Abstract
Small extracellular vesicles (sEV) have emerged as a novel mode of intercellular material transport and information transmission. It has been suggested hormones may regulate the production and function of sEV. However, the specific impact of growth hormone-releasing hormone (GHRH) on pituitary sEV production and the role of sEV in the regulation of the GHRH-GH-IGF axis has not been previously reported. The results of the present study demonstrated that GHRH increased the production of pituitary sEV by promoting the expression of Rab27a. More importantly, GHRH induced alterations in protein and miRNA levels within GH3-sEV components. Notably, GH3-sEV with GHRH treatment exhibited the enhanced ability to impede BRL 3A cell proliferation and the expression of IGF-1. Conclusively, for the first time, we corroborate the influence of GHRH on pituitary sEV, thereby presenting novel evidence for how sEV participates in the balance of the GHRH-GH-IGF axis. Importantly, this study provides new insight into a novel balance mechanism mediated by sEV within the endocrine system.
Graphical Abstract

Supplementary Information
The online version contains supplementary material available at 10.1186/s12951-024-02857-y.
Keywords: GHRH, Pituitary small extracellular vesicles, Hepatocyte, miR-375-3p, IGF-1
Introduction
Small extracellular vesicles (sEV) are heterogeneous nanoscale vesicles secreted by almost all cells in the body [1], displaying a characteristic cup-shaped structure under transmission electron microscopy. Initially, sEV were considered as cellular metabolic byproducts or cellular "garbage". However, researchers have now recognized their significance as crucial cellular modulators in recent years [2–4]. Importantly, they mediate cell communication, which is a ubiquitous mechanism for intercellular interactions [5]. It has been discovered that sEV could serve as a carrier for a diverse array of substances, including proteins, lipids, and messenger RNA (mRNA), non-coding RNA (ncRNA), such as microRNA (miRNA) [6–9]. Furthermore, they are increasingly recognized for their regulatory function in the transportation of functional miRNA to target cells [10–14]. A substantial body of research now indicates that sEV play a widespread role in substance transport, information transmission, and immune response regulation [2, 4, 7].
Interestingly, while hormones and sEV are chemically distinct, it appears that they play pivotal roles in intercellular information transmission [15]. Moreover, numerous studies indicate that hormones can influence the secretion and functionality of sEV [16–18]. Furthermore, a recent study has shown that adenoma pituitary sEV may serve as nonhormonal pituitary-derived messengers mediating cellular signaling [19]. These limited studies hint sEV may be a regulator in endocrine network.
The pituitary gland, a central gland of the endocrine system within the animal body and human being, participates in regulating multiple physiological processes [20–22]. It has been well known the hypothalamic-pituitary-liver (GHRH-GH-IGF) axis plays an important role in regulating animal growth and development. Moreover, homeostasis of this axis is of particular importance physiologically. As well known, GH is both positively and negatively regulated to maintain its homeostasis. GH is regulated by the antagonistic actions of GHRH and somatostatin [23, 24], and the negative feedback of GH release through IGF-1 also play an important role [25, 26].
Up to now, researches have focused on exploring sEV from different types of pituitary adenoma [19, 27–29]. Our preliminary research has already demonstrated that the swine anterior pituitary can produce and release sEV, and contains functional non-coding RNA [30]. As a novel potential pathway for endocrine and paracrine signaling, the understand of sEV in the physiologically normal pituitary remains rare. As we all know, the growth hormone-releasing hormone (GHRH), a hypothalamic hormone, positively affects growth hormone (GH) synthesis and secretion. Nevertheless, there is a dearth of reported studies exploring the influence of GHRH on pituitary sEV.
In this study, we aimed to investigate the effects of GHRH on the secretion and functionality of pituitary sEV, along with the associated mechanisms. We examined the changes in the sEV composition induced by GHRH, and the changes of their impact on IGF-1 and hepatocyte proliferation. The findings of this study provide valuable insights into the interplay between hormones and sEV, highlighting the role of pituitary sEV as a novel regulatory factor in the Hypothalamic-pituitary-liver axis.
Materials and methods
Cell culture and GHRH treatment
The rat pituitary cell line GH3 (ATCC) was cultured in F12 (Gibco, USA) medium supplemented with 2.5% fetal bovine serum (FBS) (Gibco USA), 15% horse serum (Hyclone, China) and 1% penicillin/ streptomycin (Gibco USA). The cell line of rat liver cells (BRL 3A) was cultured in in DMEM (Gibco, USA) medium supplemented with 10% FBS (Gibco USA) and 1% penicillin/streptomycin (Gibco USA). Porcine primary pituitary cell culture was performed as described in the previous studies [31–34]. Before treatment, the cells were starved in serum-free medium overnight. During treatment with GHRH, the cell culture medium was replaced with a fresh EV-depleted serum medium. EV-depleted serum was prepared by subjecting serum to ultracentrifugation (Optima XE, Beckman Coulter) at 120,000×g for 18 h at 4 °C according to the literature [35].
sEV isolation and analysis
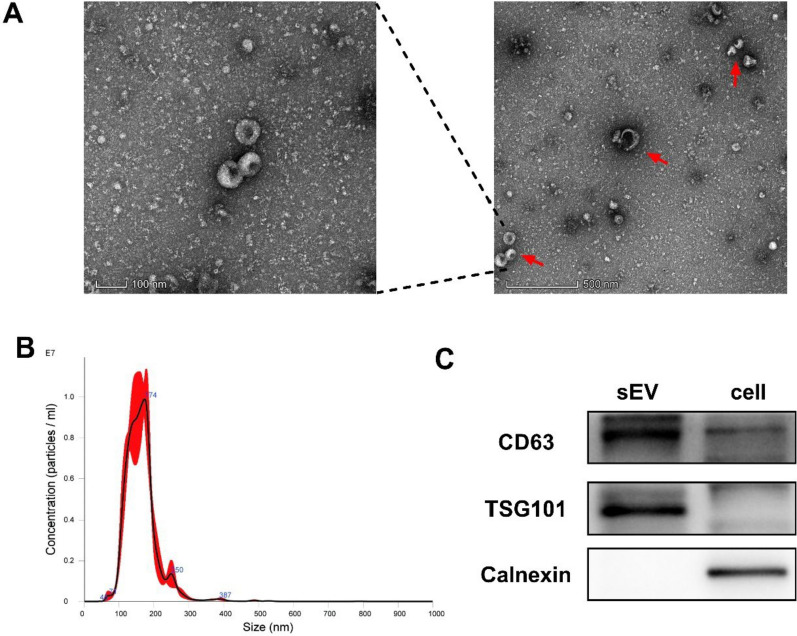
sEV from cell culture supernatant (50 mL per group) were isolated using a differential ultracentrifugation method as described [36–38]. The obtained pellet was resuspended in PBS and stored at –80 °C. We assessed sEV protein concentration using the BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). The morphology of sEV was examined with the transmission electron microscope. A drop of sEV suspension (about 10 μL) was fixed on a formvar-coated copper grid for 2 min, washed briefly in ultrapure water, negatively stained with 1% uranyl acetate, and observed by transmission electron microscopy (TEM; JEM-2000EX; Jeol, Tokyo, Japan) at an acceleration voltage of 80 kV. Size distribution, concentration, and intensity of sEV were analyzed by a NanoSight LM10 instrument (NanoSight, Amesbury, UK). The sEV markersCD63 and TSG101 were used as positive control whereas the endoplasmic reticulum protein Calnexin was used as a negative control in Western blot analysis.
Cell transfection
According to the sequence of Rab 27a mRNA, three target Rab27a mRNA siRNA with different sites were designed to avoid the off-target effect. The target siRNAs and control siRNA (NC) were purchased from and synthesized by the GenePharma company. GH3 cells were transfected with NC or siRab27a using Lipofectamine 2000 (Invitrogen, USA).
Cell viability assay
Cell proliferation was evaluated using Cell counting kit-8 (CCK8; EZBioscience, EZB-CK8) method, 5-ethynyl-2ʹ-deoxy uridine (EdU; Beyotime Biotechnology, C0071S) incorporation assay. Firstly, the rate of cell proliferation was determined with CCK-8 kit according to the manufacturer’s instructions. The number of viable cells was assessed by measuring the absorbance at 450 nm using a Synergy 2 Multi-Mode Reader (Bio Tek Instruments, Inc., Winooski, VT, USA). Secondly, DNA synthesis was examined with EdU incorporation assay to evaluate cell proliferation. The EdU positive cells were counted and normalized by the total number of Hoechst 33,342 stained cells.
Quantitative real-time PCR analysis
Total RNA and miRNA were extracted from cells and sEV using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The concentration of RNA and miRNA were measured by NanoDrop 2000 (Wilmington, DE, USA). The cDNAs were obtained by Color Reverse Transcription Kit (with gDNA remover) (EZBioscience, Roseville, CA, USA). All qPCR experiments were performed on a real-time PCR machine (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and specific primers of the genes of interest, and amplification efficiencies were checked by standard curves. Gene expression was quantified by the comparative cycle threshold (Ct) method. The target gene expression was normalized to those of β-actin, while the relative miRNA levels were normalized to the U6 or cel-miR-39 in sEV and calculated as 2 − (ΔΔCT).
Western Blot
Total protein of indicated tissues, cells or sEV was extracted using RIPA lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) containing 1 mM phenyl methane sulfonyl fluoride (PMSF). The protein concentrations were determined using the BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Equal amounts of total protein were separated by SDS-PAGE and transferred to a PVDF membrane in a tris–glycine methanol buffer. Primary antibodies used include anti-CD63 (Sangon Biotech), anti-TSG101 (Zen Biotech), Calnexin (Sangon Biotech), anti-PCNA (Zen Biotech), anti-GH (Santa Cruz), anti-Rab27a (Sangon Biotech), anti-IGF-1 (Zen Biotech), anti-Jak2 (Zen Biotech), anti-Stat5 (Zen Biotech), anti-p-Stat5 (Zen Biotech) and anti-Tubulin (Bioworld). Membranes were washed and incubated with either anti-mouse or anti-rabbit antibody conjugated to horseradish peroxidase (HRP) for 30 min to 1 h at room temperature. The membranes were incubated with ImmobilonTM Western Chemiluminescent HPR Substrate (Millipore, Burlington, WA, USA) and scanned with a FlourChem M Fluorescent Western Imaging System (Protein Simple, Santa Clara, CA, USA).
ELISA
The concentrations of GH and IGF-1 were measured with ELISA kits purchased from Nanjing Jiancheng Bioengineering Institute. ELISA was performed according to the manufacturer’s instructions. Color alterations in the wells were read using the 96-well microplate reader (BioTek Instruments).
Label-free quantitative proteomic analysis
Data-Dependent Acquisition Lable-free quantitative proteomic analysis was carried out by Lianchuan Biotechnology Company (Hangzhou, China). Briefly, the samples were homogenized using a tissue lyser (60 Hz, 2 min) followed by 15 min centrifugation (20,000g, 4 °C) and harvest the supernatant. After quality control of protein extraction, trypsin was added and the samples were incubated at 37 °C. The enzymatically digested peptides were desalted and dried peptide fractions were redissolved in pure water and stored at − 20 °C. Using Reversed-Phase High-Pressure Liquid Chromatography (HPLC) fractionation to obtain dried peptide samples. Then the samples redissolved with 0.1% FA followed by 10 min centrifugation (20,000×g) and supernatant was collected and injected into a self-loading C18 column. Separation was performed by Thermo Scientific EASY-nLC™ 1200 system at a flow rate of 300 nL/min. MaxQuant (version 2.1.4.0) software was used to analyze the DDA label-free MS/MS data and statistical analysis was performed in R (version 4.0.0). The raw protein intensity will be normalized by method "medium", Hierarchical clustering was performed using pheatmap package. Principal component analysis (PCA) was performed using metaX package. T test was used for statistical differential analysis and a cut of p-value < = 0.05 and fold change > = 1.2 was used to select statistically differential expressed proteins.
Statistics analysis
All experimental results are presented as the mean ± S.E.M. Student’s t-test was used for two group comparison, and one-way ANOVA was used among more than two groups. p < 0.05 was considered as statistically significant. *p < 0.05; **p < 0.01.
Results
GHRH promotes the produce of sEV from GH3 cells
GH3 pituitary cells were used to investigate the effect of GHRH on pituitary sEV production. GH3-sEV was harvested by ultracentrifugation conducted as described in the methods guided by MISEV2018 [39, 36, 40]. Transmission electron microscopic (TEM) images showed that GH3-sEV had classic cup-shaped morphology (Fig. 1A). The distribution curve of the particle size of GH3-sEV was determined by nanoparticle tracking analysis (NTA) (Fig. 1B). Western Blot revealed that GH3-sEV was highly positive for sEV-associated protein makers CD63 and TSG101, but negative for Calnexin (Fig. 1C). All of these indicates the features of sEV.
Fig. 1.
Identification of GH3 sEV. A Analysis of GH3-sEV by transmission electron microscopy. B Nanoparticle tracking analysis (NTA) of size distribution of isolated GH3-Sev. C Western Blot analysis showing the biomarkers of sEV including CD63 and TSG101. Calnexin was used as a negative control
We then added different concentration gradients of GHRH to the culture medium of GH3 cells. The results showed that 40 nM GHRH could promoted GH synthesis and secretion (Fig. 2A). At the same, NTA showed that the concentration (particles/ml) and intensity (a.u.) of GH3-sEV with GHRH treatment were significantly increased compared with the control group (Fig. 2B). Furthermore, GHRH could also elevate the level of CD63 in sEV of the same protein amount (Fig. 2C). These data suggest that GHRH promotes the production of sEV from GH3 cells.
Fig. 2.
GHRH promotes the produce of sEV from GH3 cells. A Effect of different doses of GHRH on GH mRNA and GH secretion after 12 h by qRT-PCR and ELISA, respectively. B GH3-sEV isolated from the control and GHRH-treated group were investigated by NTA for concentration (particles/ml) and intensity (a.u.) (n = 3). C CD63 protein level under 40 nM GHRH stimulation, Ponceau S was applied to represent the amounts of loaded total protein (n = 4). (*p < 0.05, **p < 0.01)
GHRH may regulate the production of pituitary sEV via Rab27a
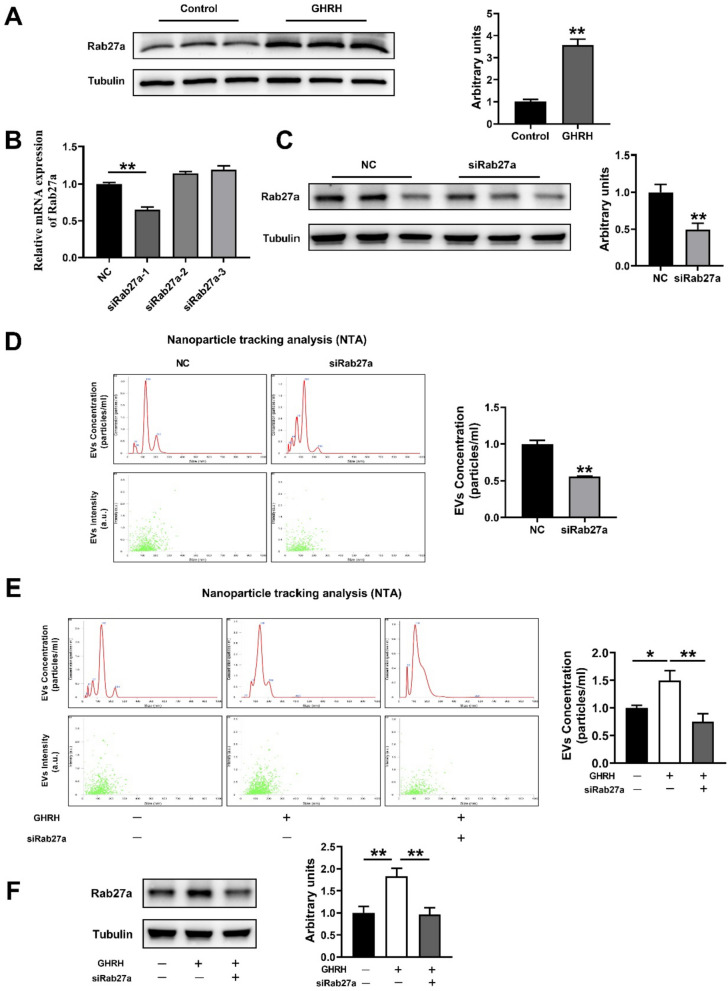
We next explore the potential underlying mechanism for GHRH to promote sEV production. It has been confirmed that Rab27a, a Rab family member, plays a central role in sEV secretion [41, 42]. Interestingly, our results showed that Rab27a expression was profoundly increased by GHRH (Fig. 3A). To further verify whether the process of GHRH-promoted pituitary sEV production is regulated by Rab27a, we transfected siRab27a to GH3 cells. As expected, one of three designed candidate siRab27a, siRab27a-1, significantly decreased the mRNA and protein expression of Rab27a (Fig. 3B, C). NTA showed that the concentration and intensity of GH3-sEV was significantly decreased after Rab27a suppression (Fig. 3D). Interestingly, the inclusion of siRab27a reversed the facilitation of GHRH on the concentration and intensity of GH3-sEV (Fig. 3E), and the expression of Rab27a in GH3 cells as well (Fig. 3F).
Fig. 3.
GHRH increased the sEV production from GH3 via Rab27a. A The effect of GHRH on the Rab27a protein of GH3 cells. B The expression of Rab27a mRNA after transfection with siRab27a 1–3. C The expression of Rab27a protein after transfection with siRab27a-1. D NTA analysis for sEV concentration and intensity of the NC and siRab27a group. E NTA analysis for sEV concentration and intensity of NC, GHRH and GHRH + siRab27a-1 groups, and F The expression of Rab27a protein
Additionally, we further validated the above results in primary pituitary cells. Swine anterior pituitary primary cells were isolated as described previously [31, 32, 43],and were identified by cell morphology (Fig. 4A) and immunofluorescence staining with antibodies against the pituitary-specific marker Pit-1 (Fig. 4B). Then sEV was harvested by ultracentrifugation conducted as above, and was characterized by TEM (Fig. 4C), NAT (Fig. 4D) and western blotting analysis (Fig. 4E). We further compared the production of sEV from swine anterior pituitary primary cells and the expression of Rab27a in cells with GHRH treatment combining with siRab27a-1, and the results are exactly the same with that of GH3 cells (Fig. 4F, G). Taken together, these results show that GHRH is able to promote pituitary sEV production via Rab27a.
Fig. 4.
GHRH increased the sEV production from swine primary pituitary cell via Rab27a. A Cell morphology of primary swine pituitary cell. B Pit1 immunofluorescence staining. C Image of transmission electron microscopy. D NTA analysis for sEV derived from primary swine pituitary cell. E Western Blot analysis showing the positive biomarkers of sEV including CD63 and TSG101, negative biomarker Calnexin. F NTA analysis for concentration and intensity, and G The expression of Rab27a protein in primary swine pituitary cell
GHRH-treated GH3-sEV inhibits cell proliferation and IGF-1 expression more robustly in BRL 3A cells
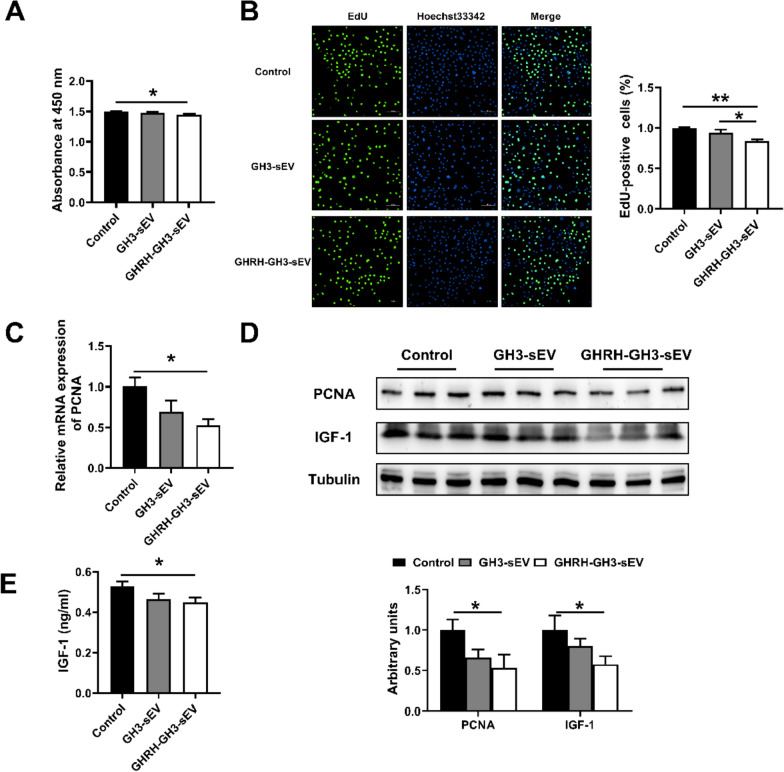
To further reveal if GHRH treatment change the effect of GH3-sEV on hepatocyte and IGF-1, GH3 sEV and GHRH treated GH3-sEV were added to the culture medium of BRL 3A cells. The cell proliferation was evaluated with CCK8 kits and EdU staining, further confirmed by the detection of PCNA mRNA and protein expression. Interestingly, GHRH-treated GH3-derived sEV significantly inhibited BRL 3A cell proliferation compared with GH3-sEV (Fig. 5A–D). At the same time, GHRH-treated GH3-sEV obviously decreased the protein levels of IGF-1 within cells as well as in supernatant (Fig. 5D, E). Moreover, we obtained the same results using sEV from swine primary pituitary cells (Supplement Figure 1). These results suggest that GHRH-treated GH3-sEV inhibits cell proliferation and IGF-1 expression more robustly than GH3-sEV in BRL 3A cells.
Fig. 5.
GHRH-treated GH3-sEV inhibits cell proliferation and IGF-1 expression more robustly in BRL 3A cells. A The result of CCK8 kits. B The result of EdU-positive cells. C The expression of PCNA mRNA. D The levels of PCNA and IGF-1 protein in BRL 3A cells. E The levels of IGF-1 in cell supernatant
GHRH alters the composition of GH3-sEV
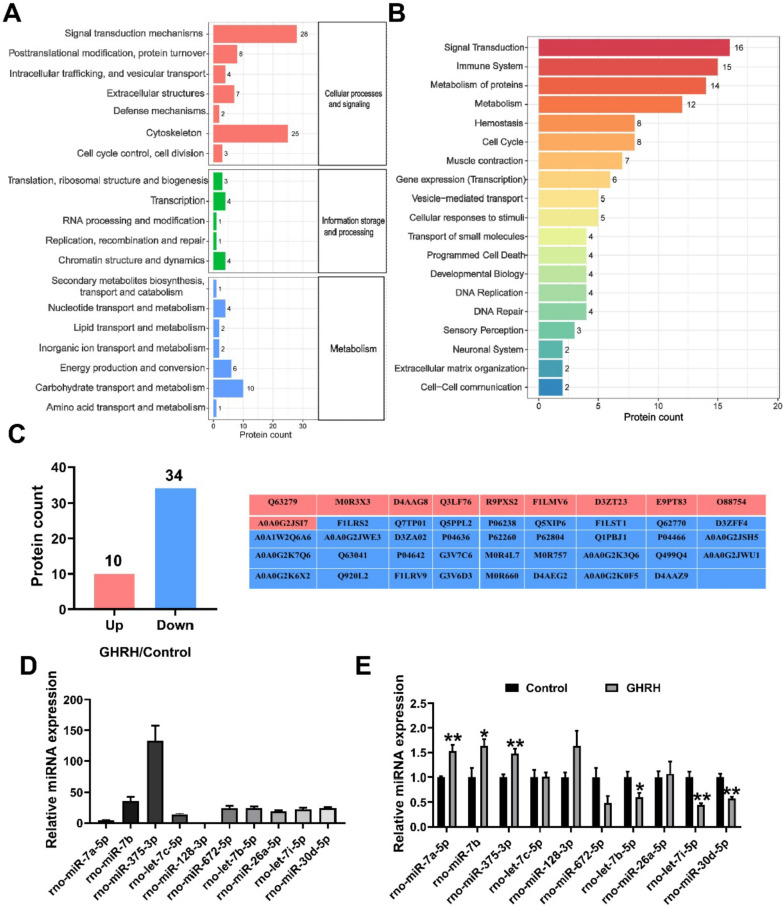
Protein and miRNA are important functional molecules in sEV. We hypothesize that GHRH treatment alter the components of the GH3-sEV. To investigate potential protein disparities between GH3-sEV generated with and without GHRH treatment, label-free quantitative analysis was performed, followed by bioinformatics analysis to probe the potential functional role of identified proteins. The EggNOG analysis revealed that most of these proteins function in intracellular signaling, metabolism and transport processes (Fig. 6A). Additionally, Reactome pathway analysis indicated that proteins with GH3-sEV are enriched in signaling, immune system, metabolism, cell cycle, small molecule transport pathways (Fig. 6B). A total of 139 proteins were quantified, with 126 in the GH3-sEV group and 119 in the GHRH-GH3-sEV group. Among these, 10 proteins were up-regulated and 34 were down-regulated in the GHRH-GH3-sEV compared to GH3-sEV (Fig. 6C). It was discovered that the expression of proteins such as YWHAE, DCUN1D5, and MFGE8, which play roles in regulating the cell cycle, cell growth and the IGF transport pathway, were significantly down-regulated in GH3-sEV after GHRH treatment [44–48].
Fig. 6.
GHRH alters the protein and miRNA composition of GH3-sEV. GO A EggNOG analysis. B Reactome analysis. C The protein changes between GHRH-GH3-sEV and GH3-sEV. D Identification of top 10 miRNAs in GH3-sEV. E The comparison of miRNAs in GHRH-GH3-sEV and GH3-sEV
Additionally, prior RNA sequencing of GH3-sEV was conducted and the expression levels of the top 10 miRNAs were verified by using qRT-PCR (Fig. 6D). Subsequently, results showed a significant increase in the levels of miR-7a-5p, miR-7b, and miR-375-3p, and a significant decrease in the levels of let-7b-5p, let-7i-5p, and miR-30d-5p in GHRH-GH3-sEV relative to GH3-sEV (Fig. 6E). The results above suggest that GHRH induces alterations in the composition of GH3-sEV components, both protein and miRNA.
GHRH-GH3-sEV may regulate cell proliferation and IGF-1 via its increased miR-375-3p
To probe the possible mechanisms by which GHRH-GH3-sEV influence BRL 3A cell proliferation and the expression of IGF-1, we assessed the changes in miRNA levels within the BRL 3A cells. The miRNA with the highest level in GH3-derived sEV was miR-375-3p, and more importantly, its level was significantly increased after GHRH treatment as shown (Fig. 6E). Interestingly, in sEV donor BRL 3A cells, the expression of miR-375-3p were also significantly higher in the GHRH-GH3-sEV treatment compared to the control and GH3-sEV treatment (Fig. 7A). Previous studies have indicated that miR-375-3p is involved in the regulation of cell proliferation [49, 50]. Based on these findings, it is proposed that miR-375-3p play a crucial regulatory role in this process.
Fig. 7.
GHRH-GH3-sEV may regulate cell proliferation and IGF-1 via its increased miR-375-3p. A The expression of miR-375-3p in BRL 3A cells in different treatments. B Relative expression of miR-375-3p following transfection with NC and miR-375-3p mimics. C The cell proliferation results detected with CCK8 kits and D EdU-positive assay. E The expression of PCNA and IGF-1 mRNA and F protein expression in BRL 3A cells. G The IGF-1 protein levels in the BRL 3A cells supernatant. H The effect of miR-375-3p mimics on Jak2/ Stat5 pathway proteins. I The effect of GHRH-GH3-sEV on Jak2/Stat5 pathway proteins
To further confirm the function of miR-375-3p, we transfected BRL 3A cells with NC and miR-375-3p mimics and the efficiency of miR-375-3p transfection was confirmed by the dramatically upregulated miR-375-3p in BRL 3A cells (Fig. 7B). More importantly, miR-375-3p overexpression prominently inhibited proliferation of BRL 3A cells (Fig. 7C–F). At the same time, we found a marked reduction in the expression of IGF-1 protein in cells and supernatant (Fig. 7F–G).
Previous studies have shown that miR-375-3p directly targets the 3'UTR of Jak2, which involved in the regulation of IGF-1 expression through the Jak/Stat pathway [51–56]. Therefore, we examined the expression of Jak2 after transfection of miR-375-3p mimics. The results showed that JAK2 protein levels was significantly decreased and phosphorylation levels of Stat5 was also robustly inhibited (Fig. 7H). To determine whether GHRH-GH3-sEV regulate IGF-1 through Jak2/Stat5, we examined the change of the protein levels with different treatments. Interestingly, GHR-GH3-sEV attenuates the expression of Jak2 and p-Stat5 more robustly than GH3-sEV (Fig. 7I). The results above indicate that GHRH-GH3-sEV exhibits great efficacy in suppressing cellular proliferation and IGF-1 expression compared to GH3-sEV. This enhanced potency can be attributed to its ability to modulate the JaK2/Stat5 pathway via miR-375-3p.
Discussion
A growing body of research indicates that sEV can serve as a communicator to mediate material exchange and information transfer between cells and tissues [2–4], by carrying a variety of cargoes including proteins, lipids, nucleic acids, ect [7]. Interestingly, they exhibit heterogeneity because of their different sources and sorting mechanisms. Furthermore, sEV functions as significant signaling molecules in the endocrine system, which help maintain internal environment homeostasis in addition to hormones. For example, a recent investigation has revealed that hypothalamic neural stem/progenitor cells exhibit an endocrinological function by secreting extracellular vesicles miRNAs that contribute to the regulation of aging speed in the hypothalamus [57]. sEV derived from pancreatic islets play an important role in intercellular communication [58, 59]. Moreover, they also demonstrate a considerable contribution in maintaining reproductive system operation [60–62]. However, there are differences between sEV and hormones in terms of their biological constituents, biogenesis, and functional mechanisms [15]. In addition, the relationship between sEV and hormones is far from entirely clear. We summarize that on the one hand, hormones could affect the secretion of sEV and sEV also have potential to impact hormone secretion [17, 18, 63–65]; On the other hand, the combination of hormones and EVs has been shown to yield a more efficacious effect [66–71]. Balance in the hormone network plays an important role in maintaining homeostasis. There are both positive feedback and negative feedback regulation in the somatotropic axis signaling. GH is regulated by the antagonistic actions of GHRH and somatostatin, and the negative feedback of IGF-1 [23–26]. However, it is unclear whether and how there is positive or negative feedback between sEV and GH.
Our previous research has shown that sEV derived from swine anterior pituitary contain functional noncoding RNAs and they may participate in the cellular metabolic and biosynthetic process [30]. As we all know, GHRH is involved in regulating pituitary GH synthesis and release and the hypothalamus-pituitary-liver axis plays an important role in the growth regulation of animals. The question of whether GHRH affects the production of pituitary sEV and sEV function is of great interests.
If GHRH affect production of sEV of pituitary cells remains unexplored. The present study indicates that GHRH not only promotes the secretion of GH, but also facilitates the production of pituitary sEV. There are a number of studies reveals that Rab27a plays a very crucial role in sEV secretion [41, 42], and it was further confirmed in our research both in GH3 cells and primary pituitary cells. It is clear that GHRH promotes sEV production by increasing Rab27a, but the precious regulation mechanism is waiting for further exploration. Our study firstly provides evidence that GHRH promotes the yield of pituitary sEV, which is a new potential way in its biological regulation, even a new model for other hormones.
If GHRH change composition and function of GH3 derived sEV is the next key interesting point. Our results showed that GHRH could cause components alterations in GH3-sEV, both protein and miRNA. A total of 139 proteins were quantified through Label-free quantitative proteomic analysis. It showed that 10 proteins were up-regulated and 34 were down-regulated in the GHRH-GH3-sEV, comparing to GH3-sEV. Moreover, the levels of miRNAs in GH3-sEV group were altered following stimulation with GHRH. Notably, in contrast to GH3-sEV, GHRH-GH3-sEV exhibited a more robust suppression on the proliferation of BRL 3A cells, and the expression of IGF-1 as well. Upon comparing these altered proteins, it was discovered that the expression of proteins such as YWHAE, DCUN1D5, and MFGE8, which play roles in regulating the cell cycle, cell growth and the IGF transport pathway [44–48], were significantly down-regulated after treatment with GHRH, which may partially account for the suppression of GHRH-GH3-sEV. More interestingly, the expression of miR-375-3p showed a significant increase in BRL 3A cells treated with GHRH-GH3-sEV. It has been reported that miR-375-3p targets Jak2 3'UTR, which is involved in regulating IGF-1 expression through the Jak/Stat pathway [51–56]. In the present study, we validated that GHRH-GH3-sEV is a negative factor to regulate the expression of IGF-1 through the Jak2/Stat5 pathway by a way of delivering more miR-375-3p to recipient cell. Collectively, it is illustrated, for the first time, that GHRH alters protein and miRNA in pituitary derived sEV and changes its function of the inhibition of cell proliferation and IGF-1 expression in BRL 3A through miR-375-3p, and some key proteins as well.
In conclusion, our findings empirically demonstrate the influence of GHRH on pituitary sEV production and its cargos, leading to more robust inhibition of liver cell proliferation and IGF-1 expression. More importantly, this inhibition is just the opposite to GH function. GHRH elevates GH secretion and simultaneously increased pituitary sEV production to balance GH function. Thus, it is deduced that pituitary sEV plays a negative role in GHRH-GH-IGF axis. To our knowledge, it is the first time to suggest the idea that sEV is a new balance mediator in hormone regulation network, providing a theoretical foundation for a homeostatic regulatory mechanism between sEV and hormones.
Supplementary Information
Author contributions
Jiali Xiong: Writing-original draft, Methodology, Formal analysis, Data curation. Yuxuan Wang: Data curation, Validation, Supervision. Hailong Wang: Methodology, Supervision. Junyi Luo: Data curation, Project administration. Ting Chen: Methodology, Supervision. Jiajie Sun: Data curation, Conceptualization. Qianyun Xi: Conceptualization, Funding acquisition. Yongliang Zhang: Writing-review&editing, Funding acquisition, Supervision. All authors reviewed the manuscript.
Funding
This work was supported by the Key projects of the National Major R&D Program (2022YFD1300904), the National Natural Science Foundation of China (32372958, 32072812, 32072814) and Natural Science Foundation of Guangzhou Province (2021A1515011310, 2020A1515010062.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiali Xiong and Yuxuan Wang contributed equally to this work.
References
- 1.van Niel G. Shedding light on the cell biology of extracellular vesicles. Nat Revi Mol Cell Biol. 2018;19(4):213–28. [DOI] [PubMed] [Google Scholar]
- 2.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–79. [DOI] [PubMed] [Google Scholar]
- 3.Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514. [DOI] [PubMed] [Google Scholar]
- 4.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Natasha G, Gundogan B, Tan A, Farhatnia Y, Wu W, Rajadas J, Seifalian AM. Exosomes as immunotheranostic nanoparticles. Clin Ther. 2014;36(6):820–9. [DOI] [PubMed] [Google Scholar]
- 6.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39(16):7223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3(5):321–30. [DOI] [PubMed] [Google Scholar]
- 8.Fan L, Yao L, Li Z, Wan Z, Sun W, Qiu S, Zhang W, Xiao D, Song L, Yang G. Exosome-based mitochondrial delivery of circRNA mSCAR alleviates sepsis by orchestrating macrophage activation. Advanced Science. 2023;10(14):2205692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behera J, Kumar A, Voor MJ, Tyagi N. Exosomal lncRNA-H19 promotes osteogenesis and angiogenesis through mediating Angpt1/Tie2-NO signaling in CBS-heterozygous mice. Theranostics. 2021;11(16):7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaoyun K, Lin Z, Xiaoyu A, Vivek V, Ertugrul K, Dirk MH, Arshad M, Mathias B, Thorsten RD. Adipose-derived mesenchymal stem cells reduce autophagy in stroke mice by extracellular vesicle transfer of miR-25. J Extracellular Vesicles. 2020;10(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huan Z, Rui-Xin W, Xiao-Tao H, Ying A, Xin-Yue X, Hai-Hua S, Li-An W, Fa-Ming C. Periodontitis-compromised dental pulp stem cells secrete extracellular vesicles carrying miRNA-378a promote local angiogenesis by targeting Sufu to activate the Hedgehog/Gli1 signalling. Cell Prolif. 2021;54(5):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong L, Pu Y, Zhang L, Qi Q, Xu L, Li W, Wei C, Wang X, Zhou S, Zhu J, et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles promote lung adenocarcinoma growth by transferring miR-410. Cell Death Dis. 2018;9(2):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kogure A, Kosaka N, Ochiya T. Cross-talk between cancer cells and their neighbors via miRNA in extracellular vesicles: an emerging player in cancer metastasis. J Biomed Sci. 2019;26(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadi Valadi KE. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9. [DOI] [PubMed] [Google Scholar]
- 15.Xiong J, Fan Y, Wang Y, Luo J, Chen T, Sun J, Xi Q, Zhang Y. New signaling kid on the block in the endocrine system: the role of extracellular vesicles. Endocrinology. 2023;164(8):099. [DOI] [PubMed] [Google Scholar]
- 16.Crewe C, Joffin N, Rutkowski JM, Kim M, Scherer PE. An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell. 2018. 10.1530/ey.16.11.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma S, Shao S, Yang C, Yao Z, Gao L, Chen W. A preliminary study: proteomic analysis of exosomes derived from thyroid-stimulating hormone-stimulated HepG2 cells. J Endocrinol Invest. 2020;43(9):1229–38. [DOI] [PubMed] [Google Scholar]
- 18.Heiston EM, Ballantyne A, Stewart NR, La Salvia S, Musante L, Lanningan J, Erdbrügger U, Malin SK. Metabolism: Insulin infusion decreases medium-sized extracellular vesicles in adults with metabolic syndrome. Am J Physiol-Endocrinol Metab. 2022;323:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou C, Shen S, Moran R, Deng N, Marbán E, Melmed S. Pituitary somatotroph adenoma-derived exosomes: characterization of nonhormonal actions. J Clin Endocrinol Metab. 2021;107(2):379–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin MT, Ho LT, Uang WN. Effects of anterior pituitary hormones and their releasing hormones on physiological and behavioral functions in rats. J Steroid Biochem. 1983;19(12):433–8. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi YJCC. Hormones and osteoporosis update. Possible roles of pituitary hormones, TSH and FSH, for bone metabolism. Clin Calcium. 2009;19(7):977–83. [PubMed] [Google Scholar]
- 22.Weiss S, Bergland R, Page R, Turpen C, Hymer WC. Pituitary cell transplants to the cerebral ventricles promote growth of hypophysectomized rats. Proc Soc Exp Biol Med. 1978;159(3):409–13. [DOI] [PubMed] [Google Scholar]
- 23.Thorner MO, Vance ML, Hartman ML, Holl RW, Evans WS, Veldhuis JD, Van Cauter E, Copinschi G, Bowers CY. Physiological role of somatostatin on growth hormone regulation in humans. Metabolism. 1990;39(9):40–2. [DOI] [PubMed] [Google Scholar]
- 24.Frohman LA, Jansson JO. Growth hormone-releasing hormone. Endocr Rev. 1986;7(3):223–53. [DOI] [PubMed] [Google Scholar]
- 25.Berelowitz M, Szabo M, Frohman LA, Firestone S, Chu L, Hintz RL. Somatomedin-C mediates growth hormone negative feedback by effects on both the hypothalamus and the pituitary. Science. 1981;212(4500):1279–81. [DOI] [PubMed] [Google Scholar]
- 26.Schwander JC, Hauri C, Zapf J, Froesch ER. Synthesis and secretion of insulin-like growth factor and its binding protein by the perfused rat liver: dependence on growth hormone status. Endocrinology. 1983;113(1):297–305. [DOI] [PubMed] [Google Scholar]
- 27.Xiong Y, Tang Y, Fan F, Zeng Y, Liu Z. Exosomal hsa-miR-21-5p derived from growth hormone-secreting pituitary adenoma promotes abnormal bone formation in acromegaly. Transl Res. 2019;215:1–16. [DOI] [PubMed] [Google Scholar]
- 28.Zhao P, Cheng J, Li B, Nie D, Zhang Y. Toxicology: Up-regulation of the expressions of MiR-149–5p and MiR-99a-3p in exosome inhibits the progress of pituitary adenomas. Cell Biol Toxicol. 2021;37:1–19. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Liu YT, Tang H, Xie WQ, Yao H, Gu WT, Zheng YZ, Shang HB, Wang Y, Wei YX. Exosome-transmitted lncRNA H19 inhibits the growth of pituitary adenoma. J Clin Endocrinol Metab. 2019;104(12):6345–56. [DOI] [PubMed] [Google Scholar]
- 30.Xiong J, Zhang H, Zeng B, Liu J, Luo J, Chen T, Sun J, Xi Q, Zhang Y. An exploration of non-coding RNAs in extracellular vesicles delivered by swine anterior pituitary. Front Genet. 2021;12: 772753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barb C, Barrett J, Wright J, Kraeling R, Rampacek G. Opioid modulation of LH secretion by pig pituitary cells in vitro. Reproduction. 1990;90(1):213–9. [DOI] [PubMed] [Google Scholar]
- 32.Lin J, Barb C, Kraeling R, Rampacek G. Growth hormone releasing factor decreases long form leptin receptor expression in porcine anterior pituitary cells. Domest Anim Endocrinol. 2003;24(2):95–101. [DOI] [PubMed] [Google Scholar]
- 33.Ye R-S, Xi Q-Y, Qi Q, Cheng X, Chen T, Li H, Kallon S, Shu G, Wang S-B, Jiang Q-Y. Differentially expressed miRNAs after GnRH treatment and their potential roles in FSH regulation in porcine anterior pituitary cell. PLoS ONE. 2013;8(2): e57156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qi Q-E, Xi Q-Y, Ye R-S, Chen T, Cheng X, Li C-Y, Zhu X-T, Shu G, Wang L-N, Jiang Q-Y. Alteration of the miRNA expression profile in male porcine anterior pituitary cells in response to GHRH and CST and analysis of the potential roles for miRNAs in regulating GH. Growth Hormon IGF Res. 2015;25(2):66–74. [DOI] [PubMed] [Google Scholar]
- 35.Tian Y, Gong M, Hu Y, Liu H, Zhang W, Zhang M, Hu X, Aubert D, Zhu S, Wu L. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J Extra Vesicles. 2020;9(1):1697028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A. Cardiac fibroblast–derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Investig. 2014;124(5):2136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoco Cell Biol. 2006;30(1):21–9. [DOI] [PubMed] [Google Scholar]
- 38.Zhang M, Yu Q, Tang W, Wu Y, Lv J, Sun L, Shi G, Wu M, Qu J, Di C. Epithelial exosomal contactin-1 promotes monocyte-derived dendritic cell–dominant T-cell responses in asthma. J Allergy Clin Immunol. 2021;148(6):1545–58. [DOI] [PubMed] [Google Scholar]
- 39.Clotilde Théry KWW, Elena A, Maria JA, Johnathon DA, Ramaroson A, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;45:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. 2006, 30(1). [DOI] [PubMed]
- 41.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. [DOI] [PubMed] [Google Scholar]
- 42.Song L, Tang S, Han X, Jiang Z, Dong L, Liu C, Liang X, Dong J, Qiu C, Wang Y. KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nat Commun. 2019;10(1):1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye R-S, Li M, Li C-Y, Qi Q-E, Chen T, Cheng X, Wang S-B, Shu G, Wang L-N, Zhu X-T. miR-361-3p regulates FSH by targeting FSHB in a porcine anterior pituitary cell model. Reproduction. 2017;153(3):341–9. [DOI] [PubMed] [Google Scholar]
- 44.Yang YF, Lee YC, Wang YY, Wang CH, Hou MF, Yuan SF. YWHAE promotes proliferation, metastasis, and chemoresistance in breast cancer cells. Kaohsiung J Med Sci. 2019;35(7):408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo W, Li GJ, Xu HB, Xie JS, Shi TP, Zhang SZ, Chen XH, Huang ZG. In vitro biological characterization of DCUN1D5 in DNA damage response. Asian Pac J Cancer Prev. 2012;13(8):4157–62. [DOI] [PubMed] [Google Scholar]
- 46.Wang M, Fu Z, Wu J, Zhang J, Jiang L, Khazan B, Telljohann R, Zhao M, Krug AW, Pikilidou M, et al. MFG-E8 activates proliferation of vascular smooth muscle cells via integrin signaling. Aging Cell. 2012;11(3):500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gu Y, Zhang Z, Camps MGM, Ossendorp F, Wijdeven RH, Ten Dijke P. Genome-wide CRISPR screens define determinants of epithelial-mesenchymal transition mediated immune evasion by pancreatic cancer cells. Sci Adva. 2023;9(28):eadf9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Jin X, Liu B, Zhang P, Chen W, Li Q. CircRNA CBL11 suppresses cell proliferation by sponging miR-6778–5p in colorectal cancer. BMC Cancer. 2019;19(1):826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang K, Wei Z, Cao H. miR-375-3p inhibits the progression of laryngeal squamous cell carcinoma by targeting hepatocyte nuclear factor-1β. Oncol Lett. 2020;20(4):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin Y, Cheng Z, Fu X, Ji S. MicroRNA-375-3p is implicated in carotid artery stenosis by promoting the cell proliferation and migration of vascular smooth muscle cells. BMC Cardiovasc Disord. 2021;21(1):518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000;19(49):5662–79. [DOI] [PubMed] [Google Scholar]
- 52.Yin L-h, Zheng X-q, Li H-y, Bi L-x, Shi Y-f, Ye A-f, Wu J-b, Gao S-m. Epigenetic deregulated miR-375 contributes to the constitutive activation of JAK2/STAT signaling in myeloproliferative neoplasm. Leuk Res. 2015;39(4):471–8. [DOI] [PubMed] [Google Scholar]
- 53.Xu Y, Jin J, Liu Y, Huang Z, Deng Y, You T, Zhou T, Si J, Zhuo W. Snail-regulated MiR-375 inhibits migration and invasion of gastric cancer cells by targeting JAK2. PLoS ONE. 2014;9(7): e99516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sos BC, Harris C, Nordstrom SM, Tran JL, Balázs M, Caplazi P, Febbraio M, Applegate MA, Wagner K-U, Weiss EJ. Abrogation of growth hormone secretion rescues fatty liver in mice with hepatocyte-specific deletion of JAK2. J Clin Investig. 2011;121(4):1412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi SY, Martin RG, Duncan RE, Choi D, Lu S-Y, Schroer SA, Cai EP, Luk CT, Hopperton KE, Domenichiello AF. Hepatocyte-specific deletion of Janus kinase 2 (JAK2) protects against diet-induced steatohepatitis and glucose intolerance. J Biol Chem. 2012;287(13):10277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S, Wang G, Zhang M, Zhuang L, Wan X, Xu J, Wang L, Zhu X, Gao P, Xi Q. The dipeptide Pro-Asp promotes IGF-1 secretion and expression in hepatocytes by enhancing JAK2/STAT5 signaling pathway. Mol Cell Endocrinol. 2016;436:204–10. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Kim MS, Jia B, Yan J, Zuniga-Hertz JP, Han C, Cai D. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature. 2017;548(7665):52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guay C, Menoud V, Rome S, Regazzi R. Horizontal transfer of exosomal microRNAs transduce apoptotic signals between pancreatic beta-cells. Cell Commun Signal. 2015;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Figliolini F, Cantaluppi V, De Lena M, Beltramo S, Romagnoli R, Salizzoni M, Melzi R, Nano R, Piemonti L, Tetta C, et al. Isolation, characterization and potential role in beta cell-endothelium cross-talk of extracellular vesicles released from human pancreatic islets. PLoS ONE. 2014;9(7): e102521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.da Silveira JC, Veeramachaneni DN, Winger QA, Carnevale EM, Bouma GJ. Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle. Biol Reprod. 2012;86(3):71. [DOI] [PubMed] [Google Scholar]
- 61.Machtinger R, Laurent LC, Baccarelli AA. Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum Reprod Update. 2016;22(2):182–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo H, Chang Z, Zhang Z, Zhao Y, Jiang X, Yu H, Zhang Y, Zhao R, He B. Extracellular ATPs produced in seminal plasma exosomes regulate boar sperm motility and mitochondrial metabolism. Theriogenology. 2019;139:113–20. [DOI] [PubMed] [Google Scholar]
- 63.Crewe C, Joffin N, Rutkowski JM, Kim M, Zhang F, Towler DA, Gordillo R, Scherer PE. An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell. 2018;175(3):695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao M, Isaac R, Yan W, Ruan X, Jiang L, Wan Y, Wang J, Wang E, Caron C, Neben S. Cancer-cell-secreted extracellular vesicles suppress insulin secretion through miR-122 to impair systemic glucose homeostasis and contribute to tumour growth. Nat Cell Biol. 2022;24(6):954–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qian B, Yang Y, Tang N, Wang J, Sun P, Yang N, Chen F, Wu T, Sun T, Li Y. M1 macrophage-derived exosomes impair beta cell insulin secretion via miR-212-5p by targeting SIRT2 and inhibiting Akt/GSK-3β/β-catenin pathway in mice. Diabetologia. 2021;64(9):2037–51. [DOI] [PubMed] [Google Scholar]
- 66.Shao L-t. PTH (1–34) enhances the therapeutic effect of bone marrow mesenchymal stem cell-derived exosomes by inhibiting proinflammatory cytokines expression on OA chondrocyte repair in vitro. Arthritis Res Ther. 2022;24(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun C-K, Chen C-H, Chang C-L, Chiang H-J, Sung P-H, Chen K-H, Chen Y-L, Chen S-Y, Kao G-S, Chang H-W. Melatonin treatment enhances therapeutic effects of exosomes against acute liver ischemia-reperfusion injury. Am J Transl Res. 2017;9(4):1543. [PMC free article] [PubMed] [Google Scholar]
- 68.Alzahrani FA. Melatonin improves therapeutic potential of mesenchymal stem cells-derived exosomes against renal ischemia-reperfusion injury in rats. Am J Transl Res. 2019;11(5):2887. [PMC free article] [PubMed] [Google Scholar]
- 69.Xu F, Zhong JY, Lin X, Shan SK, Guo B, Zheng MH, Wang Y, Li F, Cui RR, Wu F. Melatonin alleviates vascular calcification and ageing through exosomal miR-204/miR-211 cluster in a paracrine manner. J Pineal Res. 2020;68(3): e12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ozansoy M, Ozansoy MB, Yulug B, Cankaya S, Kilic E, Goktekin S, Kilic U. Melatonin affects the release of exosomes and tau-content in in vitro amyloid-beta toxicity model. J Clin Neurosci. 2020;73:237–44. [DOI] [PubMed] [Google Scholar]
- 71.Pournaghi M, Khodavirdilou R, Saadatlou MAE, Nasimi FS, Yousefi S, Mobarak H, Darabi M, Shahnazi V, Rahbarghazi R, Mahdipour M. Effect of melatonin on exosomal dynamics in bovine cumulus cells. Process Biochem. 2021;106:78–87. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.