Abstract
γ-secretase depends on presence of presenilins (PS), Nct, Aph-1, and PEN-2 within a core complex. This endoproteolytic activity cleaves within transmembrane domains of amyloid-β precursor protein (APP) and Notch, and familial Alzheimer's disease (FAD) mutations in PS1 or PS2 genes shift APP cleavage from production of amyloid-β (Aβ) 40 peptide to greater production of Aβ42. Although studies in PS1/PS2-deficient embryonic cells define overlapping activities for these proteins, in vivo complementation of PS1-deficient animals described here reveals an unexpected spectrum of activities dictated by PS1 and PS2 alleles. Unlike PS1 transgenes, wild-type PS2 transgenes expressed in the mouse CNS support little Aβ40 or Aβ42 production, and FAD PS2 alleles support robust production of only Aβ42. Although wild-type PS2 transgenes failed to rescue Notch-associated skeletal defects in PS1 hypomorphs, a “gained” competence in this regard was apparent for FAD alleles of PS2. The range of discrete and divergent processing activities in mice reconstituted with different PS genes and alleles argues against γ-secretase being a single enzyme with intrinsically relaxed substrate and cleavage site specificities. Instead, our studies define functionally distinct γ-secretase variants. We speculate that extrinsic components, in combination with core complexes, may tailor functional variants of this enzyme to their preferred substrates.
Keywords: Alzheimer's disease, endoproteolysis, PS1, PS2, Notch
Amyloid-β (Aβ) peptide is released from Aβ precursor protein (APP), a type-1 transmembrane protein (TM1), by the sequential action of two endoproteases denoted β-secretase and γ-secretase. In familial Alzheimer's diseases (FAD), cleavage by γ-secretase is altered by rare APP mutations lying in close proximity to the cleavage site or by many mutations in the presenilin (PS) genes. Dominant mutations in presenilins all cause increased production of Aβ ending at residue 42, which is prone to aggregation and deposited within amyloid plaques in Alzheimer's disease brains. Homozygosity for loss-of-function PS1 alleles reduces γ-secretase activity and causes accumulation of C-terminal fragments (CTFs) of APP and developmental defects arising as a consequence of failed endoproteolysis and signaling from another TM1 γ-secretase substrate, Notch 1 (1, 2). For both Notch and APP, intramembraneous proteolysis results in release of CTFs, Notch intracellular domain and the APP intracellular domain, implicated in transcriptional activation of effector genes (3, 4).
γ-secretase activity resides within high-molecular-mass complexes that include at least three other proteins, nicastrin (Nct), PEN-2, and Aph-1, in addition to PS1 (5–7). γ-secretase processes a number of receptors or receptor-like TM1 proteins and exhibits cleavage site heterogeneity on model substrates like APP and Notch 1. For example, APP can yield Aβ38, Aβ39, and Aβ43, and an “ε” juxtamembrane cleavage in addition to Aβ40 and Aβ42. Notch 1 has a predominant juxtamembrane cleavage site (“site 3”) but also weaker internal sites. Whether varied cleavages reflect cleavage-site promiscuity by one complex, coexpression of physically distinct γ-secretase complexes causing endoproteoloysis at unique positions, or the concerted actions by endoproteolytic and exoproteolytic activities is unclear. Although one approach to the issue of catalytic action is by means of pharmacological interventions, we have addressed this problem by genetic complementation of PS-deficient mice. Assayed in this manner, alleles of PS1 and PS2 reveal an unexpected divergence in phenotypic activity.
Materials and Methods
Transgenes. Transgene constructs were based on a wild-type (wt) PS2 cDNA sequence (ΔGlu-235 variant) and including 365 and 100 base pairs of 5′ and 3′ flanking untranslated sequences, respectively. A parental construct bounded by XhoI sites was mutagenized in vitro to generate N141I or M239V FAD alleles. All three constructs were sequenced in their entirety to exclude the presence of spurious mutations. Because human PS2 cDNAs contain a NotI site, a derivative of the cos.tet vector (“cos.Fse.Tet”) (8) was created by digesting the parental vector with NotI, “filling-in” with deoxynucleotides in the presence of DNA polymerase and religating to thereby create Fse1 sites at the boundaries between hamster prion protein (PrP) genomic DNA and the cos6.EMBL vector. PS2 XhoI fragments were ligated into the cosFse1.Tet vector digested with SalI. Mammalian DNA inserts were liberated with Fse1 and microinjected to yield Tg founders (9). Procedures for animal husbandry and genotyping are described in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Protein Analyses. Western blot analyses were performed as described in refs. 10 and 11. For signal quantification, autoradiographic exposures of APP CTFs derived from age-matched littermates from segregating litters were scanned and analyzed by imagequant software (Molecular Dynamics). Multiple exposures were taken to confirm linearity between signal and response and sample values were then adjusted by using β-actin as an internal control. Blue native gel electrophoresis is described in Supporting Materials and Methods. Procedures for diethylamine extraction of brain homogenates, ELISAs, and antibodies for the detection of mouse Aβ and γ-secretase components are described in refs. 12–14.
Results
PS2 Transgenic Mice. PS1 mRNA has two equimolar physiological spliced forms differing in exon 4 (±Glu-231), and an analogous situation also exists for the PS2 ± Glu-235 variants (15, 16). PS2 cDNA containing a wt human PS2 coding sequence (ΔGlu-235 variant) plus residual 5′ and 3′ untranslated sequences, as well as allelic variants differing only at the position of the pathogenic N141I and M239V FAD mutations were independently inserted into a variant (“cosFse1.Tet”) of the hamster PrP cosmid vector cos.Tet (8). This vector includes 25 kb of 5′ flanking and 10 kb of intronic sequences and engenders position-independent expression (11). PrP gene expression occurs in adult CNS neurons, other adult tissues, and various embryonic structures (17, 18). Expression is apparent in many tissues from embryonic day 8.5 onwards in the instance where the cos.Tet vector was used to drive a lacZ reporter transgene (P. Tremblay and S. B. Prusiner, personal communication). PS2 transgenic mice were produced by microinjection of recombinant cosmids into FVB/N oocytes. Four founders were obtained from microinjection of wt PS2 constructs, with two lines (32790 and 32799) selected for further analysis, as were TgPS2 N141I and TgPS2 M239V lines (lines 1032 and 1379, respectively). These lines encoding mutant and wt versions of PS2 had similar levels of transgene N-terminal fragment (NTF) expression (P = 0.25, 0.42 for N141I and M239V lines vs. wt PS2 line 32799) and had similar 2-fold diminutions in endogenous mouse PS1 [arising from physiological titration of γ-secretase subunits (19)] (see Fig. 5, which is published as supporting information on the PNAS web site).
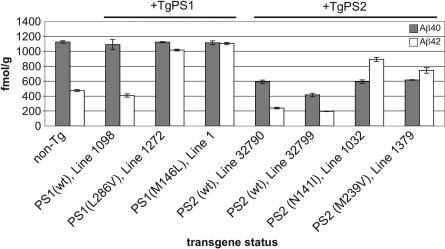
Mouse Aβ40 and Aβ42 levels were measured in brain homogenates of 2-month-old TgPS2 mice and of TgPS1 mice created by similar methodologies (10) (Fig. 1). Two effects were apparent. First, the two wt and mutant PS2 Tg lines all exhibited ≈2-fold lower levels of brain mouse Aβ40 (range 416 ± 22 to 617 ± 5 fmol/g in TgPS2 mice vs. 1,125 ± 17 fmol/g in the non-Tg, P = 2.3 × 10–5 to 3.5 × 10–5). Suppression of mouse Aβ40 was not seen with wt or mutant PS1 transgenes (P = 0.4–0.48). Second, there was a marked elevation of mouse Aβ42 in Tg lines expressing FAD mutant forms of PS2 (474 ± 13 fmol/g in the non-Tg, vs. 892 ± 27 and 745 ± 38 fmol/g, P = 0.0002 and 0.002 for N141I and M239V alleles, respectively). Aβ42 also was elevated by two PS1 FAD alleles (L286V, P = 5.5 × 10–6; M146L, P = 7 × 10–7) but was suppressed by wtPS2 (line 32790, P = 1.1 × 10–4; line 32799, P = 3.3 × 10–5). These analyses establish the baseline performance of PrP promoter-driven PS transgenes in a wt PS genetic background.
Fig. 1.
Aβ production in PS2 Tg mice. ELISA analysis of endogenous Aβ species in Tg mouse lines used in this study. TgPS1 mice have been described previously: Tg(L286V)1272 and Tg(PS1wt)1098 mice express similar levels of human PS1 mRNA and NTF, whereas Tg(M146L)line 1 mice express higher levels of mRNA such that PS1 holoprotein can be detected in addition to NTF (10, 45). Numbers of animals in each sample group are, from left to right, n = 5, 4, 3, 4, 5, 4, 6, and 3. Note that all TgPS2 lines have reduced levels of Aβ40 as compared with non-Tg mice but that this situation does not apply to TgPS1 counterparts. For Aβ40 values measured vs. non-Tg mice, results are as follows: PS2 wt line 32790, P = 2.3 × 10–5; PS2 wt line 32799, P = 3.5 × 10–6; PS2 N141I line 1032, P = 3.2 × 10–5; and PS2 M239V line 1379, P = 3 × 10–5. For Aβ40 values measured in Tg PS1 mice vs. non-Tg mice, results were as follows: P = 0.4 for PS1 wt line 1098, P = 0.48 for PS1 L286V line 1272, and P = 0.4 for PS1 M146L line 1. For Aβ42 values measured vs. non-Tg mice, there were significant reductions by wt PS2 transgenes (PS2 wt line 32790, P = 0.00011; PS2 wt line 32799, P = 3.0 × 10–5) and significant elevations by FAD PS2 transgenes (PS2 N141 line 1032, P = 2.4 × 10–4; PS2 M239V line 1379, P = 2.2 × 10–3). For Aβ42 values measured in Tg PS1 mice vs. non-Tg mice, results are as follows: P = 0.11 for PS1wt line 1098, P = 5.5 × 10–6 for PS1 L286V line 1272, and P = 7 × 10–7 for PS1 M146L line 1.
Human PS2 Transgenes Expressed in PS1 Hypomorphs and PS1-Null Mice. To assess the ability of exogenous PS1 or PS2 to complement loss of PS1, PS1-deficient mice were cross-bred with Tg mice bearing mutant and wt alleles of PS2 (Table 1). A first set of complementation studies exploited mice with a hypomorphic mutation in the mouse PS1 locus. Measured in brain and dorsal spinal cord at embryonic day 11.5, homozygotes express 0.5–1.0% of wt PS1 mRNA levels. These mice have severe axial skeletal deformities, diminished γ-secretase activity, and a commensurate accumulation of APP CTFs, but they survive to adulthood, reproduce, and have no overt neuroanatomical pertubations (20).
Table 1. Activities of PS transgenes assessed in PS1-deficient mice.
| Added gene*
|
Allele of added gene
|
CNS Aβ production†
|
Axial skeletal defects in adults
|
||
|---|---|---|---|---|---|
| Aβ40 | Aβ42 | Reference | |||
| PS1-deficient (hypomorphic) background | |||||
| None | n.a. | Very low | Very low | Yes | 20 |
| PS1 | FAD(M146L) | High | High | No | This work |
| PS2 | wt | Low | Low | Yes | This work |
| PS2 | FAD(N141I) | Very low | High | No | This work |
| PS2 | FAD(M239V) | Very low | High | No | This work |
| PS1-deficient (null) background | |||||
| None | n.a. | n.a.‡ | n.a.‡ | n.a.‡ | 21 |
| PS1 | FAD(M146L) | High | High | No§ | This work |
| PS1 | FAD(A246E) | High | High | No | 18 |
| PS2 | wt | n.a.¶ | n.a.¶ | n.a.¶∥ | This work |
| PS2 | FAD(N141I) | Very low | High | No | This work |
n.a., not applicable.
All experiments refer to mice with prion promoter containing transgenes.
Measurements of murine Aβ species in the adult mouse brain.
PS1-deficient mice die before birth.
Normal number of vertebrae and ribs but kinked tails present in some adult mice.
PS1-deficient mice bearing wt PS2 genes die before birth.
Altered number or morphology of somites is apparent in TgwtPS2/PS10/0 embryos.
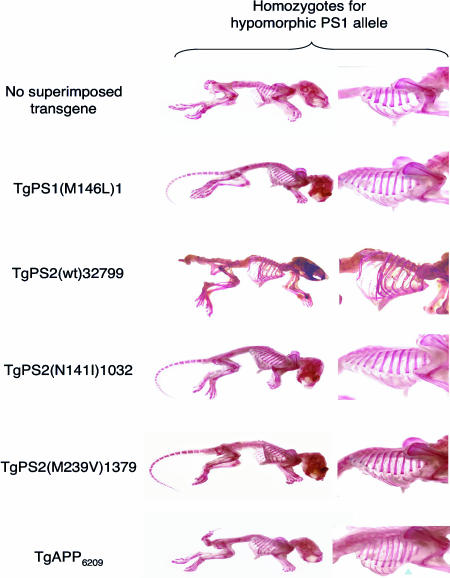
wt PS2, N141I PS2, M239V PS2, and M146L PS1 transgenic lines were crossed into the PS1 hypomorphic genetic background (Table 1 and Fig. 2). All possible genotypes were obtained from these crosses of PS0/+ × PS10/+/TgPS breeding pairs. However, in contrast to the virtually complete phenotypic rescue by the M146L mutant form of PS1 (11/11) and the mutant forms of PS2 (N141I, n = 8/8; M239V, n = 7/7), presence of the wt PS2 transgene had no discernable impact on the axial skeletal deficits present in the parental line (present in all 13 TgPS2wt.PS10/0 mice examined). A negative-control transgene derived from the same cosmid vector but encoding a wt human APP allele failed to prevent skeletal abnormalities (present in 6/6 mice). These data indicate that biological activities related to skeletal development and efficient signaling in the Notch pathway are shared between FAD mutant forms of PS1 and PS2. In contrast, wt PS2 failed to rescue the axial skeletal defects associated with drastically reduced PS1 expression.
Fig. 2.
Effects of PS1 and PS2 transgenes on the abnormal skeletal morphology present in PS1-hypomorphs. All animals shown are homozygous for the hypomorphic PS1 allele. Row 1 shows an animal with no superimposed transgene, whereas rows 2–6 represent animals with superimposed transgenes expressing mutant PS1, wtPS2, mutant PS2, or wt APP transgenes (as indicated). The disorganized and reduced number of vertebrae (10) and reduced number of ribs, fusion of ribs (arrow), and aberrant tail morphology present in PS1-null homozygotes is remedied by mutant PS1 and PS2 transgenes (these mice have 13 normal thoracic vertebrae and ribs) but not by a TgAPP695.6209 transgene (46) nor by the wt PS2 transgene (only 10–11 ribs, with the last 4 ribs partially detached).
A second series of experiments used mice completely deficient for PS1 expression (21). Here, crosses of the PS1 M146L, PS2 N141I, and PS2 wt transgenes into the PS1-deficient background resulted in only a subset of the possible genotypes reaching term (see Table 2, which is published as supporting information on the PNAS web site). Although both the PS1(M146L)1 and PS2(N141I)1032 transgene arrays were able to compensate for complete deficiency of wtPS1 and thereby yield adult PS10/0 mice, no wtPS2 transgene-positive PS10/0 adult mice were observed among 58 live births from crosses of PS10/+ × PS10/+ + TgPS2 wt line 32799 parents (P = 0.00025). Because we were unable to obtain live births of wtPS2/PS10/0 mice, we also examined animals at embryonic day 12. However, the wt PS2 transgene had no influence on the severity of the PS1-deficient embryonic phenotype (see Fig. 6, which is published as supporting information on the PNAS web site; data not shown). Adult mice of the PS2(N141I)/PS10/0 genotype have not been described previously and are prone to hydrocephaly (see Fig. 7, which is published as supporting information on the PNAS web site).
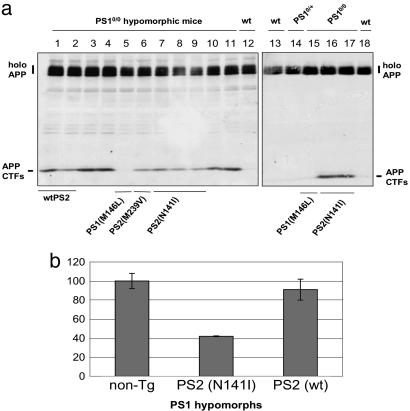
APP Processing in Transgene-Reconstituted PS1-Deficient Mice. Mouse APP CTFs. To determine whether divergent effects on Notch-associated developmental pathways were accompanied by defects in APP processing, we investigated APP CTFs and Aβ levels in the brains of transgene-complemented PS1-deficient mice. Abundant APP CTFs were found in PS1 hypomorphs, as described in refs. 20 and 22. Unexpectedly, and in contrast to a TgPS1(M146L)1 control (which reduced APP CTFs to values below a baseline established by non-Tg mice), abundant mouse APP CTFs were present in brain samples from PS1 hypomorphic mice carrying the TgPS2(N141I)1032, TgPS2(M239V)1379, and TgPS2(wt)32799 transgene arrays (Fig. 3a). By densitometry, APP CTF levels were reduced somewhat (41%) by the N141I transgene but not at all (no significant difference between the Tg and the control) by the wt PS2 transgenes (Fig. 3b). These findings were extended to include genetic complementation of the PS1-deficient genetic background (21). APP CTFs were undetectable in wt mice, in mice heterozygous for PS1-deficiency, or in mice homozygous for the PS1-null allele and rescued with TgPS1(M146L), yet remained abundant in mice homozygous for the PS1-null allele bearing a TgPS2(N141I)1032 transgene array.
Fig. 3.
Effects of PS transgenes on APP CTFs. (a) Western blot analysis of APP CTFs. Brain homogenates were electrophoresed on 10–20% tricine gradient gels and blotted with anti-APP C-terminal antibody C1/6.1 (47). Equal loading and transfer of protein was verified by staining the membranes with Ponceau red. Only the superimposed Tg PS1 (M146L) transgenes eliminated the accumulation of APP CTFs found in PS1 hypomorphic mice. (b) Quantitative analysis of APP CTFs that are presented on the left side of a.
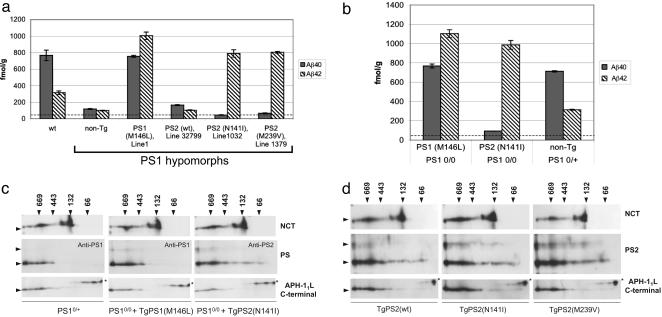
Mouse Aβ. Levels of endogenous Aβ40 and Aβ42 species in mice homozygous for the PS1 hypomorphic allele are markedly reduced with respect to wt mice (Fig. 4a) (20). Expression of a TgPS1(M146L)1 transgene array in PS1 hypomorphic mice resulted both in increased levels of total Aβ and ratios of Aβ42 to Aβ40 equal to or higher than those observed in wt nontransgenic mice (compared with wt controls; Fig. 4a) and similar to those of mice homozygous for the wt mouse PS1 locus and bearing the same TgPS1(M146L)1 transgene array (Fig. 1a). Remarkably, mutant PS2-complemented PS1-hypomorphic mice exhibited selective rescue of Aβ42 production, with their Aβ40 values being below those of PS10/0 hypomorphic mice (and close to the sensitivity limit of the ELISA). In the N141I and M239V PS2 transgene-bearing mice, Aβ42:40 ratios were 17.4:1 and 12:1, respectively. The TgPS2(wt)32799 transgene array produced a small elevation of Aβ40 to 165 ± 6 fmol/g from the 118 ± 6 fmol/g value of mice homozygous for the PS1 hypomorphic allele (P = 0.02). However, this value is considerably lower than the Aβ40 level seen in mice homozygous for the endogenous PS1 locus (≈800 fmol/g). The TgPS2(wt)32799 transgene array failed to produce a significant alteration of Aβ42 (P = 0.37). Interestingly Aβ40 levels were lower in mice bearing N141I and M239V PS2 transgenes than in comparable Tg PS2wt controls (P = 4.4 × 10–4 and 1.4 × 10–4, respectively). Assayed in the PS1 null genetic background (21), the TgPS1(M146L) transgene array produced viable adult mice with robust production of CNS Aβ40 and Aβ42 and in close agreement with previous studies of FAD PS1 alleles (18, 23). In adult mice, the TgPS2(N141I) transgene array was associated with selective production of Aβ42 (Aβ42/Aβ40 ratio of 10.5:1; Fig. 4b).
Fig. 4.
Aβ production in Tg-complemented PS-deficient mice. Aβ40 and Aβ42 levels were determined by ELISA of adult mouse brain homogenates as described in Materials and Methods. (a) Transgene rescue in mice homozygous for the PS1 hypomorphic alleles. Numbers of animals in each sample group are, from left to right, n = 3, 7, 5, 6, 3, and 5. (b) Transgene rescue in mice homozygous for the PS1-null allele. Five animals were in each sample group. Results are the mean of four measurements ± SE expressed as femtomoles of Aβ per gram of wet brain. The sensitivity of the Aβ sandwich ELISAs was 38.7 and 18.9 fmol/g analyzed for Aβ40 and Aβ42, respectively. The Aβ40-sensitivity threshold is indicated by a horizontal dotted line drawn across the bottom of each of the bar graphs. For comparisons in a of non-Tg vs. PS1(M146L), PS2wt, PS2(N141I), and PS2 (M239V) for Aβ40, P = 8 × 10–10, 0.02, 0.008, and 0.01, respectively, and for Aβ42, P = 4 × 10–7, 0.37, 10–4, and 1.8 × 10–11, respectively. (c) A 2D gel analysis of γ-secretase component proteins from mouse brain. Shown are analyses in PS1-deficient mice either heterozygous (Left) or homozygous (Center and Right) for the fully penetrant null allele. The genotypes of superimposed PS1 or PS2 transgenes are indicated. Native electrophoresis is represented in the horizontal dimension, and size markers (arrows) are indicated in kDa. Denaturing electrophoresis in the presence of SDS is shown in the vertical dimension, and size markers for the corresponding blots are, from top to bottom, 100, 45, 28, and 23 kDa (arrowheads). Antibodies used to probe the resultant blots are shown on the right side. A nonspecific band detected with the Aph-1 antibody is indicated with an asterisk. In addition to 28- to 30-kDa signals seen for PS1 or PS2 NTFs, the faint signals seen at higher molecular masses correspond to uncleaved holoprotein. (d) Analyses in Tg mice homozygous wt for the endogenous PS1 gene. Note the population of lower Mr complexes containing PS2-NTF (center to right side of PS2 blot) vs. analogous PS1-NTF signals for complexes containing either endogenous mouse PS1 or transgene-encoded human PS1.
Aβ Production in PS2-Null Mice. Results presented above suggest that wt PS2 has only a modest ability to support Aβ40 production. We sought confirmation of this observation by examining PS2-deficient mice. Here PS20/0 null mice had Aβ40 and Aβ42 levels indistinguishable from wt animals (see Fig. 8, which is published as supporting information on the PNAS web site) (24, 25). Previous experiments have demonstrated that manipulation of endogenous PS2 did not result in an increase in levels of endogenous mouse PS1 NTFs (26). However, we reasoned that under conditions where PS1 protein is available in excess over wt levels, levels of functional PS1 might be higher in PS2-deficient mice than in mice with endogenous levels of PS2. To address this hypothesis, the PS1 M146L transgene array was crossed into PS2-deficient mice, thereby revealing an effect of gene dosage (Fig. 8). As PS2 copy-number diminished, Aβ40 levels increased. In sum, PS1 and PS2 have different abilities to support Aβ40 production, and, whereas complementation experiments used a transgene encoding the ΔGlu-235 PS2 (Fig. 4), the PS2-null allele prevents production of all spliced forms of PS2 mRNA (24). Thus, it is likely that physiological spliced forms of PS2 proteins ± Glu-235 behave similarly.
Because biogenesis of γ-secretase is associated with progression of PS through a hierarchy of high-molecular-mass complexes, 2D analyses were performed on brain homogenates. These analyses revealed that a subset of human PS2 NTFs accumulated at lower molecular masses in the native electrophoretic dimension than mouse or human PS1 NTFs (i.e., PS2 NTF-immunoreactivity was present between the 443- and 66-kDa markers). This result was apparent in mice homozygous null or wt for endogenous PS1. This form of PS2 NTF-immunoreactivity may represent partially assembled γ-secretase and suggests different assembly (or disassembly) pathways for PS2 vs. PS1 in the CNS. Of greater importance, all types of PS proteins expressed in a PS1-deficient background were incorporated into high-molecular-mass complexes, with mobilities ≥443-kDa size-marker (Fig. 4 c and d). These forms comigrated with the predominant signals for Aph-1L and mature forms of Nicastrin. The most active species of γ-secretase are associated with the highest molecular mass forms (27). We interpret these data to indicate that diverse activities of wt and mutant PS alleles are not due to trivial failures in recruitment into the highest-molecular-mass complexes.
Discussion
In Vivo Assay of PS by Transgene Rescue. Functional redundancy between PS1 and PS2 was first suggested by a shared role in FAD and sequence homology, including conservation of the transmembrane aspartate residues that may mediate the catalytic activity of γ-secretase (28). Previous studies in the developing mouse embryo and in Caenorhabditis elegans have emphasized functional redundancy, with differences between the phenotype of the two PS knockouts often attributed to the higher expression levels of PS1. Our analyses of PS1 and PS2 alleles stand in contrast to this notion (Table 1).
Divergent effects on the production of Aβ40 and Aβ42 are of particular interest. Comparisons of PS1- and PS2-deficient blastocyst-derived cells suggest an important shared contribution to Aβ40 production, although PS2 containing γ-secretase complexes from blastocyst-derived cells may have lower specific activity than analogous PS1 complexes (29). Transgenic studies in a PS1-deficient background (Fig. 4a) revealed little γ-40 activity mediated by either wt or FAD mutant PS2 in the CNS, consistent with unaltered Aβ40 levels in PS2-deficient mice (ref. 24; Fig. 8). Failure of wt PS2 to support significant Aβ production is consistent with the observation that PS2 transgenes in a wt PS1 background suppresses Aβ40 production (refs. 30–32; Fig. 1). Because transgene-encoded PS2 depresses steady-state levels of endogenous PS1 (Fig. 5 a and c; ref. 19), it follows that displacement of “γ-40-secretase active” PS1 from γ-secretase complexes by transgene-encoded PS2 lacking significant γ-40 activity will be accompanied by a drop in Aβ40 production. Notably, a 2-fold reduction in endogenous mouse PS1 NTFs driven by wt human PS2 transgene expression was paralleled by a 2-fold reduction in Aβ40 level (Figs. 1 and 5). Additionally, our analyses do not reveal a role for wt PS2 in CNS Aβ42 production, because Aβ42 levels were depressed in two Tg lines (Fig. 1) and remained low when Tg wt PS2 expressed in PS1 hypomorphs (Fig. 4a). Thus, APP in the CNS is not an adept substrate for wt PS2-containing γ-secretase. In yeast, an enhanced APP-based γ-secretase reporter assay revealed >10 greater activity for PS1 vs. PS2 transgenes (P.M. and D.W., unpublished data).
FAD Mutations and the Mechanism of γ-Secretase Action. A fundamental yet unresolved issue concerns the action of PS FAD mutations. Assessed on other TM1 substrates or in the context of C. elegans these missense lesions and in-frame splicing mutations are loss-of-function alleles (33–35). These findings seems plausible because the mutations might all compromise tertiary structure needed for activity. However, to varying degrees, all mutations retain ability to support Aβ40 production and have “gained” ability to support Aβ42 production. Starting from assumptions that PS are catalytic subunits (36), one possibility is that wt PS1-containing γ-secretase complexes are active for Aβ40 and Aβ42 production, but when complexes include mutant PS1 they lose ability to generate Aβ40 because the active site deteriorates toward a conformation adept at producing Aβ42. This deterioration might involve the disposition of putative catalytic aspartate residues with regards to the Aβ42–43 peptide bond. However, in studies presented here and in analyses of TgPS1 × TgAPP mice assayed for human Aβ species (37), Aβ40 levels were unaltered by FAD PS1 mutations, indicating a true gain-of-function for Aβ42 production. Also, the hypothesis of deterioration in the conformation of an efficient wt catalytic site (suggested by some with regards to FAD vs. wt PS1) is incompatible with the performance of PS2 FAD alleles in the CNS. This incompatibility arises because the “starting point” defined by wt PS2 is not efficient with regards to APP processing, being barely capable of supporting the production of Aβ40 or Aβ42, and in some instances even suppressing production (Figs. 1 and 4a). Instead, we observe that FAD alleles of PS2 have a dramatic gain-of-function, a selective increase in Aβ42 production over PS2 wt levels. FAD PS2 alleles also gain another function with regards to wt PS2, namely ability to remedy Notch-pathway-associated deficits caused by absence of PS1. In this regard, the poor activity of wt PS2 is apparent when considering the performance of either human transgenes (Figs. 2 and 6) or the endogenous mouse PS2 locus present in PS1-deficient mice. The question then arises as to how to account for simultaneous gain- and loss-of-function properties of PS mutations.
The divergent properties of wtPS2, namely a well-documented ability to contribute to γ-secretase processing of Aβ and Notch 1 in blastocysts or cultured cells (38–40) (and results from parental cDNA clones used to construct Tg mice described here; M. Nishimura, personal communication) vs. the indifferent effect of PS2 in the adult CNS establishes context dependence and that PS2 expression is not sufficient for activity. Consequently, we infer that tissue-specific components regulate γ-secretase activity. Although variants of core components such as Aph-1 family members are located within distinct γ-secretase complexes (41), because these proteins determine only modest effects on APP processing (or are indistinguishable; refs. 42 and 43) they are not clear candidates for this role. More likely, lineage-specific γ-secretase components will be found above and beyond core components, potentially compatible with size differences in γ-secretase from different tissues (44). Interactions with these components may be affected by FAD mutations, resulting in pleiotropic alterations in activity (e.g., “inversion,” loss in cleavage efficacy for one type of substrate yet a simultaneous gain in cleavage efficacy for another type of substrate). Tg mice described here may comprise useful tools to explore this concept.
Supplementary Material
Acknowledgments
We thank Marc Mercken (Johnson and Johnson Pharmaceutical Research & Development, Beerse, Belgium), Gopal Thinakaran (University of Chicago, Chicago), Frédéric Checler (Institut de Pharmacologie Moléculaire et Cellulaire, Valbonne, France), and Sam Gandy (Thomas Jefferson University, Philadelphia) for supplying antibiotics and Karen Duff (Nathan Kline Institute, New York University, Orangeburg, NY) and Sarah Lincoln (Mayo Clinic, Jacksonville, FL) for a generous gift of PS2-knockout mice. This work was supported by the Canadian Institutes for Health Research, the Canadian Foundation for Innovation, the Howard Hughes Medical Research Foundation, the American Health Assistance Foundation, the National Institute on Aging, the National Institute of Neurologic Disorders and Stroke, and the Alzheimer Society of Ontario.
Author contributions: P.M., P.M.M., and D.W. designed research; P.M., P.M.M., M.A.C., S.D.S., Y.G., J.Y., M.J.M., J.C., P.H., B.S., H.P., G.L., C.E., and Y.J. performed research; P.M.M., R.R., P.S.G.-H., and G.A.C. contributed new reagents/analytic tools; P.M., S.D.S., and D.W. analyzed data; and P.M., P.M.M., R.A.N., P.E.F., G.A.C., and D.W. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Aβ, amyloid-β; APP, Aβ precursor protein; FAD, familial Alzheimer's disease; CTF, C-terminal fragment; NTF, N-terminal fragment; PS, presenilin; TM1, type-1 transmembrane protein; wt, wild type.
References
- 1.Wong, P. C., Zheng, H., Chen, H., Becher, M., Sirinathsinghji, D. J. S., Trumbauer, M. E., Chen, H. Y., Price, D. L., Van der Ploeg, L. H. T. & Sisodia, S. S. (1997) Nature 387, 288–292. [DOI] [PubMed] [Google Scholar]
- 2.De Strooper, B., Saftig, P., Craessaerts, K., Vanderstichele, H., Guhde, G., Annaert, W., Von Figura, K. & Van Leuven, F. (1998) Nature 391, 387–390. [DOI] [PubMed] [Google Scholar]
- 3.Schroeter, E. H., Kisslinger, J. A. & Kopan, R. (1998) Nature 393, 382–386. [DOI] [PubMed] [Google Scholar]
- 4.Cao, X. & Sudhof, T. C. (2001) Science 293, 115–120. [DOI] [PubMed] [Google Scholar]
- 5.Yu, G., Chen, F., Levesque, G., Nishimura, M., Zhang, D. M., Levesque, L., Rogaeva, E., Xu, D., Liang, Y., Duthie, M., et al. (1998) J. Biol. Chem. 273, 16470–16475. [DOI] [PubMed] [Google Scholar]
- 6.Yu, G., Nishimura, M., Arawaka, S., Levitan, D., Zhang, L., Tandon, A., Song, Y. Q., Rogaeva, E., Chen, F., Kawarai, T., et al. (2000) Nature 407, 48–54. [DOI] [PubMed] [Google Scholar]
- 7.Francis, R., McGrath, G., Zhang, J., Ruddy, D. A., Sym, M., Apfeld, J., Nicoll, M., Maxwell, M., Hai, B., Ellis, M. C., et al. (2002) Dev. Cell 3, 85–97. [DOI] [PubMed] [Google Scholar]
- 8.Scott, M. R., Köhler, R., Foster, D. & Prusiner, S. B. (1992) Protein Sci. 1, 986–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westaway, D., DeArmond, S. J., Cayetano-Canlas, J., Groth, D., Foster, D. & Prusiner, S. (1994) Cell 76, 117–129. [DOI] [PubMed] [Google Scholar]
- 10.Citron, M., Westaway, D., Xia, W., Carlson, G. A., Diehl, T., Levesque, G., Johnson-Wood, K., Lee, M., Seubert, P., Davis, A., et al. (1997) Nat. Med. 3, 67–72. [DOI] [PubMed] [Google Scholar]
- 11.Scott, M., Foster, D., Mirenda, C., Serban, D., Coufal, F., Wälchli, M., Torchia, M., Groth, D., Carlson, G., DeArmond, S. J., et al. (1989) Cell 59, 847–857. [DOI] [PubMed] [Google Scholar]
- 12.Savage, M. J., Trusko, S. P., Howland, D. S., Pinsker, L. R., Mistretta, S., Reaume, A. G., Greenberg, B. D., Siman, R. & Scott, R. W. (1998) J. Neurosci. 18, 1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phinney, A. L., Drisaldi, B., Schmidt, S. D., Lugowski, S., Coronado, V., Liang, Y., Horne, P., Yang, J., Sekoulidis, J., Coomaraswamy, J., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 14193–14198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu, Y., Chen, F., Sanjo, N., Kawarai, T., Hasegawa, H., Duthie, M., Li, W., Ruan, X., Luthra, A., Mount, H. T., et al. (2003) J. Biol. Chem. 278, 7374–7380. [DOI] [PubMed] [Google Scholar]
- 15.Sherrington, R., Froelich, S., Sorbi, S., Campion, D., Chi, H., Rogaeva, E., Levesque, G., Rogaev, E., Lin, C., Liang, Y., et al. (1996) Hum. Mol. Genet. 5, 985–988. [DOI] [PubMed] [Google Scholar]
- 16.Rogaev, E. I., Sherrington, R., Wu, C., Levesque, G., Liang, Y., Rogaeva, E. A., Ikeda, M., Holman, K., Lin, C., Lukiw, W. J., et al. (1997) Genomics 40, 415–424. [DOI] [PubMed] [Google Scholar]
- 17.Manson, J., West, J. D., Thomson, V., McBride, P., Kaufman, M. H. & Hope, J. (1992) Development (Cambridge, U.K.) 115, 117–122. [DOI] [PubMed] [Google Scholar]
- 18.Davis, J. A., Naruse, S., Chen, H., Eckman, C., Younkin, S., Price, D. L., Borchelt, D. R., Sisodia, S. S. & Wong, P. C. (1998) Neuron 20, 603–609. [DOI] [PubMed] [Google Scholar]
- 19.Thinakaran, G., Harris, C. L., Ratovitski, T., Davenport, F., Slunt, H. H., Price, D. L., Borchelt, D. R. & Sisodia, S. S. (1997) J. Biol. Chem. 272, 28415–28422. [DOI] [PubMed] [Google Scholar]
- 20.Rozmahel, R., Huang, J., Chen, F., Liang, Y., Nguyen, V., Ikeda, M., Levesque, G., Yu, G., Nishimura, M., Mathews, P., et al. (2002) Neurobiol. Aging 23, 187–194. [DOI] [PubMed] [Google Scholar]
- 21.Shen, J., Bronson, R. T., Chen, D. F., Xia, W., Selkoe, D. J. & Tonegawa, S. (1997) Cell 89, 629–639. [DOI] [PubMed] [Google Scholar]
- 22.Chen, F., Yang, D. S., Petanceska, S., Yang, A., Tandon, A., Yu, G., Rozmahel, R., Ghiso, J., Nishimura, M., Zhang, D. M., et al. (2000) J. Biol. Chem. 275, 36794–36802. [DOI] [PubMed] [Google Scholar]
- 23.Qian, S., Jiang, P., Guan, X.-M., Singh, G., Trumbauer, M. E., Yu, H., Chen, H. Y., Van der Ploeg, L. H. T. & Zheng, H. (1998) Neuron 20, 611–617. [DOI] [PubMed] [Google Scholar]
- 24.Steiner, H., Duff, K., Capell, A., Romig, H., Grim, M. G., Lincoln, S., Hardy, J., Yu, X., Picciano, M., Fechteler, K., et al. (1999) J. Biol. Chem. 274, 28669–28673. [DOI] [PubMed] [Google Scholar]
- 25.Herreman, A., Hartmann, D., Annaert, W., Saftig, P., Craessaerts, K., Serneels, L., Umans, L., Schrijvers, V., Checler, F., Vanderstichele, H., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 11872–11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen, F., Tandon, A., Sanjo, N., Gu, Y. J., Hasegawa, H., Arawaka, S., Lee, F. J., Ruan, X., Mastrangelo, P., Erdebil, S., et al. (2003) J. Biol. Chem. 278, 19974–19979. [DOI] [PubMed] [Google Scholar]
- 27.Gu, Y., Sanjo, N., Chen, F., Hasegawa, H., Petit, A., Ruan, X., Li, W., Shier, C., Kawarai, T., Schmitt-Ulms, G., et al. (2004) J. Biol. Chem. 279, 31329–31336. [DOI] [PubMed] [Google Scholar]
- 28.Kimberly, W. T., Xia, W., Rahmati, T., Wolfe, M. S. & Selkoe, D. J. (2000) J. Biol. Chem. 275, 3173–3178. [DOI] [PubMed] [Google Scholar]
- 29.Lai, M. T., Chen, E., Crouthamel, M. C., DiMuzio-Mower, J., Xu, M., Huang, Q., Price, E., Register, R. B., Shi, X. P., Donoviel, D. B., et al. (2003) J. Biol. Chem. 278, 22475–22481. [DOI] [PubMed] [Google Scholar]
- 30.Oyama, F., Sawamura, N., Kobayashi, K., Morishima-Kawashima, M., Kuramochi, T., Ito, M., Tomita, T., Maruyama, K., Saido, T. C., Iwatsubo, T., et al. (1998) J. Neurochem. 71, 313–322. [DOI] [PubMed] [Google Scholar]
- 31.Tomita, T., Tokuhiro, S., Hashimoto, T., Aiba, K., Saido, T. C., Maruyama, K. & Iwatsubo, T. (1998) J. Biol. Chem. 273, 21153–21160. [DOI] [PubMed] [Google Scholar]
- 32.Qi, Y., Morishima-Kawashima, M., Sato, T., Mitsumori, R. & Ihara, Y. (2003) Biochemistry 42, 1042–1052. [DOI] [PubMed] [Google Scholar]
- 33.Levitan, D., Doyle, T. G., Brousseau, D., Lee, M. K., Thinakaran, G., Slunt, H., Sisodia, S. S. & Greenwald, I. (1996) Proc. Natl. Acad. Sci. USA 93, 14940–14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumeister, R., Leimer, U., Zweckbronner, I., Jakubek, C., Grunberg, J. & Haass, C. (1997) Genes Funct. 1, 149–159. [DOI] [PubMed] [Google Scholar]
- 35.Marambaud, P., Wen, P. H., Dutt, A., Shioi, J., Takashima, A., Siman, R. & Robakis, N. K. (2003) Cell 114, 635–645. [DOI] [PubMed] [Google Scholar]
- 36.Wolfe, M. S., Xia, W., Ostaszewski, B. L., Diehl, T. S., Kimberly, W. T. & Selkoe, D. J. (1999) Nature 398, 513–517. [DOI] [PubMed] [Google Scholar]
- 37.Krezowski, J., Blessum, D., Ebeling, C., Pistick, R., Schenk, D., Westaway, D., Younkin, L., Younkin, S. G., Hsiao Ashe, K. & Carlson, G. A. (2004) Hum. Mol. Genet. 13, 1989–1997. [DOI] [PubMed] [Google Scholar]
- 38.Herreman, A., Serneels, L., Annaert, W., Collen, D., Schoonjans, L. & De Strooper, B. (2000) Nat. Cell Biol. 2, 461–462. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, Z., Nadeau, P., Song, W., Donoviel, D., Yuan, M., Bernstein, A. & Yankner, B. A. (2000) Nat. Cell Biol. 2, 463–465. [DOI] [PubMed] [Google Scholar]
- 40.Palacino, J. J., Berechid, B. E., Alexander, P., Eckman, C., Younkin, S., Nye, J. S. & Wolozin, B. (2000) J. Biol. Chem. 275, 215–222. [DOI] [PubMed] [Google Scholar]
- 41.Shirotani, K., Edbauer, D., Prokop, S., Haass, C. & Steiner, H. (2004) J. Biol. Chem. 279, 41340–41345. [DOI] [PubMed] [Google Scholar]
- 42.Ma, G., Li, T., Price, D. L. & Wong, P. C. (2005) J. Neurosci. 25, 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serneels, L., Dejaegere, T., Craessaerts, K., Horre, K., Jorissen, E., Tousseyn, T., Hebert, S., Coolen, M., Martens, G., Zwijsen, A., et al. (2005) Proc. Natl. Acad. Sci. USA 102, 1719–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hebert, S. S., Serneels, L., Dejaegere, T., Horre, K., Dabrowski, M., Baert, V., Annaert, W., Hartmann, D. & De Strooper, B. (2004) Neurobiol. Dis. 17, 260–272. [DOI] [PubMed] [Google Scholar]
- 45.Janus, C., D'Amelio, S., Amitay, O., Chishti, M. A., Strome, R., Fraser, P., Carlson, G. A., Roder, J. C., St. George-Hyslop, P. & Westaway, D. (2000) Neurobiol. Aging 21, 541–549. [DOI] [PubMed] [Google Scholar]
- 46.Hsiao, K. K., Borchelt, D. R., Olson, K., Johannsdottir, R., Kitt, C., Yunis, W., Xu, S., Eckman, C., Younkin, S., Price, D., et al. (1995) Neuron 15, 1203–1218. [DOI] [PubMed] [Google Scholar]
- 47.Mathews, P. M., Jiang, Y., Schmidt, S. D., Grbovic, O. M., Mercken, M. & Nixon, R. A. (2002) J. Biol. Chem. 277, 36415–36424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.