ABSTRACT
Mammarenaviruses include several highly virulent pathogens (e.g., Lassa virus) capable of causing severe hemorrhagic fever diseases for which there are no approved vaccines and limited treatment options. Mammarenaviruses are enveloped, bi-segmented ambisense RNA viruses. There is limited knowledge about cellular proteins incorporated into progeny virion particles and their potential biological roles in viral infection. Pichinde virus (PICV) is a prototypic arenavirus used to characterize mammarenavirus replication and pathogenesis. We have developed a recombinant PICV with a tri-segmented RNA genome as a viral vector platform. Whether the tri-segmented virion differs from the wild-type bi-segmented one in viral particle morphology and protein composition has not been addressed. In this study, recombinant PICV (rPICV) virions with a bi-segmented (rP18bi) and a tri-segmented (rP18tri) genome were purified by density-gradient ultracentrifugation and analyzed by cryo-electron microscopy and mass spectrometry. Both virion types are pleomorphic with spherical morphology and have no significant difference in size despite rP18tri having denser particles. Both virion types also contain similar sets of cellular proteins. Among the highly enriched virion-associated cellular proteins are components of the endosomal sorting complex required for transport pathway and vesicle trafficking, such as ALIX, Tsg101, VPS, CHMP, and Ras-associated binding proteins, which have known functions in virus assembly and budding. Other enriched cellular proteins include peripheral and transmembrane proteins, chaperone proteins, and ribosomal proteins; their biological roles in viral infection warrant further analysis. Our study provides important insights into mammarenavirus particle formation and aids in the future development of viral vectors and antiviral discovery.
IMPORTANCE
Mammarenaviruses, such as Lassa virus, are enveloped RNA viruses that can cause severe hemorrhagic fever diseases (Lassa fever) with no approved vaccine and limited therapeutic options. Cellular proteins incorporated into progeny virion particles and their biological roles in mammarenavirus infection have not been well characterized. Pichinde virus (PICV) is a prototypic mammarenavirus used as a surrogate model for Lassa fever. We used cryo-electron microscopy and proteomic analysis to characterize the morphology and protein contents of the purified PICV particles that package either two (bi-segmented) or three (tri-segmented) genomic RNA segments. Our results demonstrate a similar virion morphology but different particle density for the bi- and tri-segmented viral particles and reveal major virion-associated cellular proteins. This study provides important insights into the virus-host interactions that can be used for antiviral development and optimizing arenavirus-based vaccine vectors.
KEYWORDS: Pichinde virus, arenavirus, virion structure, virion-associated proteins, cryoelectron microscopy, cryotomography, mass spectrometry
INTRODUCTION
Mammarenaviruses, belonging to the Arenaviridae family, include a group of diverse pathogens that cause various human diseases, including severe hemorrhagic fevers with high mortality rates. Mammarenaviruses are geographically, serologically, and phylogenetically divided into the Old World (OW) and the New World (NW) groups. The OW mammarenaviruses are found in Africa, Asia, and Europe, except for the lymphocytic choriomeningitis virus (LCMV), which is distributed globally. The NW mammarenaviruses are found in North and South America (1). Both the OW and NW groups contain human pathogenic viruses that are capable of causing severe hemorrhagic fever diseases. These include OW viruses like Lassa virus (LASV) and Lujo virus (LUJV), as well as NW viruses like Machupo virus (MACV) and Junin virus (JUNV) (2). The most significant mammarenavirus pathogen, LASV, is estimated to cause about half a million infections and thousands of deaths annually (2). Except for Candid#1, a live-attenuated JUNV vaccine that is licensed for use only in Argentina and is not FDA-approved (3), no vaccines are currently available for other arenavirus pathogens. Ribavirin is a non-specific antiviral that can be used to treat arenaviral infections but with limitations (4). Supportive care remains the main treatment for arenaviruses.
The genome of mammarenaviruses is typically bi-segmented and encodes four viral proteins in an ambisense coding strategy. Each RNA segment contains two non-overlapping open reading frames (ORFs) in opposite orientations. The large (L) segment encodes for the zinc-binding matrix protein (Z) and the RNA-dependent RNA polymerase (L). The small (S) segment encodes for the glycoprotein precursor (GPC) and the nucleocapsid protein (NP) (1). Mammarenaviruses start the infection by binding to host cell receptor(s) (5) and entering through receptor-mediated endocytosis (5). Viral glycoprotein (GP) complex mediates receptor binding and membrane fusion. After uncoating, the viral genome is released into the cytoplasm, where viral RNA replication and transcription occur. The viral glycoprotein is translated as a GPC precursor and proteolytically cleaved by cellular proteases into the stable signal peptide (SSP), the receptor-binding GP1 subunit, and the transmembrane GP2 subunit during the conventional intracellular protein trafficking and maturation process (5). The mature GP complex on the plasma membrane consists of homotrimers of SSP/GP1/GP2 (5). Viral genomic RNAs are encapsidated by NP and associated with the viral polymerase L to form the viral ribonucleoprotein (vRNP) complex (1). The Z protein mediates viral assembly and budding at the plasma membrane by interacting with the viral GP complex (6) and vRNPs (7) and recruiting cellular components of the endosomal sorting complex required for transport (ESCRT) pathway (8–10), which promotes membrane bending and abscission to release progeny virion particles from the infected cell (11, 12). Previous electron microscopic studies have detected electron-dense particles in mammarenavirus virions (1), but few studies have examined the virion structures at high resolution or characterized virion components. A proteomic study of live-attenuated JUNV vaccine (Candid #1) virions has identified hundreds of virion-associated human proteins, among which ribosomal proteins, Ras superfamily proteins, and ESCRT proteins are highly represented (13). Whether other mammarenaviruses, such as the prototypic arenavirus Pichinde virus (PICV), share a similar cellular protein composition remains to be examined.
PICV is a nonpathogenic NW mammarenavirus isolated from rice rats in Colombia, South America, and has been used to study different aspects of arenaviral biology and pathogenesis in a surrogate guinea pig model for Lassa fever (14–18). The reverse genetics system to generate recombinant wild-type and mutant PICVs has allowed us to identify viral virulence determinants and novel viral immune evasion mechanisms (19–22). Various recombinant virus systems have been developed for LCMV (23, 24) and other arenavirus pathogens LASV (25), LUJV (26), JUNV (27, 28), MACV (29), and Guanarito virus (GTOV) (30), which have greatly facilitated our understanding of arenavirus biology and virulence and the development of antivirals and vaccines (31). For example, the use of recombinant JUNV vaccine Candid#1 in experimental vaccination led to the identification of the mechanism for viral attenuation (28), which will improve vaccine safety and efficacy. Arenaviruses such as LCMV and PICV have also been developed as viral vectors for delivering antigens and/or therapeutic molecules (32, 33). The bi-segmented RNA genome (L and S segments) was genetically engineered into a tri-segmented genome (L, S1, and S2 segments), of which S1 and S2 can each accommodate an additional ORF (34–37). We have cloned various ORFs representing different antigens into the tri-segmented PICV vector (rP18tri) to generate vaccine candidates, which have been shown to elicit strong and protective immunity against the respective pathogens (38–41). Yet, we do not know how an additional RNA segment might affect viral particle morphology and protein compositions.
In this study, we purified recombinant PICV virions with bi-segmented (rP18bi) and tri-segmented (rP18tri-G to express the enhanced green fluorescent protein [eGFP] reporter gene) genomes by density gradient ultracentrifugation, examined the virion morphology via cryo-electron microscopy (EM), and characterized virion-associated proteins by mass spectrometry (MS). Our results demonstrate that the bi- and tri-segmented PICV virions have different densities but similar morphology, diameter, and protein compositions. Highly enriched virion-associated cellular proteins include components of the biological processes required for mammarenavirus assembly and budding, such as the ESCRT pathway, vesicle trafficking, and Ras superfamily proteins. This study improves our understanding of virus-host interactions in mammarenavirus biology and aids in the future development of mammarenavirus vaccine vectors and antiviral discovery.
MATERIALS AND METHODS
Viruses and cells
Baby hamster kidney fibroblasts (BHK-21; ATCC CCL-10) and Vero C1008 (Vero E6; ATCC CRL-1587) cells were cultured in growth medium (Dulbecco’s Modified Eagle’s Medium, Corning) supplemented with 10% fetal bovine serum (Sigma), L-Glutamine 100× (Genesee Scientific), Penicillin/Streptomycin (Invitrogen-Life Technologies), and non-essential amino acids (Invitrogen). Cells were cultivated at 37°C with 5% CO2. The recombinant bi-segmented PICVs (rP18bi) and the tri-segmented PICV encoding the GFP reporter gene (rP18tri-G) were generated by the reverse genetics system as described before (19) and propagated on BHK-21 cells.
Virus purification and concentration
Eleven 10 cm2 cell culture plates of BHK-21 cells (90% confluence) were infected with rP18bi or rP18tri-G at a multiplicity of infection (MOI) of 0.01 for 48 h at 37°C and 5% CO2. The cellular supernatants were centrifuged at 3,000 rpm for 15 min to pre-clear samples which were filtered through a 0.2 µm sterile cellulose nitrate disposable filter (Thermo Scientific).
The filtered supernatant was transferred into ultracentrifuge tubes (Beckman Coulter) to lay on top of an 8% Optiprep density gradient medium (Sigma) in sodium chloride-Tris-EDTA (STE) buffer (10 mM Tris, 1 mM EDTA, and 100 mM NaCl). The samples were ultracentrifuged at 130,404 relative centrifugal force (RCF) for 2 h at 4°C using a SW28 rotor in an Optima L-90K Ultracentrifuge (Beckman Coulter). The virus pellet was resuspended in STE buffer, laid on top of a discontinuous Optiprep gradient (with a density of 15%, 20%, 30%, and 40%) in an ultracentrifugation tube (Beckman Coulter), and ultracentrifuged with the SW 41Ti rotor at 268,320 RCF for 3 h at 4°C. A tuberculin syringe was used to puncture the side of the ultracentrifuge tube to collect the appropriate band(s) containing the biological samples. Aliquots of virion samples were stored at −80°C for plaque assay and protein and RNA analyses. For cryo-electron microscopy preparation, viruses were inactivated by adding paraformaldehyde (Sigma-Aldrich) to a final 1% concentration and incubated at ambient temperature for 1 h or 15 min at 37°C. Both inactivated and non-inactivated viruses were added to 11 mL of STE buffer and ultracentrifuged for 1.5 h at 229,581 RCF at 4°C using the SW 41Ti rotor. The inactivated virus pellet was resuspended in STE buffer and used immediately for cryo-electron microscopy. Aliquots of virion samples were analyzed by mass spectrometry.
Viral titer quantification and viral RNA reverse transcription-quantitative PCR (RT-qPCR)
Infectious PICV particles in the purified virus preparations were quantified by plaque assay as described (19, 42). To quantify the amount of viral RNAs present in the purified virion preparations, viral RNA was extracted from 20 µL of the purified virion samples using the Quick-RNA viral kit (Zymo Research) according to the manufacturer’s instructions. Quantitative RT-qPCR was conducted using Luna Universal One-Step RT-qPCR kit (New England Biolabs) with primers specific to PICV NP gene sequence (5´CCCGGACAGAGAAATCCTTATG3´ and 5´CTCCCTTGAACTTGAGACCTTG3´), GPC gene sequence (5´TTGGTGATGGCTGTCCGAAG3´ and 5´ACCCAGTCTCACCCATTTGT3´), and L gene sequence (5´GAAGACAGCAGCAGTTCCTAA3´ and 5´GAGAGAACCTCCTTCTCCAAATC3´). The RT-qPCR conditions were 55°C for 10 min, 95°C for 1 min, 40 cycles of 95°C for 10 s and 60°C for 30 s, and one cycle of 60°C for 1 min.
2D cryo-electron microscopy
Lacey carbon-coated 300 mesh grids (Electron Microscopy Sciences) were glow-discharged and loaded onto the Leica GP2 plunger. Samples were applied to the carbon side of the grid. A 1 µL volume of concentrated 10 nm gold fiducials in bovine serum albumin (BSA) (Electron Microscopy Sciences) was applied to the back side of the grid. Grids were blotted from the back side and plunge-frozen in liquid ethane immediately after blotting. Grids were stored in liquid nitrogen until data collection. The grids were imaged using a Tecnai F30 field emission gun transmission electron microscope (FEI Company) operating at 300 kV. The captured images were acquired at a nominal magnification of 39,000× with an electron dose of approximately 35 electrons/Å2, utilizing a Gatan 4k-by-4k CCD camera (Gatan Inc.). These images were subsequently binned by a factor of 2. Defocus values varied within the range of −3 to −7 µm. The 2D cryomicroscopy images were analyzed using ImageJ. The longest axis was measured and recorded as the major axis of the virus particle. The next longest axis perpendicular to the major axis was measured as the minor axis. Virus particle diameter was recorded as the average between both axes (i.e., major and minor axes). Sphericity was recorded as the ratio between the two axes. Statistical analyses were performed in GraphPad Prism 9.5.1 using an unpaired t-test to determine the differences in the viral particle groups.
3D cryotomography
Tilt series acquisition was performed on an FEI Titan Krios G2 (Thermo Fisher Scientific) electron microscope, equipped with a Gatan Biocontinuum K3 Direct Electron Detector and GIF filter, operated at 300 kV. Tilt series were recorded using Thermo Fisher Scientific Tomography 5 software with a pixel size of 1.085 Å. Defocus values ranged from −3 to −6 µm. A 100 µm objective aperture was inserted. A dose-symmetric collection scheme was employed with a collection range of +/−60 degrees at 3-degree increments for a total dose of 130 e/Å2. Movies of 30 frames were divided into six fractions per tilt using correlated double sampling in super-resolution mode. Zero loss imaging was used for all tilt series with a slit width of 20 eV. A total of 16 tilt series were collected. Gain and dark differences were applied during acquisition. Frames for each tilt angle in a tilt series were drift-corrected using MotionCor2 version 1.5.0 (43). Tilt series were aligned using AreTomo version 1.3.4 (44) fiducial-less patch tracking, and subsequent tomograms were generated via simultaneous algebraic reconstruction technique (SART). For tomogram visualization, nonlinear anisotropic diffusion (NAD) filtering was applied using IMOD version 4.11.21 (45) with a K value of 5 over 10 iterations.
Mass spectrometry
The protein concentration of the purified virions was analyzed via the Pierce BCA Protein Assay kit (Thermo Scientific) with STE as the diluent. Purified virus was lysed in a 1:1 ratio of virions to lysis buffer [7 M urea, 2 M thiourea, 0.4 M Tris pH 8, 20% acetonitrile, 10 mM tris(2-carboxyethyl)phosphine (TCEP), 40 mM chloroacetamide] and submitted to the University of Minnesota Center for Metabolomics and Proteomics (CMSP) facility for trypsin enzymatic digestion, peptide extraction, and MS analysis using their standard operating procedures.
The Reference Sequence protein database for Mesocricetus auratus (because the rP18bi and rP18tri-G viruses were being propagated in Baby hamster’s kidney BHK-21 cells) was downloaded from the National Center for Biotechnology Information on 19 September 2023 and merged with a common lab contaminant protein database (46), and four Pichinde virus proteins: L (GenBank no. ABU39911), Z (GenBank no. ABU39906), NP (GenBank no. ABU39909), and GPC (GenBank no. ABU39904). A total of 53,451 protein sequences were included in the analysis. Peptide tandem MS was processed using SEQUEST (Thermo Scientific) in Proteome Discoverer 3.0. The precursor mass recalibration node was applied with a precursor mass tolerance of 20 ppm and product ion tolerance of 0.8 Da with fixed carbamidomethyl (CAM) modification of cysteine 57.0215 m/z. The SEQUEST (47) database search parameters were enzyme trypsin full specificity, two missed cleave sites, and precursor tolerance 15 ppm, fragment ion tolerance 0.6 Da. CAM cysteine (+57.021 Da) was specified as a fixed modification. The dynamic modifications were acetylation of protein N-terminus (+42.011 Da), oxidation of methionine (+15.995 Da), conversion of glutamine to pyroglutamic acid (−17.027 Da), methionine loss at the protein N-terminus (−131.040 Da), methionine loss + acetylation at the protein N-terminus (−89.030 Da), and deamidation of asparagine or glutamine (+0.984 Da). A 1% protein and peptide false discovery rate and two peptide minimum were applied as filters on the protein report using the Percolator algorithm (48) in Proteome Discoverer 3.0.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Purified viruses were lysed with complete radioimmunoprecipitation assay (RIPA) buffer [phosphate-buffered saline (PBS) 1× (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4), 50 mM HEPES, 150 mM NaCl, 1% NP40 alternative, 0.5% sodium deoxycholate] with protease phosphatase inhibitors (10 µg/mL phenylmethylsulfonyl fluoride, 30 µL/mL aprotinin, 10 mM sodium orthovanadate, 10 µg/mL pepstatin, and 10 µg/mL leupeptin). Both cell lysates and purified viruses were boiled for 5 min in 1× SDS-PAGE loading buffer [300 mM Tris-Cl (pH 6.8), 600 mM β-mercaptoethanol, 12% sodium dodecyl sulfate, 60% glycerol, and 0.6% bromophenol blue] and resolved in a NuPAGE 4 to 12% gradient gel (Invitrogen) or 6% polyacrylamide gel. Silver staining was performed using Pierce Silver Stain for Mass Spectrometry kit (Thermo Scientific) according to the manufacturer’s procedures. Protein bands of interest were cut out of the gel and submitted to the University of Minnesota (UMN) CMSP facility for protein identification.
Western blot analysis of virus-infected cells and virion particles
Purified virion particles and virus-infected cells were lysed in a complete RIPA buffer and analyzed in a NuPAGE 4 to 12% gradient gel (Invitrogen) as described above. Proteins were transferred to a 0.45 µm nitrocellulose membrane (Bio-Rad) via the semi-dry transfer Trans-Blot Turbo system (Bio-Rad) per the manufacturer’s instructions. The membrane was blocked with 5% dry skim milk and 5% normal goat serum in Tris-buffered saline with 0.1% Tween 20 (VWR Scientific) for an hour at ambient temperature. The membrane was probed with guinea pig anti-PICV antiserum (1:500), rabbit anti-NP antiserum (1:5,000), rabbit anti-PDCD6IP (VWR, 12422-1-AP; 1:500), or mouse anti-β-actin (Sigma-Aldrich, A5441; 1:5,000) overnight at 4°C. The membrane was washed and then probed with horseradish peroxidase (HRP)-conjugated goat anti-guinea pig secondary antibody (Sigma, AP108P; 1:5,000), HRP-conjugated goat anti-rabbit secondary antibody (Thermo Scientific, 31460; 1:5,000), or HRP-conjugated goat anti-mouse secondary antibody (VWR, 102646-160; 1:5,000) at room temperature for an hour. After washing, the membrane was applied with SuperSignal West Pico Chemiluminescent substrate (Thermo Scientific) for imaging on an iBright FL1500 system (Thermo Fisher Scientific).
RESULTS AND DISCUSSION
Purification of bi-segmented and tri-segmented PICV particles
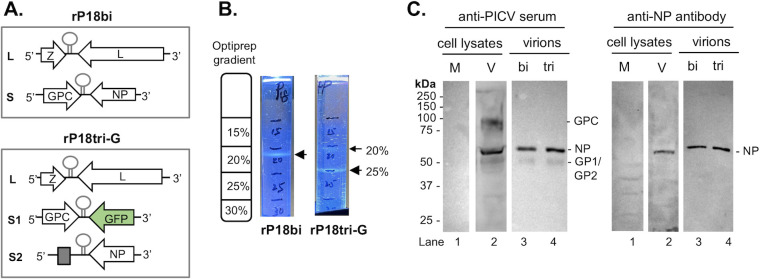
Wild-type mammarenaviruses have a bi-segmented RNA genome, while recombinant viruses that have been developed as viral vectors for vaccines and immunotherapy, such as LCMV and PICV vector systems, have tri-segmented genomes (35, 39). To determine whether mammarenaviruses with bi- or tri-segmented genomes differ in size and protein composition, we aimed to characterize rP18bi (bi-segmented PICV) and rP18tri-G (tri-segmented PICV) (Fig. 1A) virion particles through cryo-electron microscopic and proteomic analyses. We cultivated the respective viruses in BHK-21 cells and purified them through density gradient ultracentrifugation. The rP18bi virions formed a single band at the 20% density gradient, while the rP18tri-G virions showed a major band between the 20% and 25% fractions (labeled as the 25% band) and a relatively minor band between the 15% and 20% fractions (labeled as the 20% band) (Fig. 1B). Thus, the majority of the rP18tri-G virions have a buoyant density that is greater than that of the rP18bi virions. Each virion preparation was performed independently at least three times, for which consistent patterns of virion bands were observed in the density gradient. The overwhelming majority of particles in the purified bands are viral particles with only a few vesicles visible by EM (Fig. S1), supporting the purity of the virus preparations. We also confirmed that these virus preparations contained infectious viruses by plaque assaying. Western blot analysis using anti-PICV serum and anti-NP antibody detected the presence of viral NP and GP1/GP2 proteins in both the rP18bi and rP18tri-G virions (Fig. 1C). However, the GPC precursor protein was detected only in virus-infected cells, consistent with previous publications showing that the unprocessed GPC precursor is largely excluded from the released virion particles, which preferentially incorporated the GP1/GP2 cleaved glycoproteins (49, 50).
Fig 1.
Purification of bi-segmented and tri-segmented recombinant rPICV virions. (A) Genomic structure of rPICVs with a bi-segmented genome (rP18bi) and a tri-segmented genome (rP18tri-G). The rP18bi genome has L and S RNA segments, each encoding two viral genes in opposite orientations. The rP18tri-G genome consists of L, S1, and S2 segments, of which S1 encodes GPC and GFP, while S2 encodes NP only. (B) Sedimentation patterns of rP18bi and rP18tri-G virions after density gradient ultracentrifugation. The major bands of the rP18bi and rP18tri-G virion particles are shown by large arrows, and a minor band for rP18tri-G is shown by a small arrow. (C) Detection of Pichinde viral proteins in the purified virions and virus-infected cells by Western blot analysis with guinea pig anti-PICV antiserum (left panel) and rabbit anti-NP antibody (right panel). M, mock-infected cells. V, virus-infected cells. The detected viral proteins are shown.
We next quantified the viral RNA level of the purified viral preparations by qRT-PCR with primers specific to L, NP, and GPC genes, respectively (Table 1). The L:NP, L:GPC, and NP:GPC ratios were calculated for each virus preparation, and were used to estimate the L:S or L:S1:S2 ratio. The rP18bi virion preparation has an L:S ratio of ~1:2, which is consistent with prior knowledge of arenavirus virions (1). The rP18tri-G 20% viral preparation (minor) has more L segments than S1 (GPC) and S2 (NP) segments, with an L:S1:S2 ratio of ~2:1:1, indicating that a majority of viral particles in this preparation may contain two segments, L and S1 or L and S2. The rP18tri-G 25% preparation (major) are dense particles that have more S1 than L and S2 segments, with an L:S1:S2 ratio of ~1:2:1, which reflects the prevalence of the S1 segment over L and S2 in this pool of viral particles. This information may help design tri-segmented arenavirus vectors to optimize the delivery of antigens or immune genes.
TABLE 1.
The levels of viral RNAs (L, GPC, and NP) in each viral preparation
| L copy# per mL | GPC copy# per mL | NP copy# per mL | L:GPC ratio | L:NP ratio | GPC:NP ratio | |
|---|---|---|---|---|---|---|
| rP18bi | 2.63 × 1012 | 4.54 × 1012 | 4.36 × 1012 | 0.6 | 0.6 | 1.1 |
| rP18tri-G 20% | 2.44 × 1012 | 1.30 × 1012 | 1.64 × 1012 | 1.9 | 1.5 | 0.8 |
| rP18tri-G 25% | 1.31 × 1012 | 2.30 × 1012 | 1.29 × 1012 | 0.6 | 1.0 | 1.8 |
2D cryo-EM and 3D cryotomographic analyses of purified viral particles
Paraformaldehyde-inactivated PICV particles were subjected to cryo transmission electron microscopic analysis to determine the 2D morphology of the rP18bi and rP18tri-G virion particles. Viral particles from the single rP18bi band and the two bands (20% and 25%) of rP18tri-G were used for the analysis. The rP18bi and the rP18tri-G 25% (major) had average diameters with standard deviations of 77.70 ± 22.82 nm and 75.11 ± 18.18 nm, respectively, and were not statistically different. The rP18tri-G 20% (minor) particles were significantly smaller than the other two fractions, with average particle diameter and standard deviation of 67.19 ± 17.83 nm (Fig. 2A). The average sphericity values and standard deviations for the rP18bi, rP18tri-G (major), and rP18tri-G (minor) preparations are 0.76 ± 0.14, 0.82 ± 0.12, and 0.85 ± 0.12, respectively (Fig. 2B). Despite differences in sphericity, these values were still indicative of round virion particles (Fig. 2C). These results of similar diameter and shape for the PICV virions are consistent with other findings in the tri-segmented LCMV virions (35). Thus, the bi- and tri-segmented PICV virions have different density gradients, but they both are pleomorphic and have spherical or elliptical morphology of similar size.
Fig 2.
EM analysis of rP18bi and rP18tri-G virion particles. Violin plots of rP18bi (n = 386) and rP18tri-G 20% (n = 199) and 25% (n = 342) fractions for particle diameters (A) and particle sphericity (B). Solid lines represent median values and dashed lines represent the 25% and 75% percentiles. Statistical analysis was conducted with GraphPad. (C) Cryo-electron micrographs of representative virion particles. Electron-dense dots are gold beads added to the sample for alignment in tomography. (D) Representative tomographic slices from 3D reconstructed PICV virions depicting putative ribosomal densities (orange arrowhead), Z protein layer (green), envelope glycoproteins (blue), and putative RNPs (pink). The inset was rotated along the x- and y-axis to better illustrate densities. Scale bar = 200 angstroms.
PICV particles were also subjected to 3D cryotomography to reconstruct high-resolution 3D volumes and to analyze the structures inside the virions. The tomograms recapitulated the 2D cryo-EM results and showed similar diameters and shapes for the PICV virions (Fig. 2D). Glycoproteins were observed protruding from the viral membrane with distinctive stalk and head regions as previously described for multiple mammarenaviruses (51). A density consistent with the matrix layer formed by the Z protein was observed lining the internal side of the virus membrane. Scattered density representing RNP complexes was detected abundantly within the viral particle, possibly accounting for the characteristic “sandy” appearance of arenavirus virions under EM (1). Electron-dense particles of ribosome size were only present in a few virions at a low number but absent in a majority of PICV virions, suggesting that the incorporation of whole ribosomes, even if they are true ribosomes, may occur rarely.
Proteomic analysis of virion-associated proteins
MS analysis was conducted to determine viral and cellular protein components of both the rP18bi and rP18tri-G virion particles. Two independently purified batches of the rP18bi (Prep A and Prep B) and a batch of the rP18tri-G major fraction (Prep C) were submitted for MS analysis. After removing proteins of lab contaminants (46), a total of 510 proteins, including 4 PICV viral proteins and 506 cellular proteins of hamster origin (the viruses were propagated in hamster BHK-21 cells), were identified from three viral preparations, with peptide-spectrum matches (PSMs) ranging from 1 to 773 (Table S1). The identified proteins have a high level of overlap among the three viral preparations, as shown in a Venn diagram (Fig. 3). Seventy-three percent (374 of 510) of the proteins are common in all three viral preparations (Preps). The level of variation, which is 18% between Preps A and B, 24.5% between Preps A and C, and 12% between Preps B and C, is independent of the virus identity (rP18bi vs rP18tri-G). Together, these results suggest that the bi- and tri-segmented PICV virions have similar composition of cellular proteins.
Fig 3.

A comparison of cellular proteins identified from three virion Preps is shown in the Venn diagram. Prep A and Prep B are two independently purified rP18bi virion preparations. Prep C is the major band of rP18tri-G virions (25% fraction) after density gradient ultracentrifugation.
As expected, all four viral proteins (Z, L, NP, and GPC) were detected in each virion preparation with high PSMs (Table 2), which confirms the identity of these viral preparations as being of the PICV particles. Viral NP protein has the highest PSMs among all identified viral and cellular proteins, consistent with its being the most abundant viral protein in a virion particle. The other three viral proteins, L, GPC, and Z, are also among the top 22 proteins when they were ranked by PSMs.
TABLE 2.
Identification of four PICV viral proteins in the rP18bi (Preps A and B) and rP18tri-G (Prep C) virions by proteomic analysis
| PICV protein | Size (kDa) | Percent coverage | # Unique peptides | Prep A (# PSMs) |
Prep B (# PSMs) |
Prep C (# PSMs) |
|---|---|---|---|---|---|---|
| NP | 62.8 | 47 | 30 | 516 | 773 | 512 |
| L | 251.5 | 20 | 48 | 44 | 62 | 87 |
| GPC | 57.8 | 14 | 9 | 33 | 31 | 33 |
| Z | 10.8 | 39 | 3 | 20 | 33 | 22 |
Virion-associated cellular proteins consist mainly of membrane proteins
As a low PSM value suggests low confidence in peptide identification, we applied a filter of PSMs > 4 to all three virion preparations and identified 131 PICV virion-associated cellular proteins (Table S2), of which 83 were previously identified in the JUNV virion particles (13) and/or LASV VLPs (52) . All 131 cellular proteins, despite being identified from PICVs cultivated in hamster BHK-21 cells, are conserved across animal species, including humans. The conservation suggests that these virion-associated proteins have a general biological significance that can be applied to mammarenaviruses of human and other rodent isolates. The majority of these proteins (69%) are membrane-associated, and nearly half (46.5%) are localized to the plasma membrane (Table 3), consistent with the fact that PICV is an enveloped virus and that it assembles and releases at the plasma membrane (53). Many proteins are involved in intracellular transport and membrane re-organization, such as 30 endosomal proteins and 19 cytoskeleton proteins, which are incorporated into virion particles likely through their interactions with viral structural components during the transport, assembly, and budding of new PICV particles. Thirty-one cellular proteins do not have an obvious association with membranes or cytoskeleton, including ribosomal proteins and histones. These soluble cellular proteins are unlikely to be randomly packaged into virion particles, as the eGFP reporter protein was expressed at a high level in cells infected with the rP18tri-G virus (39), but it was not detected inside the rP18tri-G virion particles.
TABLE 3.
Subcellular location of cellular proteins identified in rPICV virions with PSMs > 4
| Total cellular proteins (PSMs > 4) | 131 |
|---|---|
| Membrane | 91 |
| Cell membrane | 61 |
| Endosomes | 30 |
| Cytoskeleton | 19 |
| Cytoplasm | 29 |
| Nucleus | 2 |
The plasma membrane-localized proteins include cellular receptors, ion channels, transporters, tetraspanins, integrins, enzymes, and signaling molecules (Table 4). Cspg4 and Ptgfrn are transmembrane proteins highly enriched in the virion (PSMs from 49 to 85), but their functional roles in PICV replication are unknown. Interestingly, transferrin receptor 1 (TfR1), a known receptor for New World Clade B mammarenavirus (54), was identified in the PICV virions, but not alpha-dystroglycan, which is a known receptor for Old World and New World Clade C mammarenaviruses (5). PICV is a New World Clade A mammarenavirus whose receptor has not been identified. The significance of TfR1 being packaged into PICV virion particles remains to be characterized.
TABLE 4.
Function groups of the enriched cellular proteins identified in rPICV virions
| Functional groups | Proteins identified in rPICV virions |
|---|---|
| ESCRT pathway | Chmp1b, Chmp2a, Chmp4b, Pdcd6ip (ALIX), Tsg101, Vps37b, Vps4b, Cep55, Ist1 |
| Ras-related proteins | Rab1a, Rab5a, Rab5b, Rab5c, Rab7a, Rab8a, Rab8b, Rab10, Rab11b, Rab13, Rab35, Rac1, Rala, Ralb, Rap1b, Rras2 |
| Membrane re-organization, vesicle transport | Arf3, Ehd1, Ehd2, Ehd3, Ehd4, Ezr, Flot1, Flot2, Msn, Pacsin2, Sdc1, Sdc2, Sdc4, Sdcbp, Sh3gl1, YBX1 |
| Cytoskeleton organization | Cfl1, Ezr, Krt15, Msn, Sdcbp, Tagln2, ThymB4X, Tmsb10 |
| Receptors and signaling molecules | Cd36, Cd44, Cd81, Cd82, Cd151, Epha2, Tfr1, Gnai2, Gnb1(GNB2), Itch, Mxra8, |
| Transporters | Atp1a1, Atp2b1, Cacna2d1, Slc1a4, Slc1a5, Slc3a2, Slc44a2, Slc7a5, Slc16a1, Stom |
| Tetraspanins | Tspan5, Tspan7, Cd81, CLDN3 |
| Other transmembrane proteins | Cspg4, Ptgfrn, Adam10, Cd63, Gja1, Jam3, Kirrel, Tmem2 (Cemip2) |
| Extracellular matrix (ECM) and ECM-binding proteins such as integrins | Anxa2, Anxa6, Cd81, Igsf8, Itga3, Itga5, Itgav, Itgb1-a, Mfge8, Mxra8 |
| Chaperones | Dnaja1, Dnaja2, HSP90AA1, HSP90AB1, HSPA1A, HSPA5, HSPA8, ST13 |
| Protein biosynthesis | Eef2, Eif4a1(ENP3), Pa2g4, PAIP1, PKM, Rack1(TRIM7, Gnb2l1), Rpl11, Rpl13, Rpl13a, Rpl15, Rpl17, Rpl18, Rpl3, Rpl4, RPL6, Rpl7, Rpl7a, Rps16, Rps18, RPS2, Rps27a, Rps3, Rps3a, Rps6, Rps8 |
The ESCRT pathway, membrane trafficking, and cytoskeleton re-organization
Highly enriched in the PICV virions are proteins of the endosomal sorting complexes required for the transport (ESCRT) pathway, which is often hijacked by enveloped RNA viruses to drive virus budding off the cellular membrane (12, 55). We have identified proteins in the ESCRT-I complex (Tsg101, VPS28, VPS37, and MVB12), the ESCRT-III complex (Ist1, CHMP proteins 1 to 7), the ESCRT-associated protein Pdcd6ip (ALIX), the AAA ATPase VPS4, and Cep55 in the PICV virions (Table 4). The most abundant ESCRT pathway protein is Pdcd6ip (ALIX), which has a higher PSM value (≥102) than three of the viral proteins (L, GPC, and Z). Pdcd6ip (ALIX) is also highly enriched in JUNV virion particles (13) and has been shown to allow the incorporation of NP into viral-like particles of Mopeia virus by interacting with both NP and Z (9). These results suggest a major role for Pdcd6ip (ALIX) in mammarenavirus assembly and budding, which needs to be further validated and can be explored as a potential cellular target for antiviral development.
It has previously been shown that the viral matrix protein Z is the major mediator of arenaviral assembly and budding by interacting with viral GPC and vRNP complex (6, 8) and recruiting the cellular ESCRT complex (8). The Z protein uses its C-terminal late (L) domains (PSAPPYEP for PICV) to interact with components of the ESCRT complex to drive membrane re-organization for virus budding (56). The mechanism of the L domains mediating virus budding has been well characterized in retroviruses such as HIV-1 and has been demonstrated for several other enveloped RNA virus families, including mammarenaviruses, paramyxoviruses, and filoviruses (55). We have previously shown that rPICV mutant viruses carrying mutations within the L domain(s) of the Z protein are attenuated (57), suggesting an important role of the cellular ESCRT pathway in PICV replication. The Z protein also uses other amino acid sequences or motifs outside the L domain(s) to facilitate virus budding, although the molecular mechanisms have not been well characterized (58, 59). After the early formation of the ESCRT complex, Cep55 and Ist1 (60, 61), which function to promote abscission at the end of cytokinesis during the normal cell cycling process, are likely recruited by the budding virion to promote its release from the infected cells.
Multiple cellular proteins with known functions in membrane organization (e.g., Ehd proteins, Sdc proteins), endosomal transport (e.g., Msn), intracellular vesicle trafficking (e.g., ARF1, Pacsin2), and cytoskeleton re-organization (e.g., Cfl1, Sdcbp, and ThymB4X) are also enriched in the PICV particles. Proteins of endosomes and cytoplasmic trafficking vesicles are likely involved in the intracellular transport of viral glycoprotein GPC and possibly the membrane-associated viral matrix protein Z (6, 8, 13, 62) to the plasma membrane. Cytoskeleton re-organization proteins, such as actin-binding thymosins, Transgelin-2, cofilin-1, keratin, and ezrin, may be involved in transporting the vRNP complex from the cytoplasmic replication site to the plasma membrane for assembly and budding. Syntenin proteins, such as Sdcbp and syndecan (SDC) proteins (e.g., Sdc1, 2, and 4), are known to mediate exosome biogenesis (63), a similar biological process used by viruses for budding. Finally, many Ras superfamily proteins, including 11 Ras-associated binding (Rab) proteins, which are key regulators of cellular vesicle trafficking (64), are found in the rP18bi and rP18tri virions. In summary, these rPICV virion particles are enriched for cellular proteins that are likely to play important roles in arenavirus assembly and budding, some of which have been demonstrated by previous studies (8, 9, 13) while others will be validated in future studies.
Chaperones
Heat shock proteins (HSPs) are highly enriched in PICV virions. A chaperone protein, HSPA8, was identified with a high PSM value (≥133), second only to the viral NP protein. HSPA8 is a key regulator of protein homeostasis involved in various cellular processes, including autophagy (65, 66). HSPA8 has been shown to interact with various viral proteins or RNAs to facilitate viral replication (67–70). A total of eight chaperone proteins were identified in the PICV virions, including HSPA8, HSPA1A, and HSPA5 from the Hsp70 family, Dnaja1 and Dnaja2 from the Hsp40 family, and HSP90AA1 and HSP90AB1 from the Hsp90 family (Table 4). In fact, HSPs are the most frequently identified protein class in different types of virion particles (52), which include mammarenaviruses PICV (this study) and JUNV (13), filoviruses (Ebola and Marburg), lentiviruses and retroviruses such as HIV-1 and Moloney murine leukemia virus, vesicular stomatitis virus, porcine reproductive and respiratory syndrome virus, and respiratory syncytial virus, as well as DNA viruses such as Kaposi’s sarcoma-associated herpesvirus, and vaccinia virus (52). HSPs play crucial roles in guiding the different conformational states of proteins, such as synthesis, folding, assembly, disassembly, translocation, and degradation, to maintain homeostasis (71). Viruses have been known to exploit the functions of cellular HSPs to facilitate their own lifecycles (71). The functional significance and the molecular mechanism of these chaperones in mammarenavirus replication warrant further characterization.
Ribosomal proteins
We have also found 11 ribosomal large subunit proteins and eight small subunit proteins in the PICV virions, along with several other translational factors such as eukaryotic elongation factor 2 (Eef2), initiation factor 4A-1 (Eif4a1), and polyadenylate-binding protein 1 (PAIP1) (Table 4). These findings are consistent with the previous proteomic studies with LASV virus-like particles (VLPs), JUNV VLPs, and infectious JUNV particles (13, 52). In fact, ribosomal proteins are among the most frequently identified cellular proteins in enveloped RNA virions (52). As obligate intracellular parasites, viruses rely on the host cellular translational machinery, especially the ribosomes and translation factors, to synthesize the viral proteins. Viruses have evolved various mechanisms of exploiting specific ribosomal proteins to compete against host protein biosynthesis in the infected cells (72). It is unknown, however, whether the rPICV virion-associated ribosomal proteins are part of the functional ribosomes that are incorporated into arenavirus virions and what their biological relevance is to viral infection. Our high-resolution 3D cryotomography (Fig. 2D), however, suggests that whole ribosomes in PICV virions are rare. A study to identify functional ribosomes from LASV VLPs by in vitro translation did not generate successful results, implying incomplete or immature ribosomes (52). The biological significance of the ribosomal proteins incorporated into mammarenavirus particles remains to be determined.
Analysis of virion-associated proteins by SDS-PAGE and protein identification
We analyzed protein components of the purified rP18bi and rP18tri-G virions by SDS-PAGE. Both virion samples displayed nearly identical protein patterns by silver staining (Fig. 4A), consistent with their similar protein composition as revealed by the proteomic analysis (Fig. 3). We next excised the discernable bands from each of the gels and submitted 16 samples (E1–E10, E11–E17) from the rP18bi preparation for protein identification by MS (Fig. 4B). Viral proteins L (250 kDa), NP (63 kDa), GPC (65 kDa), GP1/GP2 (40 kDa), and Z (14 kDa) were identified with high PSMs in respective samples with the expected sizes. Though the unprocessed GPC protein was detected in E8 and E9 protein bands, the E13 sample (at 38 kDa) has the highest PSMs across the samples, suggesting that the processed GP1/GP2 represent the major forms of the glycoproteins on the viral particles (5).
Fig 4.
Analysis of virion-associated proteins by SDS-PAGE and protein identification. (A) Purified rP18bi (lane 1) and rP18tri-G (lane 2) virions were analyzed by 4%–12% SDS-PAGE followed by silver staining. The four viral proteins with the expected sizes were shown. (B) The protein bands were excised from the rP18bi protein gel as shown in brackets and arrows, and submitted for MS analysis and protein identification. The major proteins identified in each sample are listed on the right with the viral proteins shown in red.
Most of the bands from the virion particles resolved on SDS-PAGE are of cellular origin (Fig. 4B). A total of 295 cellular proteins were identified from all 16 samples analyzed, of which 178 (60%) were also found in the proteomic analysis of the rPICV virions (Table S1), demonstrating the reproducibility of our results from two different methods. Cellular proteins detected with high PSMs include clathrin heavy chain 1, the major protein of coated pits and vehicles, Cspg4, a type I transmembrane protein located at the apical plasma membrane, PDCD6IP (ALIX) of the ESCRT complex, chaperone proteins such as Hspa8, gap-junction protein Gja1, syntenin-1 (Sdcbp) in membrane trafficking, several ribosomal proteins, surface receptor molecules, and Rab family proteins.
In addition, we performed a Western blot analysis of cell lysates and purified PICV particles using the available viral and cellular protein antibodies (Fig. 5), which produced consistent results as the proteomic analysis. Viral NP, GPC precursor, and the GP1/GP2 cleaved subunits were detected in virus-infected cell lysates, while viral NP and GP1/GP2 were also detected in virion particles. PDCD6IP (ALIX), which was highly enriched in virions by proteomic analysis, was readily detected in both cell lysates and virion particles by Western blotting. In contrast, β-actin, which was not identified in virions by proteomic analysis, was only detected in cell lysates but not in virions by Western blotting.
Fig 5.

Analysis of cell lysates and virion particles by Western blotting. Total cell lysates from BHK-21 cells after mock (M), rP18bi (bi), or rP18tri (tri) infection together with the purified rP18bi and rP18tri virion particles were analyzed by Western blotting with antibodies against Pdcd6ip (ALIX), β-actin, and PICV. The detected proteins are shown.
Taken together, our proteomic analyses of Pichinde virion-associated proteins before and after protein separation by gel electrophoresis identified a comprehensive and reproducible list of both the expected viral proteins and the enriched virion-associated cellular proteins that include the protein components of the cellular ESCRT pathway, ribosomal proteins, chaperones, and Ras-related proteins.
Summary
Our study is the first to provide both morphological and proteomic analyses of infectious arenavirus virion particles with bi- and tri-segmented genomes and has revealed new insights into arenavirus virion formation to facilitate the development of antivirals and arenavirus vectors. We used density gradient ultracentrifugation to prepare highly purified recombinant PICV particles with either a bi-segmented (rP18bi) or a tri-segmented (rP18tri-G) RNA genome. These particles were then used to determine the virion morphology by cryo-EM and 3D cryo-tomography, to characterize the virion-associated protein compositions by mass spectrometry, and to assess viral genomic RNA contents by qRT-PCR. Our results showed a similar size and morphology of PICV virion particles with two or three RNA segments, implying a capacity limit for foreign genes to be encoded on a tri-segmented vector. The majority of tri-segmented PICV virions also package more S1 than L or S2 RNA segment with an L:S1:S2 ratio of ~1:2:1, which will guide the design of arenavirus vectors for an optimized antigen/gene delivery. Lastly, our study revealed a comprehensive list of PICV virion-associated cellular proteins, including those previously identified in JUNV particles such as the ESCRT components involved in arenavirus budding as well as new protein candidates to be investigated in future studies. These virion-enriched cellular proteins provide important insights into the complex interactions between arenavirus and its host cell components during the formation of progeny virion particles that can be exploited as potential new targets for antiviral development.
ACKNOWLEDGMENTS
This research was funded by the National Institutes of Health (NIH) award 1R01AI131586 to H.L. and Y.L. H.M. was supported in part by an NIH pre-doctoral T32 fellowship AI83196 and by the University of Minnesota Doctoral Dissertation Fellowship.
We thank LeeAnn Higgins and Todd Markowski of the Center for Metabolomics and Proteomics (CMSP) at the University of Minnesota for providing services related to proteomics data generation. The Orbitrap Eclipse instrumentation platform used in this work was purchased through the High-end Instrumentation Grant S10OD028717 from the NIH.
Contributor Information
Luiza Mendonça, Email: luiza@umn.edu.
Yuying Liang, Email: liangy@umn.edu.
Rebecca Ellis Dutch, University of Kentucky College of Medicine, Lexington, Kentucky, USA.
DATA AVAILABILITY
The data supporting the findings of this study are available within the article and the supplemental material. Raw data that support the findings of this study are available from the corresponding authors upon reasonable request.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/jvi.00799-24.
Cryo-electron micrograph of the rP18bi virion particles after density gradient ultracentrifugation.
List of virion-associated proteins identified by mass spectrometry.
List of virion-enriched cellular proteins.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Buchmeier MJ, de la Torre JC, Peters CJ. 2020. Arenaviridae: the viruses and their replication, p 784–811. In Knipe DM, Howley PM (ed), Fields virology, 7th ed, Vol. 1. Wolters Kluwer, Philadelphia. [Google Scholar]
- 2. Brisse ME, Ly H. 2019. Hemorrhagic fever-causing arenaviruses: lethal pathogens and potent immune suppressors. Front Immunol 10:372. doi: 10.3389/fimmu.2019.00372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saito T, Reyna RA, Taniguchi S, Littlefield K, Paessler S, Maruyama J. 2023. Vaccine candidates against arenavirus infections. Vaccines (Basel) 11:635. doi: 10.3390/vaccines11030635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCormick JB, King IJ, Webb PA, Scribner CL, Craven RB, Johnson KM, Elliott LH, Belmont-Williams R. 1986. Lassa fever. Effective therapy with ribavirin. N Engl J Med 314:20–26. doi: 10.1056/NEJM198601023140104 [DOI] [PubMed] [Google Scholar]
- 5. Pennington HN, Lee J. 2022. Lassa virus glycoprotein complex review: insights into its unique fusion machinery. Biosci Rep 42:BSR20211930. doi: 10.1042/BSR20211930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Capul AA, Perez M, Burke E, Kunz S, Buchmeier MJ, de la Torre JC. 2007. Arenavirus Z-glycoprotein association requires Z myristoylation but not functional RING or late domains. J Virol 81:9451–9460. doi: 10.1128/JVI.00499-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iwasaki M, de la Torre JC. 2018. A highly conserved leucine in mammarenavirus matrix Z protein is required for Z interaction with the virus L polymerase and Z stability in cells harboring an active viral ribonucleoprotein. J Virol 92:e02256-17. doi: 10.1128/JVI.02256-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perez M, Craven RC, de la Torre JC. 2003. The small RING finger protein Z drives arenavirus budding: Implications for antiviral strategies. Proc Natl Acad Sci USA 100:12978–12983. doi: 10.1073/pnas.2133782100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shtanko O, Watanabe S, Jasenosky LD, Watanabe T, Kawaoka Y. 2011. ALIX/AIP1 is required for NP incorporation into Mopeia virus Z-induced virus-like particles. J Virol 85:3631–3641. doi: 10.1128/JVI.01984-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Casabona JC, Levingston Macleod JM, Loureiro ME, Gomez GA, Lopez N. 2009. The RING domain and the L79 residue of Z protein are involved in both the rescue of nucleocapsids and the incorporation of glycoproteins into infectious chimeric arenavirus-like particles. J Virol 83:7029–7039. doi: 10.1128/JVI.00329-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Strecker T, Eichler R, Meulen J ter, Weissenhorn W, Dieter Klenk H, Garten W, Lenz O. 2003. Lassa virus Z protein is a matrix protein and sufficient for the release of virus-like particles [corrected]. J Virol 77:10700–10705. doi: 10.1128/jvi.77.19.10700-10705.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolff S, Ebihara H, Groseth A. 2013. Arenavirus budding: a common pathway with mechanistic differences. Viruses 5:528–549. doi: 10.3390/v5020528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ziegler CM, Eisenhauer P, Kelly JA, Dang LN, Beganovic V, Bruce EA, King BR, Shirley DJ, Weir ME, Ballif BA, Botten J. 2018. A proteomics survey of Junín virus interactions with human proteins reveals host factors required for arenavirus replication. J Virol 92:e01565-17. doi: 10.1128/JVI.01565-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jahrling PB, Hesse RA, Rhoderick JB, Elwell MA, Moe JB. 1981. Pathogenesis of a Pichinde virus strain adapted to produce lethal infections in guinea pigs. Infect Immun 32:872–880. doi: 10.1128/iai.32.2.872-880.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lan S, Shieh W-J, Huang Q, Zaki SR, Liang Y, Ly H. 2020. Virulent infection of outbred Hartley guinea pigs with recombinant Pichinde virus as a surrogate small animal model for human Lassa fever. Virulence 11:1131–1141. doi: 10.1080/21505594.2020.1809328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shieh W-J, Lan S, Zaki SR, Ly H, Liang Y. 2020. Pichinde virus infection of outbred Hartley guinea pigs as a surrogate animal model for human Lassa fever: histopathological and immunohistochemical analyses. Pathogens 9:579. doi: 10.3390/pathogens9070579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brisse M, Fernández-Alarcón C, Huang Q, Kirk N, Schleiss MR, Liang Y, Ly H. 2022. Hearing loss in outbred Hartley guinea pigs experimentally infected with Pichinde virus as a surrogate model of human mammarenaviral hemorrhagic fevers. Virulence 13:1049–1061. doi: 10.1080/21505594.2022.2087948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liang Y, Lan S, Ly H. 2009. Molecular determinants of Pichinde virus infection of guinea pigs--a small animal model system for arenaviral hemorrhagic fevers. Ann N Y Acad Sci 1171 Suppl 1:E65–E74. doi: 10.1111/j.1749-6632.2009.05051.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lan S, McLay Schelde L, Wang J, Kumar N, Ly H, Liang Y. 2009. Development of infectious clones for virulent and avirulent Pichinde viruses: a model virus to study arenavirus-induced hemorrhagic fevers. J Virol 83:6357–6362. doi: 10.1128/JVI.00019-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang Q, Shao J, Lan S, Zhou Y, Xing J, Dong C, Liang Y, Ly H. 2015. In vitro and in vivo characterizations of pichinde viral nucleoprotein exoribonuclease functions. J Virol 89:6595–6607. doi: 10.1128/JVI.00009-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McLay L, Lan S, Ansari A, Liang Y, Ly H. 2013. Identification of virulence determinants within the L genomic segment of the Pichinde arenavirus. J Virol 87:6635–6643. doi: 10.1128/JVI.00044-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar N, Wang J, Lan S, Danzy S, McLay Schelde L, Seladi-Schulman J, Ly H, Liang Y. 2012. Characterization of virulence-associated determinants in the envelope glycoprotein of Pichinde virus. Virology (Auckl) 433:97–103. doi: 10.1016/j.virol.2012.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sánchez AB, de la Torre JC. 2006. Rescue of the prototypic arenavirus LCMV entirely from plasmid. Virology (Auckl) 350:370–380. doi: 10.1016/j.virol.2006.01.012 [DOI] [PubMed] [Google Scholar]
- 24. Flatz L, Bergthaler A, de la Torre JC, Pinschewer DD. 2006. Recovery of an arenavirus entirely from RNA polymerase I/II-driven cDNA. Proc Natl Acad Sci U S A 103:4663–4668. doi: 10.1073/pnas.0600652103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Albariño CG, Bird BH, Chakrabarti AK, Dodd KA, Erickson BR, Nichol ST. 2011. Efficient rescue of recombinant Lassa virus reveals the influence of S segment noncoding regions on virus replication and virulence. J Virol 85:4020–4024. doi: 10.1128/JVI.02556-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bergeron É, Chakrabarti AK, Bird BH, Dodd KA, McMullan LK, Spiropoulou CF, Nichol ST, Albariño CG. 2012. Reverse genetics recovery of Lujo virus and role of virus RNA secondary structures in efficient virus growth. J Virol 86:10759–10765. doi: 10.1128/JVI.01144-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Albariño CG, Bergeron E, Erickson BR, Khristova ML, Rollin PE, Nichol ST. 2009. Efficient reverse genetics generation of infectious junin viruses differing in glycoprotein processing. J Virol 83:5606–5614. doi: 10.1128/JVI.00276-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seregin AV, Yun NE, Miller M, Aronson J, Smith JK, Walker AG, Smith JN, Huang C, Manning JT, de la Torre JC, Paessler S. 2015. The glycoprotein precursor gene of Junin virus determines the virulence of the Romero strain and the attenuation of the Candid #1 strain in a representative animal model of Argentine hemorrhagic fever. J Virol 89:5949–5956. doi: 10.1128/JVI.00104-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patterson M, Seregin A, Huang C, Kolokoltsova O, Smith J, Miller M, Smith J, Yun N, Poussard A, Grant A, Tigabu B, Walker A, Paessler S. 2014. Rescue of a recombinant Machupo virus from cloned cDNAs and in vivo characterization in interferon (αβ/γ) receptor double knockout mice. J Virol 88:1914–1923. doi: 10.1128/JVI.02925-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taniguchi S, Maruyama J, Saito T, Littlefield K, Reyna RA, Manning JT, Huang C, Saijo M, Paessler S. 2024. Development of reverse genetics system for Guanarito virus: substitution of E1497K in the L protein of Guanarito virus S-26764 strain changes plaque phenotype and growth kinetics. J Virol 98:e0196423. doi: 10.1128/jvi.01964-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martínez-Sobrido L, de la Torre JC. 2016. Reporter-expressing, replicating-competent recombinant arenaviruses. Viruses 8:197. doi: 10.3390/v8070197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vrba SM, Kirk NM, Brisse ME, Liang Y, Ly H. 2020. Development and applications of viral vectored vaccines to combat zoonotic and emerging public health threats. Vaccines (Basel) 8:1–31. doi: 10.3390/vaccines8040680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stachura P, Stencel O, Lu Z, Borkhardt A, Pandyra AA. 2023. Arenaviruses: old viruses present new solutions for cancer therapy. Front Immunol 14:1110522. doi: 10.3389/fimmu.2023.1110522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dhanwani R, Ly H, Liang Y. 2017. Recombinant tri-segmented Pichinde virus as a novel live viral vaccine platform, p 169–179. In Ferran MC, Skuse GR (ed), Recombinant virus vaccines. Springer New York, New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Emonet SF, Garidou L, McGavern DB, de la Torre JC. 2009. Generation of recombinant lymphocytic choriomeningitis viruses with trisegmented genomes stably expressing two additional genes of interest. Proc Natl Acad Sci U S A 106:3473–3478. doi: 10.1073/pnas.0900088106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oestereich L, Wurr S, Becker-Ziaja B, Bockholt S, Pahlmann M, Cadar D, Kümmerer BM, Günther S, Kerber R. 2022. Establishment of recombinant trisegmented Mopeia virus expressing two reporter genes for screening of mammarenavirus inhibitors. Viruses 14:1869. doi: 10.3390/v14091869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zaza AD, Herbreteau CH, Peyrefitte CN. 2018. Description and characterization of a novel live-attenuated tri-segmented Machupo virus in Guinea pigs. Virol J 15:99. doi: 10.1186/s12985-018-1009-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kirk NM, Huang Q, Vrba S, Rahman M, Block AM, Murphy H, White DW, Namugenyi SB, Ly H, Tischler AD, Liang Y. 2023. Recombinant Pichinde viral vector expressing tuberculosis antigens elicits strong T cell responses and protection in mice. Front Immunol 14:1127515. doi: 10.3389/fimmu.2023.1127515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dhanwani R, Zhou Y, Huang Q, Verma V, Dileepan M, Ly H, Liang Y. 2016. A novel live Pichinde virus-based vaccine vector induces enhanced humoral and cellular immunity after a booster dose. J Virol 90:2551–2560. doi: 10.1128/JVI.02705-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ahmed S, Parthasarathy D, Newhall R, Picard T, Aback M, Ratnapriya S, Arndt W, Vega-Rodriguez W, Kirk NM, Liang Y, Herschhorn A. 2023. Enhancing anti-viral neutralization response to immunization with HIV-1 envelope glycoprotein immunogens. NPJ Vaccines 8:181. doi: 10.1038/s41541-023-00774-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kumari S, Chaudhari J, Huang Q, Gauger P, De Almeida MN, Ly H, Liang Y, Vu HLX. 2023. Assessment of immune responses to a trivalent pichinde virus-vectored vaccine expressing hemagglutinin genes from three co-circulating influenza A virus subtypes in pigs. Vaccines (Basel) 11:1806. doi: 10.3390/vaccines11121806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dhanwani R, Huang Q, Lan S, Zhou Y, Shao J, Liang Y, Ly H. 2018. Establishment of bisegmented and trisegmented reverse genetics systems to generate recombinant Pichindé viruses. Methods Mol Biol 1604:247–253. doi: 10.1007/978-1-4939-6981-4_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zheng SQ, Palovcak E, Armache J-P, Verba KA, Cheng Y, Agard DA. 2017. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods 14:331–332. doi: 10.1038/nmeth.4193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zheng S, Wolff G, Greenan G, Chen Z, Faas FGA, Bárcena M, Koster AJ, Cheng Y, Agard DA. 2022. AreTomo: an integrated software package for automated marker-free, motion-corrected cryo-electron tomographic alignment and reconstruction. J Struct Biol X 6:100068. doi: 10.1016/j.yjsbx.2022.100068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kremer JR, Mastronarde DN, McIntosh JR. 1996. Computer visualization of three-dimensional image data using IMOD. J Struct Biol 116:71–76. doi: 10.1006/jsbi.1996.0013 [DOI] [PubMed] [Google Scholar]
- 46. Frankenfield AM, Ni J, Ahmed M, Hao L. 2022. Protein contaminants matter: building universal protein contaminant libraries for DDA and DIA proteomics. J Proteome Res 21:2104–2113. doi: 10.1021/acs.jproteome.2c00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eng JK, McCormack AL, Yates JR. 1994. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom 5:976–989. doi: 10.1016/1044-0305(94)80016-2 [DOI] [PubMed] [Google Scholar]
- 48. Käll L, Canterbury JD, Weston J, Noble WS, MacCoss MJ. 2007. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat Methods 4:923–925. doi: 10.1038/nmeth1113 [DOI] [PubMed] [Google Scholar]
- 49. Wright KE, Spiro RC, Burns JW, Buchmeier MJ. 1990. Post-translational processing of the glycoproteins of lymphocytic choriomeningitis virus. Virology (Auckl) 177:175–183. doi: 10.1016/0042-6822(90)90471-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gorzkiewicz M, Cramer J, Xu HC, Lang PA. 2023. The role of glycosylation patterns of viral glycoproteins and cell entry receptors in arenavirus infection. Biomed Pharmacother 166:115196. doi: 10.1016/j.biopha.2023.115196 [DOI] [PubMed] [Google Scholar]
- 51. Neuman BW, Adair BD, Burns JW, Milligan RA, Buchmeier MJ, Yeager M. 2005. Complementarity in the supramolecular design of arenaviruses and retroviruses revealed by electron cryomicroscopy and image analysis. J Virol 79:3822–3830. doi: 10.1128/JVI.79.6.3822-3830.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gale TV, Horton TM, Hoffmann AR, Branco LM, Garry RF. 2019. Host proteins identified in extracellular viral particles as targets for broad-spectrum antiviral inhibitors. J Proteome Res 18:7–17. doi: 10.1021/acs.jproteome.8b00204 [DOI] [PubMed] [Google Scholar]
- 53. Lehmann-Grube F, Popescu M, Schaefer H, Gschwender HH. 1975. LCM virus infection of cells in vitro. Bull World Health Organ 52:443–456. [PMC free article] [PubMed] [Google Scholar]
- 54. Radoshitzky SR, Abraham J, Spiropoulou CF, Kuhn JH, Nguyen D, Li W, Nagel J, Schmidt PJ, Nunberg JH, Andrews NC, Farzan M, Choe H. 2007. Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature New Biol 446:92–96. doi: 10.1038/nature05539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Welker L, Paillart J-C, Bernacchi S. 2021. Importance of viral late domains in budding and release of enveloped RNA viruses. Viruses 13:1559. doi: 10.3390/v13081559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fehling SK, Lennartz F, Strecker T. 2012. Multifunctional nature of the arenavirus RING finger protein Z. Viruses 4:2973–3011. doi: 10.3390/v4112973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang J, Danzy S, Kumar N, Ly H, Liang Y. 2012. Biological roles and functional mechanisms of arenavirus Z protein in viral replication. J Virol 86:9794–9801. doi: 10.1128/JVI.00385-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ziegler CM, Eisenhauer P, Bruce EA, Beganovic V, King BR, Weir ME, Ballif BA, Botten J. 2016. A novel phosphoserine motif in the LCMV matrix protein Z regulates the release of infectious virus and defective interfering particles. J Gen Virol 97:2084–2089. doi: 10.1099/jgv.0.000550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Urata S, Yasuda J, de la Torre JC. 2009. The Z protein of the new world arenavirus tacaribe virus has bona fide budding activity that does not depend on known late domain motifs. J Virol 83:12651–12655. doi: 10.1128/JVI.01012-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tedeschi A, Almagro J, Renshaw MJ, Messal HA, Behrens A, Petronczki M. 2020. Cep55 promotes cytokinesis of neural progenitors but is dispensable for most mammalian cell divisions. Nat Commun 11:1746. doi: 10.1038/s41467-020-15359-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bajorek M, Morita E, Skalicky JJ, Morham SG, Babst M, Sundquist WI. 2009. Biochemical analyses of human IST1 and its function in cytokinesis. Mol Biol Cell 20:1360–1373. doi: 10.1091/mbc.e08-05-0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Agnihothram SS, Dancho B, Grant KW, Grimes ML, Lyles DS, Nunberg JH. 2009. Assembly of arenavirus envelope glycoprotein GPC in detergent-soluble membrane microdomains. J Virol 83:9890–9900. doi: 10.1128/JVI.00837-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Friand V, David G, Zimmermann P. 2015. Syntenin and syndecan in the biogenesis of exosomes. Biol Cell 107:331–341. doi: 10.1111/boc.201500010 [DOI] [PubMed] [Google Scholar]
- 64. Corbeel L, Freson K. 2008. Rab proteins and Rab-associated proteins: major actors in the mechanism of protein-trafficking disorders. Eur J Pediatr 167:723–729. doi: 10.1007/s00431-008-0740-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stricher F, Macri C, Ruff M, Muller S. 2013. HSPA8/HSC70 chaperone protein: structure, function, and chemical targeting. Autophagy 9:1937–1954. doi: 10.4161/auto.26448 [DOI] [PubMed] [Google Scholar]
- 66. Bonam SR, Ruff M, Muller S. 2019. HSPA8/HSC70 in immune disorders: a molecular rheostat that adjusts chaperone-mediated autophagy substrates. Cells 8:849. doi: 10.3390/cells8080849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sztuba-Solinska J, Diaz L, Kumar MR, Kolb G, Wiley MR, Jozwick L, Kuhn JH, Palacios G, Radoshitzky SR, J. Le Grice SF, Johnson RF. 2016. A small stem-loop structure of the Ebola virus trailer is essential for replication and interacts with heat-shock protein A8. Nucleic Acids Res 44:gkw825. doi: 10.1093/nar/gkw825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang Y, Zhao M, Zhao L, Geng Y, Li G, Chen L, Yu J, Yuan H, Zhang H, Yun H, Yuan Y, Wang G, Feng J, Xu L, Wang S, Hou C, Yang G, Zhang N, Lu W, Zhang X. 2023. HBx-induced HSPA8 stimulates HBV replication and suppresses ferroptosis to support liver cancer progression. Cancer Res 83:1048–1061. doi: 10.1158/0008-5472.CAN-22-3169 [DOI] [PubMed] [Google Scholar]
- 69. Yu D-S, Weng T-H, Hu C-Y, Wu Z-G, Li Y-H, Cheng L-F, Wu N-P, Li L-J, Yao H-P. 2019. Corrigendum: chaperones, membrane trafficking and signal transduction proteins regulate zaire ebola virus trVLPs and interact with trVLP elements. Front Microbiol 10:2975. doi: 10.3389/fmicb.2019.02975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhu P, Lv C, Fang C, Peng X, Sheng H, Xiao P, Kumar Ojha N, Yan Y, Liao M, Zhou J. 2020. Heat shock protein member 8 is an attachment factor for infectious bronchitis virus. Front Microbiol 11:1630. doi: 10.3389/fmicb.2020.01630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Santoro MG, Amici C, Rossi A. 2009. Role of heat shock proteins in viral infection, p 51–84. In Pockley AG, Calderwood SK, Santoro MG (ed), Prokaryotic and eukaryotic heat shock proteins in infectious disease. Springer Netherlands, Dordrecht. [Google Scholar]
- 72. Miller CM, Selvam S, Fuchs G. 2021. Fatal attraction: the roles of ribosomal proteins in the viral life cycle. Wiley Interdiscip Rev RNA 12:e1613. doi: 10.1002/wrna.1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cryo-electron micrograph of the rP18bi virion particles after density gradient ultracentrifugation.
List of virion-associated proteins identified by mass spectrometry.
List of virion-enriched cellular proteins.
Data Availability Statement
The data supporting the findings of this study are available within the article and the supplemental material. Raw data that support the findings of this study are available from the corresponding authors upon reasonable request.