Abstract
Background
The objective of the following retrospective review was to perform a cost-effectiveness analysis of the use of molecular testing of indeterminate thyroid nodules compared to current management practices in Nova Scotia, Canada.
Methods
All cases of cytologically indeterminate thyroid nodules from January 1st, 2014 to December 31st, 2018 were reviewed. All interventions related to an indeterminate thyroid nodule were recorded. Patients were excluded if less than 18 years old if no further information regarding medical management was electronically available beyond the diagnosis of an indeterminate thyroid nodule, history of radiation, or previous thyroid surgery prior to diagnosis of an indeterminate thyroid nodule in the remaining lobe. Microcosting was performed to determine the cost of all relevant interventions including repeat fine needle aspiration biopsy, ultrasound, thyroid surgery(s), and molecular testing. Institution-specific transition state probabilities were calculated and used to build a cost-effectiveness analysis model. Model output was an incremental cost-effectiveness ratio, defined as the ratio of cost difference to effectiveness difference between routine molecular testing and the current management strategy, yielding cost per surgery avoided.
Results
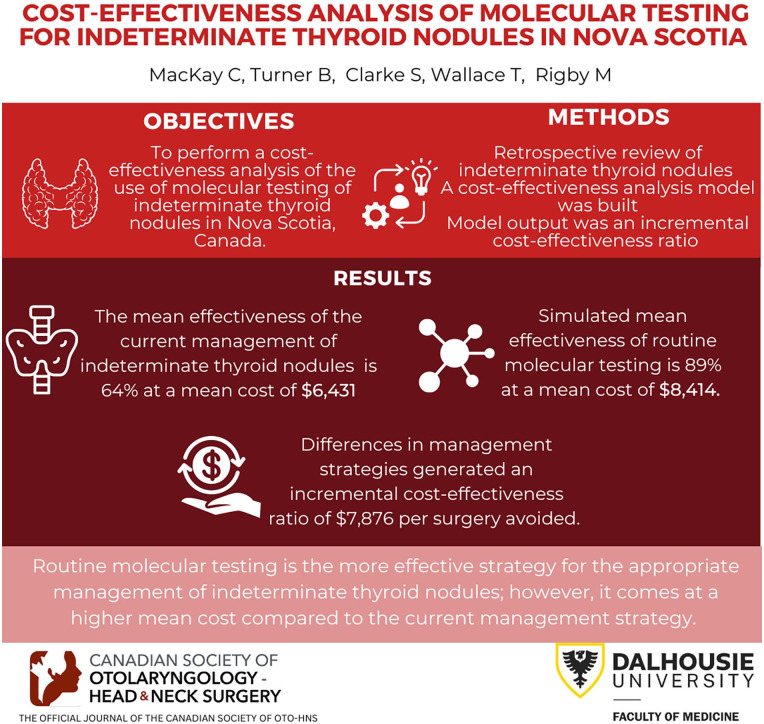
The mean effectiveness of the current management of indeterminate thyroid nodules in Nova Scotia based on the American Thyroid Association guidelines is 64% at a mean cost of $6431, while the simulated mean effectiveness of routine molecular testing is 89% at a mean cost of $8414. Differences in management strategies generated an incremental cost-effectiveness ratio of $7876 per surgery avoided.
Conclusion
Routine molecular testing is the more effective strategy for the appropriate management of indeterminate thyroid nodules; however, it comes at a higher mean cost compared to the current management strategy. As the cost of molecular testing continues to decrease, and the cost of OR resources continues to rise, molecular testing is likely to become the optimal strategy in Nova Scotia.
Keywords: thyroid nodule, molecular testing, cost-effectiveness
Graphical abstract.
Introduction
Thyroid nodules are incredibly common among the general population, notably among women, and are estimated to be present in 30% to 60% of the general population. 1 Despite their high prevalence, only a small minority (5%) are estimated to be malignant. 1 Thyroid nodules are frequently an incidental finding on chest imaging, prompting further investigation with a dedicated ultrasound.
Developed in 2017 by the American College of Radiology (ACR) to standardize the ultrasonographic findings of thyroid nodules, the thyroid imaging, reporting, and data system (TI-RADS) classification provides a recommendation for fine needle aspiration (FNA) biopsy, ultrasound surveillance, or no follow-up based on nodule size, shape, and presence of high-risk sonographic features. 2 Surveillance or surgical management is suggested per the American Thyroid Association (ATA) guidelines based on The Bethesda System for Reporting Thyroid Cytopathology. 3 Repeat FNA may be performed in nodules with indeterminate cytopathology at the physician’s discretion or followed with serial ultrasounds to assess for interval growth. 3 Alternatively, molecular testing (MT) can be performed peri-operatively to better stratify malignancy risk prior to planned diagnostic thyroid surgery as results may lead a surgeon to recommend upfront total thyroidectomy in high-risk individuals. 3 Previous work at our institution found that younger patients, patients with Bethesda IV nodules, and patients with an indeterminate nodule >3 cm were significantly more likely to choose to undergo diagnostic thyroid surgery. 4 Despite a low malignancy risk, many patients still experience decisional conflict regarding surveillance versus surgery.4,5
Several commercially available FNA biopsy tests can be used to better stratify the risk of malignancy in indeterminate thyroid nodules (ITNs) by identifying genetic mutations associated with increased malignancy risk. ThyroSeq v3 uses next-generation DNA and RNA sequencing to identify an array of genetic mutations, including PTEN, RAS, PAX8/PPARG, RET/PTC, and BRAF V600E, some of which carry a >90% risk of malignancy.6,7 Using the McGill Algorithm, an analysis of the use of ThyroSeq v3 in 50 patients with ITNs identified a 54% decrease in diagnostic surgery. 8 Alternatively, Afirma and RosettaGX Reveal use analysis of messenger (mRNA) and microRNA (miRNA), respectively, to detect gene mutations that increase the risk of malignancy in an ITN. However, molecular testing is not perfect and only evaluation of a surgical specimen by pathology can definitively rule out malignancy. Various studies have sought to determine the sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) of each molecular test for identifying nodules that are at increased risk of malignancy. A newer version of the Afirma molecular testing kit, Afirma Genomic Sequencing Classifier (GSC), has been found to have a 95% sensitivity and a 96% NPV 9 ; however, reported findings of test specificity vary from 68% to 94.3% and a variable PPV of 47% to 60%.9,10
ThyGenX, the initial oncogenic screening panel of the Interpace Diagnostics, Inc. molecular testing, which also uses next-generation DNA and RNA sequencing, has been reported to have 71% sensitivity, 69% specificity, 81% NPV, and 56% PPV. 11 Interpace Diagnostics miRNA classifier ThyraMIR is performed should the initial ThyGenX oncogenic screening panel identify a genetic mutation with a weak malignancy association. ThyGenX and ThyraMIR together have a 95% sensitivity and a 90% specificity for detecting genetic mutations that increase nodule malignancy rate. 11 Per recommendation 13 of the ATA guidelines, patients who undergo molecular testing should be counseled regarding the limitations of the testing, as a negative test does not guarantee benign disease on thyroid pathology. 3
There is notable variability in the cost of the commercial molecular tests mentioned above. 7 Afirma BRAF mutation analysis with medullary thyroid carcinoma (MTC) detection is 975 USD, while Afirma Gene Expression Classifier (GEC) + MTC, the most expansive genetic panel option is priced at 6400 USD. RosettaGX Reveal and ThyroSeq are 3700 and 4056 USD, respectively. 7 The cost of certain molecular tests has decreased significantly in recent years. The most up-to-date and current out-of-pocket cost for patients for ThyGenX molecular testing in Canada, coordinated through TAP Molecular, is 1140.74 CAD (900 USD) (TAP Molecular, Quebec, personal communication). Although patients frequently experience decisional conflict, few patients in Nova Scotia have elected to proceed with molecular testing because of the cost.
With higher nursing shortages and increasing demands on the Canadian healthcare system, the use of microsimulation modeling may become more critical in guiding future healthcare policy. Compared to state-transition cohort modeling, which works off the Markov assumption that transitions between health states depend only on the current health state at that particular moment in time, microsimulation allows comparison of the natural history of a disease course within a particular region and accounts for individual variability in disease to determine the effectiveness of an intervention specific to a given population. 12 Both the governments of Quebec and Ontario have performed similar cost-effectiveness analyses, using quality-adjusted life years (QALY) to make recommendations on provincial funding of molecular testing.13,14 Although their findings were consistent with other reports with respect to increasing the probability of avoiding unnecessary surgery, the province of Ontario’s Health Technology Assessment findings suggested that molecular testing was favored not to be cost-effective at current pricing. 14
Methods
Ethics approval for the study was obtained from the Nova Scotia Health Authority Research Ethics Board (ROMEO #1024802). All cases of cytologically ITNs diagnosed in Nova Scotia between January 1st, 2014 and December 31st, 2018 were analyzed. The health records of 733 patients were analyzed to identify all relevant records pertaining to the management of the ITN(s). All management events pertaining to the indeterminate nodule, including repeat FNA(s), ultrasounds, thyroid surgery, or molecular testing (if applicable), were recorded. Transition states within current practice were defined as the probability of moving from one health state to another: for example, from ultrasound to ultrasound (surveillance) or biopsy to surgery (diagnostic hemithyroidectomy). Patients were excluded from the analysis of transition state probabilities if there was no information available or subsequent management beyond a diagnosis of an indeterminate nodule. In addition, patients were excluded if <18 years of age, if they had a history of radioactive iodine, external beam radiation to the neck, previous thyroid surgery prior to the diagnosis of ITN in the remaining lobe if a Bethesda V or VI diagnosis was made simultaneously in a different nodule, or if surgical pathology noted a histology other than papillary thyroid cancer. Six patients paid out-of-pocket for molecular testing and therefore were excluded from analysis due to the confounding effect on subsequent management. FNA biopsies performed in a different thyroid nodule as a consequence of ultrasound surveillance were included in the transition state probability calculation. If a repeat FNA of the ITN or a neck mass was suspicious or positive for malignancy, subsequent management was not included in the probability calculation.
Institution-specific data were used to generate transition probabilities for the current management strategy arm of the model. Transition probabilities for the molecular testing arm of the model were informed by extrapolation from our data set as well as values from the literature from centers that have previously trialed or implemented various brands of molecular testing (Table 1). Sensitivity, specificity, NPV, and PPV for ThyraMir/ThyGenX were obtained from Lupo et al. 11
Table 1.
Molecular Testing Transition State Probabilities, Operating Room Time (min), and Molecular Testing Cost Range.
| Transition state | Range | Source |
|---|---|---|
| Surgery after molecular testing | 0.38-0.50 | 8, 15, 16 |
| Malignancy in surgical pathology | 0.44-0.91 | 8, 15, 16 |
| Completion thyroidectomy | 0.3-0.6 | Institutional data and expert opinion |
| Hemithyroidectomy OR time (min) | 60-230 | Institutional data |
| Total thyroidectomy OR time (min) | 155-360 | Institutional data |
| Cost of molecular testing (CAD) | 800-1600 | Range based on the variability of MT cost |
Abbreviation: MT, molecular testing.
The total cost of thyroid nodule management was determined through microcosting from the perspective of a single-payer provincial system. Cost of thyroid ultrasound, radiologist performed ultrasound-guided FNA, hemithyroidectomy, completion thyroidectomy, and total thyroidectomy were obtained from Nova Scotia Health Authority administration. The cost of ThyGenX/ThyraMIR molecular testing was obtained from TAP Molecular, Quebec (personal communication; Table 2).
Table 2.
Costs of Interventions in the Management of ITNs in Nova Scotia, Canada, Obtained Through Microcosting.
| Event | Cost (CAD) |
|---|---|
| Ultrasound | 119.25 |
| Ultrasound-guided FNA | 381.00 |
| Hemithyroidectomy | 9320.53 |
| Completion thyroidectomy | 9509.41 |
| Total thyroidectomy | 13,956.36 |
| Molecular testing | 1100 |
Abbreviations: FNA, fine needle aspiration; ITN, indeterminate thyroid nodule.
Complications were rare postoperatively in the retrospective review and therefore were not included explicitly in the model. They are accounted for in the cost of surgery, specifically in the length of stay postoperatively.
The cost-effectiveness microsimulation model was built and run using R statistical software (v 3.6.1; R Core Team 2019) based on a framework developed by Krijkamp et al. 12 One million simulated patients per diagnostic arm were run over 12 cycles based on a 3-month cycle length. Cycle length and number of cycles were chosen based on event rates from our retrospective data. At a cycle length of 3 months, the majority of patients experienced only 1 event per cycle. No patients had transition events beyond 3 years, and therefore 12 cycles were run to match these data. The number of unnecessary surgeries avoided was selected as the marker of effectiveness as justified by Dharampal et al 17 in their recent CEA of thyroid nodules conducted in Alberta, Canada. These authors argue that QALYs, a measurement of the consequences of a diseased state, is not an optimal outcome when analyzing the cost-effectiveness of a diagnostic test or intervention in an asymptomatic health condition that is less likely to affect one’s global health state, downstream health, or result in significant time away from work. Unnecessary surgery was defined as patients with ITN who received surgery but had a benign result on final pathology. Patients were assigned a utility of 0 if they had unnecessary surgery, a utility of 1 if a malignant nodule was found on final pathology or they were appropriately never operated on. The primary outcome of the model was an incremental cost-effectiveness ratio (ICER), defined as the ratio of cost difference to effectiveness difference between 2 diagnostic management strategies, yielding cost per surgery avoided.
According to the Guidelines for the Economic Evaluation of Health Technologies: Canada—Fourth Edition 18 models that span more than 1 year should be run multiple times with discounting values of 0%, 1.5%, and 3% to account for uncertainty. The results of the model with discounting set at 1.5% are reported as the reference model per the above Guidelines. The results of all non-reference models are reported in Appendix A.
Due to the wide variation in transition probabilities in the literature (Table 1), a probabilistic sensitivity analysis (PSA) was conducted to account for this variability. PSA analysis was conducted in R using the DARTH framework and the dampack package. 19 The PSA consisted of 10,000 simulations in which 15 model parameters were varied; parameters varied included the probability of surgery after molecular testing (and its reciprocal), probability of malignancy after any thyroid surgery (and its reciprocal), probability of completion thyroidectomy if malignancy on diagnostic lobectomy (and its reciprocal), operating room time of hemi- and total thyroidectomy (which modified the cost of hemi-, total, and completion thyroidectomy in both arms), cost of molecular testing, and utility of papillary thyroid microcarcinoma (PTMC; Appendix A). The cost of surgery was included in the PSA as the length of surgery varied by patient, thus affecting operating room-related costs. As the utility of surgery for PTMC is also equivocal in the literature, the reference model was run with the utility as a PSA distribution between 0 and 1. Non-reference models were also run with the utility set at 0 and 1, respectively, for comparison (Appendix A).
Results
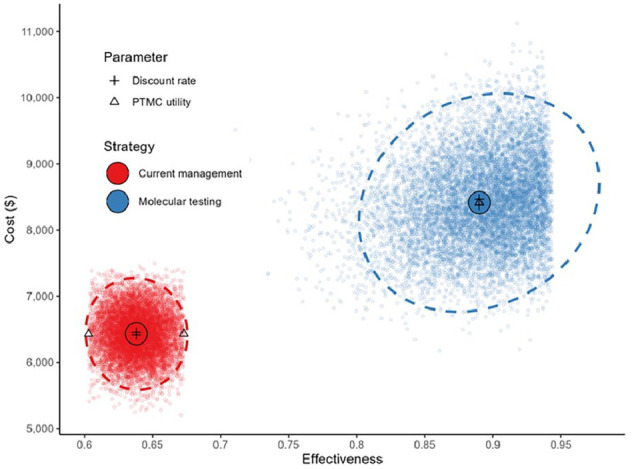
The mean cost of current management of indeterminate nodules in Nova Scotia, Canada is $6431 with a mean effectiveness of 64%, while the simulated mean cost of molecular testing of ITNs was $8414 with a mean effectiveness of 89% (Figure 1). Differences in cost and effectiveness between the 2 management strategies yielded an ICER of $7876 per surgery avoided.
Figure 1.
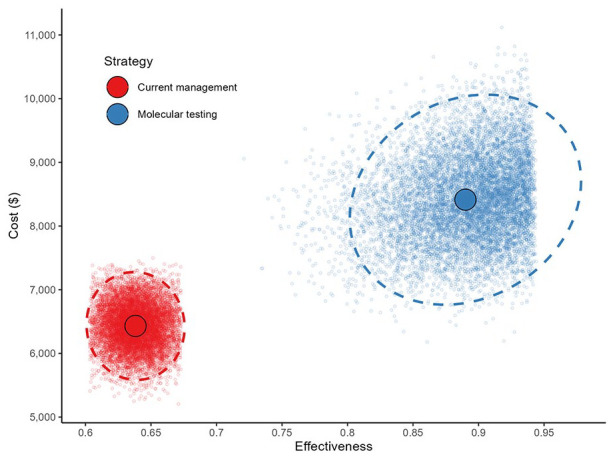
Probabilistic sensitivity analysis based on 10,000 simulations.
At willingness to pay (WTP) thresholds of $8000, $10,000, or $15,000 per unnecessary surgery avoided the probability that MT is the most cost-effective strategy was 53.8%, 82.2%, or 99.3%, respectively (Figure 2).
Figure 2.
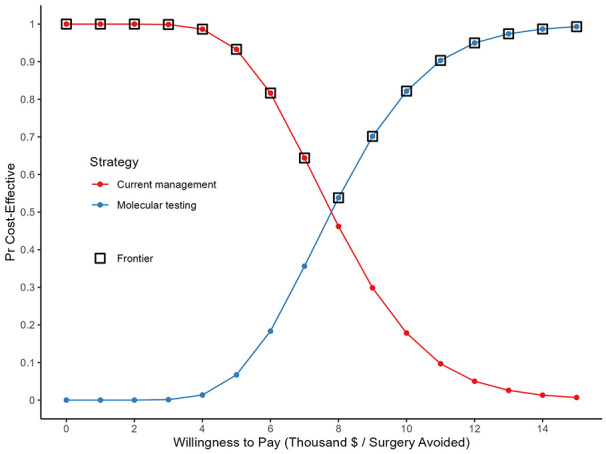
Cost-effectiveness acceptability curves of molecular testing and current management strategy in the management of indeterminate thyroid nodules.
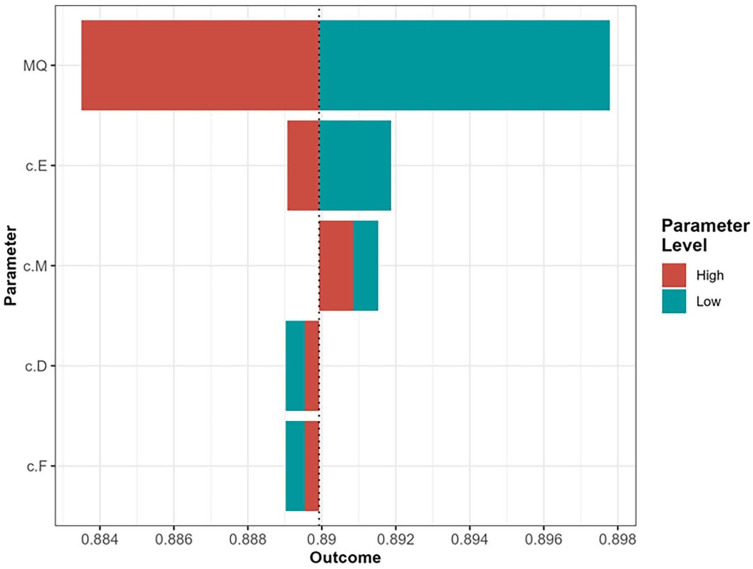
One-way sensitivity analysis was performed on all varied parameters. This analysis concluded that, within all clinically relevant ranges, molecular testing was the optimal strategy. Therefore, we did not perform 2-way sensitivity analysis. A tornado plot of the 1-way sensitivity analyses showed that the rate of surgery following molecular testing had the greatest influence on the model (Appendix A).
Discussion
In our model, molecular testing of indeterminate nodules consistently remained the more effective strategy for avoiding unnecessary surgery; however, it was consistently the more costly strategy. Our microsimulation is a robust model developed using institution-specific data with over 700 patients, rather than utilizing a base case model, thus providing a strong provincial picture of the landscape of the management of indeterminate nodules in Nova Scotia. Adherence to the ATA guidelines resulted in effectiveness at avoiding unnecessary surgery of 64%, which speaks to a strong history of counseling patients on management options for ITNs, given the potential risks and benefits of surgery and the option for noninvasive ultrasound surveillance given lower rates of malignancy, particularly in Bethesda III thyroid nodules. Our model accounts for additional FNA biopsies performed on other thyroid nodules identified in patients who opted for ultrasound surveillance. However, it should be noted that our data may inappropriately represent the most current probability of completion thyroidectomy. Since 2019, Nova Scotia Health Pathology and Laboratory Medicine has been running BRAF V600E mutation testing on hemithyroidectomy surgical specimens positive for differentiated thyroid cancer that were staged T1/2 and/or N0/Nx or N1a with less than or equal to 5 lymph nodes involved. This updated protocol may increase the probability of completion thyroidectomy at our institution in the period after the retrospective analysis. In addition, the use of routine molecular testing in the future may better delineate which patients may benefit from upfront total thyroidectomy, reducing the number of completion thyroid surgeries. An analysis by Carty et al 20 of 389 patients with Bethesda IV nodules found the routine use of ThyroSeqv2 or ThryoSeqv3 identified all patients with intermediate to high risk and medullary thyroid cancers preoperatively.
As was succinctly outlined by Dharampal et al 17 in their simulated analysis of molecular testing for indeterminate nodules in Alberta, Canada, we also feel the use of unnecessary surgery avoided better represents the effectiveness of molecular testing compared to QALYs. Five-year surveillance of an indeterminate nodule is unlikely to cause significant time away from work, will be asymptomatic if small, and will cause varying degrees of anxiety among patients. For patients who elect to undergo diagnostic lobectomy, postoperative complications that can affect long-term quality of life (permanent hypocalcemia requiring lifetime supplementation, 21 and permanent vocal cord palsy22,23) are rare. We agree that QALY is a poor metric of the effectiveness of molecular testing in our model, given our primary outcome is to determine the utility of a diagnostic test in avoiding a more costly and invasive intervention in an essentially asymptomatic condition.
PTMC (defined as tumors 1 cm or less in size) represents a challenge when determining a utility to assign. These are indolent tumors with low rates of clinical progression; the 2015 ATA guidelines suggest that active surveillance may be appropriate for some patients. This is not routinely done outside of select centers. 3 Given this information, neither a value of 1 nor 0 was felt to be appropriate for patients with PTMC on final pathology, and it was therefore included as one of the variables in the PSA. Comparison models were run with the utility set at either 0 or 1 but the resulting ICERs were not meaningfully different (Appendix A).
Although our nodule characteristics for study inclusion were identical to the base case characteristics used by Dharampal et al, our notably higher current practice effectiveness at avoiding unnecessary surgery is due to several variables, including our time horizon, and our lower probability of proceeding to surgery after a diagnosis of indeterminacy. Despite an equal incidence of thyroid malignancy of 13% between provinces, 24 Dharampal et al report a probability of proceeding to diagnostic lobectomy over a 1-year time frame of 0.74, while the probability of having diagnostic thyroid surgery at our center over 5 years was 0.52. This is reflective of a strong history of counseling patients regarding conservative management options in nodules favored to be benign. There may be several factors that influence this disparity, including the proportion of surgeons who practice under alternate funding arrangements versus a fee-for-service model. 25 It is notable that the schedule of benefits for thyroid surgery for fee-for-service physicians in Alberta, Canada is more than 3 times that of Nova Scotia physicians. In addition, differences from province to province in rates of malignancy within indeterminate nodules will affect the probability of diagnostic surgery within a cost-effectiveness analysis. Given the following model is based on institution-specific data, the effect of malignancy rate on cost-effectiveness is inherently built into the model.
Molecular testing itself is not without limitations; there is considerable variability among current molecular tests on the market, which include Afirma GSC, ThyroSeqv3, and ThyraMIR/ThyGeNEXT. However, the more recent versions of these molecular tests have sensitivities above 90% and specificities above 80%.9-11,16,26 Regardless, molecular testing remains financially out-of-reach for the majority of Canadians within a public healthcare system that currently does not cover this risk stratification tool. Not only is effectiveness affected by the individual cost of each molecular test but also heavily influenced by the thyroid nodule malignancy rate within the local population. 27 A cost-effectiveness analysis by Wu et al using routine GEC molecular testing was both the more costly and more effective management strategy. As the simulated malignancy rate varied from 15% to 35%, molecular testing became the more effective management strategy at a lower overall cost. 27 In addition, as malignancy rates and institutional practices vary by local regions within a country, certain molecular tests may prove more beneficial in reducing unnecessary surgery; tests with high NPV may be more useful as a rule-out test in regions with a low threshold for surgery while a limited genetic panel may be more useful in ruling out high-risk mutations that require more extensive upfront surgery. 28
Although a comprehensive analysis with data from over 700 patients, our study is not without limitations. Our study is limited by the availability of patient data within the electronic medical record due to the absence of a single integrated province-wide system. All cytopathology specimens in Nova Scotia were captured in our retrospective review as all specimens are analyzed at Nova Scotia Health’s tertiary care hospital in Halifax, Nova Scotia; however, there is no integrated medical record system that captures all clinic reports and hospital records from community hospitals, thus several patients were excluded from analysis as there was no additional electronic information beyond a diagnosis of an indeterminate nodule. As the probabilities in the model are based on institution-specific data, they are affected by the potential tendencies of surgeons toward surgery versus conservative management. In addition, our institution-specific probabilities are altered by our rate of unsatisfactory FNA; a retrospective review performed in 2013 of 1491 thyroid FNA biopsies performed at Queen Elizabeth II Health Sciences Centre found an unsatisfactory rate of 28.9%, while the rate of Bethesda III and IV cytopathology was 45% and 18.8%, respectively. 29 Within our study, 23.7% of patients underwent a repeat FNA after a diagnosis of indeterminate while interestingly, 27 patients underwent 1 or more FNAs prior to a diagnosis of indeterminate. It is unknown whether rates of unsatisfactory thyroid FNA biopsies at our institution have improved since 2013, and to what extent lower rates of unsatisfactory biopsies would result in significant changes in transition state probabilities, and therefore effectiveness within our model.
As the cost of molecular testing remains out-of-reach for many patients, our cost-effectiveness analysis has potential implications in advising local healthcare policy. Governments must examine whether provincial coverage of molecular testing and therefore 2 to 2.5 hours of operating room time per thyroid surgery avoided is worth a valuation of nearly $8000 to reduce the current surgical backlog. 30 As noted above, Chen et al 8 noted a 54% decrease in diagnostic surgery at their institution when routinely using molecular testing for ITNs. Applying the same findings to the 219 patients at our institution who underwent hemithyroidectomy during our 5-year study period, a 50% reduction in diagnostic surgery would represent 220 to 275 hours of newly available OR time for other surgical procedures. Should the provincial government elect to provide universal coverage, it should be noted that patients may have a tendency toward molecular testing before diagnostic surgery or alternatively, given anxiety about malignancy, still prefer diagnostic surgery in a thyroid nodule favored to be benign on molecular testing. Interestingly, of 6 patients (excluded from the model as this would not represent a single-payer perspective) who underwent molecular testing from 2014 to 2018 at our institution, 4 patients had no known genetic mutations, of whom 1 still elected to have diagnostic surgery. Patient autonomy and malignancy anxiety contrast the assumptions made in other published cost-effectiveness analyses. 31 Routine BRAF testing alone on cytologically indeterminate nodules may provide a more economical alternative to molecular testing by potentially avoiding 2-stage surgery.
Conclusion
Our cost-effectiveness model comparing routine molecular testing of ITNs to current management strategies yielded an ICER of $7876 per surgery avoided. Although modeling of routine molecular testing suggests a potential effectiveness of 89% at a mean cost of $8414, close adherence to the ATA guidelines at our institution has resulted in an effectiveness of 64% with a mean cost of $6431 per patient under current management. At a WTP threshold of $8000, MT would be the most cost-effective strategy 53.8% of the time. As WTP increases, so does the probability that MT will be the most cost-effective strategy. For routine molecular testing to become the dominant management strategy, both economically and for patients, several model parameters, particularly the rate of surgery after MT and the cost of MT would need to decrease beyond the range tested from the literature. An additional downstream benefit of routine molecular testing would include an estimated 220 to 275 hours over 5 years of newly available OR time. Finally, the ability of molecular testing to identify high-risk mutations prior to surgery that would make total thyroidectomy the recommended treatment option, and therefore decrease rates of hemithyroidectomy plus completion thyroidectomy, has the potential to decrease the cost per surgery avoided. Due to a wide range of reported practice patterns in the literature, molecular testing may be a much more cost-effective strategy than currently modeled if the ability to identify patients who may benefit from upfront total thyroidectomy is as high as the reported testing performance metrics.
Acknowledgments
The authors would like to acknowledge M. Greenan and T. Hogg for their assistance in obtaining the procedure costs.
Appendix A
Table A1.
Distributions and Hyperparameters of Parameters Included in Probabilistic Sensitivity Analysis.
| Parameter | Distribution | Mean | SD | Lower limit | Upper limit |
|---|---|---|---|---|---|
| Probability of surgery following MT | Truncated normal | 0.42 | 0.05 | 0.38 | 0.50 |
| Length of hemithyroidectomy | Truncated normal | 163 | 26.6 | 60 | 230 |
| Length of total thyroidectomy | Truncated normal | 242 | 46.6 | 155 | 360 |
| Cost of MT | Truncated normal | 1200 | 120 | 800 | 1600 |
| Probability of malignancy following surgery in MT arm | Truncated normal | 0.875 | 0.10 | 0.44 | 0.91 |
| Probability of a completion hemithyroidectomy following malignancy in MT arm | Truncated normal | 0.45 | 0.1 | 0.30 | 0.60 |
| Utility of papillary thyroid microcarcinoma | Truncated normal | 0.5 | 0.25 | 0 | 1 |
Abbreviation: MT, molecular testing.
Table A2.
Results of Non-Reference Case Discounting Rates Compared to the Reference Case Rate of 1.5% (Bold).
| Discount rate | Mean cost ($) | Mean effectiveness | ICER ($/Sx avoided) |
|---|---|---|---|
| 0% | 7979.687 | ||
| Current management | 6455.919 | 0.638 | |
| MT | 8466.800 | 0.890 | |
| 1.5% | 7876.348 | ||
| Current management | 6430.515 | 0.638 | |
| MT | 8413.664 | 0.890 | |
| 3.0% | 7777.749 | ||
| Current management | 6415.357 | 0.638 | |
| MT | 8373.680 | 0.890 |
Abbreviations: ICER, incremental cost-effectiveness ratio; MT, molecular testing.
Table A3.
Results of Non-Reference Case Papillary Thyroid Microcarcinoma Utility Compared to the Reference Case with Utility Drawn from a Distribution (Bold).
| PTMC utility | Mean cost ($) | Mean effectiveness | ICER ($/Sx avoided) |
|---|---|---|---|
| 0 | 6909.927 | ||
| Current management | 6430.515 | 0.603 | |
| MT | 8413.664 | 0.890 | |
| PSA distribution | 7876.348 | ||
| Current management | 6430.515 | 0.638 | |
| MT | 8413.664 | 0.890 | |
| 1 | 9138.935 | ||
| Current management | 6430.515 | 0.673 | |
| MT | 8413.664 | 0.890 |
Abbreviations: ICER, incremental cost-effectiveness ratio; MT, molecular testing; PSA, probabilistic sensitivity analysis; PTMC, papillary thyroid microcarcinoma.
Figure A1.
An annotated version of Figure 1 displays the results of varying the discount rate and utility of papillary thyroid microcarcinoma (PTMC) parameters (Tables A2 and A3).
Figure A2.
Tornado plot showing the results of the 1-way sensitivity analysis. Parameters closer to the top of the plot have a greater influence on the outcome. MQ, probability of surgery following molecular testing; c.E, cost of total thyroidectomy; c.M, cost of molecular testing; c.D, cost of hemithyroidectomy; c.F, cost of completion thyroidectomy.
Footnotes
List of Abbreviations: ACR American College of Radiology
ATA American Thyroid Association
CEA Cost-effectiveness analysis
FNA Fine needle aspiration
GEC Gene expression classifier
GSC Gene sequence classifier
ICER Incremental cost-effectiveness ratio
ITNs Indeterminate thyroid nodules
miRNA microRNA
mRNA messenger RNA
MT Molecular testing
NPV Negative predictive value
PPV Positive predictive value
PSA Probabilistic sensitivity analysis
QALY Quality-adjusted life years
WTP Willingness to pay
Author Contributions: Colin MacKay was involved in data acquisition, statistical analysis, model construction, data interpretation, and revision of the manuscript. Brooke Turner was involved in data acquisition and interpretation, drafting, and revision of the manuscript. Scott Clarke was involved in data acquisition. Timothy Wallace was involved in data acquisition and assisted with the revision of the manuscript. Matthew H. Rigby was involved in study conceptualization and study supervision and assisted with data acquisition, analysis, interpretation, preparation, and revision of the manuscript.
Availability of Data and Materials: The data sets used and analyzed for the following study are available from the corresponding author upon reasonable request.
Consent for Publication: Consent for publication is not applicable.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval and Consent to Participate: Ethics approval for the study was obtained from the Nova Scotia Health Authority Research Ethics Board (ROMEO #1024802).
ORCID iDs: Colin MacKay  https://orcid.org/0000-0002-5079-1862
https://orcid.org/0000-0002-5079-1862
Matthew H. Rigby  https://orcid.org/0009-0008-2900-4626
https://orcid.org/0009-0008-2900-4626
References
- 1. Grani G, Sponziello M, Pecce V, Ramundo V, Durante C. Contemporary thyroid nodule evaluation and management. J Clin Endocrinol Metab. 2020;105(9):2869-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tessler FN, Middleton WD, Grant EG, et al. ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS Committee. J Am Coll Radiol. 2017;14(5):587-595. [DOI] [PubMed] [Google Scholar]
- 3. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuta V, Forner D, Azzi J, et al. Treatment choices in managing Bethesda III and IV thyroid nodules: a Canadian multi-institutional study. OTO Open. 2021;5(2):2473974X211015937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taylor BA, Hart RD, Rigby MH, Trites J, Taylor SM, Hong P. Decisional conflict in patients considering diagnostic thyroidectomy with indeterminate fine needle aspirate cytopathology. J Otolaryngol Head Neck Surg. 2016;45:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ohori NP. Molecular testing and thyroid nodule management in North America. Gland Surg. 2020;9(5):1628-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nishino M, Nikiforova M. Update on molecular testing for cytologically indeterminate thyroid nodules. Arch Pathol Lab Med. 2018;142(4):446-457. [DOI] [PubMed] [Google Scholar]
- 8. Chen T, Gilfix BM, Rivera J, et al. The role of the ThyroSeq v3 molecular test in the surgical management of thyroid nodules in the Canadian public health care setting. Thyroid. 2020;30(9):1280-1287. [DOI] [PubMed] [Google Scholar]
- 9. Patel KN, Angell TE, Babiarz J, et al. Performance of a genomic sequencing classifier for the preoperative diagnosis of cytologically indeterminate thyroid nodules. JAMA Surg. 2018;153(9):817-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Endo M, Nabhan F, Porter K, et al. Afirma gene sequencing classifier compared with gene expression classifier in indeterminate thyroid nodules. Thyroid. 2019;29(8):1115-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lupo MA, Walts AE, Sistrunk JW, et al. Multiplatform molecular test performance in indeterminate thyroid nodules. Diagn Cytopathol. 2020;48(12):1254-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krijkamp EM, Alarid-Escudero F, Enns EA, Jalal HJ, Hunink MGM, Pechlivanoglou P. Microsimulation modeling for health decision sciences using R: a tutorial. Med Decis Making. 2018;38(3):400-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Institut national d’excellence en santé et en services sociaux (INESSS). Advisability of Using Molecular Tests to Clarify the Diagnosis of Thyroid Nodules with Indeterminate Cytology [Internet]. INESSS; 2021. Accessed 2023 June 23, 2023. https://www.inesss.qc.ca/fileadmin/doc/INESSS/Rapports/Technologies/INESSS_ThyroSeq_Avis.pdf [Google Scholar]
- 14. Ontario Health (Quality). Molecular testing for thyroid nodules of indeterminate cytology: a health technology assessment. Ont Health Technol Assess Ser. 2022;22(2):1-111. [PMC free article] [PubMed] [Google Scholar]
- 15. Kay-Rivest E, Tibbo J, Bouhabel S, et al. The first Canadian experience with the Afirma® gene expression classifier test. J Otolaryngol Head Neck Surg. 2017;46:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steward DL, Carty SE, Sippel RS, et al. Performance of a multigene genomic classifier in thyroid nodules with indeterminate cytology. JAMA Oncol. 2019;5(2):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dharampal N, Smith K, Harvey A, Paschke R, Rudmik L, Chandarana S. Cost-effectiveness analysis of molecular testing for cytologically indeterminate thyroid nodules. J Otolaryngol Head Neck Surg. 2022;51(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidelines for the economic evaluation of health technologies: Canada. 4th ed. CADTH; 2017. [Google Scholar]
- 19. Alarid-Escudero F, Knowlton G, Easterly C, Enns EA. Decision Analytic Modeling Package (dampack) [Internet]. 2021. Accessed April 20, 2023. https://cran.r-project.org/web/packages/dampack/citation.html
- 20. Carty SE, Ohori NP, Hilko DA, et al. The clinical utility of molecular testing in the management of thyroid follicular neoplasms (Bethesda IV nodules). Ann Surg. 2020;272(4):621-627. [DOI] [PubMed] [Google Scholar]
- 21. Jeong JY, Song CM, Ji YB, Park JH, Kim DS, Tae K. Incidence and risk factors of hypoparathyroidism and hypocalcemia after hemithyroidectomy. Langenbecks Arch Surg. 2023;408(1):298. [DOI] [PubMed] [Google Scholar]
- 22. Gunn A, Oyekunle T, Stang M, Kazaure H, Scheri R. Recurrent laryngeal nerve injury after thyroid surgery: an analysis of 11,370 patients. J Surg Res. 2020;255:42-49. [DOI] [PubMed] [Google Scholar]
- 23. Joliat GR, Guarnero V, Demartines N, Schweizer V, Matter M. Recurrent laryngeal nerve injury after thyroid and parathyroid surgery. Medicine (Baltimore). 2017;96(17):e6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Government of Canada SC. Changing trends in thyroid cancer incidence in Canada: a histologic examination, 1992 to 2016 [Internet]. 2020. Accessed June 17, 2023. https://www150.statcan.gc.ca/n1/pub/82-003-x/2020001/article/00002/tbl/tbl01-eng.htm [DOI] [PubMed]
- 25. Gosden T, Pedersen L, Torgerson D. How should we pay doctors? A systematic review of salary payments and their effect on doctor behaviour. QJM. 1999;92(1):47-55. [DOI] [PubMed] [Google Scholar]
- 26. Jug R, Parajuli S, Ahmadi S, Jiang XS. Negative results on thyroid molecular testing decrease rates of surgery for indeterminate thyroid nodules. Endocr Pathol. 2019;30(2):134-137. [DOI] [PubMed] [Google Scholar]
- 27. Wu JX, Lam R, Levin M, Rao J, Sullivan PS, Yeh MW. Effect of malignancy rates on cost-effectiveness of routine gene expression classifier testing for indeterminate thyroid nodules. Surgery. 2016;159(1):118-129. [DOI] [PubMed] [Google Scholar]
- 28. Rajab M, Payne RJ, Forest VI, Pusztaszeri M. Molecular testing for thyroid nodules: the experience at McGill University teaching hospitals in Canada. Cancers (Basel). 2022;14(17):4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Williams BA, Bullock MJ, Trites JR, Taylor SM, Hart RD. Rates of thyroid malignancy by FNA diagnostic category. J Otolaryngol Head Neck Surg. 2013;42(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Government of Nova Scotia, Canada. Government of Nova Scotia [Internet]. 2014. Accessed May 20, 2023. https://waittimes.novascotia.ca/procedure/thyroid-or-parathyroid-surgery#waittimes
- 31. Nicholson KJ, Roberts MS, McCoy KL, Carty SE, Yip L. Molecular testing versus diagnostic lobectomy in Bethesda III/IV thyroid nodules: a cost-effectiveness analysis. Thyroid. 2019;29(9):1237-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]