Abstract
Background
Pneumococcal meningitis, a vaccine-preventable disease caused by Streptococcus pneumoniae (Spn) is the leading bacterial meningitis in under five children. In April 2014, Uganda introduced routine immunization with 10-valent Pneumococcal Conjugate Vaccine (PCV10) for infants. The target coverage for herd immunity is ≥ 90% with three doses (PCV10-dose 3). We assessed the effect of PCV10 introduction and coverage on the trends of pneumococcal meningitis in under five children.
Methods
We analyzed laboratory-confirmed pediatric bacterial meningitis (PBM) data at two high-volume WHO-accredited sentinel surveillance hospitals in Kampala City and Gulu District, from 2003 to 2022. We used confirmed cases to estimate the minimum incidence of pneumococcal meningitis in the host districts and calculated annual incidence of pneumococcal meningitis per one million populations, and the proportion of confirmed PBM attributable to Spn. We divided the study period into 2003–2013 (pre-PCV10) and 2014–2022 (post-PCV10), and conducted interrupted time series analysis using autoregressive integrated moving average models for the effect of PCV10 on trends of pneumococcal meningitis and PBM attributable to Spn. We analyzed reported PCV10 data in DHIS2 from 2014 to 2022 for annual PCV10-dose 3 coverage.
Results
Among the 534 confirmed PBM cases, 331(62%) were pneumococcal meningitis; 227(69%) from Gulu District and 104(31%) from Kampala City. The majority (95%) of the isolates were not serotyped. The majority (57%) were male and unimmunized (98%); median age = 14(IQR = 6–27) months with most (55%) aged ≥ 12 months. The case-fatality rate was 9%. During Pre-PCV10 period, the overall incidence of pneumococcal meningitis in the host districts increased; slope change = 1.0 (95%CI = 0.99999, 1.00001) but declined in post-PCV10 period (2014–2022) by 92% from 86 cases /1,000,000 in 2014 to 7/1,000,000 in 2022, slope change= -1.00006 (95%CI=-1.00033, -0.99979). Whereas there was an immediate decline in the proportion of confirmed PBM attributable to Spn in the host districts, level change=-1.84611(95%CI=-1.98365,-1.70856), an upward trend was recorded from 2016 to 2022, slope change = 1.0 (95%CI = 0.99997, 1.00003). During 2015–2022, PCV10-dose 3 coverage was largely > 90% for Gulu District and 52–72% for Kampala City.
Conclusion
The PCV10 routine immunization program reduced the incidence of pneumococcal meningitis in Kampala City and Gulu District. There was no effect on the confirmed PBM proportionately attributable to Spn. Kampala City persistently recorded PCV10-dose3 coverage < 90%. We recommend enhancing serotyping and periodic nasopharyngeal carriage surveys to ascertain the maximum vaccine effectiveness and monitor Spn serotypes, and strengthening routine immunization in Kampala City.
Keywords: Pneumococcal meningitis, Streptococcus pneumoniae, Children under five, 10-valent pneumococcal conjugate vaccine, Uganda
Background
Streptococcus pneumoniae (Spn) is the cause of pneumococcal meningitis (Spn meningitis), a vaccine-preventable life-threatening disease. Pneumococcal meningitis together with other invasive pneumococcal diseases (IPD) caused by Spn including septicemia, and osteomyelitis [1] account for approximately one million deaths every year, most among children < 5 years [2]. Pneumococcal meningitis (Spn meningitis) has a case-fatality rate of about 50% in developing countries, and accounts for nearly half of deaths due to IPD. Additionally, 20% of the survivors develop serious long-term neurological complications such as hearing loss, epilepsy, cognitive dysfunction, impaired vision and speech [3, 4]. Approximately 90 serotypes of Spn exist worldwide, but seven serotypes (types 1, 5, 6 A, 6B, 14, 19 F, and 23 F) cause most (≥ 70%) of the Spn meningitis [2, 3].
The World Health Organization (WHO) recommends two pneumococcal conjugate vaccines (PCV) for immunization against IPD in Africa: the 10-valent (PCV10, composed of antigens from serotypes 1, 4, 5, 6B, 7 F, 9 V, 14, 18 C, 19 F, and 23 F) and the 13-valent (PCV13, composed of antigens from serotypes 1, 3, 4, 5, 6 A, 6B, 7 F, 9 V, 14, 18 C, 19 A, 19 F, and 23 F) vaccines [5]. Both PCV10 and PCV13 have been shown to offer protection against over 70% of serotypes associated with IPD in children < 5 years [2]. Beyond directly protecting recipients against serotype-specific IPD, the pneumococcal conjugate vaccine also indirectly protects unimmunized children and adults in close contact in the community by reducing Spn carriage in the close population [1]. A study in selected African countries revealed that PCV10 had significantly reduced the proportion of confirmed Spn meningitis cases due to serotypes included in PCV10 from 77.8% in 2011 to 37.1% in 2016 [5]. Other studies in Kenya and Gambia demonstrated reduction in the incidence of IPD due to any Spn serotype in children under 5 years by 81% and 56%, after the introduction of PCV10 and PCV13 respectively [6, 7].
Uganda lies in the meningitis belt of Africa and is prone to meningitis outbreaks.
From 2001 to 2006, Spn meningitis, caused by at least 30 different serotypes, was the leading type of bacterial meningitis in children < 5 years in Uganda [8]. PCV10 covered 14 (47%) of the serotypes responsible for IPD in Uganda at the time of introduction [8].
In April 2014, the Uganda Ministry of Health (MoH) introduced PCV10 into the routine vaccination program for children < 1 year. The vaccine is given in three doses, starting at the age of 6 weeks and with an interval of 4 weeks between the next two doses, in line with the 3p + 0 PCV immunization schedule [9]. In May 2023, the national coverage for the 3rd dose of PCV10 among children < 1 year stood at 86% [10]. This was below the World Health Organization (WHO) and national target of 90% required for herd immunity [11]. In addition, studies have found that immunity among children vaccinated under the 3p + 0 PCV schedule wanes over time, and is significantly reduced after the first year of life [1, 12], raising questions about the effectiveness of the vaccine in the long-term.
To date, no studies have been done to determine the impact of PCV10 on the trend of pneumococcal meningitis in Uganda since its introduction into the routine immunization program in 2014. Additionally, there was no population-based surveillance system for pneumococcal meningitis in Uganda, as of 2023. In absence of such a community based surveillance system, WHO recommends use of data from sentinel surveillance sites as alternatives to assess the impact of PCV [1].
We described the characteristics and clinical outcomes, trends in incidence and proportion of Spn meningitis among children < 5 from 2003 to 2022, and PCV10 coverage for sentinel surveillance host districts in Uganda to inform programming.
Methods
Study design, setting, and data source
We conducted a descriptive analysis of PBM surveillance data at the two active large volume WHO-accredited sentinel meningitis surveillance sites in Uganda from
2003–2022. The sites are located at Mulago National Referral Hospital in Kampala City, Central Uganda, and St. Mary’s Lacor Hospital in Gulu District, Northern Uganda. We also analyzed the national and district PCV10 coverage data for Uganda from the District Health Information Software 2 (DHIS2), 2014–2022.
Paediatric bacterial meningitis surveillance at sentinel sites
On July 12, 2001, Uganda established the first pediatric bacterial meningitis (PBM) sentinel surveillance site for children < 5 at Mulago National Referral Hospital in Kawempe Division, Kampala Capital City. Two years later, another PBM sentinel surveillance site was established at St. Mary’s Lacor Hospital in Gulu District. At each of the sites, individual case data is captured using a standardized electronic case investigation form. The form captures personal identification and demographic characteristics, medical and immunization history, clinical information, and laboratory investigations.
At both sites, a suspected case of pediatric bacterial meningitis was defined as sudden onset, in a child aged 0–59 months, of fever (axillary temperature > 380C or rectal temperature > 38.50C) and ≥ 1 of the following: seizures other than febrile seizures, neck stiffness, bulging fontanel, altered consciousness, or other meningeal signs. Probable bacterial meningitis was defined as a suspected meningitis case with cerebrospinal fluid (CSF) examination showing at least one of the following: turbid or cloudy appearance, leukocytosis (> 100 cells/mm3) or leukocytosis (10–100 cells/mm3) AND either an elevated protein (> 100 mg/dl) or decreased glucose (< 100 mg/mm3). Confirmed meningitis was defined as suspected case with a laboratory-confirmed bacterial pathogen in CSF or blood in a child with a clinical syndrome consistent with bacterial meningitis [13]. The laboratory diagnostic methods used to confirm bacterial meningitis at the sentinel sites are quality-assured culture, latex agglutination, Binax test for Spn, and Polymerase Chain Reaction (PCR) testing with or without serotyping of isolates. Serotyping is conducted to identify the circulating serotypes of the causative bacterial agent in the population for purposes of surveillance. In this study, we used laboratory confirmed cases.
Surveillance for the 10-valent pneumococcal meningitis vaccination coverage in Uganda
Aggregate PCV10 coverage data for children < 1 year is routinely reported by health facilities through DHIS2, the electronic database for the Health Management Information System (HMIS) in Uganda. The data is reported in the monthly outpatient report form (HMIS 105), and is disaggregated by dose as per the schedule. In DHIS2, the health facilities are grouped under their respective sub-counties and districts [14]. To assess the coverage of pneumococcal conjugate vaccine (PCV10) in the host districts, we included aggregate records of PCV10 coverage for each of the three doses for all children < 1 year, reported in DHIS2, 2014–2022.
Study population
To describe the characteristics and clinical outcomes, and trends of Spn meningitis among children < 5, we included all records for children aged 2–59 months with laboratory-confirmed bacterial meningitis, by culture, Binax, latex agglutination or Polymerase Chain Reaction (PCR), 2003–2022. Children < 2 months were excluded because the bacterial etiology of meningitis in that age group is different from that of the older age group. Additionally, optimal vaccine induced immunity from PCV requires at least 4 weeks after the dose to develop, yet the first dose is given at 6 weeks of age [12]. We additionally excluded children with mixed bacterial causes identified in their CSF.
Study variables, data abstraction, and analysis
We abstracted data on demographic characteristics, clinical signs and symptoms, laboratory test results, PCV vaccination status and doses and disease outcomes from individual electronic case investigation forms. We also abstracted PCV-10 vaccination data for the sentinel surveillance site host districts from District Health Information Software Version2 (DHIS2) from 2014 to 2022, using pivot tables. Additionally, we abstracted the population census data for the host districts from the Uganda Bureau of Statistics (UBOS) and the estimated proportion of surviving infants (4.3%) to determine the denominator by year for Gulu District, and Kawempe Division in Kampala City [15]. Kawempe Division was considered to represent Kampala City because it’s the primary catchment area for Mulago National Referral Hospital PBM sentinel surveillance site, and we assumed that almost all the pediatric bacterial meningitis seek care at the site.
We analyzed the data using Microsoft Excel, Epi Info, version 7.2.5.0 and Stata version 12. We categorized age of cases into three meaningful categories (2–11, 12–23, and 24–59 months) based on known differences in their susceptibility to pneumococcal meningitis. We calculated proportions and rates for categorical variables including sex, vaccination status, and causative bacterial agent, age group, presenting signs and symptoms and outcomes. We computed case-fatality rate as the percentage of children 2–59 months with pneumococcal meningitis that died and disaggregated it by age group. Additionally, we calculated the median age of the cases.
Since PBM sentinel surveillance system was not population-based, we used laboratory confirmed pneumococcal meningitis cases to estimate the minimum incidence of pneumococcal meningitis in Gulu District and Kampala City’s Kawempe Division. The estimated incidence of pneumococcal meningitis in Kawempe Division was used as proxy for the incidence in Kampala City.
We divided the study period into 2003–2013 (pre-PCV10) and 2014–2022 (post-PCV10). We conducted interrupted time series analysis using autoregressive integrated moving average (ARIMA) models with robust standard errors, with a single interruption corresponding to 2014 (the year of introduction of PCV10 into Uganda’s immunization program) to assess the effect of routine PCV10 immunization. The study outcomes were the annual incidence of pneumococcal meningitis and proportion of pediatric bacterial meningitis attributable to Streptococcus pneumoniae. We plotted a line graph of the annual incidence of pneumococcal meningitis and proportion of pediatric bacterial meningitis attributable to Streptococcus pneumoniae to assess for stationarity and seasonality. Additionally, we used the Durbin-Watson test to identify autocorrelation in the observations for both annual incidence of pneumococcal meningitis and the proportion of pediatric bacterial meningitis attributable to Spn. Since there was no autocorrelation between the observed annual incidences of pneumococcal meningitis (p-value = 0.34), and proportions of pediatric bacterial meningitis attributable to Spn (p-value = 0.46), we did not apply any autocorrelation lag. Whereas the time series for both the incidence and proportion of PBM attributable to Spn displayed no clear seasonal patterns, they were non-stationary. We applied a differencing lag of 2 for incidence and a lag of 1 for the proportion of PBM attributable to Spn time series to achieve stationarity. Additionally, a moving average lag of 2 was applied based on the estimated time lag required to realize for the maximum effect of pneumococcal conjugate vaccines on pneumococcal disease epidemiology [1]. We used the stepwise approach to estimate both the immediate (level change) and long-term (slope change) effects of PCV10 introduction on incidence of Spn meningitis and proportion of PBM attributable to Spn. We used the Akaike information criteria (AIC) to select the best fitting ARIMA models, and the final models were validated as white noise by checking the model residuals for autocorrelation using the Durbin-Watson test.
We also calculated the coverage for the third dose of PCV10 (PCV10-dose 3) as the percentage of surviving infants that had received all the three doses of PCV10; a measure of full immunization against Spn. We used line graphs to display trends of incidence of pneumococcal meningitis and PCV10-dose 3 coverage over time.
Results
Characteristics of children aged 2–59 months with confirmed pneumococcal meningitis at the pediatric bacterial meningitis sentinel surveillance sites, Uganda, 2003–2022
We identified a total of 12,528 cases of bacterial meningitis among children aged 2–59 months, admitted at Mulago National Referral Hospital in Kampala City, and St. Mary’s Hospital, Lacor in Gulu District, from 2003 to 2022. The majority of cases, n = 12,152 (97%) had CSF analysis done; 534 (4%) were laboratory confirmed cases, 852 (7%) were probable and 11,142 (89%) were suspected. Among those confirmed, 331(62%) were cases of pneumococcal meningitis but only 15(5%) had Spn serotype results. Overall, the majority, n = 281 (85%) of the pneumococcal meningitis cases were admitted in the pre-PCV10 period and this was consistent upon stratification by host district, sex and age group. Most of the cases n = 227 (69%) were identified from Gulu District, and the majority, n = 189 (57%) were male. The median age was 14 months (IQR = 6–28 months) and there was no significant difference in the median age in the post-PCV10 and pre-PCV10 eras as revealed by overlapping interquartile ranges. Notably, most of the cases, n = 183 (55%) were aged 12–59 months (Table 1).
Table 1.
Characteristics of children aged 2–59 months with pneumococcal meningitis in the pediatric bacterial meningitis sentinel surveillance host districts, Uganda, 2003–2022
| Variable | Total Cases (%) | Pre-PCV10 | Post-PCV10 | ||
|---|---|---|---|---|---|
| Cases | Percentage | Cases | Percentage | ||
| Sentinel surveillance site host district | |||||
| Gulu District | 227(69) | 202 | 89 | 25 | 11 |
| Kampala City | 104(31) | 79 | 76 | 25 | 24 |
| Sex | |||||
| Male | 189(57) | 154 | 81 | 35 | 19 |
| Female | 142(43) | 127 | 89 | 15 | 11 |
| Age (in months) | |||||
| 2–11 | 148(45) | 124 | 84 | 24 | 16 |
| 12–23 | 83(25) | 70 | 84 | 13 | 16 |
| 24–59 | 100(30) | 87 | 13 | 13 | 13 |
| Median age | 14 (IQR = 6–27 months) | 14 (IQR = 6–28 months) | 12 (IQR = 6–24 months) | ||
Clinical presentation and outcomes of children aged 2–59 months with confirmed pneumococcal meningitis attending sentinel surveillance hospitals, Uganda, 2003–2022.
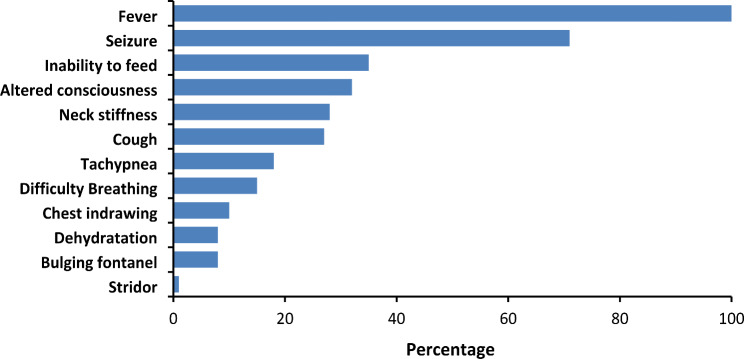
In addition to fever, majority (71%) of the cases presented with seizures, followed by inability to feed (35%), and altered consciousness (32%). Bulging of the fontanel (8%) was the least common meningeal symptom (Fig. 1). Overall, among Spn meningitis cases with documented outcomes at discharge (91%), the case fatality rate (CFR) was 9% (26/302). The CFR was highest among children aged
Fig. 1.
Clinical presentation of children < 5 years with confirmed pneumococcal meningitis, Gulu and Kampala City, Uganda, 2003–2022 (N = 331)
24–59 months (12%) followed by those aged 2-11months (9%); children aged 12–23 months had the lowest (4%) case fatality rate. The average case-fatality rate for each of these two age-groups was more than twice as high compared to those aged 12–23 months.
Effect of 10-valent pneumococcal conjugate vaccine on trends in incidence of Spn meningitis among children < 5 in pediatric bacterial meningitis sentinel surveillance host districts, Uganda, 2003–2022.
Trend in incidence of pneumococcal meningitis before introduction of the PCV10 routine immunization program (2003–2013)
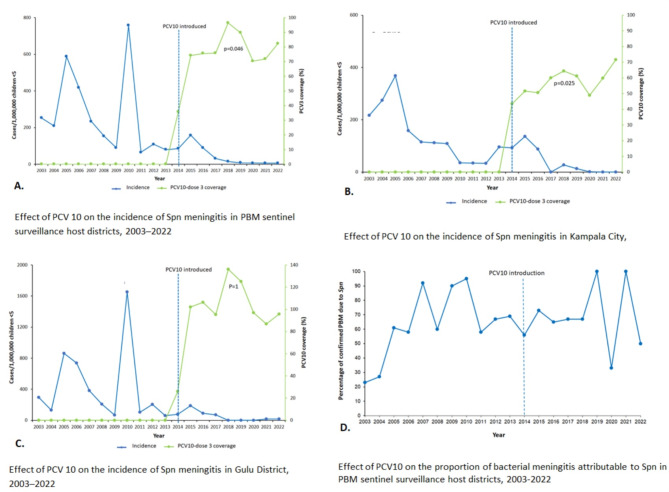
Prior to the introduction of PCV10, the overall incidence of pneumococcal meningitis among children < 5 years in the pediatric bacterial meningitis surveillance site host districts increased; slope change = 1.0 (95%CI = 0.99999, 1.00001) (Table 2; Fig. 2A). Specifically, the increase in incidence of pneumococcal meningitis was recorded in Gulu District; slope change = 0.99995 (95%CI = 0.99985, 1.00006) from 2003 to 2013 (Table 2; Fig. 2C). This was probably driven by pneumococcal meningitis outbreaks in Gulu District during 2006–2007, and 2016 reflected by peaks in those years (Fig. 2A and C). In Kampala City, there was no significant change in the incidence of pneumococcal meningitis among children < 5 years; slope change = 0.11206 (95%CI=-2.80445, 3.02862), p = 0.94 during the pre-PCV1O period (Table 2; Fig. 2-B).
Table 2.
Interrupted time series analysis for effect of PCV10 on the incidence of pneumococcal meningitis among children < 5 in PBM sentinel surveillance host districts, Uganda, from 2003–2022
| District | Pre-PCV10 (2003–2013) | Post-PCV10 (2014–2022) | ||||
|---|---|---|---|---|---|---|
| Trend (slope change) | Immediate (level change) | Trend (slope change) | ||||
| Estimated change (95%CI) | P-value | Estimated change (95%CI) | P-value | Estimated change (95%CI) | P-value | |
| Kampala City | 0.11206 (-2.80445,3.02862) | 0.94 | -1.85145 (-1.92094,-1.782) | < 0.001 | -0.99997(-1.00002,-0.99997) | < 0.001 |
| Gulu District | 0.99995 (0.99985,1.00006) | < 0.001 | -0.19783 (-1.31913,0.92346) | 0.729 | -0.99992 (-1.00115,-0.99869) | < 0.001 |
| Overall | 1.0 (0.99999,1.00001) | < 0.001 | 0.00024(-0.00029,-0.00033) | 0.881 | -1.00006 (-1.00033,-0.99979) | < 0.001 |
Fig. 2.
Trends in Spn meningitis incidence and proportion of PBM due to Spn among children aged 2–59 months in the pediatric bacterial meningitis sentinel surveillance host districts, 2003–2022
Trend in the incidence of pneumococcal meningitis after introduction of the PCV10 routine immunization program (2014–2022)
Overall, the introduction of PCV10 into the routine immunization program resulted in a sustained decline in the incidence of pneumococcal meningitis in the sentinel surveillance host districts; slope change= -1.00006 (95%CI=--1.00033,-0.99979), p < 0.001. In Kampala City, there was an immediate effect of PCV10 on the incidence of pneumococcal meningitis, two years post-introduction; level change=-1.85145 (95%CI =-1.92094, -1.782), p < 0.001 and a sustained decline in incidence during 2016–2022; slope change=-0.99997(95%CI =-1.00002,-0.99997), p < 0.001 (Table 2; Fig. 2B). Similarly, Gulu District recorded a sustained decline in incidence post introduction of PCV10; slope change= -0.99992 (95%CI=-1.00115,-0.99869). However, unlike Kampala City, there was no immediate change in incidence post introduction in Gulu District; level change= -0.19783 (95%CI=-1.31913, 0.92346), P = 0.73. Overall, the incidence of pneumococcal meningitis declined by 92% from 86 cases /1,000,000 in 2014 to 7/1,000,000 in 2022 (Fig. 2-B). Specifically, in Kampala City, the incidence declined by 100% from 93 cases/1,000,000 in 2014 to 0/1,000,000 in 2022 (Fig. 2-B). Similarly, Gulu District posted an 81% reduction from 77 cases to 15/1,000,000 (Fig. 2-C).
Effect of PCV10 on trends in the proportion of confirmed bacterial meningitis attributable to Spn, pediatric bacterial meningitis sentinel surveillance host districts, Uganda, 2003–2022
In the pre-PCV10 period, there was no significant change in the proportion of confirmed bacterial meningitis attributable to Spn in the pediatric bacterial meningitis sentinel surveillance host districts; slope change = 0.446 (95%CI=-0.10296, 0.99496), p = 0.11. Post-PCV10, there was an immediate decline in the proportion of PBM attributable to Spn during 2015–2016, level change=-1.84611(95%CI=-1.98365, -1.70856), p < 0.001 before a rebound increase during 2017–2022, slope change = 1.0 (95%CI = 0.99997, 1.00003), p < 0.001 (Table 3; Fig. 2-D). Notably, all the confirmed cases of pediatric bacterial meningitis in 2019, n = 2, and 2021, n = 2, were due to streptococcus pneumoniae but the isolates were not serotyped.
Table 3.
Interrupted time series analysis for effect of PCV10 on the proportion of pediatric bacterial meningitis attributable to Streptococcus pneumoniae among children < 5 in PBM sentinel surveillance host districts (combined), Uganda, from 2003–2022
| Pre-PCV10 (2003–2013) | Post-PCV10 (2014–2022) | ||||
|---|---|---|---|---|---|
| Trend (slope change) | Immediate (level change) | Trend (slope change) | |||
| Estimated change (95%CI) | P-value | Estimated change | P-value | Estimated change (95%CI) | P-value |
| 0.446 (-0.10296,0.99496) | 0.111 | -1.84611(-1.98365,-1.70856) | < 0.001 | 1.0 (0.99997,1.00003) | < 0.001 |
Trend in the coverage of pneumococcal conjugate vaccine in pediatric bacterial meningitis sentinel surveillance host districts, Uganda, 2014–2022
The coverage for the third dose of PCV10 in the host districts in the year of PCV10 introduction (2014) was below the 90% WHO target at 36%. Subsequently, an upward trend coverage was recorded to a range of 70–96% during 2015 to 2022 (p = 0.046) (Fig. 2-A). Similarly in Kampala City, PCV10-dose 3 coverage posted an upward trend but remained below the WHO target with a range of 52–72% (p = 0.025) (Fig. 2-B). Whereas the PCV10-dose 3 coverage in Gulu District was largely above 90% from 2015 to 2022, it had no trend and was > 100% for four of the eight years post-introduction, suggesting data quality gaps possibly arising from a smaller than actual denominator due to periodic refugee influx (Fig. 2-C).
Discussion
Our study reveals that pneumococcal meningitis is still an important public health threat in Uganda; accounting for 62% of pediatric bacterial meningitis in sentinel site host districts from 2003 to 2022. The serotyping rate for Streptococcus pneumoniae isolates was 5%, way less than the WHO target of 90%. Apart from fever, most of the Spn meningitis cases presented with seizures and average case-fatality rate was 9%; highest among children aged 24–59 months.
Overall, the introduction of PCV10 into the routine immunization program caused a downward trend in incidence of pneumococcal meningitis in children < 5 years in the PBM sentinel surveillance host-districts, from 2003 to 2022. Whereas the incidence of pneumococcal meningitis in the sentinel surveillance host districts generally increased Pre-PCV10, the districts recorded a sustained downward trend in incidence within two years of PCV10 introduction.
However, there was no effect of PCV10 routine immunization on trend in the proportion of laboratory confirmed bacterial meningitis attributable to Spn despite an immediate decline in the first two years post-PCV10. Nevertheless, both host districts recorded very few cases of pneumococcal meningitis overall from 2018 onwards.
The coverage for the third dose of PCV10 (PCV10-dose 3) in Gulu District surpassed the 90% WHO target after only two years of its introduction into the national routine immunization program, and was sustained from 2016 to 2022. However, the PCV10-dose 3 coverage in Kampala City was persistently below the WHO target indicating poor utilization of the vaccine.
The above findings suggest that PCV10 has been effective in reducing the incidence of pneumococcal meningitis in Uganda, and are consistent with other studies done in Kenya and other Sub-Saharan countries [6, 7, 16]. Whereas the majority of cases presented with seizures, suggestive of severe disease at admission, the observed case average case fatality rate was generally below what has been reported in other studies done in Africa [5, 16]. This is likely attributed to improvements in bacterial meningitis treatment services at PBM sentinel surveillance hospitals.
The case fatality rate was higher for children aged 12–23 months compared to those aged 24–59 months. The higher case-fatality rate among the younger children is comparable with what other studies have found [4, 5].
Despite the overall reduction in the incidence of Spn meningitis post-introduction of PCV10 into the routine immunization program in Uganda, the proportion of laboratory-confirmed pediatric bacterial meningitis due to of Spn has remained high and generally increased after two years post-introduction. This raises questions on the possibility of serotype replacement by non-PCV10 Spn serotypes in communities where children have been vaccinated with the current PCV vaccine. Unfortunately, Spn serotyping rates at the sentinel sites were grossly suboptimal with only 5% of the isolates serotyped, way below the WHO target of 90% [13].
Our findings on the PCV10-dose 3 coverage were consistent with coverage estimates by the WHO and United Nations Children’s Fund (UNICEF) during 2014–2022 [17]. The coverage for Gulu District was largely above the WHO target of 90% and > 100% during four of the eight years under review. This suggests under targeting during micro planning, resulting into a smaller denominator or data quality gaps such as over-reporting [18].
Despite lower and suboptimal PCV10-dose 3 coverage in Kampala City during 2014 to 2022, the incidence of Spn meningitis in the City also steadily declined. This finding is not surprising because studies done in Kenya and the US found that PCV coverage between 65 and 75% is sufficient to induce significant herd immunity against invasive pneumococcal diseases including Spn meningitis [6, 19]. Additionally, unlike Kampala City, Gulu District directly lies in the meningitis belt with a higher prevalence of streptococcus pneumoniae. Furthermore, the Northern Region where Gulu District lies frequently receives refugees from South Sudan which also lies in the meningitis belt. This could explain the observed differences in incidence of pneumococcal meningitis across the host sites.
We also noted that compared to 2016–2019, there was lower PCV10-dose 3 coverage in the host districts during 2020–2022. This could be attributed to knock on effect of the COVID-19 pandemic on routine immunization services across these districts. These findings are consistent with what other studies have found on the disruptive effect of the COVID-19 pandemic and its control measures such as lockdowns on the routine immunization programs [20, 21].
Study limitations
Uganda’s pediatric bacterial meningitis sentinel surveillance system is not population-based and thus we might have underestimated the incidence of pneumococcal meningitis in the host districts since not all patients may have sought care at the sentinel hospitals. Additionally, we included only laboratory confirmed cases in our analysis which might have resulted in further underestimation due to variations in confirmation rates. Nevertheless, we believe our results reflect trend in incidence of Spn meningitis in the host districts given that most of the patients in these areas seek care from the sentinel sites. Additionally, we considered a long study period catering for any potential variations in health seeking behaviors and laboratory confirmation rates.
We were unable to determine the trend of vaccine type Spn meningitis since only 5% of the isolates from confirmed cases were serotyped and several years had no single isolate serotyped. Notwithstanding the absence of Spn serotype data, we demonstrated that the overall incidence of Spn meningitis due to any serotype declined since the introduction of PCV into the routine immunization.
Conclusion
The introduction of the 10-valent pneumococcal conjugate vaccine into Uganda’s routine immunization program significantly reduced the incidence of pneumococcal meningitis in Kampala City and Gulu District. There was no effect on the proportion of bacterial meningitis attributable to streptococcus pneumoniae. The coverage for the third dose of PCV10 in Kampala City was persistently suboptimal (< 90%), while Gulu District had poor immunization data quality.
Recommendations
Ministry of Health working with Kampala Capital City Authority should strengthen the routine immunization program in Kampala City and consider further investigations to ascertain factors associated with the persistent suboptimal PCV10 coverage in the City. The findings would guide targeted interventions aimed at improving not only routine PCV10 immunization coverage but also coverage for other vaccines.
Ministry of Health and partners should consider further support to the pediatric bacterial meningitis sentinel surveillance hospitals to increase the rate of Spn isolate serotyping to meet the WHO target of 90% and enable ascertainment of the trend of Spn meningitis due to PCV10 and non-PCV10 serotypes. This would help explain the serotypes responsible for the persistently high proportion of bacterial meningitis attributable to Spn despite the declining trend and inform further interventions.
We also recommend that the ministry considers conducting a nation-wide nasopharyngeal carriage survey for Streptococcus pneumoniae to assess the effectiveness of PCV in reducing pneumococcal carriage, the distribution of the different serotypes and rule out replacement by non-PCV10 serotypes in the population. This is necessary since some regions including eastern and western Uganda have no active pediatric bacterial meningitis surveillance. Together with enhanced serotyping rates at sentinel sites, the findings would guide decision-making processes on the introduction of new PCV vaccine formulations or schedules.
Ministry of Health and Gulu District local government should consider conducting immunization data quality assessment for Gulu District to ascertain the reasons for the reported coverage in excess of 100%. The assessment should focus on whether the district and health facilities are using the correct denominator during micro-planning, and over reporting. This is important in ensuring that the observed district performance does not mask poor coverage at lower community level.
Further investigations to understand the predictors of mortality among children < 5 years with pneumococcal meningitis may help inform the Ministry of Health on appropriate interventions to improve treatment outcomes.
Acknowledgements
We appreciate the WHO Uganda country office, Mulago National Referral Hospital and St. Mary’s Hospital Lacor for providing us access to the pediatric bacterial meningitis surveillance database and technical support during data collection and cleaning. We also thank the Ministry of Health, the Uganda Public Health Fellowship Program, and the Public Health Emergency Operations Centre for their technical support and the US CDC for funding this investigation.
Abbreviations
- CDC
United States Centers for Disease Prevention and Control
- CFR
Case Fatality Rate
- CSF
Cerebrospinal fluid
- DHIS2
District Health Information Software Version-2
- HMIS
Health Management Information System
- IPD
Invasive Pneumococcal Disease
- IQR
Interquartile range
- MoH
Ministry of Health
- Spn
Streptococcus pneumoniae
- PCR
Polymerase Chain Reaction
- PCV
Pneumococcal Conjugate Vaccine
- PCV10
10-valent Pneumococcal Conjugate Vaccine
- PCV10-dose 3
Third dose of PCV
- UBOS
Uganda Bureau Of Statistics
- UNICEF
United Nations Children’s Fund
- WHO
World Health Organization
Author contributions
YN, AK, RA, IA, FN, BK, RM, LB and DK designed the study and contributed to the data collection and analysis. YN led the writing of the manuscript. YN, BK, RM, LB, MB, DK and ARA participated in manuscript writing and review to ensure scientific integrity and intellectual content. All the authors contributed to the final draft of the manuscript. All the authors have read and approved the final manuscript.
Funding
and disclaimer.
The project was supported by the President’s Emergency Plan for AIDS (PEPFAR) through the United States Centers for Disease Control and Prevention Cooperative Agreement number GH001353-01 through the Makerere University School of Public Health to the Uganda Public Health Fellowship Program, Ministry of Health. The findings and conclusions in this report are those of the authors and do not necessarily represent the official views of the US Centers for Disease Control and Prevention, the Department of Health and Human Services, the Agency for Toxic Substances and Disease Registry, the Makerere University School of Public Health, or the Ministry of Health.
Data availability
The datasets on which our findings are based belong to the Uganda Public Health Fellowship Program and WHO. For confidentiality reasons, the datasets are not publicly available. However, the datasets are available upon reasonable request from the corresponding author with permission from the Uganda Public Health Fellowship Program and WHO.
Declarations
Ethical considerations
We used retrospective surveillance data and did not directly engage any participant for this study. The Ministry of Health Uganda provided administrative clearance to conduct this study as part of the routine national public health surveillance data analysis for action. The US Centers for Disease Control and Prevention (CDC) provided the non-research determination (NRD) for non-human subjects. In agreement with the International Guidelines for Ethical Review of Epidemiological Studies by the Council for International Organizations of Medical Sciences (1991) and the Office of the Associate Director for Science, US CDC/Uganda, it was determined that this activity was not human subject research and that its primary intent was to improve public health practice or disease control. This activity was reviewed by the US CDC and was conducted consistent with applicable federal law and CDC policy. §§See, e.g., 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. § 241(d); 5 U.S.C. § 552a; 44 U.S.C. § 3501 et seq. The protocol was approved by the US CDC human subjects review board (The National Institute for Occupational Safety and Health Institutional Review Board) and the Uganda Ministry of Health and the study performed in accordance with the Declaration of Helsinki. All individualized surveillance records were anonymized and kept confidential.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.mondiale de la Santé O, Organization WH. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper–February 2019 Vaccins antipneumococciques conjugués chez les nourrissons et les enfants de moins de 5 ans: note de synthèse de l’OMS–février 2019. Weekly Epidemiological Record = Relevé épidémiologique hebdomadaire. 2019;94(08):85–103.
- 2.WHO, Pneumococcal Disease. 2023. https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/pneumococcal-disease
- 3.WHO. Pneumococcal meningitis-Togo 2023 [updated 11-4-2023]. https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON455
- 4.Oligbu G, Collins S, Sheppard CL, Fry NK, Slack M, Borrow R, et al. Childhood deaths attributable to invasive pneumococcal disease in England and Wales, 2006–2014. Clin Infect Dis. 2017;65(2):308–14. [DOI] [PubMed] [Google Scholar]
- 5.Mwenda JM, Soda E, Weldegebriel G, Katsande R, Biey JN-M, Traore T, et al. Pediatric bacterial meningitis surveillance in the World Health Organization African region using the invasive bacterial vaccine-preventable disease surveillance network, 2011–2016. Clin Infect Dis. 2019;69(Supplement2):S49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammitt LL, Etyang AO, Morpeth SC, Ojal J, Mutuku A, Mturi N, et al. Effect of ten-valent pneumococcal conjugate vaccine on invasive pneumococcal disease and nasopharyngeal carriage in Kenya: a longitudinal surveillance study. Lancet. 2019;393(10186):2146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackenzie GA, Hill PC, Jeffries DJ, Hossain I, Uchendu U, Ameh D, et al. Effect of the introduction of pneumococcal conjugate vaccination on invasive pneumococcal disease in the Gambia: a population-based surveillance study. Lancet Infect Dis. 2016;16(6):703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kisakye A, Makumbi I, Nansera D, Lewis R, Braka F, Wobudeya E, et al. Surveillance for Streptococcus pneumoniae meningitis in children aged < 5 years: implications for immunization in Uganda. Clin Infect Dis. 2009;48(Supplement2):S153–61. [DOI] [PubMed] [Google Scholar]
- 9.UNEPI. Immunization guidelines 2022. https://www.health.go.ug/wp-content/uploads/2019/11/UNEPI-Immunization-guidelines.pdf
- 10.Health Mo. 2023 [updated 15-6-2023]. https://hmis.health.go.ug/dhis-web-data-visualizer/index.html#/
- 11.Immunization WHODo. Immunization in practice: a practical guide for health staff. World Health Organization; 2015.
- 12.Swarthout TD, Henrion MY, Thindwa D, Meiring JE, Mbewe M, Kalizang’Oma A, et al. Waning of antibody levels induced by a 13-valent pneumococcal conjugate vaccine, using a 3 + 0 schedule, within the first year of life among children younger than 5 years in Blantyre, Malawi: an observational, population-level, serosurveillance study. Lancet Infect Dis. 2022;22(12):1737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Organization WH. Overview of VPD surveillance principles 2018 [cited 2023 August 2023]. https://cdn.who.int/media/docs/default-source/immunization/vpd_surveillance/vpd-surveillance-standards-publication/who-surveillancevaccinepreventable-01-overview-r2.pdf?sfvrsn=932d9e08_8&download=true
- 14.Health UMo. Coverage for key antigens 2023. https://hmis.health.go.ug/dhis-web-dashboard/#/
- 15.Statistics UBo, Population, Censuses. 2023. https://www.ubos.org/explore-statistics/20/
- 16.Tagbo BN, Bancroft RE, Fajolu I, Abdulkadir MB, Bashir MF, Okunola OP, et al. Pediatric bacterial meningitis surveillance in Nigeria from 2010 to 2016, prior to and during the phased introduction of the 10-valent pneumococcal conjugate vaccine. Clin Infect Dis. 2019;69(Supplement2):S81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.UNICEF Wa. Uganda: WHO and UNICEF estimates of immunization coverage: 2022 revision 2023. https://www.google.com/search?q=Uganda%3A+WHO+and+UNICEF+estimates+of+immunization+coverage%3A+2022+revision&oq=Uganda%3A+WHO+and+UNICEF+estimates+of+immunization+coverage%3A+2022+revision&gs_lcrp=EgZjaHJvbWUyBggAEEUYOTIGCAEQRRg6MgYIAhBFGDzSAQgxMzk5ajBqN6gCALACAA&sourceid=chrome&ie=UTF-8
- 18.Organization WH. Immunization in practice: a practical guide for health staff, 2015 update 2015. https://iris.who.int/handle/10665/193412
- 19.Loughlin AM, Hsu K, Silverio AL, Marchant CD, Pelton SI. Direct and indirect effects of PCV13 on nasopharyngeal carriage of PCV13 unique pneumococcal serotypes in Massachusetts’ children. Pediatr Infect Dis J. 2014;33(5):504–10. [DOI] [PubMed] [Google Scholar]
- 20.Ali I. Impact of COVID-19 on vaccination programs: adverse or positive? Hum Vaccines Immunotherapeutics. 2020;16(11):2594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lassi ZS, Naseem R, Salam RA, Siddiqui F, Das JK. The impact of the COVID-19 pandemic on immunization campaigns and programs: a systematic review. Int J Environ Res Public Health. 2021;18(3):988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets on which our findings are based belong to the Uganda Public Health Fellowship Program and WHO. For confidentiality reasons, the datasets are not publicly available. However, the datasets are available upon reasonable request from the corresponding author with permission from the Uganda Public Health Fellowship Program and WHO.