Abstract
Background
Following a stroke, brain activation reorganisation, movement compensatory strategies, motor performance and their evolution through rehabilitation are matters of importance for clinicians. Two non-invasive neuroimaging methods allow for recording task-related brain activation: functional near-infrared spectroscopy (fNIRS) and electroencephalography (fEEG), respectively based on hemodynamic response and neuronal electrical activity. Their simultaneous measurement during movements could allow a better spatiotemporal mapping of brain activation, and when associated to kinematic parameters could unveil underlying mechanisms of functional upper limb (UL) recovery. This study aims to depict the motor cortical activity patterns using combined fNIRS-fEEG and their relationship to motor performance and strategies during UL functional tasks in chronic post-stroke patients.
Methods
Twenty-one healthy old adults and 21 chronic post-stroke patients were recruited and completed two standardised functional tasks of the UL: a paced-reaching task where they had to reach a target in front of them and a circular steering task where they had to displace a target using a hand-held stylus, as fast as possible inside a circular track projected on a computer screen. The activity of the bilateral motor cortices and motor performance were recorded simultaneously utilizing a fNIRS-fEEG and kinematics platform.
Results and conclusions
Kinematic analysis revealed that post-stroke patients performed worse in the circular steering task and used more trunk compensation in both tasks. Brain analysis of bilateral motor cortices revealed that stroke individuals over-activated during the paretic UL reaching task, which was associated with more trunk usage and a higher level of impairment (clinical scores). This work opens up avenues for using such combined methods to better track and understand brain-movement evolution through stroke rehabilitation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12984-024-01461-3.
Keywords: Neuroplasticity, Sensorimotor cortex, fNIRS, fEEG, Reaching and tracing tasks, Stroke, Upper limb
Background
Due to its prevalence, functional non-recovery of the paretic upper limb (UL) is a critical concern in stroke rehabilitation [1]. UL functional recovery is mainly attributed to plastic reorganization within the human brain [2, 3], and post-stroke patients often demonstrate abnormal brain activation in comparison to healthy individuals. When using the paretic hand, patients with stroke show increased contralesional and ipsilesional sensorimotor network activation compared to healthy individuals [4], as well as increased activations of contralesional primary motor cortex and bilateral premotor and supplementary motor areas [5]. During the process of functional paretic arm recovery, there is a progressive evolution towards a more “normal” lateralization of the primary sensorimotor cortex [6–10], which underlines the potential of monitoring brain reorganization to predict patients’ responses to rehabilitation [11]. Brain reorganization is classically assessed by functional magnetic resonance imaging (fMRI), mostly in the supine position and during moderately functional tasks such as thumb-finger opposition or elbow flexion-extension [12]. To monitor brain activations under more ecological conditions, i.e., during upright, unrestrained, functional tasks, it is possible to use portable brain imagery techniques such as functional near infrared spectroscopy (fNIRS) and functional electroencephalography (fEEG).
The fNIRS method detects variations in blood-oxygen level-dependant response, as in fMRI [13], and can do so under more ecological conditions [14]. FNIRS measures both oxygenated (HbO2) and deoxygenated (HbR) hemoglobin in the cerebral cortex blood vessels, and has been previously used to measure sensorimotor network activation during UL movements in healthy young adults [15, 16], older healthy adults [16, 17] and stroke patients [18, 19]. In fully UL functional tasks, such as reaching, studies have identified a bilateral sensorimotor cortex (SM1) activation pattern [16, 20]. Nevertheless, to the best of our knowledge, only one recent study investigated SM1 activation in a stroke population using fNIRS during a reaching task under ecological conditions [18]. They found enhanced ipsi/contralesional SM1 activation in the stroke patients despite poorer motor performance in reaching and grasping.
The fEEG method detects direct variations in electrical currents at the scalp due to local electric fields produced by neuronal activity [21]. Event-related power changes within specific frequency bands (alpha-mu – 8 to 13 Hz and beta – 14 to 29 Hz) reflect the balance between excitation and inhibition in the sensorimotor network [22], classically with an event-related desynchronization (ERD, i.e. power decrease) at movement execution and an event-related synchronization (ERS, i.e. power increase) at rest [23]. In patients with stroke, a number of studies have shown a relationship between the magnitude of the ERD in the lesioned hemisphere and the paretic UL function [24–26].
Coupling fNIRS and fEEG could provide a better spatio-temporal view of SM1 brain activation patterns in both hemispheres [27]. However, to better understand SM1 activity during fully functional UL tasks, it is important to complement functional brain imaging with kinematic assessments [16]. During forward-reaching tasks, stroke patients often exhibit non-mandatory trunk compensation, i.e. even if they can do with their paretic UL alone, they favour trunk flexion to the detriment of arm use [28, 29]. Unfortunately, this non-use of the paretic UL [30] can lead to maladaptive brain plasticity [31] and hinder functional recovery [32]. Overall, it is now clear that non-mandatory trunk compensation and associated non-use have an impact on the plastic reorganisation of the brain (for a review, see [33]). Thus, investigating how trunk compensation affects SM1 activations during different functional UL tasks (detailed description of UL tasks in Sect. “Experimental design”) may help to understand the mechanisms underlying functional recovery [18].
The primary aim of the present study was to investigate bilateral SM1 activation during functional UL tasks in people with and without stroke. We hypothesised increased SM1 activation in the stroke cohort, both in the ipsilesional and contralesional hemispheres and particularly during performance of the paretic UL. Additionally, we investigated the effect of stroke on the relationship between brain activation patterns and motor performance. Our hypothesis was that individuals in the stroke group would perform worse when using their paretic arm, and that SM1 activation in the injured hemisphere would be positively correlated with task performance.
Materials and methods
Participants
The study cohort consisted of 21 post-stroke patients and 21 healthy adults. For the stroke group, the inclusion criteria were to: (i) be aged between 18 and 90 years old, (ii) be at more than 3 months of a first cerebrovascular accident of any aetiology (hemorrhagic or ischemic; participants with several strokes were excluded), and (iii) have an UL motor impairment with FM-UE ≥ 15 [34]. The non-inclusion criteria were to: (i) have hemineglect or severe attentional problems (omission of more than 15 bells on the Bell’s test; [35], (ii) have aphasia of comprehension dysfunction (Boston Diagnostic Aphasia Examination < 4/5; [36], and (iii) have severe cognitive dysfunction (Mini Mental State Examination-MMSE < 24; [37]. To be included, the healthy adults had to be aged between 60 and 90 years old (to fit with the stroke group age) and to be right-handed assessed by the Edinburgh Handedness Inventory [38]. Exclusion criteria were the existence of neurological (including a history of traumatic brain injury) or motor disorders at the level of the upper limb (history of tendinous disease, arthritis, surgery). Healthy participants were recruited via local association, while stroke ones were recruited at the beginning of a rehabilitation protocol (ReArm project, Clinical trial identifier: NCT04291573, 2nd March 2020).
Table 1 provides detailed participant information, including gender, age, lesioned side, laterality, and clinical scores (refer to the clinical assessments section for additional details). For the stroke group, Table 2 presents all patients’ demographic data and clinical history. At the time of the experiment, patients were not included in any intensive acute rehabilitation, and were just following maintenance therapy depending on their needs.
Table 1.
Characteristics of the participants for each group (n = 21)
| Characteristics | Healthy group | Stroke group |
|---|---|---|
| Age (years) (SD) | 73.1 (± 6.7) | 64.4 (± 10.2) |
| Sex (female/male) | 11/10 | 6/15 |
| Handedness score (SD) | 0.96 (± 0.08) | - |
| Paretic arm (right/left) | - | 8/13 |
| FM-UE | - | 48.7 (± 5.9) |
| WMFT | - | 57.3 (± 9.8) |
| BBT ratio | - | 54.0 (± 25.1) |
BBT ratio = (paretic score / non-paretic score) * 100. Group comparison showed a significant difference in age (T-test, p = .002) and a non-significant difference in sex (Chi-square, p = .116)
Table 2.
Demographic information, clinical data and lesion information
| P | Age | Sex | Hemisphere lesioned | Months since stroke | HD before stroke | Paretic arm | Lesion localisation | Vascular territory | Type of stroke | Sensitivity deficit | Spasticity | MMSE | FM-UE | BI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | M | L | 8 | R | R | subcortical | Brainstem | Is | yes | no | 27 | 45 | 85 |
| 2 | 61 | M | R | 73 | R | L | cortical | MCA | Is | yes | no | 30 | 55 | 95 |
| 3 | 52 | M | R | 84 | R | L | cortical | MCA | Is | yes | yes | 24 | 51 | 90 |
| 4 | 63 | M | L | 88 | L | R | cortical | MCA, ACA | Is | yes | no | 28 | 44 | 95 |
| 5 | 70 | M | R | 98 | R | L | cortical | MCA | Is | yes | no | 29 | 51 | 100 |
| 6 | 73 | F | R | 223 | R | L | cortical | ACoA | H | no | no | 27 | 53 | 95 |
| 7 | 63 | F | R | 21 | R | L | cortical | - | Is | no | yes | 28 | 27 | 90 |
| 8 | 57 | F | R | 10 | R | L | cortical | PO lobe | H | no | no | 25 | 60 | 90 |
| 9 | 74 | M | R | 49 | R | L | cortical | MCA | Is | no | no | 28 | 50 | 85 |
| 10 | 37 | M | R | 32 | L | L | subcortical | MCA, ACha | Is | no | yes | 29 | 46 | 95 |
| 11 | 68 | M | R | 43 | R | L | cortical | MCA | Is | yes | no | 24 | 47 | 95 |
| 12 | 76 | M | L | 93 | R | R | subcortical | None (H stroke) | H | yes | no | 29 | 41 | 90 |
| 13 | 62 | F | R | 12 | R | L | cortical | MCA | Is | yes | no | 28 | 45 | 85 |
| 14 | 49 | F | R | 25 | R | L | cortical | MCA | Is | - | yes | 27 | 54 | 95 |
| 15 | 82 | M | L | 45 | R | R | - | - | Is | yes | - | 29 | 58 | 100 |
| 16 | 72 | M | L | 4 | R | R | subcortical | ACha | Is | no | no | 29 | 44 | 90 |
| 17 | 66 | M | L | 9 | R | R | subcortical | ACha | Is | no | no | 30 | 38 | 95 |
| 18 | 73 | M | L | 9 | R | R | subcortical | brainstem | Is | no | yes | 30 | 36 | 25 |
| 19 | 71 | F | L | 18 | R | R | subcortical | None (H stroke) | H | yes | no | 25 | 57 | 95 |
| 20 | 62 | M | R | 3 | R | L | subcortical | None (H stroke) | H | yes | no | 29 | 46 | 90 |
| 21 | 60 | M | R | 9 | R | L | subcortical | MCA | Is | no | no | 27 | 43 | 90 |
Abbreviations M, male; F, female; R, right; L, left; HD, hand-dominance; MCA, middle cerebral artery; ACoA, anterior communicating artery; ACA, anterior cerebral artery; ACha, anterior choroidal artery; PO, parieto-occipital; Is, Ischemic; H, Hemorrhagic; MMSE, mini mental state evaluation (score/30); FM, Upper Limb Fugl-Meyer (score/66); BI, Barthel index (score/100). The severity of the motor impairment was evaluating using the FM-UE in accordance with the motor impairment classification in clinical and research settings [39]
In accordance with the Declaration of Helsinki, this study was approved from the French Research Ethics Committee, (Comité de Protection des Personnes-CPP SUD-EST II, N°ID-RCB: 2019-A00506-51, http://www.cppsudest2.fr/) for the stroke patients, and from the local Ethics Committee of the EuroMov DHM laboratory for the healthy subjects (EuroMov IRB, number 1912B). All participants provided informed written consent prior participation in the study.
Experimental design
Each participant engaged in an hour-long session in a quiet isolated room. The participants were equipped with the fNIRS-fEEG neuroimaging systems and performed two functional UL tasks while seated: a paced reaching arm task and a circular steering task. The setup permitted synchronized recording of UL kinematics and SM1 activity (fNIRS and fEEG) using lab streaming layer (LSL, https://github.com/labstreaminglayer/App-LabRecorder). More comprehensive details about the functional motor task methodology can be found in our recent methodological paper (see Fig. 5 in [40]).
Upper-limb function assessments
All participants performed the two functional UL tasks, as detailed in earlier studies [16, 40]. We chose and developed the functional proximal UL tasks that could provide relevant kinematics parameters to understand the movement reorganization (i.e., trunk compensation, movement time, accuracy, speed, performance [28, 29, 41]). There was a gap in the literature at this level, as most of the research projects on task-related brain activity were focused on for distal tasks or tasks that did not used the entire UL (i.e., from trunk to wrist). Since full mobility of the UL is necessary in everyday life activities, it was applicable to use standardized UL movement tasks such as reaching tasks and circular trajectory tracking tasks [16, 42]. Moreover, in the context of stroke, proximal movements, such as arm reaching, can be used to assess patients at the beginning of the recovery process and patient with greater impairment. Indeed, since the process functional recovery has been shown to be proximo-distal direction, patients are most likely to first recover at the level of the proximal UL movements. The reaching task, with maximal condition (maximal arm use with trunk restrained) and spontaneous condition (spontaneous arm use), was previously developed to identify trunk compensation [28], and we further designed the task with a paced rhythm (5 movements per 20s) to particularly enable fNIRS recordings of brain activity changes. The addition of the circular steering task was done in order to allow for a proximal UL performance-based task focused on speed rather than accuracy thus allowing brain activity to be extrapolated to performance.
Paced reaching task
Participants were seated on a chair fitted with armrests and were instructed to reach a target (a table tennis ball) placed in front of them at a height of 80 cm and a distance which facilitated the complete extension of the arm. A Kinect sensor (V2, Microsoft, USA), sampled at 30 Hz, was positioned 1.70 m above and 1.60 m away from the target. Participants had to reach the target by extending their arm in two conditions: (i) spontaneous condition (i.e., spontaneous arm use, SAU), and (ii) maximal condition (i.e., maximal arm use, MAU), wherein their shoulders were constrained to minimize trunk movements. Each block consisted in five movements per 20-second block, timed to 4s vocal prompts (“go” for 2s; “stop” for 2s) and was interspaced by 20s of rest. After a familiarization block with each arm, participants completed three blocks using their non-dominant/paretic hand, followed by three blocks using their dominant/non-paretic hand in the spontaneous condition. Then, participants repeated the task for three blocks with each hand under the maximal condition.
Circular steering task
This task was based on the speed-accuracy trade-off [43]. Participants were seated on a chair in front of a horizontal graphic tablet (A3 size; Wacom, Kazo, Japan) equipped with a stylus affixed to a mouse pad, facing a 24-inch vertical screen projecting a circular target (33-inch circumference) with a 2 cm tunnel. A Kinect was placed above the graphic tablet at the height of 1.70 m. The task was delivered using a lab-made software, the LSL-Mouse (https://github.com/KarimaBak/LSL-Mouse). Participants were instructed to move a cursor as fast as possible in a clockwise direction. During the familiarization phase, participants were instructed to accelerate if movement trajectory errors (any instances outside the 2 cm circular tunnel boundaries) were below 15% (based on pilot testing). The task comprised three blocks for each arm (20s of task with 20s of rest), commencing with their non-dominant/paretic hand.
Clinical assessments of paretic upper limb impairment
In conjunction with the functional kinematics and brain evaluation, patients’ UL motor function was appraised through clinical evaluations. We utilized several recognized and validated tests, including the FM-UE [34, 39], the Box and Block test (BBT [44]), the Wolf-motor function test (WMFT [45]), the Barthel Index (BI [46]), and the Proximal-arm non-use test (PANU [28, 29]). Comprehensive details of these evaluations are described in the cited references.
The FM-UE assesses upper limb motor impairment, while the BBT measures arm and hand grasping function. WMFT evaluates upper limb function, and the BI measures overall functional recovery (independent function in activities of daily living). The PANU test quantifies the amount of shoulder and elbow movements that a post-stroke individual does not use spontaneously, but can use when forced to do so. These tests collectively provide a comprehensive overview of the paretic UL’s functional capacity and impairment (for the FM-UE) level in stroke patients.
Brain activity (fNIRS and fEEG)
Participants wore a custom neoprene head cap equipped with a combined fEEG-fNIRS system to monitor brain activity within the left and right SM1 regions during both functional motor tasks. We utilized a wireless Starstim fNIRS integration system (Starstim8, Neuroelectrics, Barcelona, Spain; Octamon+, Artinis Medical Systems, Elst, The Netherlands) to measure fEEG and fNIRS signals. Details regarding the placement of the 16 channels, comprising 4 fNIRS and 4 fEEG channels per SM1 hemisphere, are outlined in a previous article (see Fig. 1 in [16]).
The 8 fEEG electrodes were positioned in and around the SM1 cortices: C4, FC2, FC6, CP2 in the right hemisphere and C3, FC1, FC3, CP1 in the left hemisphere, in alignment with the international 10–10 system. The electrodes (NG Geltrode, Neuroelectrics, Spain) were filled with electro-gel (Signa Gel®). Using an ear clip, reference electrodes (CMS, DRL) were placed over the right earlobe. The fEEG signals were sampled at a rate of 500 Hz. We controlled the wifi- fEEG device via a software interface (Neuroelectrics Instrument Controller, NIC v 2.0).
For the fNIRS recording, we used a continuous-wave system employing two wavelengths to capture changes in HbO2 and HbR overlying the left and right SM1, sampling at 10 Hz. The two receivers were positioned at the C1 and C2 locations of the 10–10 fEEG system, with four transmitters placed 3 cm from the receivers using plastic holders. The fNIRS Bluetooth device was managed through a software interface (Oxysoft, v3.2.51.4, Artinis Medical Systems, Elst, The Netherlands).
Following the equipment setup, participants were asked to perform a wrist extension task to verify if the movement induced a hemodynamic response in the SM1.
Data analysis
Task performance
The paced reaching and circular steering task kinematics analysis was undertaken based on previous work [28, 29, 47] and LSL-Kinect software (LSL-KinectV2: https://github.com/KarimaBak/LSL-KinectV2). For the paced reaching task, we calculated the proximal-arm non-use (%) and the hand mean velocity (mm/s). For both tasks, we calculated as trunk compensation parameter, the range of trunk anterior flexion (°) representing the use of the trunk to realize the reaching movement. And, we calculated, as arm use parameters the range of elbow extension (°) representing the use of whole arm to perform the movement.
We assessed the speed-accuracy trade-off during the circular steering task using the Index of Performance (IPe in bits/s [48]). We calculated the Index of Effective Task Difficulty (IDe) with the formula: 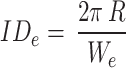 , where R represents the subject’s mean circular path radius, and We denotes the effective path width. We determined We using MacKenzie’s formula [49]:
, where R represents the subject’s mean circular path radius, and We denotes the effective path width. We determined We using MacKenzie’s formula [49]: 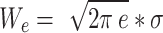 , where σ is the standard deviation of the radius. We then computed IPe by dividing IDe by the movement time (MT). In addition, we calculated the speed as laps per second and accuracy as bias (We/W, following [49]) of the movement.
, where σ is the standard deviation of the radius. We then computed IPe by dividing IDe by the movement time (MT). In addition, we calculated the speed as laps per second and accuracy as bias (We/W, following [49]) of the movement.
Brain activity (fNIRS and fEEG)
We processed all fNIRS raw data using the HOMER toolbox in MATLAB (Homer2 NIRS processing package, [50]) with the files generated by the Lab Recorder (xdf files). Pre- and post-processing steps are detailed in a previous study [16] and a flowchart presenting these steps is available in the supplementary materials (file 1). We used the relative changes (Δ) in peak HbO2 concentration as an indicator of brain activity.
We analysed all fEEG data using the EEGLAB toolbox on MATLAB ([51], version 2021.1), with the files generated by the Lab Recorder (xdf files). Details of pre- and post-processing steps are provided in a previous study [16]. We calculated the event-related spectral perturbations (ERSP) in the alpha (8–13 Hz) and beta (14–29 Hz) rhythms, revealing average power changes in these specific time frequencies. This information provides insight into event-related desynchronization (ERD; power decrease in a specific frequency band relative to baseline, i.e., rest) and synchronization (ERS; power increase in a specific frequency band relative to the task). For fEEG and fNIRS analyses, parameters were averaged by tasks (paced reaching; circular steering), hand condition (dominant / non-paretic; non-dominant / paretic), and hemisphere (contralateral / ipsilesional; ipsilateral / contralesional).
Statistical analyses
Statistical analyses were performed using R software (version 4.2.1) and the ggplot2 [52], dplyr [53] and rstatix [54] packages. Parametric tests were employed following the validation of data normality via the Shapiro-Wilk test and visual examination of Q-Q plots. Effects sizes were indicated using the partial eta square (η2p), with small (0.02), medium (0.13), and large (0.26) effect sizes noted [55, 56]. A threshold of p < .05 was used for statistical significance. If necessary, pairwise comparisons were conducted using t-tests, with the Benjamini-Hochberg procedure applied for p-value correction in multiple tests [57]. Significant effects were interpreted only when of sufficient intensity (η2p > .02). All values are presented as mean (SD) unless stated otherwise. In the absence of three-level interaction effects, only two-level interaction effects were reported for each factor combination. Note that the degrees of freedom of the analysis are varied across variables due to differing exclusion rates for subjects.
Tasks performance and kinematics
The movement parameters for the circular steering task (IPe, speed, accuracy, range of trunk anterior flexion, range of elbow extension) were evaluated through a mixed ANOVA, which included group (healthy and stroke) as a between-subject factor, and hand (non-paretic/dominant and paretic/non-dominant hand) as a within-subject factor. Similarly, a mixed ANOVA was employed for the paced-reaching task (PANU, mean velocity, range of trunk anterior flexion, range of elbow extension), incorporating group (healthy and stroke) as a between-subject factor and hand (non-paretic/dominant and paretic/non-dominant hand) and condition (spontaneous- SAU and maximal- MAU) as within-subject factors.
Cortical activations
For the analysis of fNIRS peak of ΔHbO2 and fEEG Alpha and Beta ESRPs, a mixed ANOVA was applied with group (healthy and stroke) as a between-subject factor, and hand (non-paretic / dominant and paretic / non-dominant hand), condition (spontaneous- SAU and maximal- MAU, paced-reaching task), and hemisphere (contralateral / ipsilesional; ipsilateral / contralesional) as within-subject factors.
Brain-movement relationship
In our investigation of the association between performance in the circular steering task and brain activation (fNIRS peak ΔHbO2) across the groups, we consistently applied Spearman rank partial correlation analysis. This approach was chosen to account for the non-normal distribution of some variables and to maintain consistency across the analysis, thus enhancing comparability of our findings. We choose to keep only moderate effects to avoid false effects, thus, we just present correlation with at least a rs2 > 0.25. Only those effects were reported to facilitate the results presentation. As statistics were undertaken with a non-parametric Spearman rank correlation, no regression lines were built on the figure representing the correlations as they would be misleading.
Results
Tasks performance and kinematics
Circular steering task
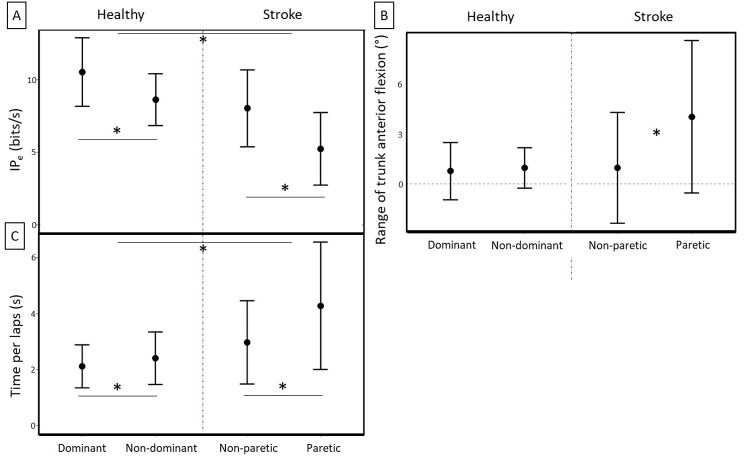
On the circular steering task (Fig. 1), we found a higher performance (IPe) in the healthy group and with the dominant hand / non paretic hand for both groups (Group: F(1,40) = 20.52, p = .000, η2p = .34; Hand: F(1,40) = 53.00, p = .000, η2p = .57) with no Group × Hand interaction (F(1,40) = 1.97, p = .169, η2p = .05). For the speed component (i.e., time per lap), we found a Group x Hand interaction (F(1,40) = 5.83, p = .020, η2p = .13). Post-hoc analysis showed that the time per lap difference between paretic/non-dominant and non-paretic/dominant hand, was significantly higher for the stroke group, with a longer time per lap with the paretic arm (Healthy: η2p = .25; Stroke: η2p = .34). Moreover, it shows that the time per lap was significantly shorter in the healthy group, whatever the hand. For the accuracy component (i.e., bias), we did not find any significant effects (healthy / dominant: bias 183 (± 56.6); healthy / non-dominant: bias = 189 (± 49.5); stroke / non-paretic: bias = 202 (± 77.9); stroke / paretic: bias = 233 (± 96.3).
Fig. 1.
Circular steering task performances and strategies (mean ± SD) for the two groups and according to hand trial. (A) Index of performance (IPe); (B) Range of trunk anterior flexion; and (C) Time per lap. (* for statistically significant differences at p < .05)
On the circular steering task, we found that the trunk compensations were higher in the stroke group when performing with the paretic hand (Group x Hand interaction: F(1,35) = 8.95, p = .005, η2p = .20). For both groups, the range of elbow extension was significantly higher with the dominant / non -paretic hand (F(1,35) = 8.28, p = .007, η2p = .19).
Paced reaching task
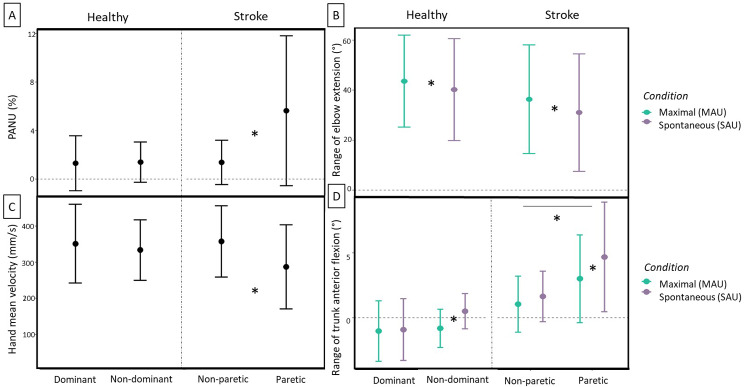
On the paced reaching task (see Fig. 2), we found a Group x Hand interaction on the PANU, range of trunk flexion and hand mean velocity (PANU: F (1,37) = 8.85, p = .005, η2p = .19; range of trunk flexion: F (1,37) = 5.01, p = .031, η2p = .12; hand mean velocity: F (1,37) = 4.93, p = .033, η2p = .12). The range of trunk anterior flexion, and PANU were higher for the stroke paretic hand and at the same time the hand mean velocity was lower. For the range of trunk anterior flexion, we found a Hand x Condition interaction showing that the range of anterior trunk flexion was lower in the maximal condition for the non-dominant / paretic hand (F (1,37) = 4.88, p = .033, η2p = .12). We also found a condition effect on the range of elbow extension, for both groups, it was higher in the maximal condition (F (1,37) = 7.11, p = .011, η2p = .16).
Fig. 2.
Paced reaching task upper limb movement strategies (mean ± SD) for the healthy and stroke groups and according to hand trial and condition (for condition interaction and/or effects). (A) Proximal-arm non-use, PANU; (B) Range of elbow extension; (C) Hand mean velocity; and (D) Range of trunk anterior flexion. (* for statistically significant differences at p < .05)
Brain activity
Brain activity (fNIRS: peak of ΔHbO2; fEEG: ERD and ERS) during paced reaching and circular steering tasks are presented in Fig. 3 (fNIRS) and Fig. 4 (fEEG) and the statistical results are detailed in the supplementary materials for group, hand, hemisphere, and condition effects and two-way interaction effects with each factor combinations (see Supplementary material files 2 and 3). The significant three-levels interactions are reported in the text.
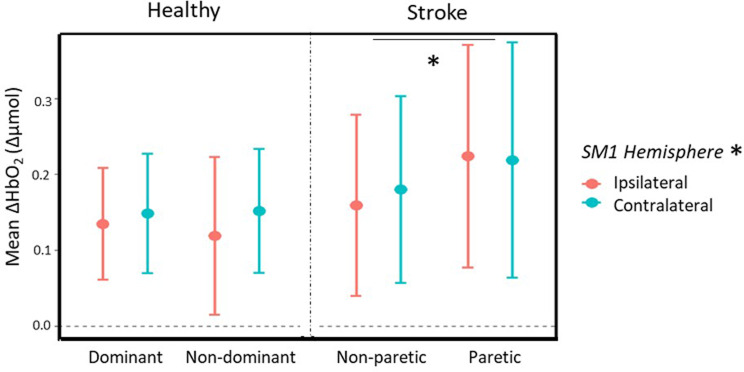
Fig. 3.
Paced reaching task fNIRS mean ΔHbO2 peak (mean ± SD) for the healthy and stroke groups as a function of hand and hemisphere (ipsilateral in orange; contralateral in cyan). * For statistically significant differences at p < .05: hand effect in the stroke group and hemisphere effect for all groups and conditions
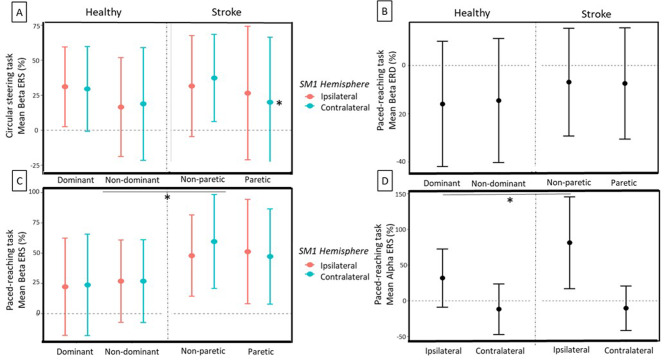
Fig. 4.
fEEG Beta event-related spectral perturbation (ERSP) (mean ± SD) for the healthy and stroke groups. Circular steering task: lower Beta event-related synchronization (ERS) in contralateral (ipsilesional) hemisphere of the stroke group after paretic arm movement (A). Paced reaching task: Beta event-related desynchronisation (ERD) with a tendency to lower ERD in the stroke group (B), higher Beta ERS in the stroke group for both hemispheres (C), and a higher Alpha ERS for the stroke group in the Ipsilateral hemisphere. (* for statistically significant differences at p < .05)
fNIRS
Circular steering
The analysis of the mean ΔHbO2 peak during the circular steering task showed no significant effects.
Paced reaching task
The analysis of the mean ΔHbO2 peak during the paced reaching task showed a higher activation for stroke group with the paretic hand (Group x Hand: F (1,36) = 4.51, p = .041, η2p = .11) and a higher activation in the contralateral side compared to the ipsilateral one for both groups (Hemisphere: F (1,36) = 6.45, p = .016, η2p = .15). Nevertheless, the three-way interaction Group x Hand x Hemisphere (F (1,36) = 2.82, p = .102, η2p = .07) showed a trend for difference between the two hemispheres or the ipsilateral (contralesional) hemisphere being higher than the contralateral (ipsilesional) side for paretic hand use in the stroke group.
fEEG
Circular steering task
On the circular steering task (Fig. 4A), we found for Beta ERS a 3-way interaction Group x Hand x Hemisphere (F (1,25) = 5.02, p = .034, η2p = .17). Post-hoc comparisons revealed that, for the stroke group, there was a Hand x Hemisphere interaction (F (1,14) = 7.56, p = .016, η2p = .35) showing a lower post-movement synchronization in the contralateral (ipsilesional) hemisphere when performing with the paretic hand (see Fig. 4A). The analysis of the mean ERSP did not show any main or interaction effect of Group on Alpha and Beta ERD nor Alpha ERS.
Paced reaching task
On the paced reaching task, there was a Group x Brain interaction for the Beta ERD (F (1,23) = 4.98, p = .036, η2p = .19). Although there was a tendency to a Group effect (F (1,29) = 3.88, p = .051, η2p = .03) showing a smaller Beta desynchronization in the stroke group (Fig. 4B), the post-hoc comparisons between the different modalities of the Group x Brain interaction were too low to emerge, and thus are not shown in Fig. 4B. For the Alpha ERD we did not find any significant main or interaction effect. For the Beta ERS, we found a Hand x Condition interaction showing that for the stroke group, the post-movement Beta synchronization was higher for the maximal condition (F (1,21) = 8.80, p = .007, η2p = .30; see Fig. 4C). We also found a Group x Hand x Hemisphere interaction (F (1,21) = 5.08, p = .035, η2p = .20). Post-hoc comparison revealed a Hand x Brain interaction for the stroke group (F (1,27) = 14.9, p = .001, η2p = .36) showing a higher Beta ERS with the dominant / non-paretic hand in the stroke group. For Alpha ERS, we found a Group x Hemisphere interaction (F (1,16) = 4.53, p = .049, η2p = .22). Post-hoc comparisons revealed that there was a Group effect in the ipsilateral hemisphere (F (1,53) = 28.8, p = .000, η2p = .35), showing a higher post-movement Alpha synchronization in the ipsilateral hemisphere of the stroke group in comparison to the healthy group (see Fig. 4D). There was also a Hand x Hemisphere interaction (F (1,16) = 6.28, p = .023, η2p = .28). Post-hoc comparisons revealed a Hand effect in the contralateral hemisphere (F (1,51) = 4.70, p = .035, η2p = .08), with a higher post-movement synchronization in the non-dominant / paretic hand.
Brain-movement-clinical scores relationship in the stroke group
Brain-movement relationship
Circular steering task
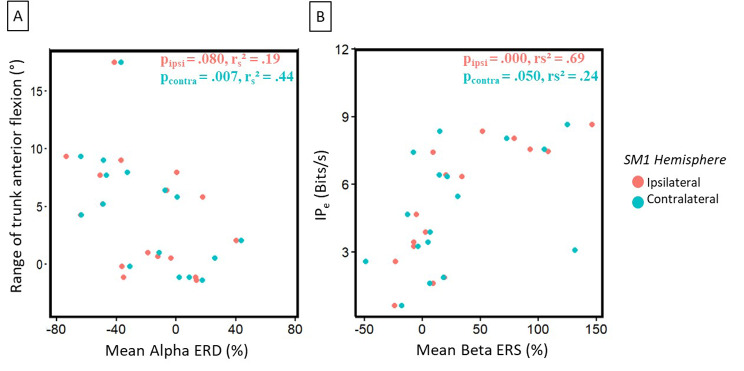
The spearman rank correlation analysis for the circular steering task with paretic hand showed that an increased use of the trunk was associated with a higher movement Beta desynchronization on the contralateral (ipsilesional) hemisphere (p = .007, rs2 = 0.44) and a tendency in the ipsilateral (contralesional) side (p = .080, rs2 = 0.19, see Fig. 5A). We also found that for a higher IPe and time per lap in the circular steering task, there was a higher post-movement Beta synchronization in the ipsilateral (contralesional) hemisphere and a tendency in the contralateral one (IPe - Ipsilateral: p = .000, rs2 = 0.69; Contralateral: p = .050, rs2 = 0.24; Time per lap – Ipsilateral: p = .000, rs2 = 0.49; Contralateral: p = .031, rs2 = 0.28, see Fig. 5B).
Fig. 5.
Stroke group correlation between (A) Alpha ERD and the trunk use, and (B) Beta ERS and the index of effective performance (IPe) during the circular steering task with the paretic arm
Paced reaching task
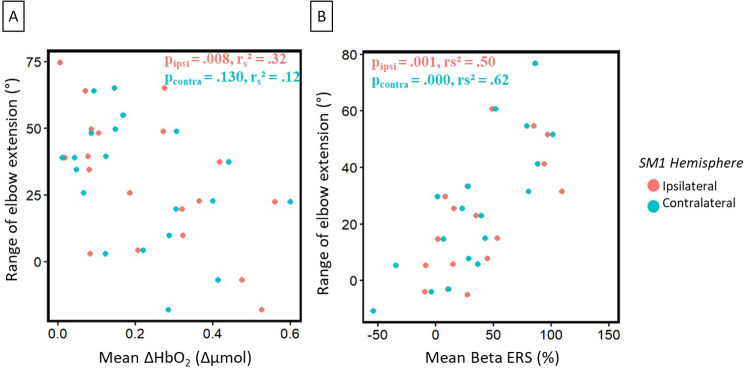
When reaching in the maximal condition, we found that elbow extension was negatively correlated with the ipsilateral hemisphere (contralesional) peak of ΔHbO2 (p = .008; rs2 = 0.32, see Fig. 6A) but not for with the contralateral one (p = .130; rs2 = 0.12). We also found that the slower to do the maximal reaching have a higher post-movement synchronization in the ipsilateral hemisphere (p = .003, rs2 = 0.47). We found that for a higher spontaneous elbow extension the Beta post-movement synchronisation was higher in both hemispheres (Ipsilateral: p = .001, rs2 = 0.50; Contralateral: p = .000, rs2 = 0.62, see Fig. 6B). On the same conditions, the Alpha post-movement synchronization in the ipsilateral hemisphere was also positively correlated to elbow extension (p = .009, rs2 = 0.37).
Fig. 6.
Stroke group correlation between (A) Peak of ΔHbO2 and elbow extension and (B) Mean Beta ERS and elbow extension during the paced-reaching task with the paretic arm
Brain-clinical scores relationship
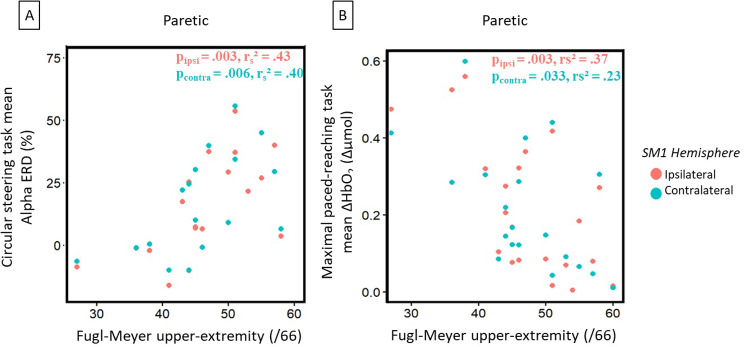
For the correlation between the brain parameters and the clinical scores, we found that a more marked Alpha ERD on the circular steering task was associated to a lower FM-UE (Non-paretic hand - Ipsilateral: p = .000, rs2 = 0.62; Non-paretic hand – Contralateral: p = .000, rs2 = 0.55; Paretic hand - Ipsilateral: p = .003, rs2 = 0.43; Paretic hand – Contralateral: p = .006, rs2 = 0.40, see Fig. 7A).
Fig. 7.
Stroke group correlation between (A) Alpha ERD during the circular steering task and the FM-UE test and, (B) Peak of ΔHbO2 during the maximal paced-reaching task and the FM-UE test
For the maximal condition of the reaching task with the paretic hand, we found a negative correlation between the peak of ΔHbO2 and FM-UE, showing that for a better clinical score there was a lower ipsilateral (contralesional; p = .003, rs2 = 0.37) and contralateral (ipsilesional: p = .033, rs2 = 0.23) peak of ΔHbO2 (see Fig. 7B).
Discussion
This study investigated the impact of chronic stroke on the bilateral SM1 electrical (fEEG) and hemodynamic (fNIRS) responses during unilateral proximal UL movements. We concurrently recorded bilateral SM1 activity via combined fNIRS/ fEEG, along with UL movements using kinematic tracking. Two previously established UL functional tasks were employed: a paced-reaching task and a circular steering task designed to interrogate the speed-accuracy trade-off [16, 28]. Our main finding was a greater increase in bilateral SM1 activity (fNIRS-peak ΔHbO2) for the paretic UL than non-paretic one during the paced-reaching task. Regarding the movement modifications, we observed that stroke patients showed slower speeds, increased trunk compensation, and decreased spontaneous use of the elbow-shoulder joint, particularly on the paretic side.
For the paced-reaching task, a greater increase in bilateral SM1 activation was detected with fNIRS during the movement with the paretic hand which tended to coincide with reduced fEEG Beta desynchronization at the onset of movement. These results might indicate compensatory brain mechanisms designed to mitigate the effects of stroke on movement execution. The fEEG findings presented a lower SM1 excitability in the stroke group, which was associated to an increased activation of the fNIRS SM1 when moving the paretic hand. This aligns with previous studies suggesting reduced brain asymmetry and increased activity as potential mechanisms of post-stroke motor recovery [58, 59]. These findings can also be compared to the study by [18] which analysed fNIRS activity during a modified Box & Block forward reaching test in stroke patients compared to a healthy cohort. Despite the inferior performance, stroke patients demonstrated increased lesioned SM1 activity during paretic arm reaching.
For the circular steering task, our findings indicated a reduced performance in the stroke group, while task-related fNIRS peak and fEEG-ERD remained comparable across both groups. This task relies heavily on visuomotor control: continuous monitoring and rectification of the trajectory while moving as fast as possible, embodying the concept of the continuous speed-accuracy trade-off [60]. Given these extensive requirements on sensorimotor control networks, this task is a good measure of neural efficiency, i.e., the amount of neural resources required to execute a given task [61]. In this context, the hypothesis of neural efficiency postulates that individuals with higher cognitive ability exhibit lower energy consumption in the brain for equivalent tasks [62]. Given the decreased performance in the stroke cohort and similar brain activity levels, it could be inferred that these individuals exhibit reduced neural efficiency when performing the circular steering task. However, as our study was confined to the SM1 region, we cannot draw a definitive conclusion regarding overall neural efficiency. Indeed, the circular steering task demands a significant level of visuomotor control, and previous research has suggested that the prefrontal area plays a substantial role in controlling such movements [63]. However, despite the potential impairment of neural efficiency in SM1, the absence of significant brain modification in stroke patients may be explained by considering the circular steering task’s nature. Indeed, the task required maximal performance from the healthy subjects as well. Moreover, our previous study found no effects of healthy aging on the level of fNIRS SM1 activity in this task, as older adults engaged both SM1 to compensate for their reduced neural efficiency [16]. We can thus hypothesize that when performance is maximized (“as fast as possible”) for all participants, brain activity will reach its maximum, and compensatory mechanisms may rely on alternative neural pathways, such as the prefrontal areas [63]. Our previous work also indicated no significant effects of healthy aging on any brain or kinematics parameters during the reaching task. This lack of effect underscores the notion that, the reaching task was, for our healthy adults, considerably simpler and less demanding than the circular steering task. However, in the present study, we observed modifications in brain activity during the paced-reaching task, potentially due to its complexity for post-stroke patients demonstrating motor compensation to complete the task successfully as in the circular steering task.
Focusing on the stroke-induced alterations in movement control, we observed the deployment of compensatory strategies by stroke patients to accomplish both functional tasks using their paretic UL. Specifically, in the circular steering task, stroke patients employed their trunk to facilitate task completion with their paretic hand, concomitantly showing reduced use of the elbow-shoulder joints. Similarly, during the reaching task, we detected evidence of proximal-arm non-use (i.e., non-mandatory trunk compensation) when the task was performed with the paretic hand. Additionally, the velocity of the paretic hand was reduced, a finding of particular interest given our use of paced reaching, indicating that the stroke patients were moving slowly to follow the paced rhythm. This result could be explained by the existence of strong correlations between clinical scores and velocity implying that the patients’ movement difficulties may be attributed to their level of impairment. This observation is consistent with prior studies demonstrating that the speed of the paretic movement is slower than that of the non-paretic movement [64]. This also aligns with our findings from the circular steering task, indicating reduced movement speed in the stroke group, particularly for the paretic arm.
A secondary aim of this study was to explore the association between brain and movement kinematics. These results are a first step for a better understanding of the underlying mechanisms of post-stroke motor recovery but as exploratory have to be treated with caution. We identified an association between trunk use and fEEG Alpha desynchronization in the circular steering task. This could imply that trunk use necessitates mobilizing increased neuronal resources across both hemispheres. Further, we detected alterations in post-movement Beta synchronization associated with motor performance. Specifically, a higher IPe correlated with increased ERS. We could hypothesize that, in this task, the high demand level is sustained by the highest-performing subjects, who are also likely to move fastest. It is well established that increased speed correlates with higher neural activity [65], implying that the ratio between the movement and rest period could be higher. Regarding the reaching task, we observed different effects depending on whether the task was performed spontaneously or maximally. In the spontaneous reaching task, our findings mirror those of the circular task, with higher post-movement synchronization observed in better performers. Conversely, the positive correlation between movement time and Alpha ERS in the maximal condition is more challenging to explain. One could hypothesize that the enhanced synchronization for slower performers might be explained by the extended duration of neural demand they experience during the task. As they move slower, their SM1 will be engaged for a longer time (i.e., the paced reaching task typically entails 2s of movement and 2s of rest), leading to higher synchronization in the ERSP. Nevertheless, the negative correlation between fNIRS brain activity and elbow extension could be akin to the circular steering task, could suggest an over-activation in lower performers who engage their trunk to facilitate movement. Another hypothesis could be that in the maximal condition, we instruct patients to use their elbow-shoulder joints maximally. Consequently, those who employ these joints less frequently will likely require more resources and increased brain activity. Thus, we could observe either the effect of trunk use or the effect of effort. However, our measurements cannot discern which hypothesis is closer to the truth (i.e., a measure of perceived effort could have been beneficial).
Lastly, an exploratory aim of this study was to investigate the association between stroke patients’ clinical scores and the corresponding brain parameters. We observed meaningful correlations that underscore the potential of fNIRS and fEEG methodologies in the context of stroke rehabilitation [18, 66, 67]. First, we found that a more pronounced Beta desynchronization at movement was linked to a lower score on the FM-UE. It is in line with prior research illustrating that a more significant event-related desynchronization in the sensorimotor cortex correlates with an enhanced demand for concentration and excitatory drive of pyramidal cells during task execution [68]. For example, studies on grip tasks during rehabilitation have shown that with progression and motor improvement, there is a reduced requirement for cortical engagement and effort to perform the grip task [69]. Secondly, the inverse correlation between fNIRS brain activity and FM-UE indicates that a lower clinical score corresponds with an increased SM1 activation during the execution of the paced-reaching task. This is plausible considering the kinematics of the task. Indeed, we found an association between higher elbow extension and higher WMFT scores (data not shown). Which could suggest that patients who utilize their arm extension more during the reaching task will have higher clinical scores, and conversely for patients using more trunk compensation to do the task. It is also known that elbow extension negatively correlates with trunk compensation [28, 47]. Consequently, patients with greater upper limb deficits may rely more on their trunk to reach the target, leading to larger brain activity in response to the increased demand for the trunk.
The methodology for the seated reaching and circular steering tasks proposed in this paper, including joint kinematics assessment of UL proximal movements and brain SM1 activity, seems well-suited for a pathological population. The combined fEEG and fNIRS methods provide detailed information about the neural and hemodynamic mechanisms underlying movement [70, 71]. Moreover, using these two controlled tasks allows for an ecological evaluation of movement within the context of functional recovery, enabling an assessment as close as possible to daily living activities [29, 43, 47]. And the analysis of movement parameters selected, such as speed, accuracy, and compensation strategies, could indicate the evolution of motor recovery [33]. Moreover, as previous studies suggest using brain laterality as an indicator of motor recovery [72], our evaluation method could be useful in routine assessments to better characterize patients’ conditions. In this study, we identified differences at the level of the kinematics and of the brain suggesting that the method developed was suitable for evaluating the stroke effects. Additionally, by combining these tools, we identified correlations between brain parameters, movement kinematics, and clinical scores. For example, in this paper, we found a brain/movement correlation for trunk use, which is important in post-stroke rehabilitation evaluation [33]. This approach might eventually allow us to identify neural markers of trunk compensation or other movement strategies, though more studies are needed to confirm this. The objective of the present study was to evaluate the effects of stroke on the brain and kinematic strategies using a newly developed method. As such, we currently lack sufficient information to provide prognostic strategies for motor rehabilitation, indeed knowing the change in the brain and kinematics following rehabilitation is necessary to answer this problem. Nevertheless, we hypothesize that this method will enable a deeper evaluation of the effects of rehabilitation methods used in clinical settings. For example, the currently running ReArm project is using this method to evaluate the effects of transcranial electrical brain stimulation (HD-tDCS) and virtual reality therapy on post-stroke upper-limb motor recovery [40].
This study has several limitations. Firstly, while age and gender matching were not strictly adhered to, the differences observed should not significantly impact the results. Specifically, the gender ratio was more balanced in the healthy group compared to the stroke group (healthy group: 11 women, stroke group: 8 women). However, statistical analysis showed no significant gender difference between the groups (chi-square test, p = .116). Therefore, gender is not considered a limitation affecting our findings. Previous literature [73–75] and our prior work [16] further support that sex does not significantly influence the variables we study (fEEG ERD-ERS; fNIRS) in the context of the functional tasks examined. Regarding age, there is a significant difference between the groups (mean age for healthy group = 72 years, stroke group = 64 years; t-test, p = .002). However, the stroke group is younger on average than the healthy group. This is relevant because the effects we are investigating, such as the decline in performance and lateralization, are typically associated with aging. Thus, the younger average age in the stroke group would likely reduce, rather than exaggerate, the differences between the groups. Moreover, correlation analyses showed no significant relationship between age and the studied variables, indicating that the age range is insufficient to show age effects on these variables, supporting our decision not to include age as a covariate in the ANOVA models. Finally, the available literature is indicating that age is not a predictor of the functional recovery [76–78], particularly our previous work did not show any effect of age on most of the brain variables studied here [16]. Additionally, in this study we did not take into account the role of associated cognitive disorders (in particular visuospatial disorders, for example, which certainly interfere a great deal with the circular task) and sensory disorders (also very important for the circular task, which relies heavily on proprioception), as well as spasticity, which interferes a great deal with elbow extension and compensatory movements by the trunk. Lastly, the reaching task, paced at a consistent rhythm for all participants, could present a significant limitation. This speed constraint could lead to an augmented use of compensatory movements in stroke patients to reach the ball at the required speed [79].
Conclusion
In conclusion, this study provides insight into the impacts of stroke on task-related brain activity and kinematics during unilateral upper limb movements that engage full UL joint movements (i.e., shoulder, elbow, wrist). Our findings highlight the brain and movement compensations associated with a chronic post-stroke population. Additionally, we demonstrate the utility of a combined fNIRS-fEEG recording approach, which correlates with kinematic and clinical scores. The concurrent evaluation of brain and kinematic parameters in ecological settings offers complementary information about the execution of paretic movements, allowing for extracting specific components for targeted intervention during rehabilitation. Moreover, these measures can enrich routine clinical assessments in ecological settings. As perspectives, the ReArm project, of which this study is a part, aims to discern the effects of rehabilitation on these specific brain and kinematic parameters. Furthermore, we aim to investigate their applicability in routine evaluation to facilitate more personalized rehabilitation strategies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Flowchart of the fEEG and fNIRS pre-processing and processing steps. Method based on previous study. (Muller 2023). Abbreviations ERSP, event-related spectral perturbation; ERS, event-related synchronization; ERD, event-related desynchronization; RP, relative power; Pn, power spectrum; ΔHbO2, variation of oxygenated blood
Supplementary Material 2: Statistical results of the ANOVA on the fEEG and fNIRS brain parameters for the circular steering task
Supplementary Material 3: Statistical results of the ANOVA on the fEEG and fNIRS brain parameters for the paced-reaching task
Acknowledgements
We acknowledge Valérie Driss from the Clinical Research and Epidemiologist unit from Montpellier University Hospital for assisting the neuroimaging and kinematics acquisition data.
Abbreviations
- BBT
Box and Block Test
- BI
Barthel Index
- ERD
Event Related Desynchronization
- ERS
Event Related Synchronization
- ERSP
Event Related Spectral Perturbation
- FM-UE
Fugl-Meyer Upper-Extremity
- IDe
Index of Task Effective Difficulty
- IPe
Index of effective performance
- fEEG
Functional Electroencephalography
- fNIRS
Functional Near-infrared Spectroscopy
- HbO2
Oxygenated blood
- HbR
Deoxygenated blood
- MAU
Maximal Arm Use
- PANU
Proximal Arm Non-Use
- SAU
Spontaneous Arm Use
- SD
Standard Deviation
- SM1
Primary Sensorimotor cortex
- WMFT
Wolf Motor Function Test
Author contributions
K.B. coordinated the project and obtained the funding. C.O.M. and G.F. wrote the original draft. C.O.M. acquired the data. C.O.M. and G.F. analyzed the data. C.O.M., G.F., M.M., K.B., D.M., I.L. and S.P. participated in the conceptualization of this project and in the interpretation of the data. G.D., B.X., M.M., S.P., K.B. and C.O.M. developed the experimental set-up and analysis solution for the fNIRS-fEEG signals. D.M., M.M., K.B. and G.F. developed the experimental set-up and analysis solution for the kinematics signals. M.M., D.M., S.P., I.L., M.D., J.F., B.X., G.D. and K.B. participated in the reviewing and editing of the manuscript. All authors read and approved the final manuscript.
Funding
The clinical trial was funded by the Direction générale de l’offre de soins (DGOS) of the French Ministère des Affaires Sociales et de la Santé (Programme hospitalier de recherche infirmière et paramédicale-PHRIP-2018-0731), with additional support from the LabEx NUMEV (ANR-10-LABX-0020) within the I-Site MUSE (ANR-16-IDEX-0006).
Data availability
Data will be available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted as part of the ReArm clinical trial (ID-RCB 2019-A00506-51) following ethical approval by the French Committee for the Protection of Individuals (CPP SUD-EST II) for patient inclusion. In addition, the local ethics committee approved the protocol for healthy participants (IRB-EM 1912B). All participants gave written informed consent prior to taking part in the study.
Consent for publication
All authors give their consent to publish.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miller EL, Murray L, Richards L, Zorowitz RD, Bakas T, Clark P, et al. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: a scientific statement from the American Heart Association. Stroke. 2010;41(10):2402–48. [DOI] [PubMed] [Google Scholar]
- 2.Ward NS. Compensatory mechanisms in the aging motor system. Ageing Res Rev. 2006;5(3):239–54. [DOI] [PubMed] [Google Scholar]
- 3.Calautti C, Baron JC. Functional neuroimaging studies of Motor Recovery after stroke in adults. Stroke. 2003;34(6):1553–66. [DOI] [PubMed] [Google Scholar]
- 4.Buma FE, Lindeman E, Ramsey NF, Kwakkel G. Functional neuroimaging studies of early upper limb recovery after stroke: a systematic review of the literature. Neurorehabil Neural Repair. 2010;24(7):589–608. [DOI] [PubMed] [Google Scholar]
- 5.Rehme AK, Eickhoff SB, Rottschy C, Fink GR, Grefkes C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. NeuroImage. 2012;59(3):2771–82. [DOI] [PubMed] [Google Scholar]
- 6.Feydy A, Carlier R, Roby-Brami A, Bussel B, Cazalis F, Pierot L, et al. Longitudinal study of Motor Recovery after Stroke. Stroke. 2002;33(6):1610–7. [DOI] [PubMed] [Google Scholar]
- 7.Tombari D, Loubinoux I, Pariente J, Gerdelat A, Albucher JF, Tardy J, et al. A longitudinal fMRI study: in recovering and then in clinically stable sub-cortical stroke patients. NeuroImage. 2004;23(3):827–39. [DOI] [PubMed] [Google Scholar]
- 8.Chollet F, DiPiero V, Wise RJ, Brooks DJ, Dolan RJ, Frackowiak RS. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann Neurol. 1991;29(1):63–71. [DOI] [PubMed] [Google Scholar]
- 9.Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, et al. A functional MRI study of subjects recovered from Hemiparetic Stroke. Stroke. 1997;28(12):2518–27. [DOI] [PubMed] [Google Scholar]
- 10.Dong Y, Dobkin BH, Cen SY, Wu AD, Winstein CJ. Motor cortex activation during treatment may predict therapeutic gains in paretic hand function after stroke. Stroke. 2006;37(6):1552–5. [DOI] [PubMed] [Google Scholar]
- 11.Gale SD, Pearson CM. Neuroimaging predictors of stroke outcome: implications for neurorehabilitation. NeuroRehabilitation. 2012;31(3):331–44. [DOI] [PubMed] [Google Scholar]
- 12.Van Dokkum L, Le Bars E, Mottet D, Bonafe A, Menjot de Champfleur N, Laffont I. Modified brain activations in the non-damaged hemisphere during movements of the supposed to be healthy upper-limb. Annals Phys Rehabilitation Med. 2016;59:e68. [Google Scholar]
- 13.Obrig H, Villringer A. Beyond the visible–imaging the human brain with light. J Cereb Blood Flow Metab. 2003;23(1):1–18. [DOI] [PubMed] [Google Scholar]
- 14.Gramigna V, Pellegrino G, Cerasa A, Cutini S, Vasta R, Olivadese G, et al. Near-Infrared Spectroscopy in Gait disorders: is it Time to begin? Neurorehabil Neural Repair. 2017;31(5):402–12. [DOI] [PubMed] [Google Scholar]
- 15.Derosière G, Alexandre F, Bourdillon N, Mandrick K, Ward TE, Perrey S. Similar scaling of contralateral and ipsilateral cortical responses during graded unimanual force generation. NeuroImage. 2014;85:471–7. [DOI] [PubMed] [Google Scholar]
- 16.Muller CO, Perrey S, Bakhti K, Muthalib M, Dray G, Xu B et al. Aging effects on electrical and hemodynamic responses in the sensorimotor network during unilateral proximal upper limb functional tasks. Behav Brain Res. 2023;114322. [DOI] [PubMed]
- 17.Larivière S, Xifra-Porxas A, Kassinopoulos M, Niso G, Baillet S, Mitsis GD, et al. Functional and effective reorganization of the aging brain during unimanual and bimanual hand movements. Hum Brain Mapp. 2019;40(10):3027–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim SB, Eng JJ. Increased sensorimotor cortex activation with decreased Motor Performance during Functional Upper Extremity tasks Poststroke. J Neurol Phys Ther. 2019;43(3):141–50. [DOI] [PubMed] [Google Scholar]
- 19.Delorme M, Vergotte G, Perrey S, Froger J, Laffont I. Time course of sensorimotor cortex reorganization during upper extremity task accompanying motor recovery early after stroke: an fNIRS study. Restor Neurol Neurosci. 2019;37(3):207–18. [DOI] [PubMed] [Google Scholar]
- 20.Nishiyori R, Bisconti S, Ulrich B. Motor Cortex Activity during Functional Motor skills: an fNIRS Study. Brain Topogr. 2016;29(1):42–55. [DOI] [PubMed] [Google Scholar]
- 21.Olejniczak P. Neurophysiologic basis of EEG. J Clin Neurophysiol. 2006;23(3):186–9. [DOI] [PubMed] [Google Scholar]
- 22.Nakayashiki K, Saeki M, Takata Y, Hayashi Y, Kondo T. Modulation of event-related desynchronization during kinematic and kinetic hand movements. J Neuroeng Rehabil. 2014;11:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110(11):1842–57. [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Shu X, Jia J, Wang H, Ding L, He Z, et al. Relation between Sensorimotor Rhythm during Motor Attempt/Imagery and Upper-Limb Motor Impairment in Stroke. Clin EEG Neurosci. 2022;53(3):238–47. [DOI] [PubMed] [Google Scholar]
- 25.Bartur G, Pratt H, Soroker N. Changes in mu and beta amplitude of the EEG during upper limb movement correlate with motor impairment and structural damage in subacute stroke. Clin Neurophysiol. 2019;130(9):1644–51. [DOI] [PubMed] [Google Scholar]
- 26.Kaiser V, Daly I, Pichiorri F, Mattia D, Müller-Putz GR, Neuper C. Relationship between electrical brain responses to Motor Imagery and Motor Impairment in Stroke. Stroke. 2012;43(10):2735–40. [DOI] [PubMed] [Google Scholar]
- 27.Yang M, Yang Z, Yuan T, Feng W, Wang P. A systemic review of functional near-infrared spectroscopy for stroke: current application and future directions. Front Neurol. 2019;10:58. [DOI] [PMC free article] [PubMed]
- 28.Bakhti KKA, Mottet D, Schweighofer N, Froger J, Laffont I. Proximal arm non-use when reaching after a stroke. Neurosci Lett. 2017;657:91–6. [DOI] [PubMed] [Google Scholar]
- 29.Bakhti KKA, Laffont I, Muthalib M, Froger J, Mottet D. Kinect-based assessment of proximal arm non-use after a stroke. J Neuroeng Rehabil. 2018;15(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taub E, Uswatte G, Mark VW, Morris DMM. The learned nonuse phenomenon: implications for rehabilitation. Eura Medicophys. 2006;42(3):241–56. [PubMed] [Google Scholar]
- 31.Lindberg PG, Schmitz C, Engardt M, Forssberg H, Borg J. Use-dependent up- and down-regulation of sensorimotor brain circuits in stroke patients. Neurorehabil Neural Repair. 2007;21(4):315–26. [DOI] [PubMed] [Google Scholar]
- 32.Lum PS, Mulroy S, Amdur RL, Requejo P, Prilutsky BI, Dromerick AW. Gains in upper extremity function after stroke via recovery or compensation: potential differential effects on amount of real-world limb use. Top Stroke Rehabil. 2009;16(4):237–53. [DOI] [PubMed] [Google Scholar]
- 33.Jones TA. Motor compensation and its effects on neural reorganization after stroke. Nat Rev Neurosci. 2017;18(5):267–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. [PubMed] [Google Scholar]
- 35.Gauthier L, Dehaut F, Joanette Y. The bells test: a quantitative and qualitative test for visual neglect. Int J Clin Neuropsychol. 1989;11(2):49–54.
- 36.Kaplan E, Goodglass H, Weintraub S, Goodglass H. Boston naming test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 37.Kalafat M, Hugonot-Diener L, Poitrenaud J. Standardisation et étalonnage français du ‘Mini Mental State’ (MMS) version GRÉCO. [French standardization and range for the GRECO version of the ‘Mini Mental State’ (MMS)]. Revue De Neuropsychologie. 2003;13(2):209–36. [Google Scholar]
- 38.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. [DOI] [PubMed] [Google Scholar]
- 39.Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16(3):232–40. [DOI] [PubMed] [Google Scholar]
- 40.Muller CO, Muthalib M, Mottet D, Perrey S, Dray G, Delorme M, et al. Recovering arm function in chronic stroke patients using combined anodal HD-tDCS and virtual reality therapy (ReArm): a study protocol for a randomized controlled trial. Trials. 2021;22(1):747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taub E, Uswatte G, Mark VW, Morris DM, Barman J, Bowman MH, et al. Method for Enhancing Real-World Use of a more affected arm in chronic stroke. Stroke. 2013;44(5):1383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123(5):940–53. [DOI] [PubMed] [Google Scholar]
- 43.Besson P, Muthalib M, De Vassoigne C, Rothwell J, Perrey S. Effects of multiple Sessions of Cathodal Priming and Anodal HD-tDCS on Visuo Motor Task Plateau Learning and Retention. Brain Sci. 2020;10(11):875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985;39(6):386–91. [DOI] [PubMed] [Google Scholar]
- 45.Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001;32(7):1635–9. [DOI] [PubMed] [Google Scholar]
- 46.Mahoney FI, Barthel DW. FUNCTIONAL EVALUATION: THE BARTHEL INDEX. Md State Med J. 1965;14:61–5. [PubMed] [Google Scholar]
- 47.Faity G, Mottet D, Froger J. Validity and reliability of Kinect v2 for quantifying Upper Body Kinematics during Seated reaching. Sensors. 2022;22(7):2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Accot Z. Scale effects in steering law tasks. in Proceedings of the SIGCHI conference on Human factors in computing systems. 2001.
- 49.MacKenzie IS. Fitts’ Law as a Research and Design Tool in Human-Computer Interaction. Human–Computer Interact. 1992;7(1):91–139. [Google Scholar]
- 50.Huppert TJ, Diamond SG, Franceschini MA, Boas DA. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl Opt AO. 2009;48(10):D280–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. [DOI] [PubMed] [Google Scholar]
- 52.Wickham H. ggplot2 [Internet]. Cham: Springer International Publishing; 2016 [cited 2023 Nov 8]. (Use R!).
- 53.Wickham H, François R, Henry L, Müller K, Vaughan D. dplyr: a grammar of data manipulation [Internet]. 2023 [cited 2023 Nov 8].
- 54.Kassambara A. Pipe-friendly framework for basic statistical tests [R package rstatix version 0.6.0] [Internet]. 2023 [cited 2023 Nov 8].
- 55.Bakeman R. Recommended effect size statistics for repeated measures designs. Behav Res Methods. 2005;37(3):379–84. [DOI] [PubMed] [Google Scholar]
- 56.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. [DOI] [PMC free article] [PubMed]
- 57.Benjamini Y, Hochberg Y. Controlling the false Discovery rate: a practical and powerful Approach to multiple testing. J Roy Stat Soc: Ser B (Methodol). 1995;57(1):289–300. [Google Scholar]
- 58.Tecchio F, Zappasodi F, Tombini M, Caulo M, Vernieri F, Rossini PM. Interhemispheric asymmetry of primary hand representation and recovery after stroke: a MEG study. NeuroImage. 2007;36(4):1057–64. [DOI] [PubMed] [Google Scholar]
- 59.Levin MF, Kleim JA, Wolf SL. What do Motor Recovery and Compensation. Mean Patients Following Stroke? Neurorehabil Neural Repair. 2009;23(4):313–9. [DOI] [PubMed] [Google Scholar]
- 60.Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol. 1954;47:381–91. [PubMed] [Google Scholar]
- 61.Kelly AMC, Garavan H. Human functional neuroimaging of Brain Changes Associated with Practice. Cereb Cortex. 2005;15(8):1089–102. [DOI] [PubMed] [Google Scholar]
- 62.Haier RJ, Siegel B, Tang C, Abel L, Buchsbaum MS. Intelligence and changes in regional cerebral glucose metabolic rate following learning. Intelligence. 1992;16(3–4):415–26. [Google Scholar]
- 63.Sakurada T, Hirai M, Watanabe E. Individual optimal attentional strategy during implicit motor learning boosts frontoparietal neural processing efficiency: a functional near-infrared spectroscopy study. Brain Behav. 2018;9(1):e01183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamper DG, McKenna-Cole AN, Kahn LE, Reinkensmeyer DJ. Alterations in reaching after stroke and their relation to movement direction and impairment severity. Arch Phys Med Rehabil. 2002;83(5):702–7. [DOI] [PubMed] [Google Scholar]
- 65.Tazoe T, Perez MA. Speed-dependent contribution of Callosal pathways to Ipsilateral movements. J Neurosci. 2013;33(41):16178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teo WP, Muthalib M, Yamin S, Hendy AM, Bramstedt K, Kotsopoulos E, et al. Does a combination of virtual reality, neuromodulation and neuroimaging provide a comprehensive platform for neurorehabilitation? - A narrative review of the literature. Front Human Neurosci. 2016;10:284. [DOI] [PMC free article] [PubMed]
- 67.Huo C, Xu G, Li W, Xie H, Zhang T, Liu Y, et al. A review on functional near-infrared spectroscopy and application in stroke rehabilitation. Med Novel Technol Devices. 2021;11:100064. [Google Scholar]
- 68.Monge-Pereira E, Ibañez-Pereda J, Alguacil-Diego IM, Serrano JI, Spottorno-Rubio MP, Molina-Rueda F. Use of Electroencephalography Brain-Computer Interface Systems as a Rehabilitative Approach for Upper Limb function after a stroke. Syst Rev PM&R. 2017;9(9):918–32. [DOI] [PubMed] [Google Scholar]
- 69.Gonzalez-Rosa JJ, Natali F, Tettamanti A, Cursi M, Velikova S, Comi G, et al. Action observation and motor imagery in performance of complex movements: evidence from EEG and kinematics analysis. Behav Brain Res. 2015;281:290–300. [DOI] [PubMed] [Google Scholar]
- 70.Yeung MK, Chu VW. Viewing neurovascular coupling through the lens of combined EEG–fNIRS: a systematic review of current methods. Psychophysiology. 2022;59(6):e14054. [DOI] [PubMed] [Google Scholar]
- 71.Li R, Yang D, Fang F, Hong KS, Reiss AL, Zhang Y. Concurrent fNIRS and EEG for brain function investigation: a systematic, methodology-focused review. Sensors. 2022;22(15):5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berthoz A, Weiss G. The vicarious brain, creator of worlds [Internet]. Harvard University Press; 2017 [cited 2023 Feb 3].
- 73.Yavuzer G, Küçükdeveci A, Arasil T, Elhan A. Rehabilitation of stroke patients: clinical profile and functional outcome. Am J Phys Med Rehabil. 2001;80(4):250–5. [DOI] [PubMed] [Google Scholar]
- 74.Shoemaker MJ, Mullins-MacRitchie M, Bennett J, Vryhof K, Boettcher I. Predicting Response to Rehabilitation in Elderly patients with stroke using the Orpington Prognostic Scale and selected clinical variables. J Geriatr Phys Ther. 2006;29(2):69. [DOI] [PubMed] [Google Scholar]
- 75.Hawe RL, Cluff T, Dowlatshahi D, Hill MD, Dukelow SP. Assessment of Sex differences in Recovery of Motor and sensory impairments Poststroke. Neurorehabil Neural Repair. 2020;34(8):746–57. [DOI] [PubMed] [Google Scholar]
- 76.Luk JKH, Cheung RTF, Ho SL, Li L. Does Age Predict Outcome in Stroke Rehabilitation? A study of 878 Chinese subjects. Cerebrovasc Dis. 2006;21(4):229–34. [DOI] [PubMed] [Google Scholar]
- 77.Stinear CM. Prediction of motor recovery after stroke: advances in biomarkers. Lancet Neurol. 2017;16(10):826–36. [DOI] [PubMed] [Google Scholar]
- 78.Bagg S, Pombo AP, Hopman W. Effect of Age on Functional outcomes after Stroke Rehabilitation. Stroke. 2002;33(1):179–85. [DOI] [PubMed] [Google Scholar]
- 79.Mandon L, Boudarham J, Robertson J, Bensmail D, Roche N, Roby-Brami A. Faster reaching in Chronic Spastic Stroke patients comes at the expense of arm-trunk coordination. Neurorehabil Neural Repair. 2016;30(3):209–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Flowchart of the fEEG and fNIRS pre-processing and processing steps. Method based on previous study. (Muller 2023). Abbreviations ERSP, event-related spectral perturbation; ERS, event-related synchronization; ERD, event-related desynchronization; RP, relative power; Pn, power spectrum; ΔHbO2, variation of oxygenated blood
Supplementary Material 2: Statistical results of the ANOVA on the fEEG and fNIRS brain parameters for the circular steering task
Supplementary Material 3: Statistical results of the ANOVA on the fEEG and fNIRS brain parameters for the paced-reaching task
Data Availability Statement
Data will be available from the corresponding author on reasonable request.