Abstract
Toward identifying the roles of protease-activated receptor-1 (PAR1) and other G protein-coupled receptors important for vascular development, we investigated the role of Gα13 in endothelial cells in the mouse embryo. LacZ inserted into Gα13 exon 1 was highly expressed in endothelial cells at midgestation. Endothelial-specific Gα13 knockout embryos died at embryonic days 9.5–11.5 and resembled the PAR1 knockout. Restoration of Gα13 expression in endothelial cells by use of a Tie2 promoter-driven Gα13 transgene rescued development of endothelial-specific Gα13 knockout embryos as well the embryonic day 9.5 vascular phenotype in Gα13 conventional knockouts; transgene-positive Gα13-/- embryos developed for several days beyond their transgene-negative Gα13-/- littermates and then manifested a previously uncharacterized phenotype that included intracranial bleeding and exencephaly. Taken together, our results suggest a critical role for Gα13 in endothelial cells during vascular development, place Gα13 as a candidate mediator of PAR1 signaling in this process, and reveal roles for Gα13 in other cell types in the mammalian embryo.
Keywords: angiogenesis, endothelial cell, G protein, G protein-coupled receptor, G13
Formation of blood vessels during mammalian embryonic development is a complex and highly regulated process. Angioblasts proliferate, migrate, and differentiate to form primitive vascular structures composed of endothelial cells. These structures remodel by sprouting, branching, growing, and regressing, and mature by the recruitment and differentiation of pericytes and smooth muscle cells (1–3). Studies to define the molecular signals that orchestrate vascular development have focused mainly on receptor tyrosine kinases and integrins and their ligands (1, 4–9). Less is known regarding the roles of G protein-coupled receptors (GPCRs) in this process (10–16).
Protease-activated receptor-1 (PAR1), a GPCR for thrombin, plays an important role in the formation and/or maintenance of blood vessels in mouse embryos, a role likely attributable to PAR1 function in endothelial cells (11, 14). PAR1 triggers a host of cellular responses through heterotrimeric G proteins of the Gq/11, Gi/o, and G12/13 families; which of these pathways is important for proper vascular development is unknown. Conventional knockout of the gene-encoding Gα13 caused death of mouse embryos at midgestation with a phenotype grossly similar to, but more penetrant than, the PAR1 phenotype (17). We report a key role for Gα13 in endothelial cells at midgestation, suggesting that loss of Gα13 signaling in endothelial cells may account for the embryonic phenotype associated with PAR1 deficiency. Our results also unmask roles for Gα13 in other cell types.
Materials and Methods
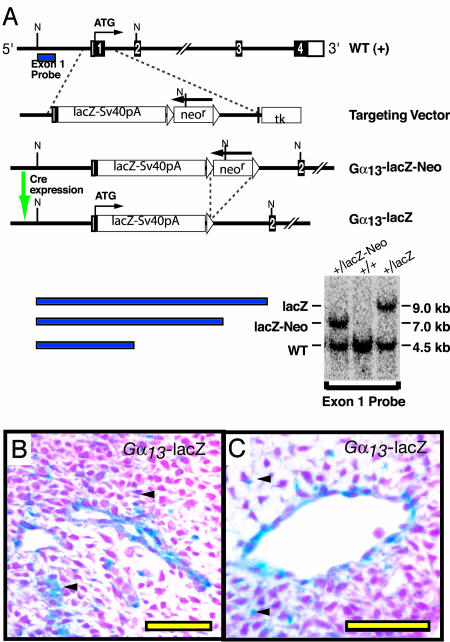
Mutant Mouse Strains. Generation of the Gα13-lacZ knockin allele. Gna13 (herein designated Gα13) gene fragments from a bacterial artificial chromosome from a 129/SvJ mouse genomic library, along with the lacZ gene, were used to construct a Gα13-lacZ knockin allele as described in Supporting Methods, which is published as supporting information on the PNAS web site, and Fig. 1. The loxP-flanked neor cassette was excised in vivo by crossing mice heterozygous for the Gα13-lacZ-Neo allele to mice carrying a β-actin promoter-Cre transgene (18).
Fig. 1.
Gα13-lacZ knockin allele. (A) Diagram of the WT Gα13 locus, the Gα13-lacZ targeting vector, and the recombined locus. Exons (coding in black, untranslated in open boxes), loxP sites (▵), and neomycin resistance (neor) and thymidine kinase negative selection (tk) cassettes are shown. The expected sizes of DNA fragments detected by Southern blot analysis by using “Exon 1” probe after an NcoI digest along with Southern blot of tail DNA from mice with the indicated Gα13 alleles are at bottom. (B and C) Analysis of tissue sections from an E10.5 Gα13+/lacZ revealed some staining throughout the mesenchyme (arrows) and stronger staining in the endothelium; intersomitic (B) and head (C) vessels are shown.
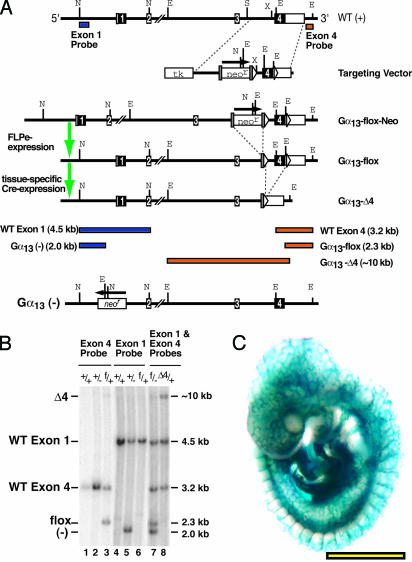
Generation of a floxed Gα13 allele. Gα13 gene fragments from BAC-125h06 were used to construct a conditional allele for Cre-lox mediated excision of Gα13 as described in Supporting Methods and Fig. 2. Cre-mediated excision of the flox allele generated a null Gα13 allele (“Δ4”), transcription of which gives rise to an mRNA fragment encoding a truncated Gα13 protein predicted to be inactive and short-lived.
Fig. 2.
Floxed Gα13 allele. (A) Diagram of WT Gα13 locus, targeting vector, recombined locus along with FLPe-excised “flox” allele, and Cre-excised “Δ4” null allele. Shaded rectangles indicate FRT sites; other conventions are as in Fig. 1. Shown at Bottom is the constitutive null Gα13 allele (“-”) described in ref. 17. Sizes of the DNA fragments detected by Southern hybridization with either Exon 1 (blue) or Exon 4 (orange) probe after NcoI/EcoRV digestion are shown in Bottom.(B) Southern blot analysis of tail DNA from adult mice with the indicated Gα13 genotypes: WT (+), exon 1 constitutive null (-), flox (f), exon 4 deletion null (Δ4). The probes used are indicated at the top. The DNA in lane 8 was obtained from a β-actin-Cre transgene-positive mouse; note the absence of the 2.3 kb Gα13 flox allele band and the presence of the ≈10.0 kb Gα13 Δ4 allele band. (C) Efficient endothelium-specific excision by Tie2-Cre. Tie2-CreTg/o;ROSA26R embryos collected at E9.5 were X-Gal stained. Virtually all endothelial cells were lacZ positive. (Scale bar: 1 mm.)
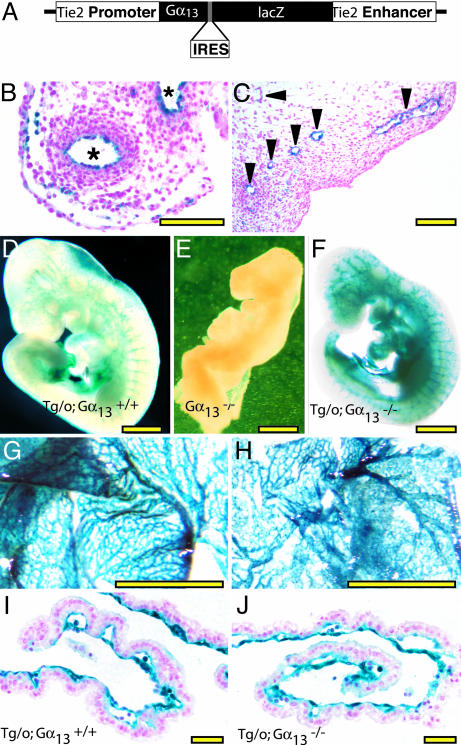
Generation of Tie2-Gα13 transgenic mice. A transgene to direct Gα13 expression to endothelium was generated by inserting Gα13 coding sequence along with an internal ribosome entry sequence (19), into pBS-Tie2-lacZ (from Thomas Sato, University of Texas Southwestern, Dallas) as described in Supporting Methods and Fig. 5. Two independent transgenic lines that showed endothelial-specific β-gal staining in embryonic day (E)8.5–11.5 embryos were selected for study. Similar results were obtained with each of the two lines.
Fig. 5.
An endothelium-specific Gα13 transgene rescues early lethality in Gα13-/- embryos. (A) Tie2 promoter/enhancer-Gα13-IRES-lacZ construct (Tie2-Gα13) used to generate mice in which Gα13 is expressed in endothelial cells. (B and C) X-Gal staining of E10.5 embryo showing expression of Tie2-Gα13 in endothelial and occasional hematopoietic cells in umbilical artery and vein (B, *) and head vessels (C, arrowheads). (D–F). Whole-mount X-Gal staining of E9.5 embryos. Tie2-Gα13-positive Gα13-/- (F) embryos were indistinguishable from Gα13+/+ (D) embryos and showed normal vascular staining. Gα13-/- embryos that lacked the transgene (E) were all grossly abnormal at this time. (Scale bar = 1 mm.) Whole-mount (G and H) and cross section (I and J) of X-Gal stained E9.5 yolk sacs. Tie2-Gα13Tg/o; Gα13+/- mice were mated to Gα13+/- mice that were homozygous for the Tie2-lacZ transgene to generate embryos with strong lacZ expression in endothelial cells. The yolk sac vasculature from both Gα13+/+ (G and I) and Gα13-/- (H and J) embryos that carried the Tie2-Gα13 transgene appeared normal. Note the striking contrast with Gα13-/- yolk sacs that lacked the transgene (Fig. 4 H and I). (Scale bars: B and C, 50 μm; D–H, 1 mm; I and J, 100 μm.)
Other Mouse Strains. Tie2-Cre and Tie2-lacZ were from T. Sato and M. Yanagisawa, respectively (University of Texas Southwestern) (20, 21), and β-actin-Cre and -FLP from J. Miyazaki (Osaka University Medical School, Osaka) (18) and S. M. Dymecki (Carnegie Institute of Washington, Baltimore) (22), respectively. The ROSA26R Cre reporter strain (23) was from The Jackson Laboratory. Gα13+/- (exon 1 deletion) mice were from M. Simon (California Institute of Technology, Pasadena, CA) (17).
Genotyping of Mutant Mouse Strains. DNA was isolated from ES cell clones, embryos, or mouse tail samples (14) and genotyped for Gα13 wild-type (WT), flox, Δ4, and (-) alleles by digestion with EcoRV and NcoI and Southern hybridization with “exon 1” and “exon 4” probes (Fig. 2). Gα13-lacZ and Gα13-lacZ-Neo alleles were genotyped by NcoI digestion and Southern hybridization with the exon 1 probe (Fig. 1). The Tie2-Gα13 and Tie2-lacZ transgenes were detected by Southern blot analysis with a lacZ cDNA probe. The Tie2-Cre and β-actin-Cre transgenes were detected either by PCR or Southern blot for Cre sequence. Southern and Northern analyses were performed by using standard techniques.
Embryo Dissection, Immunostaining, and X-Gal Staining. Noon of the day a vaginal plug was observed was defined as E0.5. After killing, the uterine horns were removed and embryonic and extraembryonic tissues were dissected free. After photographing the yolk sac and noting its properties, the yolk sac was opened, and the embryo was examined for gross phenotypes, then X-Gal was stained or immunostained in whole mount (24) or sections as described in Supporting Methods.
Isolation and Adenoviral Infection of Neonatal Endothelial Cells. Microvascular endothelial cells were isolated from the skin of Gα13flox/flox neonates. More than 95% of cells in these preparations expressed the endothelial markers PECAM1 and ICAM2 (25). Immediately after the second round of immunopurification, cells were subjected to two successive infections with ≈100 infections units/ml of Cre-GFP or GFP only adenovirus (from Hilary Beggs, University of California, San Francisco) for 24 h each (26, 27); titers were determined by using an adenoviral titer kit (Clontech). One to 6 days after the second infection, the cells were used for experiments. Gα13 mRNA expression was nearly ablated in Gα13flox/flox endothelial cells infected with the Cre adenovirus (data not shown).
Matrigel Assay. Endothelial cells (1 × 105) that had been infected with the appropriate adenovirus were plated onto a Matrigel surface and incubated 14–16 h at 37°C in DMEM with 20% FBS and 50 μg/ml endothelial cell growth supplement (BTI, Stoughton, MA) as described in Supporting Methods. photoshop 7.0 (Adobe Systems, San Jose, CA) was used to perform planimetry to determine area covered by cells.
Results
To characterize Gα13 expression in the mouse embryo, we generated a mouse bearing a Gα13 lacZ knockin allele (Gα13 lacZ allele; Fig. 1) such that lacZ transcription would mimic that of Gα13. X-Gal staining of E10.5 Gα13+/lacZ embryos revealed lacZ activity throughout the mesenchyme and stronger staining in vascular endothelial cells and dorsal neural tube (Fig. 1 and data not shown). Gα13 expression was also readily detected by Northern analysis of endothelial cells immunopurified from neonatal mice (25) and by X-Gal staining of endothelial cells from Gα13+/lacZ mice (data not shown). In the context of the phenotype of the conventional Gα13 knockout (17), these results prompted an effort to define the importance Gα13 function in endothelial cells.
We generated mice bearing a Gα13 conditional allele in which the coding portion of exon 4 was flanked by loxP sites (Fig. 2). Mice with one such “floxed” and one WT allele (Gα13+/flox) were mated to each other and to mice heterozygous for the conventional exon 1 knockout Gα13 allele (Gα13+/-) (17) to generate Gα13flox/flox and Gα13flox/- offspring. These offspring were born at or near the expected Mendelian rate and had no obvious abnormalities. Thus, insertion of loxP sites did not disrupt necessary functions of the Gα13 gene.
Gα13flox/flox mice were mated to mice carrying a β-actin promoter-driven Cre transgene (18) to generate offspring heterozygous for the Gα13 exon 4 deletion (Gα13+/Δ4). Southern blot (Fig. 2) and PCR analysis (data not shown) confirmed excision of exon 4. Gα13+/Δ4 mice were intercrossed to generate Gα13Δ4/Δ4 embryos, which died at ≈E9.5 with a phenotype indistinguishable from that reported for embryos homozygous for a Gα13 exon 1 deletion allele (Gα13-/-) (17); Gα13 Δ4/- embryos also exhibited the Gα13-/- phenotype (data not shown). Thus, Cre-mediated excision of Gα13 exon 4 resulted in a Gα13 allele that was functionally null.
We used a Tie2-Cre transgene (20, 21) to excise the Gα13 flox allele expression in endothelium. Such ablation of Gα13 function in endothelial cells resulted in a highly penetrant embryonic lethal phenotype. Gα13flox/- mice were crossed with Gα13+/- mice hemizygous for the Tie2-Cre transgene (Tie2-CreTg/o; Gα13+/-), and offspring were genotyped ≈10 days after birth (Table 1). Live Gα13flox/+ or G13+/- offspring were produced at similar rates in the presence or absence of the transgene. By contrast, Tie2-CreTg/o; Gα13flox/- mice, hereafter referred to as Gα13 endoKO, were markedly underrepresented compared with their Cre-negative counterparts (3 vs. 25; P < 0.005). The few Gα13 endoKO mice that were born were runted.
Table 1. Impaired viability of endothelial-specific Gα13 knockout mice.
| Breed | +/flox | +/- | -/- | flox/- |
|---|---|---|---|---|
| Tie2-CreTg/o | 38 | 29 | 0 | 3 |
| Tie2-Creo/o | 40 | 33 | 0 | 25 |
DNA was collected from 168 offspring from a Tie2-CreTg/o, Gα13+/- × Gα13flox/- intercross alive at ≈postnatal day 10.
The Gα13 endoKO embryonic phenotype was highly penetrant by midgestation (Table 2). Embryos from Tie2-CreTg/Tg; Gα13+/- × Gα13flox/- matings were collected at E8.5, 9.5, 10.5, and 11.5. At E8.5, the resulting Gα13-/- and Gα13 endoKO embryos were indistinguishable from their Gα13+/- and Gα13+/flox littermates in gross appearance. At E9.5, about one-half of Gα13-/- embryos were dead and all were abnormal. Gα13 endoKO embryos were also severely affected at this time; 36% were dead and 76% were affected (Table 2). By E10.5, ≈90% of Gα13-/- and 50% of Gα13 endoKO embryos were dead; all Gα13-/- embryos and 81% of Gα13 endoKO embryos were affected. By E11.5, all Gα13-/- and 88% of Gα13 endoKO embryos were dead.
Table 2. Genotype and gross phenotype of embryos from Tie2-Cre+/+; Gα13+/- × Gα13flox/- intercrosses.
|
Gα13 genotype, alive/total (% abnormal)
|
||||
|---|---|---|---|---|
| Age | flox/+ | +/- | -/- | flox/- |
| E9.5 | 27/32 (13) | 29/34 (9) | 11/21 (100) | 16/25 (76) |
| E10.5 | 21/23 (9) | 20/22 (9) | 2/19 (100) | 8/16 (81) |
| E11.5 | 17/20 (15) | 27/29 (10) | 0/13 (100) | 3/24 (92) |
Alive/total number of embryos recovered and the percent of abnormal embryos for each genotype at the indicated gestational age are shown. Alive was defined as having a heartbeat. Abnormal included embryos showing pericardial dilatation, hemorrhage, developmental delay, or absence of a heartbeat. Note that all embryos generated by this cross were Tie2-CreTg/o.
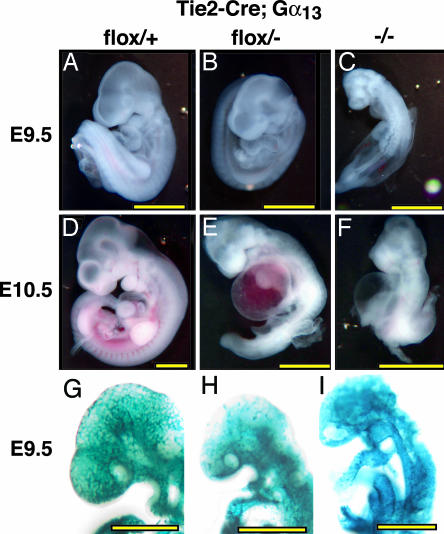
The gross features of the Gα13 endoKO phenotype were similar to, but somewhat less severe than, those of the conventional Gα13 knockout (17). Both had wrinkled yolk sacs with a paucity of blood-filled vessels as well as pale and delayed embryos with pericardial swelling and variable bleeding into cavities and tissues (Figs. 3 and 4). The onset and penetrance of developmental delay and pericardial dilatation was similar in Gα13-/- embryos and Gα13 endoKO embryos, but gross hemorrhage was more evident in the former.
Fig. 3.
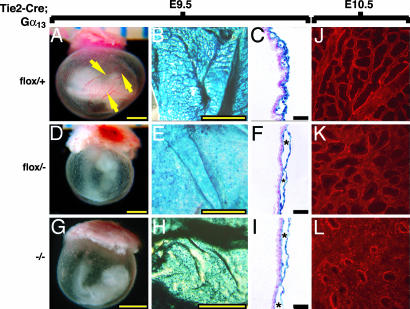
Phenotype of Gα13 endothelial-specific knockout and Gα13-/- embryos. All embryos are Tie2-CreTg/o and had a heartbeat at the time of photography. (A–F) Gross appearance of Gα13+/+ embryos (A and D), Gα13flox/- embryos (B and E), and Gα13-/- embryos (C and F). At E9.5 (A–C), Gα13-/- embryos (C) were significantly delayed compared with control embryos (A); developmental arrest appeared to occur at ≈E8.5 as reported in ref. 17. Gα13 endoKO embryos (B) had turned and appeared arrested at ≈E9.0. By E10.5 (D–F), >80% of Gα13 endoKO embryos (E) were either dead or morphologically abnormal, and all Gα13-/- embryos (F) were dead or grossly abnormal. Dilated pericardial sacs, consistent with cardiovascular failure, were a prominent feature in both. (G–I) Whole-mount X-Gal staining of Tie2-CreTg/o; ROSA26R embryos that were Gα13+/+ (G), Gα13flox/- (H), and Gα13-/- (I); embryos were collected at E9.5. Note that there is more primitive vascular plexus in the head of Gα13 endoKO (H) and global Gα13 nulls (I) compared with the control (G). (Scale bars: 1 mm.)
Fig. 4.
Phenotype of Gα13 endoKO and Gα13-/- yolk sacs. Gα13 genotypes were flox/+ (A–D), flox/- (E–H), and -/- (I–L). At E9.5, large blood-filled vessels (arrows) were seen in control (A) but not in flox/- (E) or -/- (I) yolk sacs, which were pale and dimpled. X-Gal staining of Tie2-CreTg/o; ROSA26R (B, C, F, and G) or Tie2-lacZTg/o (J and K) yolk sacs revealed an arborized structure with large and small vessels Gα13 WT (B), whereas Gα13 endoKO (F) and Gα13-/- (J) yolk sacs exhibited a more plexus-like structure that lacked large vessels. Cross sections of such yolk sacs (C, G, and K) showed blood-filled vessels spaced at regular intervals and lined with blue-stained endothelial cells in controls (C) but enlarged and often bloodless vascular spaces (*) in Gα13 endoKO (F) and Gα13-/- (I) yolk sacs. (Yellow bars: 1 mm; black bars: 100 μm.) (D, H, and L) Immunofluorescence staining of E10.5 yolk sacs for the endothelial marker PECAM1: Gα13flox/+ (D), Gα13 endoKO (H), and Gα13-/- (L).
The structure of the yolk sacs of Gα13 endoKO embryos was strikingly abnormal. At E9.5 and E10.5, WT yolk sacs contain an arborized vasculature with large and small vessels evident by gross examination and by PECAM staining. By contrast, large vessels were lacking from Gα13 endoKO yolk sacs, and unusually large vascular spaces were apparent on cross section (Fig. 4). This finding was even more obvious in Gα13-/- yolk sacs.
Whole-mount X-Gal staining of E9.5 Gα13 endoKO embryos that carried the ROSA26R excision reporter revealed a grossly normal vascular pattern in the trunk (see intersomitic vessels, branchial arch vessels, and endocardium in Fig. 3). However, head vessels showed delayed development and were disorganized compared with those seen in littermate controls (Fig. 3). Similar results were seen by immunostaining for the endothelial markers PECAM and endoglin (data not shown). Thus, endothelial cell differentiation and early vasculogenesis and patterning were relatively normal in embryos that lacked Gα13 function in endothelial cells, but subsequent remodeling of the vasculature was markedly impaired in several vascular beds.
The results described above strongly suggest that Gα13 expression in endothelial cells is required for normal vascular development in mouse embryos, but the greater severity of the Gα13-/- vs. Gα13 endoKO phenotype might reflect a necessary role for Gα13 in other cell types. To probe this question, we asked whether Gα13 expression in endothelial cells might be sufficient to rescue development of Gα13-/- embryos. We used the Tie2 promoter/enhancer (20) to drive transcription of a Gα13-IRES-lacZ cassette in endothelial cells in transgenic mice (Fig. 5). X-Gal staining of embryos from two such Tie2-Gα13 transgenic lines confirmed transgene expression in vascular structures from E8.5 onward. Staining was restricted to endothelium and endocardium and to a small fraction of hematopoietic cells, suggesting that transgene expression was appropriately cell-type specific (Fig. 5 and data not shown).
Gα13+/- mice hemizygous for the endothelial-specific Gα13 transgene (Tie2-Gα13Tg/o; Gα13+/-) were crossed to Gα13+/- mice and embryos collected at various times. Although approximately half of the transgene-negative Gα13-/- embryos recovered at E9.5 were dead and the remainder were delayed and/or otherwise abnormal, transgene-positive Gα13-/- embryos were recovered at the expected Mendelian rate, alive and indistinguishable from their Gα13+/+ and Gα13+/- littermates (Table 3 and Fig. 5). Moreover, whereas transgene-negative Gα13-/- yolk sacs showed a grossly abnormal vascular plexus (Fig. 4), transgene-positive Gα13-/- yolk sacs displayed a pattern of branching vessels indistinguishable from that seen in WT littermates (Fig. 5). Thus, expression of Gα13 in endothelial cells was sufficient to prevent vascular defects and death of Gα13-/- embryos at E9.5.
Table 3. Endothelial Gα13 transgene rescue of Gα13 early lethality: Genotype and gross phenotype of embryos from Tie2-Gα13Tg/o; Gα13+/- × Gα13+/- intercrosses.
| Alive/total embryos (% abnormal)
|
|||||||
|---|---|---|---|---|---|---|---|
|
Gα13+/+
|
Gα13+/-
|
Gα13-/-
|
|||||
| Age | Total | Tg/o | o/o | Tg/o | o/o | Tg/o | o/o |
| E9.5 | 90 | 11/11 (0) | 9/9 (0) | 23/23 (0) | 23/23 (0) | 11/11 (0) | 6/13 (100) |
| E11.5 | 104 | 15/15 (0) | 12/12 (0) | 28/28 (0) | 25/25 (0) | 13/14 (100) | 0/10 (100) |
| E12.5 | 151 | 21/22 (5) | 16/16 (0) | 49/49 (0) | 44/46 (4) | 2/18 (100) | 0/0 (NA) |
Number of alive/total recovered embryos and percent of embryos that were abnormal. “Abnormal” includes embryos showing hemorrhage, pericardial dilatation, small size, developmental delay, and/or a neural tube defect in addition to those lacking a heart beat. Note that the transgene yielded complete rescue of Gα13-/- embryos through E9.5 and nearly complete rescue at the level of viability through E11.5, at which time the rescued embryos manifested a new phenotype (see text). NA, not applicable.
Gα13-/- embryos carrying the Tie2-Gα13 transgene survived for several days beyond their transgene-negative counterparts and then developed a second phenotype characterized by exencephaly and hemorrhage within the head mesenchyme (K. Ruppel and S. Coughlin, unpublished data). To determine whether the inability of the Tie2-Gα13 transgene to completely rescue development of Gα13-/- embryos was due to a requirement for Gα13 function in another cell type vs. failure of the transgene to adequately reproduce the normal level or temporal and spatial pattern of Gα13 expression in endothelial cells, we asked whether the Tie2-Gα13 transgene would completely rescue the endothelial-specific Gα13 knockout. Tie2-Gα13Tg/o; Gα13flox/- mice were crossed to Gα13+/- mice homozygous for the Tie2-Cre transgene (Tie2-CreTg/Tg; Gα13+/-). Offspring were genotyped 10–15 days after birth (Table 4). No Gα13-/- mice were recovered, even when the endothelial-specific Gα13 transgene was present, and no Tie2-CreTg/o; Gα13flox/- pups were recovered in the absence of the transgene. By contrast, Tie2-Gα13Tg/o; Tie2-CreTg/o; Gα13flox/- mice were recovered at the expected Mendelian rate. These results suggest that the Tie2-Gα13 transgene drives Gα13 expression in a manner adequate to support the Gα13 functions in endothelial cells that are necessary for embryonic development. Thus, the contrast between the phenotypes of endothelial-specific Gα13 knockout vs. conventional Gα13 knockouts carrying the Tie2-Gα13 transgene is likely due to a necessary role for Gα13 in a cell type(s) other than endothelial cells, a role that becomes apparent only when Gα13-/- embryos are supported beyond E9.5 by the endothelial transgene.
Table 4. Tie2-Gα13 transgene rescues development of endothelial conditional Gα13 knockout mice.
| flox/+ | +/- | -/- | flox/- | |
|---|---|---|---|---|
| Tie2-Gα13Tg/o | 19 | 20 | 0 | 21 |
| Tie2-Gα13o/o | 25 | 18 | 0 | 0 |
Genotypes of 103 offspring from Tie2-Gα13Tg/o; Gα13flox/- × Tie2-CreTg/Tg; Gα13+/- intercrosses were determined at postnatal days 10–15. All carry the Tie2-Cre transgene such that all Gα13flox/- are endothelial-specific knockouts. Note the complete rescue of the endothelial-specific knockouts by the transgene.
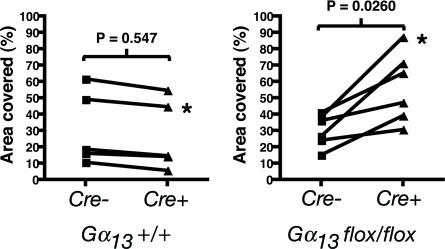
Our results strongly suggest that Gα13 signaling in endothelial cells plays a critical role in vascular development. What function(s) might it serve in this context? Gα13 links GPCRs to Rho activation and, perhaps, to other effector pathways. Toward exploring the effect of Gα13 deficiency on endothelial cell responses and behaviors, we ablated Gα13 function in cultured mouse microvascular endothelial cells. Cells were immunopurified from the skin of Gα13flox/flox neonatal mice and infected with adenovirus-directing expression of Cre and GFP or GFP alone (27). Southern and Northern blot analysis of such cultures suggested an efficiency of >95% for Gα13 excision (data not shown). Curiously, Gα13 excision had no detectable effect on Rho activation, changes in endothelial cell shape or stress fiber formation in response to PAR1 agonist, and no effect was detected in assays of endothelial cell movement on coverslips, adhesion on various matrices, or contraction of collagen gels (data not shown). There was, however, a reproducible difference in the behavior of cells cultured on Matrigel. WT or Ad-GFP Gα13flox/flox endothelial cells plated on Matrigel formed a monolayer that remodeled to become a “network” of cords and tubes (Fig. 6). Ad-Cre-GFP Gα13flox/flox endothelial cells plated at the same density formed a monolayer normally, but subsequent remodeling was markedly decreased (Fig. 6). The different remodeling behaviors depended on the Gα13 genotype in that no differences were seen in WT cells infected with the Ad-Cre-GFP vs. Ad-GFP viruses. Thus, Gα13 may be necessary for regulating cell shape, movement, and/or interaction with extracellular matrix during remodeling of endothelial structures.
Fig. 6.
Gα13-deficient endothelial cells are impaired in their ability to form a network on Matrigel. Gα13flox/flox or Gα13+/+ endothelial cells were infected with control GFP-adenovirus (Cre-) or GFP-Cre adenovirus (Cre+) then plated on Matrigel as described in Supporting Methods. The percent of the Matrigel surface covered with cells was calculated for five separate Gα13+/+ and six separate Gα13flox/flox cell preparations. Connected points indicate paired samples from a single endothelial cell preparation infected with Cre+ vs. Cre- virus. Asterisks mark the samples shown in Fig. 7, which is published as supporting information on the PNAS web site. Note that network formation decreased (and, hence, area covered by cells increased) in association with Cre expression in Gα13flox/flox but not WT endothelial cells. P values were calculated with the Mann–Whitney test.
Discussion
The roles of the >300 nonodorant GPCRs in mammals in embryonic development are relatively unexplored; the number of these receptors and partially redundant functions of some family members makes a systematic study of the necessary roles of GPCRs in development a daunting proposition. Probing for roles of GPCRs by ablation of their common G protein-signaling pathways is a more tractable alternative (17, 28, 29). This study focused on Gα13, and our results imply a key role for GPCR signaling through Gα13 in several distinct cell types and developmental processes.
The observation that Tie2-Cre-mediated excision of Gα13 caused abnormal vascular structures and embryonic death beginning at E9.5 indicates a key role for Gα13 in a Tie2-Cre-expressing lineage. Tie2-Cre can mediate excision of floxed alleles in both endothelium and in hematopoietic lineages (ref. 30 and data not shown). However, several observations point to an endothelial, rather than hematopoietic, defect as the cause of embryonic death in the Tie2-CreTg/o; Gα13flox/- embryos. Although the majority of endothelial cells in Gα13 lacZ knockin embryos showed X-Gal staining at E9.5–10.5 (Fig. 1), <2% of circulating embryonic blood cells were lacZ-positive at this time (data not shown). Thus, Gα13 may not normally be expressed in most embryonic blood cells. Similarly, X-Gal staining of E9.5 embryos bearing the Tie2-Gα13 transgene, which rescued the early vascular development defect in Gα13-/- embryos, revealed transgene expression in only a few percent of circulating blood cells. It is still formally possible that Gα13 is important in hematopoietic stem cells that represent only a small fraction of circulating cells in the embryo. However, although defects in hematopoiesis can cause death of the embryo between E10.5 and 12.5, typically such affected embryos are morphologically indistinguishable from their WT littermates except for pallor (31–35). They do not exhibit the abnormal vascular structures, failed remodeling of blood vessels, and pericardial dilatation that were already present in Gα13 endoKO embryos at E9.5). Thus, our results are most consistent with the hypothesis that Gα13 function in endothelial cells is necessary for proper vascular development.
The observation that the Tie2-Gα13 transgene completely rescued development of Gα13 endoKO embryos but not Gα13-/- embryos suggests that Gα13 function in cell types other than endothelial cells is important for embryonic development. Preliminary studies show that the exencephaly and intracranial hemorrhage exhibited by Gα13-/- embryos bearing the endothelial transgene are phenocopied by Wnt1-Cre-mediated excision of Gα13. This finding, coupled with our observation that Gα13 is expressed in the dorsal neural tube at the time and place where neural crest cells delaminate, suggests that Gα13 plays a role in the formation of neural crest or its derivatives (K. Ruppel and S. Coughlin, unpublished data).
Exactly how the loss of Gα13 function in vascular endothelial interferes with vascular development is unknown. Gα13 contributes to the regulation of the small GTPase Rho by GPCRs, and Rho plays a key role in regulation of the actin cytoskeleton and other cellular processes (36). We were unable to detect defects in GPCR-induced Rho activation, changes in cell shape, or chemokinesis of individual cells in Gα13-null endothelial cells in culture. Gα12 and Gαq, both capable of regulating Rho, were expressed in these cultures (data not shown), and partial redundancy with these or other G proteins might mask effects of Gα13 deficiency in these assays. A reproducible effect of Gα13 deficiency was seen when endothelial cells were examined for a more complex behavior: the ability to form networks on Matrigel. Null cells tended to remain organized as sheets rather than reorienting to form networks of cords and tubes. Thus, Gα13 likely helps orchestrate complex changes in cell shape, movement, and cell–cell and cell–matrix interactions required for vascular remodeling.
It is interesting to consider analogies between Gα13 phenotypes and the concertina gastrulation defect. Concertina is the Drosophila homolog of Gα13 (37). Gastrulation in fly embryos requires furrow formation and inward migration of cells at sites along the length of the embryo rather than through a single blastopore as in mouse. Concertina embryos begin a furrow by forming a zone of tightly apposed cells and do constrict the apices of some cells but not enough to form an organized groove (37). Thus, Gα13 is not necessary for initiation of gastrulation in Drosophila but plays a necessary role in coordinating or propagating changes in cell shape and movement. This result may relate to the yolk sac vascular defects seen in our studies; endothelial cells form sheets and an initial plexus but fail to properly reorganize it. Detailed studies of the cytoskeletal rearrangements in WT and Gα13-null endothelial cells and their movement during vertebrate vasculogenesis and angiogenesis are needed.
Studies of the signaling molecules that orchestrate the behavior of endothelial cells during blood vessel development have focused mainly on growth factors and receptor tyrosine kinases. Our results emphasize a role for GPCRs in this process. We previously showed that knockout of PAR1, a GPCR for thrombin, caused a partially penetrant embryonic lethality at midgestation that could be attributed to a role for PAR1 in endothelial cells (11, 14). This phenotype was similar in time of onset and general features to that observed in Gα13 endoKO embryos in this study, but the latter phenotype was more severe and penetrant. Thus, the loss of Gα13 activation by PAR1 in endothelial cells may account for the phenotype of Par1-/- embryos, but other endothelial GPCRs probably also contribute to Gα13 activation in those cells. The observation that Gα13+/- embryos showed virtually no embryonic lethality, Par1-/- embryos show ≈50% lethality, and Gα13+/- Par1-/- embryos showed ≈100% lethality is consistent with this model (K.M.R. and S.R.C., unpublished data). Knockout of S1P1, a Gi-coupled receptor for sphingosine-1 phosphate (13), caused lethality that began at ≈E12.5 and was attributable to a necessary function in endothelial cells (15). Combined deficiency of this receptor with S1P2 and S1P3, which couple to Gi, Gq, and G12/13, yielded earlier phenotypes (16). Thus, multiple GPCRs play both unique and partially redundant roles in helping to orchestrate endothelial cell function during blood vessel development, and signaling through Gα13 plays a key role in this process.
Supplementary Material
Acknowledgments
We thank Drs. Tom Sato and Mashashi Yanagisawa of the University of Texas Southwestern; J. Miyazaki of Osaka University Medical School; Marc Tessier-Lavigne of Genentech; Hilary Beggs, Louis Reichardt, and Gail Martin of the University of California, San Francisco; S.M. Dymecki, Carnegie Institute of Washington; Mel Simon of California Institute of Technology; and Andrew McMahon of Harvard University (Cambridge, MA) for specific plasmids and transgenic mouse lines (see Materials and Methods); and Pao-Tien Chuang, Didier Stainier, and Gail Martin for their critical reading of this manuscript. This work was supported in part by National Institutes of Health Grants HL65590 and HL44907 (to S.R.C.).
Author contributions: K.M.R., D.W., H.K., and S.R.C. designed research; K.M.R., D.W., H.K., A.W., Y.-W.Z., I.C., L.Y., and S.M.X. performed research; K.M.R., D.W., H.K., and S.R.C. analyzed data; and K.M.R., D.W., and S.R.C. wrote the paper.
Abbreviations: En, embryonic day n; GPCR, G protein-coupled receptor; PAR1, protease-activated receptor-1.
References
- 1.Risau, W. & Flamme, I. (1995) Annu. Rev. Cell Dev. Biol. 11, 73-91. [DOI] [PubMed] [Google Scholar]
- 2.Risau, W. (1997) Nature 386, 671-674. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet, P. (2000) Nat. Med. 6, 389-395. [DOI] [PubMed] [Google Scholar]
- 4.Sato, T. N., Tozawa, Y., Deutsch, U., Wolburg-Buchholz, K., Fujiwara, Y., Gendron-Maguire, M., Gridley, T., Wolburg, H., Risau, W. & Qin, Y. (1995) Nature 376, 70-74. [DOI] [PubMed] [Google Scholar]
- 5.George, E. L., Georges, L. E., Patel, K. R., Rayburn, H. & Hynes, R. O. (1993) Development (Cambridge, U.K.) 119, 1079-1091. [DOI] [PubMed] [Google Scholar]
- 6.Yang, J. T., Rayburn, H. & Hynes, R. O. (1993) Development (Cambridge, U.K.) 119, 1093-1105. [DOI] [PubMed] [Google Scholar]
- 7.Hellstrom, M., Kalen, M., Lindahl, P., Abramsson, A. & Betsholtz, C. (1999) Development (Cambridge, U.K.) 126, 3047-3055. [DOI] [PubMed] [Google Scholar]
- 8.Lindahl, P., Johansoon, B. R., Levéen, P. & Betsholtz, C. (1997) Science 277, 242-245. [DOI] [PubMed] [Google Scholar]
- 9.Levéen, P., Pekny, M., Gebre-Medhin, S., Swolin, B., Larsson, E. & Betsholtz, C. (1994) Genes Dev. 8, 1875-1887. [DOI] [PubMed] [Google Scholar]
- 10.Addison, C. L., Daniel, T. O., Burdick, M. D., Liu, H., Ehlert, J. E., Xue, Y. Y., Buechi, L., Walz, A., Richmond, A. & Strieter, R. M. (2000) J. Immunol. 165, 5269-5277. [DOI] [PubMed] [Google Scholar]
- 11.Connolly, A. J., Ishihara, H., Kahn, M. L., Farese, R. V., Jr., & Coughlin, S. R. (1996) Nature 381, 516-519. [DOI] [PubMed] [Google Scholar]
- 12.Yanagisawa, H., Hammer, R. E., Richardson, J. A., Williams, S. C., Clouthier, D. E. & Yanagisawa, M. (1998) J. Clin. Invest. 102, 22-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, Y., Wada, R., Yamashita, T., Mi, Y., Deng, C. X., Hobson, J. P., Rosenfeldt, H. M., Nava, V. E., Chae, S. S., Lee, M. J., et al. (2000) J. Clin. Invest. 106, 951-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin, C. T., Srinivasan, Y., Zheng, Y. W., Huang, W. & Coughlin, S. R. (2001) Science 293, 1666-1670. [DOI] [PubMed] [Google Scholar]
- 15.Allende, M. L., Yamashita, T. & Proia, R. L. (2003) Blood 102, 3665-3667. [DOI] [PubMed] [Google Scholar]
- 16.Kono, M., Mi, Y., Liu, Y., Sasaki, T., Allende, M. L., Wu, Y. P., Yamashita, T. & Proia, R. L. (2004) J. Biol. Chem. 279, 29367-29373. [DOI] [PubMed] [Google Scholar]
- 17.Offermanns, S., Mancino, V., Revel, J. P. & Simon, M. I. (1997) Science 275, 533-536. [DOI] [PubMed] [Google Scholar]
- 18.Sakai, K. & Miyazaki, J. (1997) Biochem. Biophys. Res. Commun. 237, 318-324. [DOI] [PubMed] [Google Scholar]
- 19.Plump, A. S., Erskine, L., Sabatier, C., Brose, K., Epstein, C. J., Goodman, C. S., Mason, C. A. & Tessier-Lavigne, M. (2002) Neuron 33, 219-232. [DOI] [PubMed] [Google Scholar]
- 20.Schlaeger, T. M., Bartunkova, S., Lawitts, J. A., Teichmann, G., Risau, W., Deutsch, U. & Sato, T. N. (1997) Proc. Natl. Acad. Sci. USA 94, 3058-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kisanuki, Y. Y., Hammer, R. E., Miyazaki, J., Williams, S. C., Richardson, J. A. & Yanagisawa, M. (2001) Dev. Biol. 230, 230-242. [DOI] [PubMed] [Google Scholar]
- 22.Dymecki, S. M. (1996) Gene 171, 197-201. [DOI] [PubMed] [Google Scholar]
- 23.Soriano, P. (1999) Nat. Genet. 21, 70-71. [DOI] [PubMed] [Google Scholar]
- 24.Schlaeger, T. M., Qin, Y., Fujiwara, Y., Magram, J. & Sato, T. N. (1995) Development (Cambridge, U.K.) 121, 1089-1098. [DOI] [PubMed] [Google Scholar]
- 25.Kataoka, H., Hamilton, J. R., McKemy, D. D., Camerer, E., Zheng, Y. W., Cheng, A., Griffin, C. & Coughlin, S. R. (2003) Blood 102, 3224-3231. [DOI] [PubMed] [Google Scholar]
- 26.He, T. C., Zhou, S., da Costa, L. T., Yu, J., Kinzler, K. W. & Vogelstein, B. (1998) Proc. Natl. Acad. Sci. USA 95, 2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beggs, H. E., Schahin-Reed, D., Zang, K., Goebbels, S., Nave, K. A., Gorski, J., Jones, K. R., Sretavan, D. & Reichardt, L. F. (2003) Neuron 40, 501-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Offermanns, S. & Simon, M. I. (1998) Oncogene 17, 1375-1381. [DOI] [PubMed] [Google Scholar]
- 29.Offermanns, S., Zhao, L. P., Gohla, A., Sarosi, I., Simon, M. I. & Wilkie, T. M. (1998) EMBO J. 17, 4304-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gitler, A. D., Kong, Y., Choi, J. K., Zhu, Y., Pear, W. S. & Epstein, J. A. (2004) Pediatr. Res. 55, 581-584. [DOI] [PubMed] [Google Scholar]
- 31.Tsai, F. Y., Keller, G., Kuo, F. C., Weiss, M., Chen, J., Rosenblatt, M., Alt, F. W. & Orkin, S. H. (1994) Nature 371, 221-226. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu, R., Ohneda, K., Engel, J. D., Trainor, C. D. & Yamamoto, M. (2004) Blood 103, 2560-2567. [DOI] [PubMed] [Google Scholar]
- 33.Visvader, J. E., Fujiwara, Y. & Orkin, S. H. (1998) Genes Dev. 12, 473-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujiwara, Y., Browne, C. P., Cunniff, K., Goff, S. C. & Orkin, S. H. (1996) Proc. Natl. Acad. Sci. USA 93, 12355-12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crispino, J. D., Lodish, M. B., Thurberg, B. L., Litovsky, S. H., Collins, T., Molkentin, J. D. & Orkin, S. H. (2001) Genes Dev. 15, 839-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall, A. (1998) Science 279, 509-514. [DOI] [PubMed] [Google Scholar]
- 37.Parks, S. & Wieschaus, E. (1991) Cell 64, 447-458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.