Abstract
Background
Crohn’s disease (CD) is a chronic intestinal inflammatory disorder, the etiology of which remains unknown, and is characterized by symptoms such as chronic abdominal pain, diarrhea, obstruction, and perianal lesions. Histopathology is widely regarded as the preferred method for diagnosing CD, although the histological diagnosis may lack specificity. The identification of granulomas is commonly believed to be the most reliable diagnostic indicator for CD, surpassing all other clinical features in significance. Nevertheless, research indicates that the detection rate of granulomas in CD exhibits considerable variability. Furthermore, granulomas can manifest in various specific infections including tuberculosis and Yersinia, as well as in a range of diseases characterized by macrophage reactions such as sarcoidosis and drug-induced enteritis. Granulomas associated with CD typically do not exhibit necrosis. However, the formation of caseous granulomas may occur as a result of secondary infections related to anti-CD drug treatment or perforation of the intestinal wall.
Case presentation
In this study, we present a case of a 28-year-old female patient diagnosed with CD exhibiting histologic granulomas, including both caseating and non-caseating forms, which demonstrated a positive response to medical treatment.
Conclusion
In clinical practice, various forms of granulomas may indicate diverse underlying diseases, yet lack specificity. It is suggested that the presence of caseous granulomas should not be considered as a definitive exclusion criterion for the diagnosis when clinical, endoscopic, imaging and other histopathological features are consistent with CD. This study is the first report of caseous granulomas in CD without concomitant tuberculosis infection.
Keywords: Crohn’s disease, Caseating granuloma, Tuberculosis, Specific bacterial infections, Case report
Background
Granulomas are defined by the aggregation of epithelioid cells, primarily histiocytes, and may encompass a variety of inflammatory components. The formation of granulomas is a multifaceted process resulting from the interplay between a persistent, non-degradable antigen and the host’s immune system, involving macrophages, Th1 cell response, B cell hyperactivity, circulating immune complex aggregation, and numerous biological mediators [1, 2]. Granulomatous inflammation of the gastrointestinal tract is a tissue reaction pattern that can be attributed to various potential causes, including infections, noninfectious immune reactions, and a limited number of unidentified etiologies. It is possible to find granulomas of varying morphological appearances. In addition to epithelioid and foreign body types, suppurative and necrotizing granulomas may also occur. It’s common to develop various types of intestinal granulomas, which are caused by specific bacterial infections, parasites, Crohn’s disease (CD), drug-induced reactions, gland rupture, sarcoidosis, genetic disorders, diverticulum, vasculitis-related conditions, and certain variant immunodeficiency diseases [3]. It is hypothesized that CD results from a multifaceted interplay among genetic predisposition, environmental triggers, and dysbiosis of the gut microbiota, culminating in aberrant innate and adaptive immune reactions [4]. One distinguishing characteristic of CD in comparison to other inflammatory bowel diseases is the presence of intestinal epithelioid granulomas. While granulomas can aid in the diagnosis of CD, they are neither sensitive nor specific [5]. If CD is present alongside caseous granuloma, it is typically assumed to indicate a co-infection. This case report describes a young female patient exhibiting clinical, radiological, and endoscopic features consistent with CD, along with histomorphological evidence of caseous granulomas in the bowel wall and lymph nodes. Following consultations with various medical facilities and ruling out tuberculosis as a differential diagnosis, the patient was managed clinically as a case of CD. Treatment with ustekinumab, a biological agent, resulted in symptom improvement, healing of the ulcer on endoscopy, and resolution of the anal fistula. A histopathologist has a pivotal role in identifying the aetiology of a disease or providing clinical guidance as to the likely cause. In most cases, a clinicopathological correlation is required in order to make a conclusive diagnosis. What we know about the presence of caseous granulomas in the intestine is frequently associated with tuberculosis. However, in the absence of definitive evidence of tuberculosis, caseous granulomas do not definitively rule out CD. Diagnostic efforts can be aided and complicated by conventional understanding of granulomas.
Case presentation
A 28-year-old female with a previous perianal abscess presented at the hospital due to acute abdominal pain in the right lower quadrant and low-grade fever of unknown etiology three years prior. The patient was admitted for suspected appendicitis at the local medical facility. Following this, an enhanced CT (computed tomography) scan of the abdomen revealed thickening and irregular enhancement of the wall of the proximal ileum, cecum, and adjacent ascending colon, accompanied by a surrounding mass shadow, suggesting a high likelihood of CD. The colonoscopy revealed the presence of a mass located at the terminal portion of the cecum. Subsequent biopsy of the cecum demonstrated chronic enteritis with ulcer and a small noncaseating granuloma within the muscularis mucosa. No special staining was done on the biopsy specimen. The administration of anti-infection and analgesic therapy did not yield improvement. Right hemicolectomy was undertaken due to the ineffectiveness of symptomatic treatment and the potential risk of intestinal wall perforation. Postoperative pathological findings in local hospital indicated that both CD and tuberculosis were potential diagnoses. After the surgery, abdominal pain was relieved.
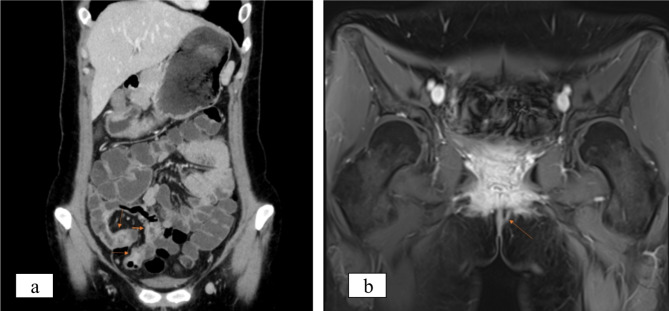
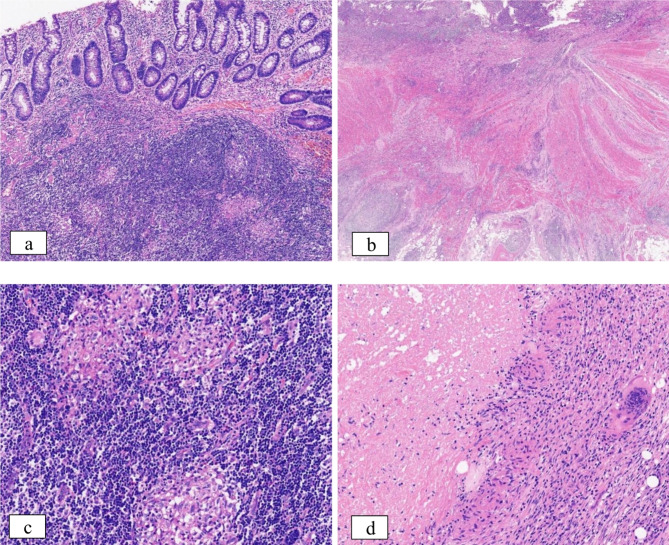
One-year post-surgery, the patient experienced an increase in stool frequency to 2–3 times per day with loose consistency. A repeat colonoscopy at the local hospital revealed histopathological findings of the ileum and rectum consistent with active chronic enteritis with ulcers and without granulomas. Upon adhering to the doctor’s recommendations, the patient underwent a five-month course of mesalazine, resulting in the formation of stools and improvement of the condition. Subsequently, the patient was admitted to our hospital this year following the identification of perianal vegetations. The laboratory analysis revealed the presence of fecal occult blood, fecal calprotectin levels exceeding 1800ug/g, and elevated levels of anti-Saccharomyces cerevisiae IgG and IgA antibodies. Standard testing for pathogenic bacteria (Shigella, Salmonella, Vibrio parahaemolyticus, Clostridium difficile) yielded negative results, while levels of Cytomegalovirus antibody IgG were found to be elevated. A re-evaluation of contrast-enhanced CT of the small intestine revealed partial thickening of the small intestine wall, consistent with the characteristic features of CD (Fig. 1a). MRI (magnetic resonance imaging) with contrast enhancement of the anal canal demonstrated the presence of an anal fistula (Fig. 1b). Endoscopic examination revealed multiple ulcers in the terminal ileum, as well as mucosal edema and ulcers at the anorectal margin, supporting the diagnosis of CD. Clinical data and disease evolution were reviewed, leading to the consideration of CD. In order to assist in establishing a definitive diagnosis, the patient underwent a pathology consultation at our hospital. The pathological examination identified irregular crypt structure, focal crypt reduction, and focal pyloric gland metaplasia, findings that are indicative of chronic enteritis (Fig. 2a). There was transmural inflammation, with the ulcer extending into the submucosa, disorganization of the muscularis propria, fibrosis of the submucosa and subserosa, and infiltration of numerous inflammatory cells, suggesting perforation at the site (Fig. 2b). Furthermore, the primary takeaway is that noncaseating granulomas are readily identifiable within the submucosal and subserosal layers (Fig. 2c), aiding in the diagnosis of CD. Within the intestinal wall, there were several multinucleated giant cells. To our surprise, histopathology once again revealed that several atypical granulomas were observed during the examination of the pathological sections. As the granulomas observed were significant in size and confluent, they are usually characterized by central necrosis and a lack of discernible structure, which is commonly referred to as caseous granulomas (Fig. 2d).
Fig. 1.
Imaging manifestations. a Enhanced CT revealed partial thickening of the wall of the small intestine. b MRI with contrast enhancement of the anal canal demonstrated the presence of an anal fistula
Fig. 2.
Histopathological evidence. a The histological examination revealed a modification in the structure of the recess, and the development of non-caseous granulomas within the submucosal lymphoid tissue was found; 10×magnification. b Inflammation throughout all layers of the intestinal wall was examined histologically; 4×magnification. c-d Granulomas. c indicates non-caseous granuloma in the submucosa of the intestinal wall; 20×magnification. d displays a caseous granuloma in the subserosa of the intestinal wall, with surrounding isolated multinucleated giant cells; 20×magnification
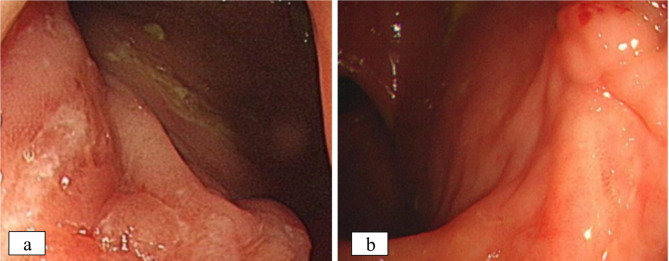
We conducted testing for the M. tuberculosis complex using genetic PCR, T-SPOT, and PPD in this study, and a Ziehl-Neelsen stain for acid-fast bacilli was performed to look for the bacilli under the microscope. All laboratory testing yielded negative results, indicating no signs of tuberculosis. In addition, no significant lesions were detected on the patient’s chest CT. There was no familial history of tuberculosis, and no other individuals were found to be infected. Given the lack of evidence for tuberculosis or other specific infections, the clinical treatment remains consistent with CD. Following comprehensive laboratory testing and meticulous examination of pathological sections, the gastroenterologist administered a three-week course of isoniazid prophylaxis, which may not have been adequate to effectively treat the patient’s tuberculosis infection, if present. A significant improvement in symptoms was observed following four courses of ustekinumab treatment. Seven months later, there was a nearly fivefold reduction in calprotectin levels. Subsequent re-examination of the enhanced MRI scan of the anal canal revealed the disappearance of the anal fistula, obvious healing of the ulcer scar under endoscopy, and a reduction in the CD activity score (SES-CD) from 9 to 1 (Fig. 3a-b). Based on the patient’s examination findings and response to treatment, the diagnosis of CD was maintained. How do anomalous caseous granulomas develop in CD? The patient’s clinical history led us to assume that the abnormal caseous granuloma in CD resulted from a secondary infection of intestinal perforation, based on a detailed clinical, radiological, and pathological evaluation. Regrettably, the patient opted not to pursue additional next-generation sequencing (NGS) testing for infection. The patient remains under continued observation.
Fig. 3.
Comparison of endoscopy findings before and after pharmacological intervention. a Endoscopy revealed the presence of multiple ulcers located at the anal margin. b Following 7 months of treatment with CD, the anal margin ulcer was successfully healed as confirmed by endoscopic examination
Disscusion
Granulomas are aggregates of activated macrophages and their derived cells that arise from prolonged exposure to antigens, with granulomatous inflammation typically resulting from either infectious or noninfectious immune reactions, as well as a number of unidentified etiologies [3]. The presence of non-caseating epithelioid granulomas in CD, which is typically seen as a characteristic feature, has been reported to range from 15 to 60%, with an average detection rate of 45% [6, 7]. Granulomas in CD exhibit diverse patterns, such as microgranulomas characterized by a limited number of histiocytes, mucinous granulomas associated with crypt rupture, nodular epithelioid granulomas lacking inflammatory infiltration and occasionally displaying Schaumann bodies, and epithelial granulomas featuring numerous aggregates of multinucleated giant cells. Necrosis and abscess formation are infrequent occurrences within granulomas [3, 8, 9]. The granulomas found in CD display a compact sarcoid-like morphology, are poorly organized, and are often smaller than 200 μm in size, appearing discrete or isolated. These granulomas have the potential to manifest in any layer of the intestinal wall [3]. The inaugural examination of the correlation between granulomas and CD behavior was documented in a publication in 1960 [10, 11]. The occurrence of granulomas in CD is linked to a more severe disease presentation. Patients with CD who present with granulomas are at a higher risk for developing penetrating complications and perianal disease [6, 11–13]. Individuals who have undergone surgical intervention for CD with the presence of granulomas are at an elevated likelihood of requiring reoperation within a six-year timeframe [11]. The presence of both caseous and non-caseating granulomas in our case prompted initial consideration of tuberculosis in accordance with conventional diagnostic protocols. There is a clinical, radiological, endoscopic, and follow-up evidence of CD. Caseous granulomas should not preclude the diagnosis of CD. What are the underlying factors contributing to the development of caseous granulomas in individuals with CD?
CD afflicted with tuberculosis infection
Tuberculosis, caused by Mycobacterium tuberculosis infection, is a significant pathogen associated with gastrointestinal granulomatous diseases. There is significant overlap between CD and intestinal tuberculosis (ITB) in terms of clinical, endoscopic, radiological, and histological features. Literature reports indicate that 5% of patients initially diagnosed with ITB were misdiagnosed as having CD and were treated with steroids, while 9% of patients initially diagnosed with CD were later found to have tuberculosis [14]. What’s more, a study has indicated that the misdiagnosis rate for CD and ITB ranges from 50 to 70% [15]. If CD is mistakenly diagnosed as ITB, the administration of unnecessary anti-tuberculosis treatment may result in drug toxicity and delay the appropriate management of CD. Likewise, the exclusive use of steroids in the treatment of tuberculosis may exacerbate the patient’s condition [14, 16]. Hence, definite distinction between CD and ITB in the intestinal tract is difficult during the course of the illness for medical professionals. The diagnosis of ITB is supported by the co-occurrence of pulmonary tuberculosis, ascites, night sweats, involvement of fewer than four intestinal segments, ring ulcers, scars, or pseudopolyps. Conversely, the presence of bloody stools, perianal lesions, chronic diarrhea, anorectal lesions, longitudinal ulcers, and cobblestone appearances are indicative of CD [17, 18]. Nevertheless, none of these indicators were specific. From a histologic standpoint, changes in the structure of crypts, such as distortion, branching, shortening, and irregular mucosal surface, are frequently seen in individuals with CD, but can also be present in cases of tuberculosis. In an article authored by Yu et al., it is apparent that individuals with ITB exhibit a greater volume and quantity of granulomas compared to those with CD, particularly those exceeding a maximum diameter of 300 μm, which are typically indicative of ITB. Additionally, patients with ITB displayed a higher density of granulomas at the same magnification, in comparison to patients with CD. Notably, the presence of more than five granulomas per tissue section was exclusively found in individuals with ITB. The author of that paper also indicated that there is a greater prevalence of confluent granulomas in patients diagnosed with ITB than with CD. Specific caseous necrosis was found in 13.3% of patients with ITB [14]. The majority of cases of CD with tuberculosis result from the reactivation of latent infection, typically manifesting around 12 weeks following initial exposure to infliximab, an anti-TNF (anti- tumor necrosis factor) biologic agent, and commonly within the initial 6 months of treatment [19]. The reactivation of tuberculosis is attributed to the induction of apoptosis in immune cells that are bound to TNF receptors by infliximab, which acts through the anchoring membrane, consequently disrupting the restrictive effect of granulomas on Mycobacterium tuberculosis [11, 20]. There has been a reported 5 to 10-fold increase in the risk of tuberculosis among patients with CD who are treated with infliximab [13]. Hence, theoretically, there exists a likelihood that CD may be complicated by tuberculosis. A 26-year-old CD patient treated with infliximab was found to have gummatous cutaneous tuberculosis as Garcia LC et al. reported [21]. In a detailed case series of CD patients who were treated with anti-TNF therapy, the incidence of extrapulmonary tuberculosis was found to be 5 out of 802 cases. This study also identified novel extrapulmonary tuberculosis locations, namely the colon, turbinate, and spleen [22]. In this particular instance, despite the presence of caseous granulomas resembling tuberculosis, the patient did not exhibit any laboratory indications of tuberculosis, and the intestinal inflammation was managed solely with mesalazine prior to surgery. Following treatment with CD medications, the patient experienced healing during endoscopy and symptom alleviation, thereby negating any evidence of tuberculosis in the patient.
CD with co-infections of other opportunistic pathogens
Patients with CD exhibit dysbiosis in their intestinal microbiota, while individuals with compromised immune systems are at an increased risk of developing opportunistic infections. For instance, a case study has documented the co-occurrence of CD with ileocolonic histoplasmosis [23]. Fungal organisms typically induce nodular granulomatous lesions that are often accompanied by necrosis. Yersinia pseudotuberculosis [24], Histoplasmosis [25], Cryptococcus neoformans [26], Coccidioides spp [26], and other fungal microorganisms have the potential to trigger necrotizing granulomas. Histoplasma species have the potential to induce the formation of caseous granulomas. These opportunistic microorganisms are capable of thriving not only in individuals with compromised immune systems, but also in those who are immunocompetent. In a comprehensive clinical examination of Ryosuke Osawa, 88% (23/26) of the immunocompromised patients had GI cryptococcosis. In addition, clinical manifestations of gastrointestinal cryptococcosis exhibited significant variations between individuals with intact immune systems and those with compromised immune function [27]. The prevalence of invasive fungal diseases attributed to TNF-a inhibitors has garnered heightened scrutiny [28–31]. If clinical suspicion of infection persists despite negative results from special stains, further investigation using polymerase chain reaction (PCR) analysis on paraffin-embedded formalin-fixed tissue may be warranted to identify pathogens such as tuberculosis, fungi, Yersinia, and others. Clinical investigations, such as stool culture or serology, have the potential to provide valuable insights. Notwithstanding, clinical and laboratory findings can establish a cause in many necrotizing granulomas that appear unexplained at the time of initial histologic examination.
Furthermore, small areas of necrosis are frequently observed in individuals with sarcoidosis [25]. Diathermy associated with surgical procedures and traumatic tissue injury are additional factors that contribute to the formation of necrotizing granulomas [32, 33]. In addition to polyangitis granulomatosis, intravascular fungal infection may also lead to the formation of granuloma with background necrosis [3].
Conclusion
In the clinical setting, the presence of intestinal caseous granulomas is often associated with the diagnosis of tuberculosis. Nevertheless, it is important to recognize that alternative etiologies, such as medication for CD, secondary opportunistic infections, or the patient’s underlying condition, may also give rise to caseous granulomas in the intestine. The diagnosis of our case was confirmed as CD with caseous granulomas, with tuberculosis being effectively ruled out through an analysis of potential sources of the granulomas. The patient lacked any known exposure to tuberculosis and exhibited no clinical laboratory evidence supporting the presence of the disease. While the patient declined further infectious testing, the efficacy of the CD treatment remains evident. Ongoing monitoring of the patient’s progress will continue. This article presents a novel interpretation that challenges the conventional understanding that the presence of caseous granulomas in the intestine does not definitively exclude the diagnosis of CD. It is imperative to consider the potential for infection complicating CD through the comprehensive integration of clinical, endoscopic, imaging, laboratory examination, and histological auxiliary staining. Moreover, the study also examines the potential occurrence of caseous granuloma in the intestinal tract.
Acknowledgements
The authors thank Prof. Shuyuan Xiao (Department of Pathology, University of Chicago Medicine, Chicago, IL, USA) for his valuable assistance on this case.
Author contributions
The manuscript was written by SQ Tao and all authors commented on previous versions of the manuscript. JH Xu and Y Chen supervised the research and modified the manuscript. W Hu and KR Shen took responsibility for reviewing endoscopy images and radiologic images. All authors read and approved the final manuscript.
Funding
Acknowledgment of grant support No funding.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval
This study was approved by the Human Research Ethics committee of the Second Affiliated Hospital of Zhejiang University School of Medicine. We appreciate the patient’s consent and support.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.James DG. A clinicopathological classification of granulomatous disorders. Postgrad Med J. 2000;76:457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hessian PA, Highton J, Kean A, Sun CK, Chin M. Cytokine profile of the rheumatoid nodule suggests that it is a Th1 granuloma. Arthritis Rheum. 2003;48:334–8. [DOI] [PubMed] [Google Scholar]
- 3.Brown I, Kumarasinghe MP. Granulomas in the gastrointestinal tract: deciphering the Pandora’s box. Virchows Arch. 2018;472:3–14. [DOI] [PubMed] [Google Scholar]
- 4.Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet. 2017;389:1741–55. [DOI] [PubMed] [Google Scholar]
- 5.Leong RWL. The significance of granulomas in Crohn’s disease and inflammatory bowel disease epidemiology in Asia. J Gastroenterol Hepatol. 2020;35:523–24. [DOI] [PubMed] [Google Scholar]
- 6.Molnar T, Tiszlavicz L, Gyulai C, Nagy F, Lonovics J. Clinical significance of granuloma in Crohn’s disease. World J Gastroenterol. 2005;11:3118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simillis C, Jacovides M, Reese GE, Yamamoto T, Tekkis PP. Meta-analysis of the role of granulomas in the recurrence of Crohn disease. Dis Colon Rectum. 2010;53:177–85. [DOI] [PubMed] [Google Scholar]
- 8.Rotterdam H, Korelitz BI, Sommers SC. Microgranulomas in grossly normal rectal mucosa in Crohn’s disease. Am J Clin Pathol. 1977;67:550–4. [DOI] [PubMed] [Google Scholar]
- 9.Lorenzi L, Bisoffi Z, Bortesi L, Zamboni G, Liut F, Villanacci V. Schaumann bodies in Crohn’s disease: a case report and review of the literature. J Crohns Colitis. 2012;6:800–3. [DOI] [PubMed] [Google Scholar]
- 10.Antonius JI, Gump FE, Lattes R, Lepore M. A study of certain microscopic features in regional enteritis, and their possible prognostic significance. Gastroenterology. 1960;38:889–905. [PubMed] [Google Scholar]
- 11.Johnson CM, Hartman DJ, Ramos-Rivers C, Rao BB, Bhattacharya A, Regueiro M, et al. Epithelioid Granulomas Associate with increased severity and progression of Crohn’s Disease, based on 6-Year Follow-Up. Clin Gastroenterol Hepatol. 2018;16:900–07. e1. [DOI] [PubMed] [Google Scholar]
- 12.Markowitz J, Kahn E, Daum F. Prognostic significance of epithelioid granulomas found in rectosigmoid biopsies at the initial presentation of pediatric Crohn’s disease. J Pediatr Gastroenterol Nutr. 1989;9:182–6. [DOI] [PubMed] [Google Scholar]
- 13.Ehlers S. Tumor necrosis factor and its blockade in granulomatous infections: differential modes of action of infliximab and etanercept? Clin Infect Dis. 2005;41(Suppl 3):S199–203. [DOI] [PubMed] [Google Scholar]
- 14.Yu H, Liu Y, Wang Y, Peng L, Li A, Zhang Y. Clinical, endoscopic and histological differentiations between Crohn’s disease and intestinal tuberculosis. Digestion. 2012;85:202–9. [DOI] [PubMed] [Google Scholar]
- 15.Weinberg SE, Mughal AM. A case of intestinal tuberculosis mimicking Crohn’s Disease: a clinical and diagnostic dilemma. Eur J Case Rep Intern Med. 2021;8:002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao XS, Wang ZT, Wu ZY, Yin QH, Zhong J, Miao F, et al. Differentiation of Crohn’s disease from intestinal tuberculosis by clinical and CT enterographic models. Inflamm Bowel Dis. 2014;20:916–25. [DOI] [PubMed] [Google Scholar]
- 17.Ma JY, Tong JL, Ran ZH. Intestinal tuberculosis and Crohn’s disease: challenging differential diagnosis. J Dig Dis. 2016;17:155–61. [DOI] [PubMed] [Google Scholar]
- 18.Rafael MA, Martins Figueiredo L, Oliveira AM, Nuno Costa M, Theias Manso R, Martins A. Gastrointestinal tuberculosis mimicking Crohn’s Disease. GE Port J Gastroenterol. 2020;27:278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carvalho LP, Tulio MA, Rodrigues JP, Bana ECT, Chagas C. Miliary Tuberculosis in a Crohn’s Disease patient: the risk beyond the screening. GE Port J Gastroenterol. 2018;26:64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lugering A, Schmidt M, Lugering N, Pauels HG, Domschke W, Kucharzik T. Infliximab induces apoptosis in monocytes from patients with chronic active Crohn’s disease by using a caspase-dependent pathway. Gastroenterology. 2001;121:1145–57. [DOI] [PubMed] [Google Scholar]
- 21.Garcia LC, Vale E, Ferrari ML, Faria LDC. Gummatous cutaneous tuberculosis associated with the use of infliximab for Crohn’s disease. Bras Dermatol. 2021;96:228–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu Y, Yu Q, Shen KR, Xu DT, Hu W, Li SY, et al. New-onset extrapulmonary tuberculosis in negative latent tuberculosis infection screening patients with Crohn’s disease under anti-TNF therapy in a tuberculosis-endemic region: a case series. J Dig Dis. 2023;24:369–75. [DOI] [PubMed] [Google Scholar]
- 23.Anderson B, Sweetser S. Ileocolonic Histoplasmosis Complicating Crohn’s Disease. Clin Gastroenterol Hepatol. 2017;15:e135–36. [DOI] [PubMed] [Google Scholar]
- 24.Aswal M, Garg A, Singhal N, Kumar M. Comparative in-silico proteomic analysis discerns potential granuloma proteins of Yersinia pseudotuberculosis. Sci Rep. 2020;10:3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukhopadhyay S, Wilcox BE, Myers JL, Bryant SC, Buckwalter SP, Wengenack NL, et al. Pulmonary necrotizing granulomas of unknown cause: clinical and pathologic analysis of 131 patients with completely resected nodules. Chest. 2013;144:813–24. [DOI] [PubMed] [Google Scholar]
- 26.Roden AC, Schuetz AN. Histopathology of fungal diseases of the lung. Semin Diagn Pathol. 2017;34:530–49. [DOI] [PubMed] [Google Scholar]
- 27.Osawa R, Singh N. Colitis as a manifestation of infliximab-associated disseminated cryptococcosis. Int J Infect Dis. 2010;14:e436–40. [DOI] [PubMed] [Google Scholar]
- 28.Munoz P, Giannella M, Valerio M, Soria T, Diaz F, Longo JL, et al. Cryptococcal meningitis in a patient treated with infliximab. Diagn Microbiol Infect Dis. 2007;57:443–6. [DOI] [PubMed] [Google Scholar]
- 29.Wallis RS, Broder M, Wong J, Beenhouwer D. Granulomatous infections due to tumor necrosis factor blockade: correction. Clin Infect Dis. 2004;39:1254–5. [DOI] [PubMed] [Google Scholar]
- 30.Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004;38:1261–5. [DOI] [PubMed] [Google Scholar]
- 31.Tsiodras S, Samonis G, Boumpas DT, Kontoyiannis DP. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc. 2008;83:181 – 94. [PubMed]
- 32.Sorensen FB, Marcussen N. Iatrogenic granulomas of the prostate and the urinary bladder. Pathol Res Pract. 1987;182:822–30. [DOI] [PubMed] [Google Scholar]
- 33.Al Khader A, Nsour E, Aldabbas R, Alneweiri A. Necrobiotic granulomas of the bowel accompanying 3-year postsurgical recurrence of colon cancer: a case report. Int J Surg Case Rep. 2020;67:200–02. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.