Abstract
The human embryonic kidney (HEK293) cell line, commonly used for recombinant adenovirus (Ad) propagation, does not express the Ad coreceptor αvβ3 or αvβ5 integrins, yet these cells are efficiently infected by Ad vectors. Here we demonstrate that Ad binds to HEK293 cells via the fiber receptor CAR and is subsequently internalized via interaction with integrin αvβ1. Function-blocking antibodies directed against αv or β1, but not β3, β5, or α5, integrin subunits block Ad infection and viral endocytosis. Therefore, αvβ1 serves as a coreceptor for Ad infection, and the lack of β3 and/or β5 but the relatively high expression of αvβ1 integrins on certain tumor cell types may explain why these cells are readily transduced by Ad vectors.
Adenovirus (Ad) host cell entry requires initial attachment to cells mediated by the fiber interaction with its cellular receptor CAR. The subsequent association of penton base with αvβ3 or αvβ5 integrins promotes Ad entry (1, 21, 23). Integrins are heterodimeric receptors for extracellular matrix proteins and cell surface counterreceptors. αv integrins (αvβ1, αvβ3, αvβ5, αvβ6, and αvβ8) mediate cell adhesion to various matrix proteins, including fibronectin, vitronectin, and fibrinogen, that contain an arginine-glycine-aspartic acid (RGD) sequence (8, 19, 24). The interaction of the Ad penton base and αv integrin triggers the activation of several signaling molecules (14) that promote actin cytoskeletal reorganization and enhance Ad internalization. Although HEK293 cells have been widely used for propagation of recombinant Ad vectors, the integrin repertoire of these cells has not been clearly established. For example, several reports indicate that HEK293 cells do not express αvβ3 and αvβ5 integrins (10, 18), while another report indicates that they do (6). Furthermore, β5 integrin-deficient mouse fibroblast cells support Ad infection, suggesting that other integrins play a role in Ad infection (11). HEK293 cells were also reported to express α5β1 and αvβ1 integrins (2), which have been identified as RGD-dependent receptors for both fibronectin and vitronectin (2, 15, 22). Considering the fact that soluble fibronectin or RGD-containing peptide reduced Ad infection (23) and that an α5β1-activating antibody has been reported to enhance Ad-mediated gene delivery to certain melanoma cells (4), it was of interest to determine whether Ad uses either αvβ1 and/or α5β1 integrins as alternative receptors for virus internalization.
The role of CAR and αv integrins in Ad infection of HEK293 cells.
To investigate whether CAR or integrins promote Ad attachment, we preincubated HEK293 cells with an excess of recombinant Ad type 2 (Ad2) fiber protein or with anti-CAR monoclonal antibody (MAb ) (RmcB), with a function-blocking Fab fragment of a penton base MAb (DAV-1) (20), or with function-blocking αvβ5 (P1F6), α5 (P1D6), or nonfunction-blocking αv (LM142) control MAb prior to measuring the specific binding of 125I-labeled Ad5 particles (Fig. 1A) as previously described (13). Pretreatment of cells with recombinant Ad fiber protein completely blocked 125I-Ad5 binding to HEK293 cells, and the RmcB antibody also partially blocked binding. These findings indicated that Ad binding to HEK293 cells is likely mediated by fiber association with CAR.
FIG. 1.
Adenovirus infection of HEK293 cells is mediated by fiber and penton base proteins. (A) Fiber protein mediates Ad binding to HEK293 cells. HEK293 cells (1.5 × 106 cells/sample, in duplicate) in suspension were preincubated with recombinant Ad2 fiber, penton base (10 μg/ml), the anti-CAR antibody (RmcB), anti-penton base antibody (DAV-1), or anti-integrin antibodies at 20 μg/ml at 4°C for 60 min. Specific Ad binding to cells was determined by using 125I-labeled Ad2 as previously described (13). (B) Penton base interaction with αv integrins promotes Ad-mediated gene delivery. HEK293 cells (106 cells/sample) were preincubated with fiber protein, anti-CAR, anti-penton base, or anti-integrin antibodies (20 μg/ml) as described above, followed by incubation with Ad.CMV.LacZ at an MOI of 1 particle/cell at 4°C for another 30 min. The cells were then warmed to 37°C for 15 min. Noninternalized virus was removed by trypsinization. Ad-mediated gene delivery was determined at 20 h postinfection by expression of β-galactosidase. Representative data from two independent experiments were plotted as the mean ± standard deviation.
Experiments were next performed to determine if CAR and/or integrins regulate Ad-mediated gene delivery (Fig. 1B). Cells were infected with Ad5.CMV.LacZ at a multiplicity of infection (MOI) of 1 and then assayed for β-galactosidase expression at 20 h postinfection. As expected from the binding studies, soluble fiber protein and anti-CAR antibody significantly inhibited Ad infection. Furthermore, anti-penton base antibody (DAV-1) and the αv integrin function-blocking antibody (L230) inhibited Ad-mediated gene delivery by approximately 40%. In contrast, the function-blocking αvβ3 (LM609) and αvβ5 (P1F6) integrin antibodies failed to significantly inhibit infection. These results suggested that other members of the αv integrin family, such as αvβ1, may facilitate Ad infection of HEK293 cells.
HEK293 cells express αvβ1 integrins.
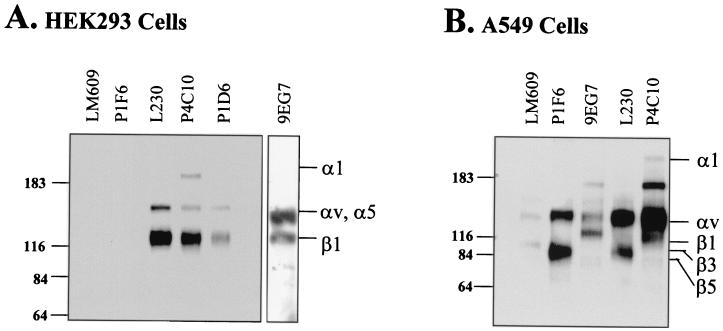
Since conflicting results have been reported with regard to αv integrin expression on HEK293 cells (6, 10, 18), we next sought to determine the repertoire of different αv integrins expressed on HEK293 cells using flow cytometry. These studies demonstrated that HEK293 cells expressed significant levels of αv, α5, and β1 integrins (Fig. 2). In contrast, αvβ3 and αvβ5 integrin expression was undetectable on HEK293 cells, whereas these αv integrins were expressed on A549 cells. To verify integrin expression, cell surface proteins of HEK293 cells were biotinylated and then solubilized with 1% Nonidet P-40. Cell lysates were then immunoprecipitated with anti-αv, anti-α5, or anti-β1 or with anti-αvβ1 integrin antibodies and then analyzed on a 6% sodium dodecyl sulfate-polyacrylamide gel under reducing conditions, followed by immunoblotting with a horseradish peroxidase-conjugated antibiotin antibody (Sigma, St. Louis, Mo.). As shown in Fig. 3A, the anti-αv antibody (L230) immunoprecipitated two proteins of approximately 155 and 121 kDa. In contrast, the anti-αvβ5 (P1F6) and anti-αvβ3 (LM609) antibodies failed to immunoprecipitate these integrins, although both antibodies immunoprecipitated the corresponding α and β chains from A549 cells (Fig. 3B). The 155- and 121-kDa as well as a 200-kDa protein were also recognized by a pan-specific β1 antibody (P4C10). The 200-kDa protein is likely the α1 integrin subunit, which is known to form a heterodimer with the β1 integrin subunit. An α5-specific antibody (P1D6) also immunoprecipitated a 155- and a 121-kDa protein. Integrin α5 also associates with the β1 subunit and exhibits mobility similar to that of the αv integrin subunit. These findings indicated that β1 integrins form heterodimeric receptors with αv and α5 but not with β3 and β5 subunits on HEK293 cells. Moreover, these findings indicated that αvβ1 is the major αv integrin on HEK293 cells. To verify this, we performed immunoprecipitation detection experiments with an αvβ1-specific antibody (9EG7). As expected, this antibody also immunoprecipitated 155- and 121-kDa proteins on HEK293 cells.
FIG. 2.
Flow cytometric analysis of integrin expression on HEK293 cells. HEK293 or A549 cells were incubated with 20 μg of anti-integrin antibodies/ml at 4°C for 60 min. Cell surface-bound antibody was detected by incubation with an R-phycoerythrin-labeled goat anti-mouse secondary antibody. Dashed line, secondary antibody control; solid line, indicated integrin antibodies.
FIG. 3.
Immunoprecipitation analyses of integrin expression on HEK293 cells. HEK293 (A) and A549 (B) cells (107 cells) were detached with 1 mM EDTA and then biotinylated with 1 mg of sulfo-N-hydroxysuccinimide-biotin. After being washed with phosphate-buffered saline four times, labeled cells were then lysed in 1 ml of lysis buffer containing 1% Nonidet P-40 and protease inhibitors. Lysates from biotinylated cells (equal to 106 cells) were immunoprecipitated with 2 μg of anti-integrin antibodies followed by capture with protein A/G beads. The immunoprecipitates were then separated on 6% sodium dodecyl sulfate gels under reducing conditions. After transfer to a polyvinylidene difluoride membrane, the filter was probed with a horseradish peroxidase-conjugated anti-biotin antibody.
αvβ1 integrins promote Ad infection of HEK293 cells.
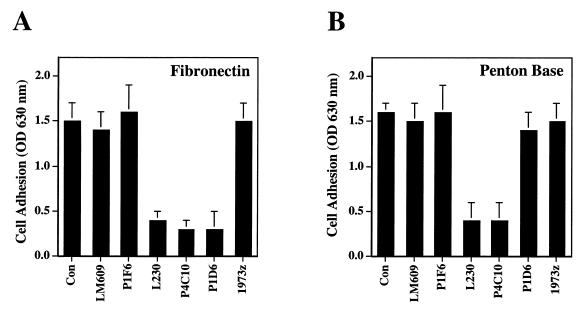
We next analyzed whether the αvβ1 integrins expressed on HEK293 cells were capable of interacting with Ad by performing cell adhesion assays in the presence or absence of function-blocking integrin antibodies. HEK293 cells adhered to fibronectin, and adhesion could be inhibited with a function-blocking anti-α5 (P1D6), anti-αv (L230), or anti-β1 (P4C10) MAb (Fig. 4). HEK293 cells also adhered to penton base; however, adhesion to penton base could only be inhibited by anti-αv or the anti-β1 antibodies. This finding indicated that integrin αvβ1 but not α5β1 serves as a receptor for Ad penton base.
FIG. 4.
Integrin-dependent cell adhesion to fibronectin (A) or penton base (B). Non-tissue culture-treated cluster plates were precoated with 10 μg of recombinant penton base or fibronectin/ml. HEK293 cells were preincubated with 20 μg of anti-integrin antibodies/ml before the cells were allowed to attach to coated plates for 15 min at 37°C. Attached cells were quantitated after fixation and stained with crystal violet. Data are presented as the mean ± standard deviation of duplicate samples. Antibodies: LM609, anti-αvβ3; P1F6, anti-αvβ5; L230, anti-αv; P4C10, anti-β1; P1D6, anti-α5; 1973z, anti-α1.
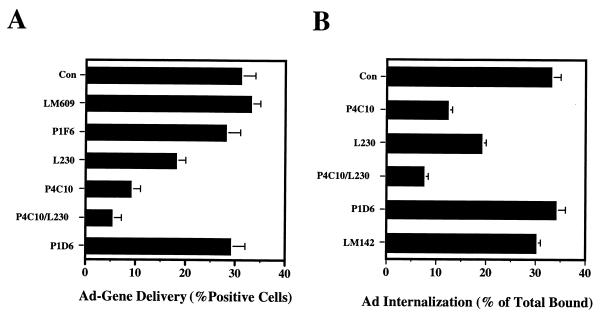
To determine whether Ad uses αvβ1 integrin for infection, we treated HEK293 cells with anti-αv integrin antibody (L230), anti-α5 antibody (P1D6), or anti-β1 integrin antibody (P4C10) and then measured Ad-mediated gene delivery to these cells (Fig. 5A). Pretreatment of HEK293 cells with anti-αv, anti-β1, or a combination of anti-αv and anti-β1 antibodies significantly blocked Ad infection. In contrast, the anti-α5 antibody alone inhibited Ad infection by less than 10%. Pretreatment with a combination of anti-β1 and anti-α5 antibodies did not further decrease gene delivery relative to that achieved with anti-β1 alone (data not shown). These data indicated that αvβ1 integrin also facilitates Ad infection of HEK293 cells.
FIG. 5.
Integrin αvβ1 promotes Ad-mediated gene delivery and virus internalization. (A) HEK293 cells in suspension were preincubated with anti-integrin antibodies for 60 min at 4°C, followed by incubation with Ad.CMV.LacZ at an MOI of 1 viral particle/cell. After being warmed to 37°C for 15 min, noninternalized virus was removed by trypsinization. Cells were plated in 6-well plates, and β-galactosidase expression was determined at 20 h postinfection (mean ± standard deviation). (B) HEK293 cells were pretreated with 20 μg of anti-integrin antibodies/ml at 4°C for 60 min, followed by the addition of 125I-labeled Ad2 (2 × 105 cpm/cell). Bound virus particles were then allowed to internalize by warming the cells at 37°C for 15 min. Internalized virions were determined by measuring their resistance to trypsinization. The data represent the percentage of trysin-resistant cpm/total cpm of specifically bound Ad ± standard deviation of duplicate samples.
Further studies investigated whether penton base interaction with integrin αvβ1 promotes Ad internalization. Cells were preincubated with function-blocking anti-integrin antibodies prior to the addition of 125I-labeled Ad2 (Fig. 5B). Virus uptake was then assayed by measuring the resistance of Ad particles to trypsin treatment. Pretreatment with anti-β1 or anti-αv antibody significantly inhibited Ad internalization by 63 and 42%, respectively, compared to that of untreated control cells. The combination of anti-αv and anti-β1 antibodies inhibited Ad internalization by 76%. In contrast, the anti-α5 or LM609 antibody (αvβ3) had little if any effect on virus entry. These studies demonstrated that αvβ1 integrin promotes Ad infection by enhancing virus internalization.
Here we demonstrated that HEK293 cells use αvβ1 integrin, instead of αvβ3 or αvβ5 integrin, for virus internalization and infection. The ability of αvβ1 to promote Ad infection may also explain why mice genetically deficient in β5 integrin are susceptible to Ad infection (11). It will be of interest to determine whether other cell types that express αvβ1 or perhaps other β1 integrins (3, 12) are also susceptible to Ad infection. In this regard, human melanoma, breast cancer, and neuroblastoma cells have been shown to express integrin αvβ1 (5, 7,9, 16). Integrin αvβ1 may also regulate the migration of human oligodendritic cells in the central nervous system (17).
Acknowledgments
We are extremely grateful to David Schlaepfer and Richard Klemke (The Scripps Research Institute) for valuable discussions and advice. We thank Joan Gausepohl and Kelly White for preparation of the manuscript.
This work was supported by the NIH (grants HL54352 and EY11431).
Footnotes
This is manuscript no. 13843-IMM from the Department of Immunology.
REFERENCES
- 1.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 2.Bodary S C, McLean J W. The integrin β1 subunit associates with the vitronectin receptor αv subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J Biol Chem. 1990;265:5938–5941. [PubMed] [Google Scholar]
- 3.Croyle A M, Janich W E, Roessler B J, Amidon G L. Role of integrin expression in adenovirus-mediated gene delivery to the intestinal epithelium. Hum Gene Ther. 1998;9:561–573. doi: 10.1089/hum.1998.9.4-561. [DOI] [PubMed] [Google Scholar]
- 4.Davison E, Diaz R M, Hart I R, Santis G, Marshall J F. Integrin α5β1-mediated adenovirus infection is enhanced by the integrin-activating antibody TS2/16. J Virol. 1997;71:6204–6207. doi: 10.1128/jvi.71.8.6204-6207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dedhar S, Gray V. Isolation of a novel integrin receptor mediating Arg-Gly-Asy-directed cell adhesion to fibronectin and type I collagen from human neuroblastoma cells. J Cell Biol. 1990;110:2185–2193. doi: 10.1083/jcb.110.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dmitriev I, Krasnykh V, Miller C R, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel D T. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedlander D R, Zagzag D, Shiff B, Cohen H, Allen J C, Kelly P J, Grumet M. Migration of brain tumor cells on extracellular matrix proteins in vitro correlates with tumor type and grade and involves αv and β1 integrins. Cancer Res. 1996;56:1939–1947. [PubMed] [Google Scholar]
- 8.Gailit J, Clark R A. Studies in vitro on the role of αv and β1 integrins in the adhesion of human dermal fibroblasts to provisional matrix proteins fibronectin, vitronectin, and fibrinogen. J Investig Dermatol. 1996;106:24144–24150. doi: 10.1111/1523-1747.ep12328177. [DOI] [PubMed] [Google Scholar]
- 9.Heyman D, Harb J, Ringeard S, Blanchard F, Lassort D, Raher S, Godard A. Upmodulation of αvβ1 integrin expression on human tumor cells by human interleukin for DA cells/leukemia inhibitory factor and oncostatin M: correlation with increased cell adhesion on fibronectin. J Cell Biochem. 1995;58:305–314. doi: 10.1002/jcb.240580305. [DOI] [PubMed] [Google Scholar]
- 10.Hu D D, Lin E, Kovach N L, Hoyer J R, Smith J W. A biochemical characterization of the binding of osteopontin to integrins αvβ1 and αvβ5. J Biol Chem. 1995;270:26232–26238. doi: 10.1074/jbc.270.44.26232. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Griffiths M, Wu J, Farese R V, Jr, Sheppard D. Normal development, wound healing, and adenovirus susceptibility in β5-deficient mice. Mol Cell Biol. 2000;20:755–759. doi: 10.1128/mcb.20.3.755-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasono K, Blackwell J L, Douglas J T, Dmitriev I, Strong T V, Reynolds P, Kropf D A, Carroll W R, Peters G E, Bucy R P, Curiel D T, Krasnykh V. Selective gene delivery to head and neck cancer cells via an integrin targeted adenoviral vector. Clin Cancer Res. 1999;5:2571–2579. [PubMed] [Google Scholar]
- 13.Li E, Stupack D, Cheresh D, Klemke R, Nemerow G. Adenovirus endocytosis via αv integrins requires phosphoinositide-3-OH-kinase. J Virol. 1998;72:2055–2061. doi: 10.1128/jvi.72.3.2055-2061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li E, Stupack D G, Brown S L, Klemke R, Schlaepfer D D, Nemerow G R. Association of p130cas with phosphatidylinositol-3-OH kinase mediates adenovirus cell entry. J Biol Chem. 2000;275:14729–14735. doi: 10.1074/jbc.275.19.14729. [DOI] [PubMed] [Google Scholar]
- 15.Marshall J F, Rutherford D C, McCartney A C, Mitjans F, Goodman S L, Hart I R. αvβ1 is a receptor for vitronectin and fibrinogen, and acts with α5β1 to mediate spreading on fibronectin. J Cell Sci. 1995;108:1227–1238. doi: 10.1242/jcs.108.3.1227. [DOI] [PubMed] [Google Scholar]
- 16.Meyer T, Marshall J F, Hart I R. Expression of αv integrins and vitronectin receptor identity in breast cancer cells. Br J Cancer. 1998;77:530–536. doi: 10.1038/bjc.1998.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milner R, Edwards G, Streuli C, Ffrench-Constant C. A role in migration for the αvβ1 integrin expressed on oligodendrocyte precursors. J Neurosci. 1996;16:7240–7252. doi: 10.1523/JNEUROSCI.16-22-07240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon K O, Nutt E M, Abraham D G, Rodan G A, Duong L T. The αvβ3 integrin regulates α5β1-mediated cell migration toward fibronectin. J Biol Chem. 1997;272:29380–29389. doi: 10.1074/jbc.272.46.29380. [DOI] [PubMed] [Google Scholar]
- 19.Smith J W, Vestal D J, Irwin S V, Burke T A, Cheresh D A. Purification and functional characterization of integrin αvβ5: an adhesion receptor for vitronectin. J Biol Chem. 1990;265:11008–11013. [PubMed] [Google Scholar]
- 20.Stewart P L, Chiu C Y, Huang S, Muir T, Zhao Y, Chait B, Mathias P, Nemerow G R. Cryo-EM visualization of an exposed RGD epitope on adenovirus that escapes antibody neutralization. EMBO J. 1997;16:1189–1198. doi: 10.1093/emboj/16.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomko R P, Xu R, Philipson L. HCAR and MAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogel B E, Tarone G, Giancotti F G, Gailit J, Ruoslahti E. A novel fibronectin receptor with an unexpected subunit composition (αvβ1) J Biol Chem. 1990;265:5934–5937. [PubMed] [Google Scholar]
- 23.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Morla A O, Vuori K, Bauer J S, Tuliano R L, Ruoslahti E. The αvβ1 integrin functions as a fibronectin receptor but does not support fibronectin matrix assembly and cell migration on fibronectin. J Biol Chem. 1993;122:235–242. doi: 10.1083/jcb.122.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]