Abstract
Gastric cancer (GC) is a highly heterogeneous and aggressive malignant tumor that seriously affects the life safety of people all over the world. Its early manifestations are subtle. The present study aimed to investigate the clinical significance of serum lipid profiles, insulin resistance markers including the triglyceride-glucose (TyG) index and the atherosclerotic index (AI), in GC patients. A retrospective analysis encompassed 215 GC patients and 827 healthy individuals. The study results show that the total cholesterol, triglycerides, low-density lipoprotein, high-density lipoprotein levels, and the TyG index of GC patients were significantly lower than those of the control group before and after propensity score matching analysis. In the GC group, the levels of CEA, CA199, CA125, and CA724 tumor markers were higher than those in the healthy control group. Patients in advanced stages exhibited lower serum levels of serum lipids and TyG index compared to those in early stages. ROC analysis revealed that the TyG index, CA125, and CA199 combination yielded the highest positive prediction rate for GC at 98.6%. TyG index is significantly associated with the risk of adverse reactions after chemotherapy (OR = 1.104, 95% CI 1.028–1.186, P < 0.01). Multiple tumor markers and the TyG index combined detection showed correlations with five adverse reactions caused by chemotherapy (r < 0.6, P < 0.05). Preoperative lipid profiles in the serum show a strong correlation with patients diagnosed with GC. Evaluating a combination of various serum lipids and cancer markers significantly improves diagnostic precision for GC and the ability to predict chemotherapy side effects.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-024-03463-w.
Keywords: Gastric cancer, Serum lipids, Triglyceride-glucose index, Diagnostic marker
Introduction
Gastric cancer (GC) is currently the fourth leading cause of cancer deaths worldwide, with approximately 1 million new cases occurring globally each year [1, 2]. The likelihood of GC surviving greatly relies on the cancer stage when diagnosed. Detecting GC early is essential to minimize disease incidence and mortality rates. Although gastrointestinal endoscopy is the gold standard for diagnosis, it is an invasive procedure requiring anesthesia and may cause physical and psychological discomfort to patients. Other imaging methods, like MRI, CT, and PET, have constraints, such as radiation harm [3, 4]. Blood markers including carcinoembryonic antigen (CEA), carbohydrate antigen (CA199), and CA125 have been acknowledged and embraced as a non-invasive diagnostic strategy for GC detection [5].
Metabolic Syndrome (MS) primarily includes impaired glucose tolerance, abnormal blood lipids, obesity, and hypertension, and there have been reports showing a correlation with insulin resistance(IR) [6]. Dyslipidemia forms a component of the metabolic syndrome and is marked by changes in serum lipid levels. In recent times, accumulating evidence indicates that serum lipids significantly influence tumor growth and advancement [7]. Several studies have demonstrated a positive association between serum lipid concentrations and the risk of gastric cancer (GC) [8]. However, the constrained understanding of the interplay between serum lipids and the clinical parameters of GC has impeded the effective utilization of serum lipids to glean more precise and significant insights for the diagnosis and treatment outcomes of GC. Additionally, there has yet to be any research conducted employing propensity score matching (PSM) to explore the relationship between serum lipids and GC.
Current evidence suggests that IR may be a risk factor for the occurrence and fatality of various cancers, including GC [9, 10]. In recent times, there has been significant attention and research on the triglyceride-glucose (TyG) index and the atherosclerotic index (AI(TC − HDLC)/HDLC)), both of which are associated with IR, diabetes, and MS [11]. Previous research has indicated that the TyG index could be utilized as indicators of cancer patients [12, 13]. However, the role of TyG index in cancer risk is still debated and its correlation with GC patients has not been established. The AI is commonly recognized as a predictor of cardiovascular disease. Nevertheless, additional investigation is required to determine the link between AI and GC. Therefore, the study evaluated the clinical feasibility of using the TyG index, AI combined with tumor antigen as a new diagnostic and predictive marker and the ability to predict chemotherapy side effects.
Methods
Study population
Individuals diagnosed with GC via histological examination at the Department of Gastrointestinal Surgery at Shanghai East Hospital from 2017 to 2021 underwent a retrospective evaluation. A total of 215 patients with GC were enrolled in this study after excluding participants who did not undergo a complete lipid profile laboratory test or those who were lost to follow-up were also excluded. In order to investigate the correlation between serum lipid levels and GC, a comparative group was selected retrospectively from a health promotion center at the same period. A total of 827 healthy participants with complete lipid profile laboratory test were enrolled as healthy control group. Ultimately, data from 215 GC patients and 827 control individuals were analyzed. This study was approved by the Ethics Committee of Shanghai East Hospital.
Data collection
Each participant filled out a questionnaire that gathered details about acute and chronic illnesses as well as their drug usage. Peripheral blood was collected for routine haematological tests and cancer antigens such as CEA and CA199. The clinicopathological classification of GC was performed according to the American Joint Committee on Cancer TNM staging system. The serum lipid profiles of all subjects were measured in the study. The serum lipid profiles were assessed using a Roche Cobas e801 chemistry analyzer (Roche Diagnostics, Mannheim, Germany). This evaluation included measurements of triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). The TyG index was derived through the formula ln [fasting TG (mg/dL) × FPG (mg/dL) / 2]. Additionally, the Atherogenic Index (AI) was analytically determined using the formula (TC - HDL-C) / HDL-C.
Statistical analysis
The data were presented as the average ± standard deviation (for normally distributed data), middle value (range of values between the 25th and 75th percentiles) (for non-parametric data), or proportion. Analysis of categorical variables was conducted by employing chi-square or Fisher exact tests, whereas continuous variables were evaluated through Student’s t-test or one-way ANOVA. An ROC curve was constructed to assess the discriminative power of the variables in predicting the occurrence of GC, and the AUC along with its 95% CI was computed. Data analysis was performed using SPSS 26.0 software, and statistical significance was defined as P < 0.05 (with two-tailed tests). The impact of serum lipids was examined through one-to-one matching of propensity scores (PSM). PSM was conducted between gastric cancer group and healthy control group to minimize the bias of baseline information. Nearest neighbor matching was performed without replacement at a 1:1 ratio and a caliper width with a 0.01 standard deviation was specified. The matched baseline information was as follows: gender, body mass index, and hypertension.
Results
Serum lipid levels, TyG are strongly associated with Gastric Cancer before and after propensity score matching
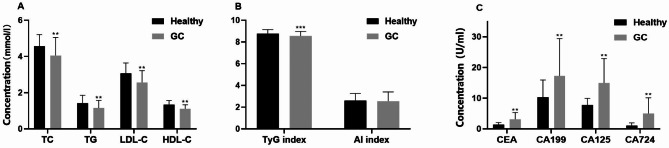
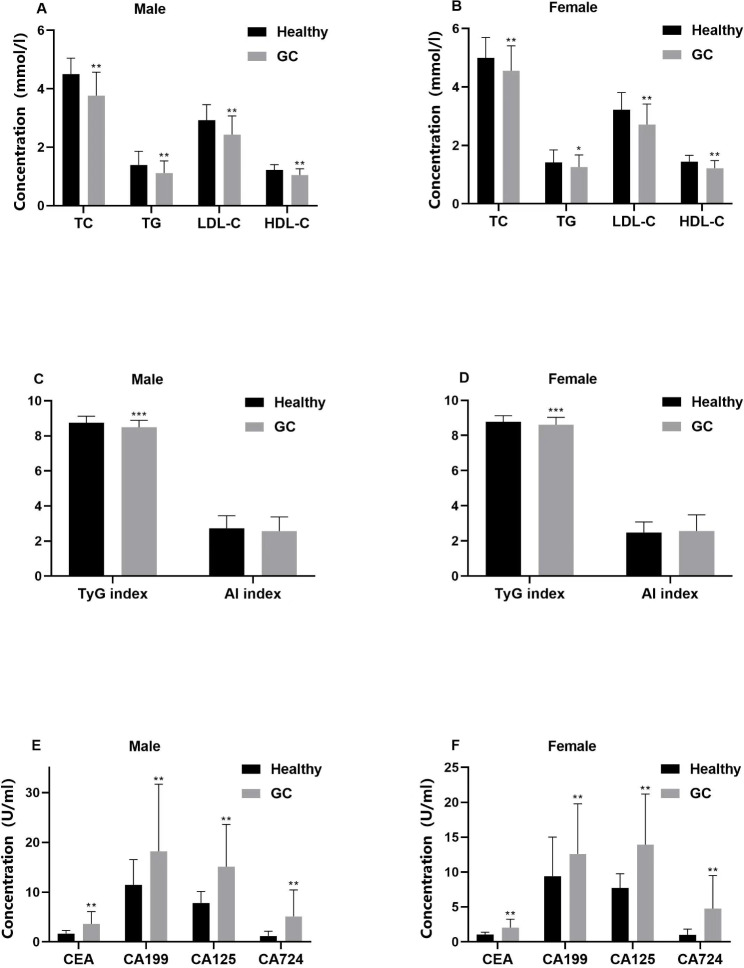
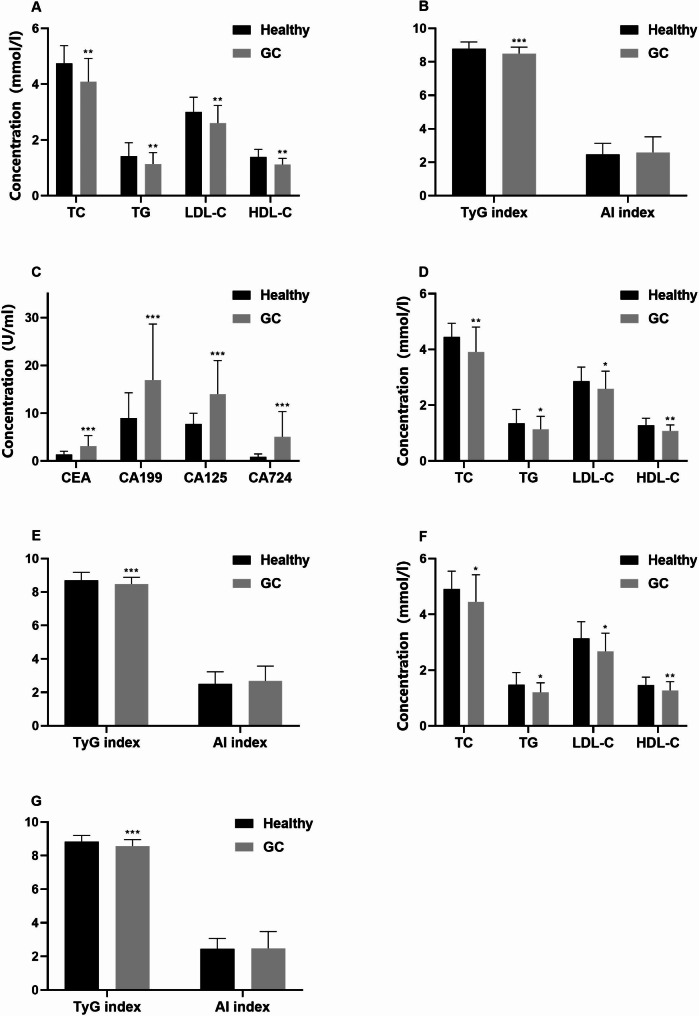
The study enrolled 215 individuals diagnosed with GC and 827 subjects with no history of illness. Variances in gender and hypertension were observed between the cohorts (Table 1). The serum concentrations of TG, TC, HDL-C, LDL-C, and TyG index were notably lower in GC patients when compared to the control group (P < 0.01). In contrast, levels of CEA, CA199, CA125, and CA724 were considerably higher in GC patients than in healthy individuals (P < 0.01) (Fig. 1). The participants were further categorized by gender into separate sets of male and female participants. Our analysis revealed that TG, TC, HDL-C, LDL-C, and TyG index remained markedly lower in both male and female GC patients when compared to their respective counterparts in the control group. Conversely, CEA, CA199, CA125, and CA724 levels were notably higher in both male and female GC patients compared to their gender-matched healthy individuals (Fig. 2). Subsequent to these outcomes, a 1:1 PSM was executed with 306 subjects being successfully matched (153 in each category). The adjusted PSM resulted in a well-balanced distribution of all variables between the two groups. An examination of the matched participants exhibited consistent results (Fig. 3).
Table 1.
Baseline characteristics
| Healthy(n = 827) | GC(n = 215) | P | ||||
|---|---|---|---|---|---|---|
| Year, median (IQR) | 65 (61.00–71.00) | 66.00 (60–72.00) | 0.649 | |||
| Gender | < 0.01 | |||||
| Male | 365 (44.14%) | 150 (69.77%) | ||||
| Female | 462 (55.86%) | 65 (30.23%) | ||||
| BMI, median (IQR), kg/m2 | 25.00 (22.90–27.00) | 23.51 (21.15–25.15) | 0.632 | |||
| Height, median (IQR), cm | 159.00 (154.00-167.00) | 162.00 (155.00-170.00) | 0.18 | |||
| Weight, median (IQR), kg | 64.00 (57.00–71.00) | 64.00 (56.00–70.00) | 0.651 | |||
| Hypertension, n (%) | ||||||
| Yes | 45 (5.44%) | 100 (46.51%) | < 0.01 | |||
| No | 782 (94.56%) | 115 (53.49%) | ||||
GC gastric cancer, BMI body mass index, IQR Interquartile range
Fig. 1.
Serum lipid, TyG index, AI, and tumor markers in GC patients and healthy participants. Serum lipids including TC, TG, HDL-C, and LDL-C (a), TyG index, and AI (b) in all research subjects. CEA, CA199, CA125, and CA724 (c), Data were presented as median (interquartile range [IQR]), analyzed by Pearson’s χ2 test. *P < 0.05, **P < 0.01, ***P < 0.001
Fig. 2.
Levels of blood lipids, TyG index, AI, and tumor markers in gastric cancer patients of different genders and healthy controls. Lipid indices include TC, TG, HDL-C, and LDL-C in male (a) and female (b), TyG index and AI in male (c) and female (d). CEA, CA199, CA125, and CA724 in Male (e) and female (f), the data to the median (interquartile range (IQR are much less)) said that using Pearson chi-square test. *p < 0.05, **p < 0.01, ***p < 0.001
Fig. 3.
Serum lipid, TyG index and AI in GC patients and healthy participants after PSM. After PSM analysis, serum lipids (a), TyG index, AI (b), and tumor markers (c) were measured in GC patients and healthy controls. Lipid measures included TC, TG, HDL, and LDL-C in male (d) and female (f), and TyG index and AI in male (e) and female (g). Data were expressed as median (interquartile range less than IQR) and analyzed using the Pearson chi-square test. *p < 0.05, **p < 0.01, ***p < 0.001
Relationships between serum lipid levels and the malignancy and invasiveness of gastric cancer
To evaluate the relationship between serum lipids and tumor stage, patients were divided into two distinct groups: one consisting of those diagnosed with relatively early-stage tumors, encompassing stages I and II, and another group composed of individuals with advanced-stage tumors, which included stages III and IV. Our findings revealed a significant reduction in the levels of TG, TC, HDL-C, LDL-C, and TyG index in patients categorized within the advanced-stage tumor group, as detailed in Table 2. Additionally, when comparing GC patients with and without lymph node metastasis, we observed that those with lymph node metastasis exhibited considerably lower levels of TG, TC, HDL-C, LDL-C, and TyG index, which is depicted in Table 3. Furthermore, the levels of TG, TC, HDL-C, LDL-C, and TyG index were found to be significantly associated with poor differentiation in GC patients, as presented in Table 4.
Table 2.
Lipid and gastric cancer staging correlation analysis
| Stage I/II(n = 88) | Stag eIII/IV (n = 123) | p | |
|---|---|---|---|
| TC (mmol/L) | 4.32(3.4–4.9) | 3.84(2.9–4.7) | 0.022 |
| TG (mmol/L) | 1.2(0.9–1.7) | 1.01(0.8–1.5) | 0.004 |
| LDL-C (mmol/L) | 2.75(2.1–3.3) | 2.44(1.8-3.0) | 0.047 |
| HDL-C (mmol/L) | 1.09(0.9–1.4) | 1.07(0.9–1.3) | 0.031 |
| TyG index | 8.63(8.3-9.0) | 8.5(8.1-9.0) | < 0.001 |
| AI | 2.46(1.9–3.5) | 2.36(1.6–3.5) | 0.32 |
TG triglyceride, TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, SCR Serum creatinine, AI atherosclerotic index, TyG triglyceride-glucose
Table 3.
Correlation analysis between blood lipids and lymph node metastasis of gastric cancer
| LN -(n = 103) | LN +(n = 112) | p | |
|---|---|---|---|
| TC (mmol/L) | 4.21 (3.3–4.9) | 3.84 (3-4.8) | 0.023 |
| TG (mmol/L) | 1.17 (0.8–1.7) | 1.03 (0.8–1.5) | 0.017 |
| LDL-C (mmol/L) | 2.56 (1.9–3.2) | 2.52 (1.8–3.3) | 0.038 |
| HDL-C (mmol/L) | 1.09 (0.9–1.4) | 1.07 (0.9–1.3) | 0.011 |
| TyG index | 8.59 (8.2–8.9) | 8.47 (8.1–8.9) | 0.003 |
| AI | 2.46 (1.9–3.5) | 2.36 (1.7–3.4) | 0.34 |
TG triglyceride, TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, SCR Serum creatinine, AI atherosclerotic index, TyG triglyceride-glucose
Table 4.
Blood lipid and gastric cancer differentiation degree of correlation analysis
| Low grade(n = 130) | High grade(n = 85) | p | |
|---|---|---|---|
| TC (mmol/L) | 4.0 (3.2–4.7) | 4.02 (3.1-5.0) | 0.014 |
| TG (mmol/L) | 0.9 (0.8–1.6) | 1.11 (0.8–1.7) | 0.003 |
| LDL-C (mmol/L) | 2.50 (1.9–3.1) | 2.56 (1.8–3.3) | 0.025 |
| HDL-C (mmol/L) | 1.08 (0.9–1.3) | 1.12 (0.9–1.4) | 0.009 |
| TyG index | 8.52 (8.1–8.9) | 8.59 (8.2-9.0) | < 0.001 |
| AI | 2.41 (1.7–3.3) | 2.42 (1.9–3.5) | 0.53 |
TG triglyceride, TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, SCR Serum creatinine, AI atherosclerotic index, TyG triglyceride-glucose
Changes in blood lipid and tumor marker levels in patients with gastric cancer after surgery
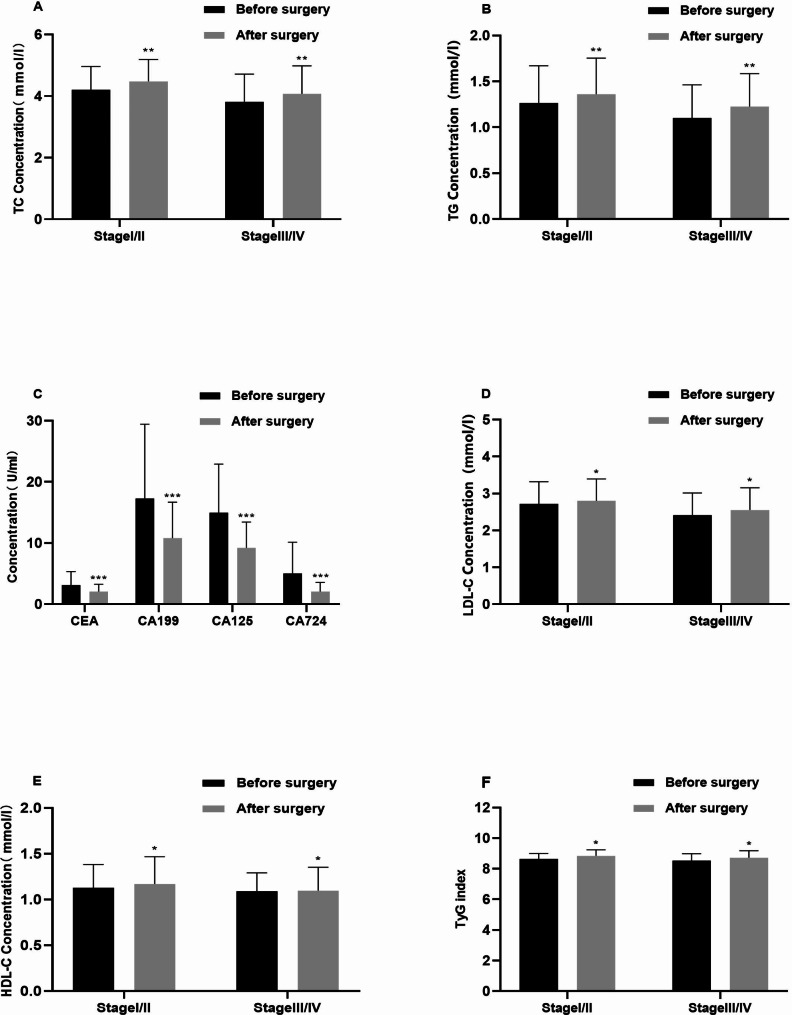
3 months after surgical intervention, GC patients experienced significant changes in lipid and glucose metabolism. Following the surgery, levels of TC, TG, LDL-C, HDL-C, and TyG index all increased in the postoperative GC group. This shift in lipid profiles indicates alterations in metabolic processes post-surgery. Additionally, a notable decrease was noted in tumor markers such as CEA, CA199, CA724, and CA125, which suggests a potential reduction in cancer-related markers after the surgical intervention. Furthermore, analyses of early-stage (I + II) and advanced-stage (III + IV) GC patients revealed significant elevations in TC, TG, LDL-C, HDL-C levels, and TyG index post-surgery(Fig. 4). This suggests that the metabolic changes observed were consistent across different cancer stages, indicating a general impact on lipid and glucose metabolism following surgical treatment.
Fig. 4.
The levels of TC (a), TG (b), LDL-C (d), HDL-C (e), TyG index, AI index (f), and tumor markers (c) in gastric cancer patients before and after surgery were compared. Data were presented as median (interquartile range less than IQR) and analyzed using the Pearson chi-square test. *p < 0.05, **p < 0.01, ***p < 0.001
Enhanced diagnostic value of serum lipid levels and serum cancer antigens in patients with GC
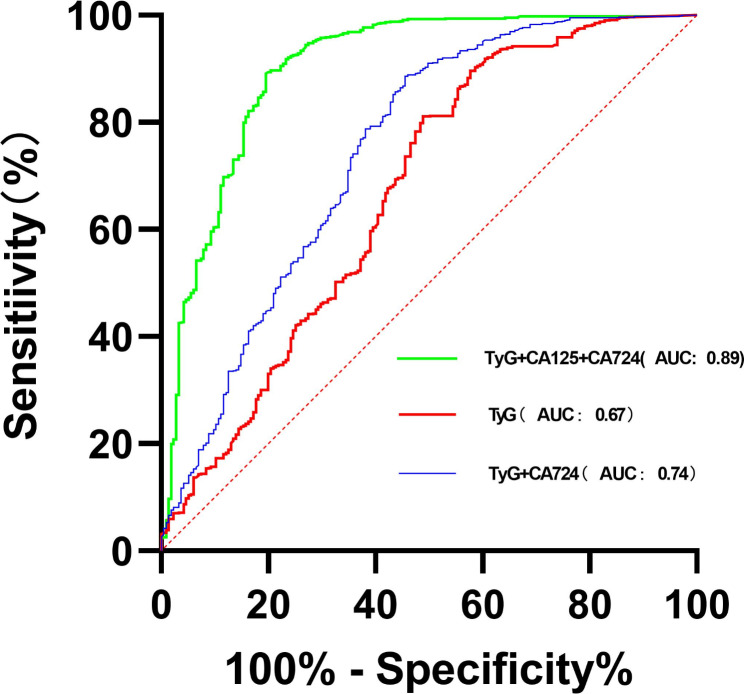
With the atypical levels of lipid levels and cancer antigens in GC patients, we assessed the effectiveness of combining the TyG index, TC, TG, LDL-C, HDL-C, CEA, CA199, CA724, and CA125 in improving diagnostic accuracy. The findings indicated that a combination of the TyG index and cancer antigens can serve as a novel diagnostic indicator for GC. Furthermore, the combination of TyG index, CA12-5, and CA19-9 demonstrated a notably high positive prediction rate of 98.6% for GC based on ROC analysis (Fig. 5). The remaining prediction rate of serum lipids plus cancer antigens can be found in supplementary Tables 1, 2, 3.
Fig. 5.
Various combined diagnostic indicators for GC patients
The TyG index at lower levels is associated with an increased vulnerability to adverse reactions following chemotherapy
Among the 215 GC patients, there were 123 advanced stage patients who received postoperative chemotherapy. All patients were followed up for 1 year. A total of 23 adverse reaction cases (including hand-foot syndrome, thrombocytopenia, anemia, neutropenia, and myelosuppression) were observed. Logistic regression analysis indicated that a lower TyG index level may be associated with an increased risk of experiencing adverse reactions following chemotherapy (OR = 1.104, 95% CI 1.028–1.186, P < 0.01)(Table 5). The adverse reactions of hand-foot syndrome, thrombocytopenia, anemia, neutropenia, and myelosuppression induced by chemotherapy did not show a significant correlation with any single tumor marker (P > 0.05). Multiple tumor markers and the TyG index combined detection showed moderate to weak correlations with five adverse reactions caused by chemotherapy (r < 0.6, P < 0.05) (Table 6).
Table 5.
Logistic analysis of blood lipids and risk of gastric cancer
| Influencing factors | Adverse reactions(n = 23) | No adverse reactions(n = 100) | Partial regression coefficient | Standard error | P | OR(95% CI) |
|---|---|---|---|---|---|---|
| TC | 3.3 ± 1.2 | 3.5 ± 1.7 | –0.182 | 0.518 | > 0.05 | 0.834(0.302–2.301) |
| TG | 0.9 ± 0.4 | 0.8 ± 0.4 | 0.076 | 0.153 | > 0.05 | 1.079(0.799–1.456) |
| HDL-C | 0.8 ± 0.3 | 0.7 ± 0.2 | 0.007 | 0.033 | > 0.05 | 1.011(0.965–1.052) |
| LDL-C | 2.3 ± 0.9 | 2.5 ± 1.1 | 0.427 | 0.478 | > 0.05 | 1.533(0.600-3.912) |
| TyG index | 8.2 ± 0.4 | 8.6 ± 0.6 | 0.099 | 0.036 | < 0.01 | 1.104(1.028–1.186) |
TG triglyceride, TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, SCR Serum creatinine, AI atherosclerotic index, TyG triglyceride-glucose
Table 6.
Correlation analysis of tumor markers combined with lipid indexes and adverse reactions of chemotherapy
| Hand-foot syndrome | Thrombocytopenia | Anemia | Neutropenia | Myelosuppression | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P | r | P | r | P | r | P | r | P | r | |
| CEA | 0.551 | 0.067 | 0.844 | 0.033 | 0.211 | 0.531 | 0.674 | 0.078 | 0.824 | 0.041 |
| CA199 | 0.119 | 0.181 | 0.123 | 0.161 | 0.331 | 0.124 | 0.899 | 0.021 | 0.312 | 0.101 |
| CA125 | 0.847 | 0.410 | 0.246 | 0.191 | 0.966 | 0.013 | 0.145 | 0.257 | 0.199 | 0.314 |
| CA724 | 0.749 | 0.311 | 0.177 | 0.224 | 0.859 | 0.215 | 0.525 | 0.248 | 0.686 | 0.067 |
| Tyg index + CA125 + CA724 | 0.004 | 0.537 | 0.011 | 0.342 | 0.009 | 0.496 | 0.01 | 0.597 | 0.037 | 0.491 |
TG triglyceride, TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, SCR Serum creatinine, AI atherosclerotic index, TyG triglyceride-glucose
Discussion
In this retrospective study, we examined the correlation between dyslipidemia and GC, while also investigating whether a combination of serum lipid levels, TyG index, and AI could enhance the accuracy of GC diagnosis. Notably, our findings indicated markedly lower levels of blood lipid markers - TC, TG, LDL-C, HDL-C, and the TyG index - in GC patients compared to healthy controls. This disparity remained statistically significant even after adjusting for propensity scores. Furthermore, the TyG index was found to be associated with GC, with lymphatic metastasis and tumor stage emerging as potential factors influencing serum lipid levels. Therefore, it was indicated that serum lipid levels and the TyG index could serve as valuable biomarkers not only for GC diagnosis but also for predicting lymph node metastasis and chemotherapy-related adverse events. Additionally, the inclusion of TyG index and cancer antigens in diagnostic procedures could significantly enhance GC detection.
The routine lipid panel, including TG, TC, HDL-C, and LDL-C, is essential for assessing lipid metabolism and its role in various metabolic pathways. There is increasing evidence pointing towards a link between lipid imbalance and cancer risk, attributed to factors such as inflammation, oxidative stress, insulin resistance, and alterations in protein function [14]. While some studies suggest that serum lipids could potentially serve as markers for cancer [15], conflicting results have been observed in previous research, especially in relation to GC. Moreover, little research has been done on the association between lipid derivatives like the TyG index and AI with GC patients, particularly with the use of PSM analysis to account for confounding factors. The association between serum lipid levels and cardiovascular diseases has been well-documented, but recent evidence indicates that these lipid components may also play a role in the development of various cancers, including GC. Contradictory findings exist regarding the relationship between serum lipid levels and GC [16–18], underscoring the need for further investigation. PSM analysis, a statistical tool that addresses bias and can simulate randomized clinical trials, has been instrumental in providing more robust evidence in research studies [19]. Remarkably, the GC group exhibited decreased serum lipid levels both prior to and following PSM examination, indicating that the connection linking low serum lipids and lipoproteins with GC might stem from metabolic factors, nutrient rivalry, and swift utilization by cancerous cells, as opposed to heightened levels functioning as a safeguarding element.
Recent studies in clinical epidemiology have emphasized a significant correlation between insulin resistance (IR) and the risk of developing cancer [9]. The TyG index, a reliable and straightforward indicator of IR, is determined by fasting levels of glucose and triglycerides. This index has been shown to be linked to a higher likelihood of developing digestive system cancers. While previous studies have not extensively explored the connection between the TyG index and GC patients, our investigation reveals a significant correlation between the two even after applying PSM. Additionally, we observed a positive association between lower TyG index levels and more advanced tumor stages in GC patients. Analysis using the ROC curve demonstrated that the area under the curve for the TyG index in predicting GC was 0.67, suggesting that this index could serve as a valuable and dependable tool in diagnosing GC. The study distinguishes itself by showing for the first time that both the TyG index can be considered reliable blood-based biomarkers for GC patients.
Current tumor markers have been widely used worldwide for cancer screening, treatment response assessment, and recurrence monitoring. Increased levels of tumor markers are linked to more progressed invasion, metastasis in lymph nodes, and reduced survival rates [20, 21]. According to our study, the GC group exhibited significantly higher levels of tumor markers compared to the control group. Following GC surgery, there was a significant decrease in tumor marker levels. However, due to the limited value of a single tumor marker in early tumor diagnosis and the high heterogeneity of GC itself, its overall sensitivity or specificity for predicting GC diagnosis is relatively low.
The diverse composition of GC makes it unlikely that a single tumor marker will be sensitive or specific enough for diagnostic testing. Therefore, in clinical practice, a combination of two or more types of biomarkers is often utilized to enhance the accuracy and effectiveness of disease detection. Our research has shown that by utilizing three serum markers together, we can achieve the highest level of predictive accuracy. Specifically, by combining the TyG index with two tumor markers, we can achieve the highest overall positive predictive rate, with the combination of TyG index + CA125 + CA724 yielding a peak value of 98.6% (P < 0.001). Additionally, among all the combinations tested, the pairing of TyG with a single marker results in a noteworthy positive predictive rate, with the combination of TyG index + CA724 reaching the highest value at 93.5% (P < 0.001).Therefore, in terms of novel strategies for predicting GC positivity, this study suggests that utilizing lipid and tumor markers as primary indicators shows great promise. In particular, it emphasizes the relationship between the TyG index and tumor markers.
There are many risks of adverse reactions in patients with advanced GC who receive postoperative chemotherapy. According to the results of this study, there is no significant correlation between a single tumor marker and chemotherapy-related adverse reactions (P > 0.05). However, the TyG index and combined detection of multiple tumor markers are positively correlated with the occurrence of five types of chemotherapy-related adverse reactions (P < 0.05). As a retrospective observational study, our study lacked an exploration of the potential mechanisms of chemotherapy side effects. We attempt to further explain the potential mechanism of TyG index as a predictive biomarker for chemotherapy side effects through literature review. As the progresses of gastric cancer, poor patient intake, increased tumor consumption, and continuous declining in nutritional status, the levels of serum lipids gradually decreased in advanced gastric cancer. Chemotherapy causes several side effects. In severe cases of chemotherapy side effects, it can be life-threatening. Some study indicated nutritional status before chemotherapy was associated with the risk of chemotherapy discontinuation [22]. In our study, we thought one of the mechanisms may be the nutritional status influencing the course of chemotherapy.
Our study may provide some value in clinical diagnosis of GC, suggesting the decrease of blood lipid level and TyG index in indicating more malignant in GC patients. However, in our study, no significant association between atherosclerotic index and GC was found, which may be related to potential confounding factors, and smaller sample sizes. As a retrospective study, the level of evidence is relatively low, and future high-quality prospective studies are needed to determine the clinical application value of the TyG index. Furthermore, the TyG index and combined detection of multiple tumor markers can provide a reference for predicting adverse reactions such as hand-foot syndrome, thrombocytopenia, anemia, neutropenia, and myelosuppression and may guide adjusting the patient’s subsequent treatment plan.
Limitations
There are various limitations to our study. To begin with, it is important to highlight that the research was carried out retrospectively at just one institution, potentially leading to selection bias. In order to fully evaluate the effectiveness of serum lipids for diagnosis, it is crucial to conduct prospective studies across different institutions. The lack of a validation group also prevents a thorough confirmation of our results. Secondly, due to the sex disparity in patients with GC, there is an imbalance in sex distribution. Nonetheless, because we performed a separate statistical study on both sexes, the effect of sex distribution imbalance on the results would be minor. PSM analysis considered only the observed covariates, not the potential bias of variables, which were not investigated in this study. For example, we did not survey the genetic situations because this was a retrospective study and there were many missing data. Moreover, due to the cross-sectional study design, we only observed a positive association of serum lipids and TyG index with GC but no causal relationship, which deserves better exploration in future studies. Finally, survival analysis based on serum lipid levels was not carried out. despite these limitations, the results clearly indicate that combining serum lipids and tumor markers can significantly enhance diagnostic accuracy for individuals with GC.
Conclusions
Preoperative lipid profiles in the serum show a strong correlation with patients diagnosed with GC. Evaluating a combination of various serum lipids and cancer markers significantly improves diagnostic precision for GC and the ability to predict chemotherapy side effects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- GC
Gastric cancer
- TyG
Triglyceride-glucose
- CEA
Carcinoembryonic antigen
- CA
Carbohydrate antigen
- MS
Metabolic Syndrome
- IR
Insulin resistance
- PSM
Propensity score matching
- AI
Atherosclerotic index
- TG
Triglyceride
- TC
Total cholesterol
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by D.Z, R.H, and X.C. The first draft of the manuscript was written by S.Z and X.J. All authors read and approved the final manuscript.
Funding
The study is funded by Pudong New Area Science and Technology Development Fund for Livelihood Research Special Project (No. PKJ2023-Y38).
Data availability
The data information used and/or analyzed during this study are available from the corresponding author (S.Z) on reasonable request.
Declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Institutional Review Committee of Shanghai East Hospital. The requirement for informed consent was waived by Ethics Committee of Shanghai East Hospital because the data were anonymous.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Di Zhang and Ren-hao Hu are contributed equally to the article.
Contributor Information
Xiao-hua Jiang, Email: jiangxiaohuash@163.com.
Shun Zhang, Email: v2zs@hotmail.com.
References
- 1.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635–48. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA-CANCER J CLIN. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 3.Douda L, Cyrany J, Tachecí I. Early gastric cancer. Vnitr Lek. 2022;68(6):371–5. [DOI] [PubMed] [Google Scholar]
- 4.Borggreve AS, Goense L, Brenkman H, Mook S, Meijer GJ, Wessels FJ, Verheij M, Jansen E, van Hillegersberg R, van Rossum P, et al. Imaging strategies in the management of gastric cancer: current role and future potential of MRI. BRIT J RADIOL. 2019;92(1097):20181044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W, Chen XL, Zhao SY, Xu YH, Zhang WH, Liu K, Chen XZ, Yang K, Zhang B, Chen ZX, et al. Prognostic significance of preoperative serum CA125, CA19-9 and CEA in gastric carcinoma. Oncotarget. 2016;7(23):35423–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. OBES REV. 2015;16(1):1–12. [DOI] [PubMed] [Google Scholar]
- 7.Hashmi S, Wang Y, Suman DS, Parhar RS, Collison K, Conca W, Al-Mohanna F, Gaugler R. Human cancer: is it linked to dysfunctional lipid metabolism? Biochim Biophys Acta. 2015;1850(2):352–64. [DOI] [PubMed] [Google Scholar]
- 8.Pih GY, Gong EJ, Choi JY, Kim MJ, Ahn JY, Choe J, Bae SE, Chang HS, Na HK, Lee JH, et al. Associations of serum lipid level with gastric Cancer Risk, Pathology, and prognosis. CANCER RES TREAT. 2021;53(2):445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arcidiacono B, Iiritano S, Nocera A, Possidente K, Nevolo MT, Ventura V, Foti D, Chiefari E, Brunetti A. Insulin resistance and cancer risk: an overview of the pathogenetic mechanisms. Exp Diabetes Res. 2012;2012:789174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto S, Nakagawa T, Matsushita Y, Kusano S, Hayashi T, Irokawa M, Aoki T, Korogi Y, Mizoue T. Visceral fat area and markers of insulin resistance in relation to colorectal neoplasia. Diabetes Care. 2010;33(1):184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez-Macedonio CP, Flores-Alfaro E, Alarcón-Romero L, Vences-Velázquez A, Castro-Alarcón N, Martínez-Martínez E, Ramirez M. CD14 and CD26 from serum exosomes are associated with type 2 diabetes, exosomal cystatin C and CD14 are associated with metabolic syndrome and atherogenic index of plasma. PEERJ. 2022;10:e13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu T, Zhang Q, Wang Y, Ma X, Zhang Q, Song M, Cao L, Shi H. Association between the TyG index and TG/HDL-C ratio as insulin resistance markers and the risk of colorectal cancer. BMC Cancer. 2022;22(1):1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Liu T, Qian L, Wang Y, Ma X, Cao L, Zhang Q, Qu J. Insulin resistance and inflammation mediate the association of abdominal obesity with colorectal cancer risk. FRONT ENDOCRINOL. 2022;13:983160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Z, Tang H, Lu S, Sun X, Rao B. Relationship between serum lipid level and colorectal cancer: a systemic review and meta-analysis. BMJ OPEN. 2022;12(6):e052373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang Z, He M, Song M. Serum lipid profiles and risk of colorectal cancer: a prospective cohort study in the UK Biobank. BRIT J CANCER. 2021;124(3):663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulmer H, Borena W, Rapp K, Klenk J, Strasak A, Diem G, Concin H, Nagel G. Serum triglyceride concentrations and cancer risk in a large cohort study in Austria. BRIT J CANCER. 2009;101(7):1202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borena W, Stocks T, Jonsson H, Strohmaier S, Nagel G, Bjørge T, Manjer J, Hallmans G, Selmer R, Almquist M, et al. Serum triglycerides and cancer risk in the metabolic syndrome and cancer (Me-Can) collaborative study. CANCER CAUSE CONTROL. 2011;22(2):291–9. [DOI] [PubMed] [Google Scholar]
- 18.Iso H, Ikeda A, Inoue M, Sato S, Tsugane S. Serum cholesterol levels in relation to the incidence of cancer: the JPHC study cohorts. INT J CANCER. 2009;125(11):2679–86. [DOI] [PubMed] [Google Scholar]
- 19.Radišauskas R, Kuzmickienė I, Milinavičienė E, Everatt R. Hypertension, serum lipids and cancer risk: a review of epidemiological evidence. MEDICINA-LITHUANIA. 2016;52(2):89–98. [DOI] [PubMed] [Google Scholar]
- 20.Vukobrat-Bijedic Z, Husic-Selimovic A, Sofic A, Bijedic N, Bjelogrlic I, Gogov B, Mehmedovic A. Cancer antigens (CEA and CA 19 – 9) as markers of Advanced Stage of Colorectal Carcinoma. Med Arch. 2013;67(6):397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhai H, Huang J, Yang C, Fu Y, Yang B. Serum CEA and CA19-9 levels are Associated with the Presence and Severity of Colorectal Neoplasia. CLIN LAB. 2018;64(3):351–6. [DOI] [PubMed] [Google Scholar]
- 22.Nomoto N, Tate S, Arai M, Iizaka S, Mori C, Sakurai K. Pretreatment nutritional status in combination with inflammation affects chemotherapy interruption in women with ovarian, fallopian tube, and Peritoneal Cancer. Nutrients. 2022;14(23):5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data information used and/or analyzed during this study are available from the corresponding author (S.Z) on reasonable request.