.2023.122880. [DOI] [Google Scholar]
- Walenta C. A.; Courtois C.; Kollmannsberger S. L.; Eder M.; Tschurl M.; Heiz U. Surface Species in Photocatalytic Methanol Reforming on Pt/TiO2(110): Learning from Surface Science Experiments for Catalytically Relevant Conditions. ACS Catal. 2020, 10 (7), 4080–4091. 10.1021/acscatal.0c00260. [DOI] [Google Scholar]
- Jiang Y.; Li S.; Wang S.; Zhang Y.; Long C.; Xie J.; Fan X.; Zhao W.; Xu P.; Fan Y.; Cui C.; Tang Z. Enabling Specific Photocatalytic Methane Oxidation by Controlling Free Radical Type. J. Am. Chem. Soc. 2023, 145 (4), 2698–2707. 10.1021/jacs.2c13313. [DOI] [PubMed] [Google Scholar]
- Yang Q.; Wang T.; Zheng Z.; Xing B.; Li C.; Li B. Constructing Interfacial Active Sites in Ru/g-C3N4–x Photocatalyst for Boosting H2 Evolution Coupled with Selective Benzyl-Alcohol Oxidation. Appl. Catal. B Environ. 2022, 315, 121575 10.1016/j.apcatb.2022.121575. [DOI] [Google Scholar]
- Yue Z.; Shao S.; Yu J.; Lu G.; Wei W.; Huang Y.; Zhang K.; Wang K.; Fan X. Improved Lignin Conversion to High-Value Aromatic Monomers through Phase Junction CdS with Coexposed Hexagonal (100) and Cubic (220) Facets. ACS Appl. Mater. Interfaces. 2024, 16 (23), 29991–30009. 10.1021/acsami.4c02315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Liu C.; Li M.; Li H.; Li Y.; Su R.; Zhang B. Photoimmobilized Ni Clusters Boost Photodehydrogenative Coupling of Amines to Imines via Enhanced Hydrogen Evolution Kinetics. ACS Catal. 2020, 10 (6), 3904–3910. 10.1021/acscatal.0c00282. [DOI] [Google Scholar]
- Wang L.; Cheng B.; Zhang L.; Yu J. In Situ Irradiated XPS Investigation on S-Scheme TiO2@ZnIn2S4 Photocatalyst for Efficient Photocatalytic CO2 Reduction. Small 2021, 17 (41), 2103447 10.1002/smll.202103447. [DOI] [PubMed] [Google Scholar]
- Simon T.; Bouchonville N.; Berr M. J.; Vaneski A.; Adrovic A.; Volbers D.; Wyrwich R.; Döblinger M.; Susha A. S.; Rogach A. L.; Jäckel F.; Stolarczyk J. K.; Feldmann J. Redox Shuttle Mechanism Enhances Photocatalytic H2 Generation on Ni-Decorated CdS Nanorods. Nat. Mater. 2014, 13 (11), 1013–1018. 10.1038/nmat4049. [DOI] [PubMed] [Google Scholar]
- Hu R.; Xie W. H.; Wang H. Y.; Guo X. A.; Sun H. M.; Li C. B.; Zhang X. P.; Cao R. Visible Light-Driven Carbon-Carbon Reductive Coupling of Aromatic Ketones Activated by Ni-Doped CdS Quantum Dots: An Insight into the Mechanism. Appl. Catal. B Environ. 2022, 304, 120946 10.1016/j.apcatb.2021.120946. [DOI] [Google Scholar]
- Zhukovskyi M.; Tongying P.; Yashan H.; Wang Y.; Kuno M. Efficient Photocatalytic Hydrogen Generation from Ni Nanoparticle Decorated CdS Nanosheets. ACS Catal. 2015, 5 (11), 6615–6623. 10.1021/acscatal.5b01812. [DOI] [Google Scholar]
- Yu W.; Zhang D.; Guo X.; Song C.; Zhao Z. Enhanced Visible Light Photocatalytic Non-Oxygen Coupling of Amines to Imines Integrated with Hydrogen Production over Ni/CdS Nanoparticles. Catal. Sci. Technol. 2018, 8 (20), 5148–5154. 10.1039/C8CY01326H. [DOI] [Google Scholar]
- Ma M.; Wang R.; Shi L.; Li R.; Huang J.; Li Z.; Li P.; Konysheva E. Y.; Li Y.; Liu G.; Xu X. Defect-Expedited Photocarrier Separation in Zn2In2S5 for High-Efficiency Photocatalytic C-C Coupling Synchronized with H2 Liberation from Benzyl Alcohol. Adv. Funct. Mater. 2024, 10.1002/adfm.202405922. [DOI] [Google Scholar]
- Wang R.; Zheng Z.; Li Z.; Xu X. Photocatalytic C-C Coupling and H2 Production with Tunable Selectivity Based on ZnxCd1-xS Solid Solutions for Benzyl Alcohol Conversions under Visible Light. Chem. Eng. J. 2024, 480, 147970 10.1016/j.cej.2023.147970. [DOI] [Google Scholar]
- Zhao S.; Song S.; You Y.; Zhang Y.; Luo W.; Han K.; Ding T.; Tian Y.; Li X. Tuning Redox Ability of Zn3In2S6 with Surfactant Modification for Highly Efficient and Selective Photocatalytic C-C Coupling. Mol. Catal. 2022, 528, 112429 10.1016/j.mcat.2022.112429. [DOI] [Google Scholar]
- Li C.; Li J.; Qin L.; Yang P.; Vlachos D. G. Recent Advances in the Photocatalytic Conversion of Biomass-Derived Furanic Compounds. ACS Catal. 2021, 11 (18), 11336–11359. 10.1021/acscatal.1c02551. [DOI] [Google Scholar]
- Wu X.; Xie S.; Zhang H.; Zhang Q.; Sels B. F.; Wang Y. Metal Sulfide Photocatalysts for Lignocellulose Valorization. Adv. Mater. 2021, 33 (50), 1–20. 10.1002/adma.202007129. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Gupta N. K.; Rose M.; Gu X.; Menezes P. W.; Chen Z. Mechanistic Insights into the Photocatalytic Valorization of Lignin Models via C–O/C–C Cleavage or C–C/C–N Coupling. Chem. Catal. 2023, 3 (2), 100470 10.1016/j.checat.2022.11.009. [DOI] [Google Scholar]
- Liu X.; Chen Z.; Lu S.; Xu B.; Cheng D.; Wei W.; Shen Y.; Ni B. J. Heterogeneous Photocatalytic Conversion of Biomass to Biofuels: A Review. Chem. Eng. J. 2023, 476, 146794 10.1016/j.cej.2023.146794. [DOI] [Google Scholar]
- Xu X.; Shi L.; Zhang S.; Ao Z.; Zhang J.; Wang S.; Sun H. Photocatalytic Reforming of Lignocellulose: A Review. Chem. Eng. J. 2023, 469, 143972 10.1016/j.cej.2023.143972. [DOI] [Google Scholar]
- Wu X.; Luo N.; Xie S.; Zhang H.; Zhang Q.; Wang F.; Wang Y. Photocatalytic Transformations of Lignocellulosic Biomass into Chemicals. Chem. Soc. Rev. 2020, 49 (17), 6198–6223. 10.1039/D0CS00314J. [DOI] [PubMed] [Google Scholar]
- Bartfai T.; Wang M. W. Positive Allosteric Modulators to Peptide GPCRs: A Promising Class of Drugs. Acta Pharmacol. Sin. 2013, 34 (7), 880–885. 10.1038/aps.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantaj J.; Jackson P. J. M.; Rahman K. M.; Thurston D. E. From Anthramycin to Pyrrolobenzodiazepine (PBD) – Containing Antibody–Drug Conjugates (ADCs). Angew. Chemie Int. Ed. 2017, 56 (2), 462–488. 10.1002/anie.201510610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu B. Y.; Moon S.; Kosif I.; Ranganathan T.; Farris R. J.; Emrick T. Deoxybenzoin-Based Epoxy Resins. Polymer. 2009, 50 (3), 767–774. 10.1016/j.polymer.2008.12.009. [DOI] [Google Scholar]
- Janni M.; Thirupathi A.; Subramanian S. V.; Peruncheralathan S.. Palladium-Catalyzed Domino Heteroarylation of Thioamides: A Simple Route to Benzothieno[2,3-b]Quinolones. Synthesis 2024, 2. 10.1055/a-2360-8167. [DOI] [Google Scholar]
- Xin Z.; Huang M.; Wang Y.; Gao Y.; Guo Q.; Li X.; Tung C.; Wu L. Reductive Carbon–Carbon Coupling on Metal Sites Regulates Photocatalytic CO2 Reduction in Water Using ZnSe Quantum Dots. Angew. Chem. 2022, 134 (31), 2–7. 10.1002/ange.202207222. [DOI] [PubMed] [Google Scholar]
- Huang Y. B.; Yang Z.; Dai J. J.; Guo Q. X.; Fu Y. Production of High Quality Fuels from Lignocellulose-Derived Chemicals: A Convenient C-C Bond Formation of Furfural, 5-Methylfurfural and Aromatic Aldehyde. RSC Adv. 2012, 2 (30), 11211–11214. 10.1039/c2ra22008c. [DOI] [Google Scholar]
- Hamza M. A.; Evans J. D.; Andersson G. G.; Metha G. F.; Shearer C. J. Ultrathin Ru-CdIn2S4 Nanosheets for Simultaneous Photocatalytic Green Hydrogen Production and Selective Oxidation of Furfuryl Alcohol to Furfural. Chem. Eng. J. 2024, 493, 152603 10.1016/j.cej.2024.152603. [DOI] [Google Scholar]
- Zhang Q.; Gu B.; Fang W. Sunlight-Driven Photocatalytic Conversion of Furfural and Its Derivatives. Green Chem. 2024, 26 (11), 6261–6288. 10.1039/D4GC00140K. [DOI] [Google Scholar]
- Kowalik P.; Bujak P.; Penkala M.; Tomaszewski W.; Lisowski W.; Sobczak J. W.; Siepietowska D.; Maroń A. M.; Polak J.; Bartoszek M.; Pron A. Ag-In-Zn-S Quaternary Nanocrystals Prepared from InCl2 Precursor: Photophysical and Spectroscopic Properties and Application as Visible Light Photocatalysts of Aromatic Aldehyde Photoreduction. Chem. Mater. 2023, 35 (16), 6447–6462. 10.1021/acs.chemmater.3c01199. [DOI] [Google Scholar]
- Gershoni-Poranne R.; Rahalkar A. P.; Stanger A. The Predictive Power of Aromaticity: Quantitative Correlation between Aromaticity and Ionization Potentials and HOMO-LUMO Gaps in Oligomers of Benzene, Pyrrole, Furan, and Thiophene. Phys. Chem. Chem. Phys. 2018, 20 (21), 14808–14817. 10.1039/C8CP02162G. [DOI] [PubMed] [Google Scholar]
- Cao H.; Rupar P. A. Recent Advances in Conjugated Furans. Chem. - A Eur. J. 2017, 23 (59), 14670–14675. 10.1002/chem.201703355. [DOI] [PubMed] [Google Scholar]
- Pan R.; Ge X.; Liu Q.; Yin H.; Guo Y.; Shen J.; Zhang D.; Chen P.; Yuan J.; Xie H.; Liu C. Synergic Delocalized-Conjugate and Electron-Deficient Effect and Mesoporous Channel Promote Photocatalytic Coupling H2 Evolution with Benzyl-Alcohol Oxidation. Adv. Funct. Mater. 2024, 34 (17), 2315212 10.1002/adfm.202315212. [DOI] [Google Scholar]
- Cornil J.; Beljonne D.; Calbert J.; Brédas J. Interchain Interactions in Organic Π-conjugated Materials: Impact on Electronic Structure, Optical Response, and Charge Transport. Adv. Mater. 2001, 13 (14), 1053–1067. . [DOI] [Google Scholar]
- Wu Z.; Huang X.; Zheng H.; Wang P.; Hai G.; Dong W.; Wang G. Aromatic Heterocycle-Grafted NH2-MIL-125(Ti) via Conjugated Linker with Enhanced Photocatalytic Activity for Selective Oxidation of Alcohols under Visible Light. Appl. Catal. B Environ. 2018, 224, 479–487. 10.1016/j.apcatb.2017.10.034. [DOI] [Google Scholar]
- Mohammadi M. Electronic Effects of the Substituted Dopants on Stability and Reactivity of Difuranosilapyridine-4-Ylidenes: DFT Approach. Struct. Chem. 2023, 34 (6), 2347–2364. 10.1007/s11224-023-02151-x. [DOI] [Google Scholar]
- Wang W.; Yang X.; Dai R.; Yan Z.; Wei J.; Dou X.; Qiu X.; Zhang H.; Wang C.; Liu Y.; Song S.; Jiao N. Catalytic Electrophilic Halogenation of Arenes with Electron-Withdrawing Substituents. J. Am. Chem. Soc. 2022, 144 (29), 13415–13425. 10.1021/jacs.2c06440. [DOI] [PubMed] [Google Scholar]
- Guo Y.; An W.; Tian X.; Xie L.; Ren Y. L. Coupling Photocatalytic Overall Water Splitting with Hydrogenation of Organic Molecules: A Strategy for Using Water as a Hydrogen Source and an Electron Donor to Enable Hydrogenation. Green Chem. 2022, 24 (23), 9211–9219. 10.1039/D2GC02427F. [DOI] [Google Scholar]
- Eisenberg J. B.; Lee K.; Yuan X.; Schmidt J. R.; Choi K. S. The Impact of Electron Donating and Withdrawing Groups on Electrochemical Hydrogenolysis and Hydrogenation of Carbonyl Compounds. J. Am. Chem. Soc. 2024, 146 (22), 15309–15319. 10.1021/jacs.4c03032. [DOI] [PubMed] [Google Scholar]
- Panigrahy B.; Aslam M.; Misra D. S.; Ghosh M.; Bahadur D. Defect-Related Emissions and Magnetization Properties of ZnO Nanorods. Adv. Funct. Mater. 2010, 20 (7), 1161–1165. 10.1002/adfm.200902018. [DOI] [Google Scholar]
- Mukhopadhyay S.; Das P. P.; Maity S.; Ghosh P.; Devi P. S. Solution Grown ZnO Rods: Synthesis, Characterization and Defect Mediated Photocatalytic Activity. Appl. Catal. B 2015, 165, 128–138. 10.1016/j.apcatb.2014.09.045. [DOI] [Google Scholar]
- Chen B.; Zheng W.; Chun F.; Xu X.; Zhao Q.; Wang F. Synthesis and hybridization of CuInS 2 nanocrystals for emerging applications. Chem. Soc. Rev. 2023, 52, 8374–8409. 10.1039/D3CS00611E. [DOI] [PubMed] [Google Scholar]
- Yue X.; Fan J.; Xiang Q. Internal Electric Field on Steering Charge Migration: Modulations, Determinations and Energy-Related Applications. Adv. Funct. Mater. 2022, 32, 2110258 10.1002/adfm.202110258. [DOI] [Google Scholar]
- Bai X.; Wang X.; Jia T.; Guo L.; Hao D.; Zhang Z.; Wu L.; Zhang X.; Yang H.; Gong Y.; Li J.; Li H. Efficient Degradation of PPCPs by Mo1–xS2–y with S Vacancy at Phase-Junction: Promoted by Innergenerate-H2O2. Appl. Catal. B Environ. 2022, 310, 121302 10.1016/j.apcatb.2022.121302. [DOI] [Google Scholar]
- Xu Q.; Zhu B.; Cheng B.; Yu J.; Zhou M.; Ho W. Photocatalytic H2 Evolution on Graphdiyne/g-C3N4 Hybrid Nanocomposites. Appl. Catal. B Environ. 2019, 255, 117770 10.1016/j.apcatb.2019.117770. [DOI] [Google Scholar]
- Liu Y.; Zhou Y.; Yu S.; Xie Z.; Chen Y.; Zheng K.; Mossin S.; Lin W.; Meng J.; Pullerits T.; Zheng K. Defect State Assisted Z-Scheme Charge Recombination in Bi2O2CO3/Graphene Quantum Dot Composites for Photocatalytic Oxidation of NO. ACS Appl. Nano Mater. 2020, 3 (1), 772–781. 10.1021/acsanm.9b02276. [DOI] [Google Scholar]
- Lu Y.; Jia X.; Ma Z.; Li Y.; Yue S.; Liu X.; Zhang J. W5+–W5+ Pair Induced LSPR of W18O49 to Sensitize ZnIn2S4 for Full-Spectrum Solar-Light-Driven Photocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2022, 32 (35), 2203638 10.1002/adfm.202203638. [DOI] [Google Scholar]
- He B.; Bie C.; Fei X.; Cheng B.; Yu J.; Ho W.; Al-Ghamdi A. A.; Wageh S. Enhancement in the Photocatalytic H2 Production Activity of CdS NRs by Ag2S and NiS Dual Cocatalysts. Appl. Catal. B Environ. 2021, 288, 119994 10.1016/j.apcatb.2021.119994. [DOI] [Google Scholar]
- Tian T.; Jin X.; Guo N.; Li H.; Han Y.; Yuan Y. CdS/Ethylenediamine Nanowires 3D Photocatalyst with Rich Sulfur Vacancies for Efficient Syngas Production from CO2 Photoreduction. Appl. Catal. B Environ. 2022, 308, 121227 10.1016/j.apcatb.2022.121227. [DOI] [Google Scholar]
- An S. J.; Kang M. J.; Lee H.; Kim H. S. Tunable Selectivity of Photocatalytic Benzyl Alcohol Transformation over Ag-Ion-Exchanged CdS Nanowires. ACS Sustain. Chem. Eng. 2023, 11 (11), 4364–4373. 10.1021/acssuschemeng.2c06758. [DOI] [Google Scholar]
- Luo N.; Nie W.; Mu J.; Liu S.; Li M.; Zhang J.; Gao Z.; Fan F.; Wang F. Low-Work Function Metals Boost Selective and Fast Scission of Methanol C-H Bonds. ACS Catal. 2022, 12 (11), 6375–6384. 10.1021/acscatal.1c06005. [DOI] [Google Scholar]
- Lucarelli C.; Galli S.; Maspero A.; Cimino A.; Bandinelli C.; Lolli A.; Velasquez Ochoa J.; Vaccari A.; Cavani F.; Albonetti S. Adsorbent-Adsorbate Interactions in the Oxidation of HMF Catalyzed by Ni-Based MOFs: A DRIFT and FT-IR Insight. J. Phys. Chem. C 2016, 120 (28), 15310–15321. 10.1021/acs.jpcc.6b05428. [DOI] [Google Scholar]
- Williams R. M.; Medlin J. W. Benzyl Alcohol Oxidation on Pd(111): Aromatic Binding Effects on Alcohol Reactivity. Langmuir. 2014, 30 (16), 4642–4653. 10.1021/la5001304. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Chen J.; Zhang Y.; Liu T.; Yao Q.; Yang L.; Zhou X. Interactions between Peracetic Acid and TiO2 Nanoparticle in Wastewater Disinfection: Mechanisms and Implications. Chem. Eng. J. 2021, 412, 128703 10.1016/j.cej.2021.128703. [DOI] [Google Scholar]
- Yao K.; Fang L.; Liao P.; Chen H. Ultrasound-Activated Peracetic Acid to Degrade Tetracycline Hydrochloride: Efficiency and Mechanism. Sep. Purif. Technol. 2023, 306, 122635 10.1016/j.seppur.2022.122635. [DOI] [Google Scholar]
- Shen P.; Hou K.; Chen F.; Pi Z.; He L.; Chen S.; Li X.; Yang Q. Ultra-Rapid and Long-Lasting Activation of Peracetic Acid by Cu-Co Spinel Oxides for Eliminating Organic Contamination: Role of Radical and Non-Radical Catalytic Oxidation. Chem. Eng. J. 2023, 463, 142344 10.1016/j.cej.2023.142344. [DOI] [Google Scholar]
- Mukherjee U.; Shah J. A.; Musaev D. G.; Ngai M. Y. Harnessing Bromo/Acyloxy Transposition (BrAcT) and Excited-State Copper Catalysis for Styrene Difunctionalization. J. Am. Chem. Soc. 2024, 146, 21271. 10.1021/jacs.4c08984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi M. Y.; Li Y. H.; Anpo M.; Tang Z. R.; Xu Y. J. Efficient Photoredox-Mediated C-C Coupling Organic Synthesis and Hydrogen Production over Engineered Semiconductor Quantum Dots. ACS Catal. 2020, 10 (23), 14327–14335. 10.1021/acscatal.0c04237. [DOI] [Google Scholar]
- Luo N.; Wang M.; Li H.; Zhang J.; Hou T.; Chen H.; Zhang X.; Lu J.; Wang F. Visible-Light-Driven Self-Hydrogen Transfer Hydrogenolysis of Lignin Models and Extracts into Phenolic Products. ACS Catal. 2017, 7 (7), 4571–4580. 10.1021/acscatal.7b01043. [DOI] [Google Scholar]
- Lin J.; Wu X.; Xie S.; Chen L.; Zhang Q.; Deng W.; Wang Y. Visible-Light-Driven Cleavage of C–O Linkage for Lignin Valorization to Functionalized Aromatics. ChemSusChem. 2019, 12 (22), 5023–5031. 10.1002/cssc.201902355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.