Abstract
Seeding activities of disease-associated α-synuclein aggregates (αSynD), a hallmark of Parkinson’s disease (PD), are detectable by seed amplification assay (αSyn-SAA) and being developed as a diagnostic biomarker for PD. Sensitive and accurate αSyn-SAA for blood or saliva would greatly facilitate PD diagnosis. This prospective diagnostic study conducted αSyn-SAA analyses on serum and saliva samples collected from patients clinically diagnosed with PD or healthy controls (HC). 124 subjects (82 PD, 42 HC) donated blood and had extensive clinical assessments, of whom 74 subjects (48 PD, 26 HC) also donated saliva at the same visits. An additional 57 subjects (35 PD, 22 HC) donated saliva and had more limited clinical assessments. The mean ages were 69.21, 66.55, 69.58, and 64.71 years for PD serum donors, HC serum donors, PD saliva donors, and HC saliva donors, respectively. αSynD seeding activities in either sample type alone or both sample types together were evaluated for PD diagnosis. Serum αSyn-SAA data from 124 subjects showed 80.49% sensitivity, 90.48% specificity, and 0.9006 accuracy (AUC of ROC); saliva αSyn-SAA data from 131 subjects attained 74.70% sensitivity, 97.92% specificity, and 0.8966 accuracy. Remarkably, the combined serum and saliva αSyn-SAA from 74 subjects with both sample types achieved better diagnostic performance: 95.83% sensitivity, 96.15% specificity, and 0.98 accuracy. In addition, serum αSynD seeding activities correlated inversely with Montreal Cognitive Assessment in males and positively with Hamilton Depression Rating Scale in females and in the < 70 age group, whereas saliva αSynD seeding activities correlated inversely with age at diagnosis in males and in the < 70 age group. Our data indicate that serum and saliva αSyn-SAA together can achieve high diagnostic accuracy for PD comparable to that of CSF αSyn-SAA, suggesting their potential utility for highly sensitive, accurate, and minimally invasive diagnosis of PD in routine clinical practice and clinical studies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40478-024-01873-1.
Keywords: Seeding activity, Alpha-synuclein, Parkinson’s disease, Biomarker, RT-QuIC
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease. Accurate diagnosis of PD remains a challenge. The clinical diagnosis of PD is based on clinical examination with a diagnostic accuracy of ~ 80% at early stages [1–4]. Definitive PD diagnosis still relies on postmortem detection of brain neuronal inclusions of misfolded and aggregated alpha-synuclein protein (αSynD), a central player in PD pathogenesis and pathological hallmark of PD and other synucleinopathies.
The recently developed αSyn seed amplification assay (αSyn-SAA) can detect subsets of αSynD forms at extremely high sensitivity and specificity utilizing the ultrasensitive real-time quaking-induced conversion (RT-QuIC) platform [5–7]. Multiple sample types have been examined by αSyn-SAA assays, including cerebrospinal fluid (CSF), olfactory mucosa, skin tissues from live or autopsied participants, salivary gland biopsies, intestinal biopsies, saliva, and blood, of which CSF, skin, and salivary gland αSyn-SAA studies have shown excellent results for PD diagnosis [8–31]. CSF αSyn-SAA demonstrated 87.7% sensitivity for PD and 96.3% specificity for healthy controls (HC) in a large rigorous three-laboratory study [16]. Skin αSyn-SAA also showed very impressive diagnostic accuracy for PD in studies by us and others [19–23]. However, the invasive sampling procedures for CSF and skin biopsy are a significant impediment to patient acceptance and routine clinical application.
Blood and saliva samples are highly desirable in clinical diagnosis for easy access and minimal invasiveness. A recent αSyn-SAA study with serum samples showed ~ 95% sensitivity for PD and Dementia with Lewy Bodies (DLB) and ~ 92% specificity for HC [30], comparable to the CSF αSyn-SAA [16]. Another serum αSyn-SAA study reported 98.8% sensitivity for PD using a modified αSyn-SAA protocol [31]. But the extraordinary performance of such serum αSyn-SAA in PD diagnosis is yet to be verified by other laboratories using samples from diverse patient cohorts. Saliva αSyn-SAA seems also of good potential in PD diagnosis, showing 76.0% sensitivity for PD and 94.4% specificity for HC in one report [26] and 83.78% sensitivity and 82.61% specificity in another [27]. None of the blood or saliva αSyn-SAA assays have been vigorously verified.
Here we report αSyn-SAA analysis of 124 serum samples and 131 saliva samples from PD and HC subjects and show that using αSynD seeding activities in both serum and saliva samples together can achieve much higher sensitivity and specificity for PD diagnosis than using either sample type alone.
Materials and methods
Subject recruitment and clinical assessment
All subject recruitment and clinical assessments were conducted by movement disorders clinicians within the Parkinson’s and Movement Disorders Center at University Hospitals Cleveland Medical Center (UHCMC) in the USA. 125 subjects (83 PD, 42 HC) were recruited in our “skin and peripheral biofluid biomarker study” from February 2020 to March 2024, of which 1 (PD) provided only saliva, 50 (34 PD, 16 HC) provided only blood, and 74 (48 PD, 26 HC) (designated the “ss-subset”) provided both blood and saliva at the same visit. Inclusion criteria included age 21–89 years, ≥ 40 years of age at PD onset, and all NIH Parkinson Disease Biomarker Program (PDBP) inclusion criteria (including no schizophrenia or other major psychiatric disorder, and not on investigational drugs) and exclusion criteria (including blood clotting disorders, on multiple antiplatelets or anticoagulants, deep brain stimulation, or another neurodegenerative disorder). PD subjects were required to meet UK Brain Bank Criteria for possible or probable PD [32, 33] and were assessed for Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) and modified Hoehn & Yahr (mH&Y). All subjects had demographics (age, age at diagnosis, disease duration, gender), Schwab & England (S&E) ) “on” or non-fluctuator, Montreal Cognitive Assessment (MoCA), Hamilton Depression Rating Scale (HAM-D), Hamilton Anxiety Rating Scale (HAM-A), Epworth Sleepiness Scale (ESS), Parkinson’s Disease Questionnaire − 39 (PDQ-39), Mayo Sleep Questionnaire to screen for REM sleep behavior disorder (RBD), vital signs, family history, and full neurological examination, orthostatic hypotension (self-reported and by vitals), self-reported cognitive impairment, hyposmia, and constipation. 64 PD subjects were re-assessed for diagnostic criteria one year later, whose clinical diagnosis was based on the one-year re-assessment. 28 HC subjects were also re-examined and verified to remain controls. An additional 56 subjects (34 PD, 22 HC) were recruited to donate saliva in our “saliva biomarker study” from May 2023 to May 2024. Inclusion criteria included age 30–95 years, no upper respiratory infection, and UK Brain Bank criteria for PD subjects. Subjects had demographics, family history, clinician-determined cognitive status, MDS-UPDRS Part 3, and a subset also had MoCA. Pregnancy, schizophrenia, negative dopamine transporter SPECT, MoCA < 10, lack of capacity to give informed consent, and neuroleptic-induced parkinsonism were exclusions in both studies.
Blood and saliva collection and sample preparations
Blood was collected and serum prepared and stored per NIH PDBP protocol. Saliva (2–6 ml) was collected after ≥ 60 min fasting and no gum-chewing, 4 h without tobacco, and 12 h without alcohol. Saliva was collected by drooling into a funnel atop a cryovial. Serum and saliva were immediately stored at -80 °C in cryovials.
Purification of recombinant αSyn
Recombinant wild-type αSyn was expressed in BL21(DE3) E. coli cells and purified using a modified boiling as described [35, 36]. BL21(DE3) cells expressing wild type αSyn were cultured in the Terrific Broth medium [(12 g per liter of Bacto-tryptone, 24 g per liter of yeast extract, 4% (vol/vol) glycerol, 17 mM KH2PO4 and 72 mM K2HPO4) with ampicillin], induced with 0.5 mM IPTG until reaching an OD₆₀₀ of 0.6. Cells were harvested, resuspended in a high-salt buffer (750 mM NaCl, 10 mM Tris, pH 7.6, 1 mM EDTA) with protease inhibitors, and lysed by sonication. The lysate was boiled for 20 min and centrifuged, and the supernatant was dialyzed in 10 mM Tris (pH 7.6) and 50 mM NaCl. Proteins were concentrated and purified via size-exclusion chromatography on a Superdex 75 column, followed by ion-exchange chromatography on a Hi-Trap Q HP column. Fractions containing pure αSyn were pooled, dialyzed into 10 mM Tris (pH 7.6) and 50 mM NaCl, concentrated, aliquoted, and stored at -80 °C.
Immunoprecipitation-based αSyn-SAA for serum and saliva samples
The Immunoprecipitation (IP)-based αSyn-SAA procedure was adapted from a previous report [30] with several modifications. Saliva samples were cleared at 7000 g at 4oC for 10 min and subjected to IP with MJFR-14 (anti-αSyn conformational antibody, Abcam, UK) to specifically capture misfolded αSyn species. Protein A/G agarose beads (Thermo Fisher Scientific, USA) were used to bind the antibody-antigen complexes, which were then recovered with DynaMag (Invitrogen, USA) and thoroughly washed to eliminate non-specific binding. The beads-bound antibody-antigen complexes were released by incubation with 0.2 M glycine (pH 2.6) for 10 min with agitation. The eluted αSyn was added with 3 volumes of 0.1 M Tris (pH 8.0). 4 volumes of chilled acetone was added, incubated at -20 °C overnight, and centrifuged at 13,000–15,000 g for 20 min. The pellet was resuspended in 30 µl of PBS (pH 7.5) (per 100 µl of the original serum or saliva sample) and used as the seeds for RT-QuIC reactions.
The αSyn-SAA assays were conducted with a protocol adapted from previous reports [11, 22, 23, 30]. The final RT-QuIC reaction mixture contained 100 mM phosphate buffer (pH 8.2), 100 mM NaCl, 20 µM Thioflavin T (ThT), and 7 µM recombinant αSyn. The RT-QuIC reactions were performed in a BMG FLUOstar Omega plate reader with double-orbital shaking at 400 rpm at 40 °C. ThT fluorescence was measured over a 93.35-hour period to detect the aggregation of αSyn. The average ThT fluorescence readings of quadruplicate reactions at the endpoint (93.35 h) were normalized as a percentage of the maximal fluorescence reading (260,000) and used as a measure of the relative αSynD seeding activity in the respective samples. For data analysis, a well was considered positive if its endpoint fluorescence reading was ≥ the mean + 4 standard deviations of all the negative control wells. The average endpoint fluorescence values of all 4-wells of a sample (if at least 2 wells were positive or all 4 wells were negative) or 3 negative wells (if only 1 well was positive) were used for ROC analysis to determine the cutoff values and for clinical correlation analysis. The cutoff values for the serum-only cohort, the saliva-only cohort, and the paired serum-saliva cohort (the ss-subset) were determined separately by the ROC analysis of each cohort based on the threshold at the highest Youden’s Index. A sample (serum or saliva) was considered positive when 2 or more of the quadruplicate wells were positive and the average endpoint fluorescence reading of the quadruplicate wells was above the cutoff.
Statistical analysis
All statistical analyses were performed with the R statistical software (version 4.4.0). Descriptive statistics (means, standard deviations, p values) for continuous variables such as age, were computed using the R package “tableone” (version 0.13.2) via two-sample t-tests. The p values for categorical variables (such as sex) were determined with the chi-squared tests. Binary features (such as hyposmia) were examined with ANOVA. For the paired serum and saliva samples, the optimal cutoff values for both sample types were identified by first systematically calculating the sensitivity, specificity, and accuracy across a range of ThT fluorescence cutoff values for each sample type (45,000–73,000 for saliva and 35,000–69,000 for serum, at 100 increments), and then plotting the accuracy against the cutoff combinations in a 3-D space utilizing the ‘plotly’ package (version 4.10.4). For more robust assessments, 95% confidence intervals and p values were calculated using bootstrap resampling with 1,000 iterations, facilitated by the ‘boot’ package (version 1.3–30). The performance of these analyses was illustrated using Receiver Operating Characteristic (ROC) curves [37] generated with the ‘ROCR’ package (version 1.0–11). Potential correlation of αSynD seeding activities with clinical features was evaluated using Pearson’s correlation coefficient and visualized through the ‘ggplot2’ package (version 3.5.1). For age subgroup analyses, 70 years was chosen as the cutoff age to ensure sufficient cases in each group for adequate statistical power.
Results
124 serum samples (82 PD, 42 HC) and 131 saliva samples (83 PD, 48 HC) were examined by αSyn-SAA using the RT-QuIC platform (Table 1). PD blood donors had a mean age of 69.21 years (range 44–88) and 45 males (54.9%); HC blood donors had a mean age of 66.55 years (range 44–81) and 11 males (26.2%) (Table 1). The PD saliva donors had a mean age of 69.58 years (range 49–87) and 46 males (55.4%), and HC saliva donors had a mean age of 64.71 years (range 30–81) and 14 males (29.2%) (Table 1).
Table 1.
Demographics and clinical features of patients with PD and HC subjects
| All cases | ss-subset | |||||
|---|---|---|---|---|---|---|
| PD | HC | PD | HC | |||
| Serum | Saliva | Serum | Saliva | Serum & Saliva | Serum & Saliva | |
| Sample, n | 82 | 83 | 42 | 48 | 48 | 26 |
| Age, mean (range), years | 69.21 (44–88) | 69.58 (49–87) | 66.55 (44–81) | 64.71 (30–81) | 68.83 (49–84) | 66.38 (44–81) |
| Male, number (%) | 45 (54.9) | 46 (55.4) | 11 (26.2) | 14 (29.2) | 25 (52.1) | 7 (26.9) |
| Disease duration, (range), years | 5.05 (0–17) | 6.18 (0–31) | NA | NA | 5.04 (0–17) | NA |
| mH&Y, mean (SD) | 2.1 (0.48) | 2.09 (0.61) | NA | NA | 2.04 (0.52) | NA |
| Self-reported hyposmia | 37 (45.1) | 21 (42.9) | 1 (2.6) | 0 (0) | 20 (41.7) | 0 (0) |
| SAA + a, Number (%) | 66 (80.5) | 62 (74.7) | 4 (9.5) | 1 (2.1) | 46 (95.8) | 1 (3.8) |
aPositive by αSyn-SAA
Detection of αSynD seeding activity in serum from PD and HC subjects
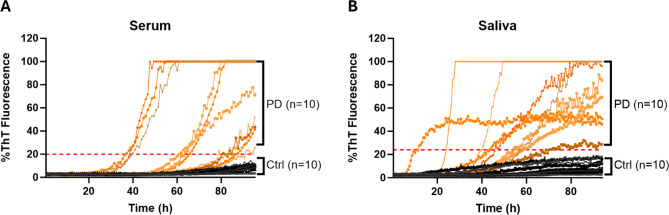
We modified the immunoprecipitation-based αSyn-SAA protocol with RT-QuIC [30] to detect αSynD seeding activities in serum and saliva samples. The αSyn-SAA reproducibility of different batches of recombinant αSyn protein was verified with 14 biopsy skin samples from known PD and healthy control subjects (7 each) (Supplementary Fig. 1). Representative RT-QuIC ThT fluorescence curves for blinded saliva and serum samples, including 10 PD and 10 HC each, indicated that the saliva and serum samples of patients with PD had overall higher ThT fluorescence readings than HC samples (Fig. 1).
Fig. 1.
Representative ThT Fluorescence Curves of αSyn RT-QuIC Assays of Serum or Saliva Samples. A. Representative curves of ThT fluorescence readings over time for αSynD RT-QuIC assays of serum samples from 10 PD and 10 HC subjects. B. Representative curves of ThT fluorescence readings over time for αSynD RT-QuIC assays of saliva samples from 10 PD and 10 HC subjects. All samples were coded and blinded for the RT-QuIC assays. The ThT fluorescence readings at the endpoint (93.35 h) were normalized to percentages of the maximal fluorescence reading (260,000) and used to measure the relative αSynD seeding activities in the respective samples. Orange lines: curves for PD samples; black lines: curves for HC subjects
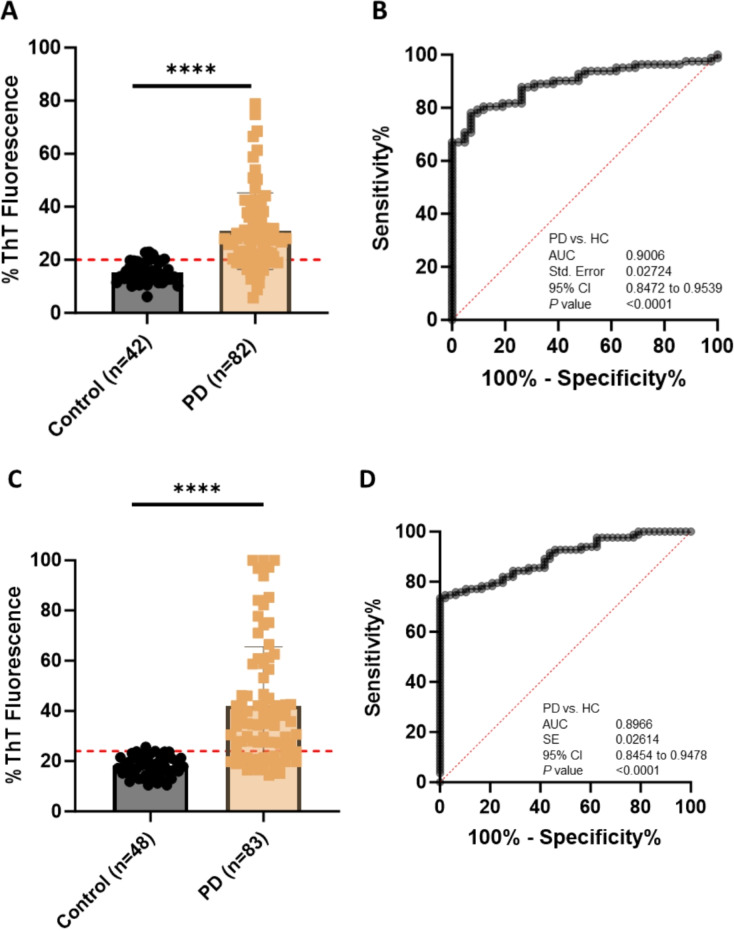
αSyn-SAA examination of 124 serum samples from 82 patients with PD (63 probable PD, 19 possible PD) and 42 HC subjects revealed 80.49% sensitivity, 90.48% specificity, and 0.9006 accuracy [AUC of ROC (same below), 95% CI, 0.8472–0.9539, p < 0.0001] for diagnosis of PD compared with clinical diagnosis (Fig. 2-A & B, Table 2). For serum samples from patients with probable PD, the sensitivity, specificity, and accuracy were 79.37%, 90.48%, and 0.8857 (95% CI, 0.8212-9502, p < 0.0001), respectively (Supplementary Fig. 2-A & B).
Fig. 2.
Comparison of αSynD Seeding Activity in Serum or Saliva Samples from Patients with PD and Healthy Controls (HC) by αSyn-SAA. Scatter graphs of RT-QulC endpoint ThT fluorescence intensities (αSynD seeding activities) in serum samples (A) or saliva samples (C) from patients with PD and HC subjects. Graphed are the average of the endpoint ThT fluorescence in quadruplicate wells of 124 serum samples (42 HC, 82 PD) or 131 saliva samples (48 HC, 83 PD) in RT-QuIC assays as a percentage of the maximum fluorescence (%ThT fluorescence). ThT fluorescence cutoff: serum, 52,105; saliva, 62,613. **** p < 0.0001. ROC curves for αSynD seeding activities in 124 serum samples (B) or 131 saliva samples (D) from patients with PD and HC subjects. SE, standard error. 95% CI, 95% confidence interval.
Table 2.
Comparison of diagnostic accuracy for PD with serum αSyn-SAA, saliva αSyn-SAA, or serum αSyn-SAA and saliva αSyn-SAA together
| All serum | All saliva | Paired Serum-Saliva (ss-subset) | |||
|---|---|---|---|---|---|
| Number of subjects | 82 PD, 42 HC | 83 PD, 48 HC | 48 PD, 26 HC | ||
| Serum alone | Saliva alone | Both serum & saliva | |||
| ThT fluorescence cutoff | 52,105 | 62,163 | 52,105 | 62,163 | serum: 52,960; saliva: 66,800 |
| Sensitivity | 80.49% | 74.70% | 85.42% | 75.00% | 95.83% |
| Specificity | 90.48% | 97.92% | 92.31% | 92.31% | 96.15% |
|
Accuracy by AUC (SE)a 95% CIb p value |
0.9006 (0.02724) 0.8472–0.9539 p < 0.0001 |
0.8966 (0.02614) 0.8454–0.9478 p < 0.0001 |
0.9623 (0.01866) 0.9258–0.9989 p < 0.0001 |
0.9046 (0.03337) 0.8392–0.9701 p < 0.0001 |
0.98 (0.011) 0.96-1.0 p < 0.001 |
astandard error; bconfidence interval
Detection of αSynD seeding activity in saliva from PD and HC subjects
αSyn-SAA examination of 131 saliva samples from 83 PD (24 probable PD and 59 possible PD) and 48 HC subjects achieved 74.70% sensitivity, 97.92% specificity, and 0.8966 accuracy (95% CI, 0.8454–0.9478, p < 0.0001) for diagnosis of patients with PD (Fig. 2-C & D, Table 2). For saliva samples from probable PD cases, the sensitivity, specificity, and accuracy were 79.66%, 97.92%, and 0.9054 (95% CI, 0.8484-09623, p < 0.0001), respectively (Supplementary Fig. 2-C & D).
PD diagnosis based on αSynD seeding activities in both serum and saliva
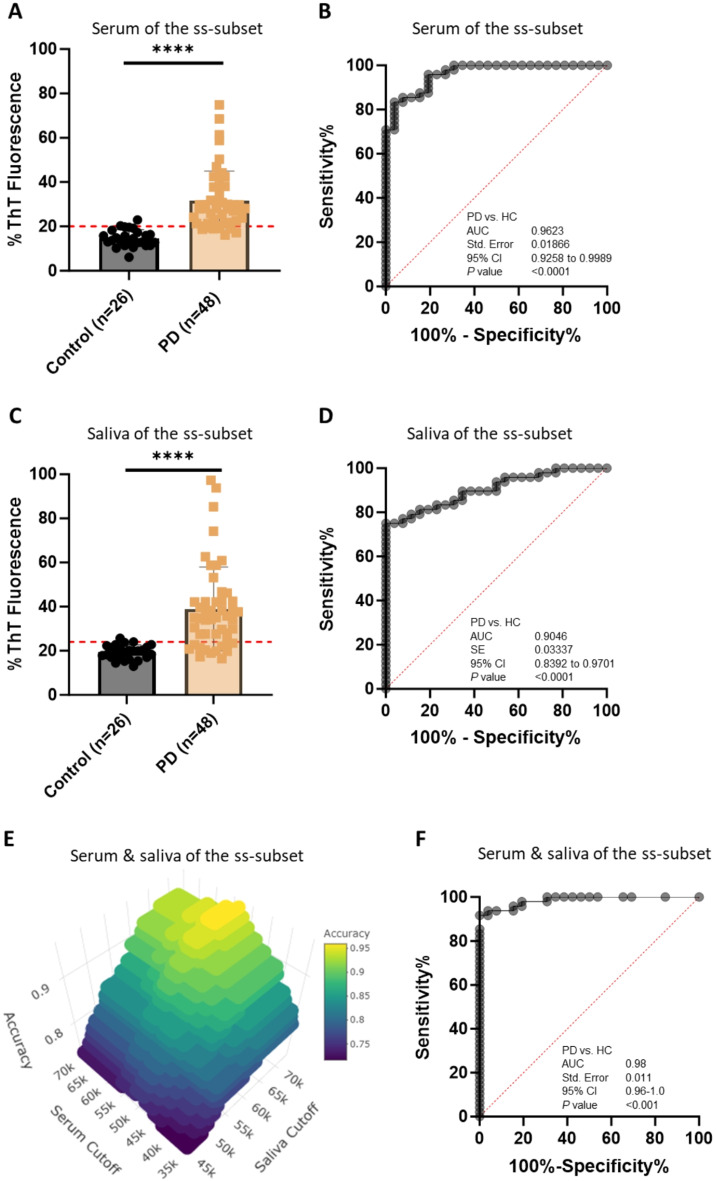
We hypothesized that PD patients with negative serum αSyn-SAA are likely to have positive saliva αSyn-SAA and vice versa. To test this hypothesis, we evaluated serum and saliva αSyn-SAA data from the “ss-subset” composed of 48 patients with PD (34 probable PD, 14 possible PD) and 26 HC subjects who provided both blood and saliva during the same visits and compared performance in PD diagnosis when the αSyn-SAA data from the two sample types were used alone or together. When the serum αSyn-SAA data were used alone, 85.42% sensitivity, 92.31% specificity, and 0.9623 accuracy (95% CI, 0.9258–0.9989, p < 0.0001) were achieved for PD diagnosis (Fig. 3-A & B); for probable PD, the sensitivity, specificity, and accuracy were 85.29%, 92.31%, and 0.9615 (95% CI, 0.9210-1.000, p < 0.0001), respectively (Supplementary Fig. 3-A & B). In comparison, when the saliva αSyn-SAA data were used alone, 75.00% sensitivity, 92.31% specificity, and 0.9046 accuracy (95% CI, 0.8392–0.9701, p < 0.0001) were achieved for PD diagnosis (Fig. 3-C & D, Table 2); for probable PD cases, the sensitivity, specificity, and accuracy were 76.47%, 92.31%, and 0.8824 (95% CI, 0.7971-0.9676, p < 0.0001), respectively (Supplementary Fig. 3-C & D).
Fig. 3.
Enhanced Diagnostic Accuracy for PD Using αSynD Seeding Activities in Both Serum and Saliva Samples from a Subset of Patients with PD and Healthy Control (HC) by αSyn-SAA. A. Scatter graph of αSynD seeding activities (RT-QuIC endpoint ThT fluorescence intensity) of serum samples in a subset of PD and HC subjects with paired serum and saliva samples. Scatter graph was plotted based on the average of the endpoint ThT fluorescence in quadruplicate wells as a percentage of the maximum fluorescence (%ThT fluorescence) in RT-QuIC assay of serum samples from 48 patients with PD and 26 HC in a subset of PD and HC subjects with both serum and saliva samples (termed serum-saliva subset or ss-subset). ThT fluorescence cutoff: 52,105. **** p < 0.0001. B. ROC curve and AUC for serum αSynD seeding activity comparisons between patients with PD and HC subjects in the ss-subset. ROC curve and AUC value were obtained based on αSynD seeding activity in serum samples from the patients with PD and HC of the ss-subset shown in panel A. C. Scatter graph of RT-QulC endpoint ThT fluorescence intensity (αSynD seeding activity) of saliva samples from patients with PD and HC in the ss-subset. Scatter graph was plotted based on αSynD seeding activities in saliva samples from the patients with PD and HC of the ss-subset shown in panel A. ThT fluorescence cutoff: 62,613. **** p < 0.0001. D. ROC curve and AUC for saliva αSynD seeding activity comparisons between the patients with PD and HC in a ss-subset. ROC curve and AUC value were obtained based on αSynD seeding activities in saliva of the patients with PD and HC of the ss-subset shown in panel C. E. 3-D Plot to identify optimal cutoff values for serum and saliva for maximum diagnostic accuracy for PD in the ss-subset shown in A and C. PD diagnostic accuracy was plotted against the RT-QuIC endpoint ThT fluorescence cutoff values of both serum and saliva in a 3-D plot, which identified the optimal endpoint ThT fluorescence cutoff settings to achieve maximal diagnostic accuracy for PD as 52,960 for serum and 66,800 for saliva. The accuracy values were calculated by varying the cutting off values for both serum and saliva with the definition that a patient was considered positive for PD only when the endpoint ThT fluorescence of both serum and saliva samples exceeded their respective cutoff values. F. ROC curve and AUC for PD diagnosis based on αSynD seeding activities in both serum and saliva of patients with PD and HC in the ss-subset. ROC curve and AUC were obtained based on calculated sensitivity and specificity values when varying the ThT fluorescence cutoff values for both serum and saliva. The sensitivity and specificity values were calculated based on the same definition of PD positivity as described in panel E. R analysis of the paired serum and saliva αSynD seeding activity data of the ss-subset was in agreement with the ROC analysis. **** p < 0.001. SE, standard error. 95% CI, 95% confidence interval
(Continued next page)
For combined serum and saliva αSyn-SAA data analysis, a patient was PD-positive if either serum or saliva αSyn-SAA was positive, and a patient was PD-negative when αSyn-SAAs were negative in both sample types. 3-D plotting with values of accuracy, serum cutoff, and saliva cutoff (Fig. 3E) revealed 52,960 and 66,800 (ThT fluorescence units) as the optimal cutoff values for serum and saliva samples, respectively. With these optimal cutoff values, 95.83% sensitivity, 96.15% specificity, and 93.75% accuracy were achieved for PD diagnosis (Table 2). If the specificity was set at 100%, 91.67% sensitivity and 91.67% accuracy were still attained. We also generated a ROC curve for the combined serum-saliva αSynD seeding activity data, which showed an accuracy of 0.98 (AUC of ROC, 95% CI, 0.96-1.0, p < 0.001) (Fig. 3F). The cumulative RT-QuIC ThT fluorescence kinetic curves displaying the mean and standard deviation (SD) over time of serum or saliva samples from patients with probable PD, patients with possible PD, and healthy controls of the ss-subset are shown in Supplementary Fig. 4.
Taken together, these results demonstrate that the diagnostic accuracy for PD using serum and saliva αSyn-SAA data together is much better than using αSyn-SAA data from either sample type alone (Table 2).
Clinical correlation of αSynD seeding activities in serum or saliva samples
We examined correlations between the αSyn-SAA status in serum or saliva samples of patients with PD with clinical features and demographic factors (Supplementary Table 1). When comparing serum αSyn-SAA positivity between PD and HC subjects, significant differences were found for Schwab & England scale (p < 0.001), PDQ-39 scores and some sub-scores [total (p < 0.05), mobility (p = 0.041), ADL (p < 0.001), and cognitive impairment (p = 0.006)], HAM-D (p = 0.025), self-reported hyposmia (p = 0.001) and constipation (p < 0.001) ( Supplementary Table 1). For saliva αSyn-SAA positivity in PD and HC subjects, the p-value findings were similar, except that significant difference was also found for age (p = 0.003) but not PDQ-39 mobility (p = 0.11) or HAM-D (p = 0.078) (Supplementary Table 1). A subset of saliva donors (34 PD and 22 HC) from our “saliva biomarker study” cohort, with a more limited clinical dataset, was only included in the analysis for age, age at diagnosis, disease duration, sex, mH&Y, MoCA, and MSD-UPDRS Part 3 (Supplementary Table 1).
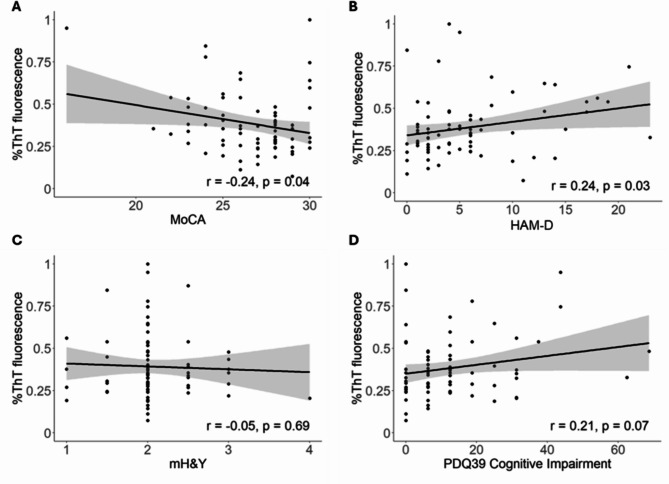
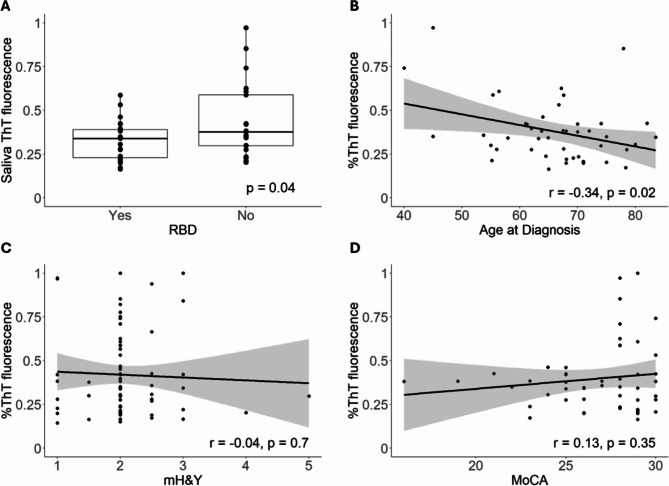
Clinical correlations with αSynD seeding activities in serum or saliva samples from patients with PD were also examined by Pearson’s correlation analysis. Serum αSynD seeding activities of patients with PD correlated significantly with MoCA (p = 0.04, inversely) and HAM-D (p = 0.03, positively), and weakly with PDQ-39 cognitive impairment (p = 0.07, positively) (Fig. 4, Supplementary Table 2). No significant correlation was found with hyposmia (p = 0.11), RBD (p = 0.21), mH&Y (p = 0.69), constipation (p = 0.98), or any other features examined (Supplementary Table 2). Saliva αSynD seeding activities of patients with PD correlated significantly with age at diagnosis (p = 0.02, inversely) and RBD (p = 0.04, inversely) (Fig. 5). No significant correlation was found between saliva αSynD seeding activities with MoCA (p = 0.35), mH&Y (p = 0.70), hyposmia (p = 0.63), constipation (p = 0.50) or any other features (Supplementary Table 2). No significant differences were found in clinical features between αSyn-SAA positive and αSyn-SAA negative PD participants for either serum or saliva samples.
Fig. 4.
Serum αSynD Seeding Activities Correlate with MoCA and HAM-D among Patients with PD. αSynD seeding activities (endpoint ThT fluorescence as a percentage of the maximum reading) in serum samples correlate inversely with MoCA score (A), positively with HAM-D score (B), and weakly positively with PDQ-39 cognitive impairment score (D), but not with modified Hoehn & Yahr (mH&Y) (C) of patients with PD. Linear regression lines with 95% confidence interval (gray shade) are shown
Fig. 5.
Saliva αSynD Seeding Activities Correlate with Age at Diagnosis and RBD among Patients with PD. αSynD seeding activities (endpoint ThT fluorescence as a percentage of the maximum reading) in saliva samples correlate inversely with RBD status (A) and age at diagnosis (B), but not with modified Hoehn & Yahr (mH&Y) (C) or MoCA (D) of patients with PD. Linear regression lines with 95% confidence interval (gray shade) are shown for B-D
Subgroup analyses by sex or age (< 70 years or ≥ 70 years) were also performed (Supplementary Tables 3 & 4). The age of 70 years was chosen to divide the age groups into two for this binary analysis for two reasons: (1) 70 is the mean age of onset for PD patients in general and the approximate mean age of our PD cohorts, and (2) age 70 would divide the cohorts into two subgroups that allow for meaningful subgroup statistical analysis. For sex subgroup analyses of patients with PD, serum αSynD seeding activities correlated inversely with MoCA in males (p = 0.01) but not in females (p = 0.70), weakly positively with PDQ-39 cognitive impairment in females (p = 0.07) but not in males (p = 0.27), and positively with HAM-D score in females (p = 0.04) but not in males (p = 0.36) (Supplementary Table 3); saliva αSynD seeding activities correlated inversely with age at diagnosis in males (p = 0.04) but not in females (p = 0.13) (Supplementary Table 4).
For age subgroup analyses of patients with PD, serum αSynD seeding activities correlated weakly inversely with MoCA in the ≥ 70 age group (p = 0.07) but not in the < 70 age group (p = 0.15), positively with HAM-A in the < 70 age group (p = 0.04) but not in the ≥ 70 age group (p = 0.40), positively with HAM-D in the < 70 age group (p = 0.01) but not in the ≥ 70 age group (p = 0.92), positively with orthostatic hypotension by vitals in the < 70 age group (p = 0.01) but not in the ≥ 70 age group (p = 0.31), inversely with ESS in the ≥ 70 age group (p = 0.03) but not in the < 70 age group (p = 0.92), and inversely with RBD in the ≥ 70 age group (p = 0.03) but not in the < 70 age group (p = 0.56) (Supplementary Table 3); saliva αSynD seeding activities correlated inversely with age at diagnosis in the < 70 age group (p = 0.01) but not in the ≥ 70 age group (p = 0.72), and inversely with Schwab & England in the ≥ 70 age group (p = 0.02) but not in the < 70 age group (p = 0.11) (Supplementary Table 4).
Discussion
Our serum and saliva αSyn-SAA data showed 0.98 in accuracy for PD diagnosis when they were considered together, comparable to that of CSF αSyn-SAA [16] and much better than when data from either sample type were used alone (Fig. 3; Table 2). This exciting finding indicates that, after further validations with larger sets of paired serum and saliva samples from more balanced cohorts of PD and HC subjects, αSynD seeding activities in serum and saliva together can be used as a valuable biomarker for highly sensitive, accurate, and minimally invasive PD diagnosis that can be implemented in routine clinical practice and also in clinical studies [38].
Importantly, high diagnostic accuracy with combined serum and saliva αSyn-SAA data was achieved only when the cutoff values for both sample types were set high for high specificity, supporting our hypothesis that serum αSyn-SAA and saliva αSyn-SAA data in patients with PD tend to be mutually complementary. It is yet to be determined whether this approach will also apply to other synucleinopathies or prodromal stage PD.
Earlier reports on serum αSyn-SAA alone showing outstanding accuracy in PD diagnosis comparable to or better than the gold-standard CSF αSyn-SAA are exciting yet need to be reproduced [30, 31]. We were unable to reproduce one prior serum αSyn-SAA report [30]. Our saliva αSyn-SAA results (74.70% sensitivity and 97.92% specificity) are largely in line with previous reports [26, 27], suggesting that saliva αSyn-SAA assay is good but inadequate for stand-alone PD diagnosis.
We detected age and/or sex-dependent correlations between serum/saliva αSynD seeding activities and some clinical characteristics, specifically MoCA, HAM-D, and RBD for serum and age at diagnosis and Schwab & England score for saliva (Figs. 4 and 5, Supplementary Tables 2–4). There was also a trend of positive correlation with PDQ-39 cognitive impairment for serum αSynD seeding activities, but the positive correlation of saliva αSynD seeding activities with RBD found in the overall analysis was not confirmed in the subgroup analysis (Supplementary Tables 2 & 4). However, none of the above correlations survived multiple correction analysis, possibly due to the modest sample sizes and the large number of clinical features included (26 in total).
Some reports described correlations of αSynD seeding activities in various sample types with certain clinical features, but they are often uncorroborated by this or other studies [10, 15, 16, 27, 31, 39, 40]. For example, CSF αSynD seeding activity was reported to correlate positively with olfactory deficit, UPDRS Part 3, and H&Y [16, 39, 40], but other studies reported otherwise [10, 15]. The previously reported correlation between saliva αSynD seeding activity and MDS-UPDRS [27] was not confirmed by our saliva αSyn-SAA data (Supplementary Tables 2& 4). Reasons for the discrepancies are unclear, but differences in study populations, clinical assessments, sample types, and αSyn-SAA parameters are possible factors.
The lack of improvement in sensitivity for the probable PD group versus the possible PD group was unexpected. One possibility is that the UK Brain Bank Criteria we utilized is less accurate than the newer MDS clinical diagnostic criteria in PD diagnosis.
Limitations
This study has several limitations: lack of validation cohort, lack of a group with a diagnosis of atypical parkinsonism, lack of confirmation by CSF or skin αSyn-SAA or gold standard neuropathological diagnosis, a slight male predominance in patients with PD and a female predominance in healthy controls, lack of assessment on the impact of PD genetics (including LRRK2), utilization of the subjective self-report of loss of smell (instead of UPSIT) and RBD (instead of polysomnography). This study serves as a proof-of-principle study, and the limitations will be addressed in follow-up larger and more extensive analyses.
Conclusions
Our study suggests that αSyn-SAA analysis of αSynD seeding activities in both serum and saliva samples together can serve as a valuable minimally invasive biomarker for highly sensitive and accurate PD diagnosis in routine clinical practice and clinical studies, and that αSynD seeding activities in serum and saliva are differentially correlated with various clinical characteristics in an age and sex-dependent manner. Further studies with larger independent cohorts of paired serum-saliva samples from more gender-balanced PD (including patients with mutations in LRRK2, GBA, and other relevant genes), non-PD synucleinopathies, and HC subjects that are validated by neuropathological diagnosis or CSF/skin CSF αSyn-SAA are needed to validate our findings. It would also be valuable to determine whether the combined serum and saliva αSyn-SAA strategy is applicable to prodromal patients, such as those with RBD and hyposmia, as well as early detection of PD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Alexis Santangelo for her skillful assistance.
Abbreviations
- αSyn
α-Synuclein
- αSynD
Disease-Associated α-Synuclein Aggregates
- αSyn-SAA
αSyn Seed Amplification Assay
- AUC
Area Under the Curve
- CI
Confidence Interval
- CSF
Cerebral Spinal Fluid
- DLB
Dementia with Lewy Bodies
- ESS
Epworth Sleepiness Scale
- HAM-A
Hamilton Anxiety Rating Scale
- HAM-D
Hamilton Depression Rating Scale
- HC
Healthy Control
- MDS-UPDRS
Movement Disorder Society-Unified Parkinson’s Disease Rating Scale
- mH&Y
modified Hoehn & Yahr
- MJFF
Michael J Fox Foundation
- MoCA
Montreal Cognitive Assessment
- NMSS
Non-Motor Symptoms Scale for Parkinson’s disease
- PD
Parkinson’s disease
- PDBP
Parkinson Disease Biomarker Program
- PDQ-39
Parkinson’s Disease Questionnaire − 39
- RBD
REM Behavior Disorder
- ROC
Receiver Operating Characteristic
- RT-QuIC
Real-Time Quaking-Induced Conversion
- S&E
Schwab & England
- SE
Standard Error
- ThT
Thioflavin T
- UHCMC
University Hospitals Cleveland Medical Center
Author contributions
Qingzhong Kong, Zerui Wang, and Steven A. Gunzler had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Qingzhong Kong, Zerui Wang, Steven A. Gunzler and Shu G. Chen are co-senior authors. Study concept and design: Qingzhong Kong, Zerui Wang, and Shu G. Chen. Acquisition, analysis, or interpretation of data: Zerui Wang, Tricia Gilliland, Hyun Jo Kim, Maria Gerasimenko, Kailey Sajewski, Manuel V. Camacho, Gurkan Bebek, Shu G. Chen, Steven A. Gunzler, and Qingzhong Kong. Drafting of the manuscript: Qingzhong Kong, Zerui Wang, Steven A. Gunzler, and Shu G. Chen. Critical revision of the manuscript for important intellectual content: Zerui Wang, Hyun Jo Kim, Gurkan Bebek, Shu G. Chen, Steven A. Gunzler, and Qingzhong Kong. Statistical analysis: Hyun Jo Kim, Gurkan Bebek, Zerui Wang, and Qingzhong Kong. Obtained funding: Qingzhong Kong, Zerui Wang, Steven A. Gunzler, and Shu G. Chen. Administrative, technical, or material support: Zerui Wang, Tricia Gilliland, Hyun Jo Kim, Maria Gerasimenko, Kailey Sajewski, Manuel V. Camacho, Gurkan Bebek, Shu G. Chen, Steven A. Gunzler, and Qingzhong Kong. Study supervision: Qingzhong Kong, Zerui Wang, Steven A. Gunzler, and Shu G. Chen.
Funding
This study was supported by grant U01 NS112010 from the National Institutes of Health (Drs. Kong, Wang, Gunzler, and Chen) and R01 NS118760 from the National Institutes of Health (Dr. Chen).
Data availability
The data supporting the findings of this study are included in tables and supplemental materials.
Declarations
Ethics approval and consent to participate
This study was approved by UHCMC Institutional Review Board. All research subjects had capacity and gave written informed consent, according to the Declaration of Helsinki.
Consent for publication
Not applicable.
Role of the funder/sponsor
The funder had no role in any of the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests
Qingzhong Kong, Steven A. Gunzler, Zerui Wang and Shu G. Chen have received grants from the National Institutes of Health. Steven A. Gunzler received grants from the Michael J Fox Foundation (MJFF) and the Parkinson Foundation, and has participated in studies funded by Biogen, Amneal, Bial, and UCB. Zerui Wang received funding from MJFF. Shu G. Chen received funding from MJFF and CurePSP. No other disclosures were reported.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zerui Wang, Email: zxw488@case.edu.
Shu G. Chen, Email: sgchen@uabmc.edu
Steven A. Gunzler, Email: Steven.Gunzler@uhhospitals.org
Qingzhong Kong, Email: qxk2@case.edu.
References
- 1.Hughes JE, Daniel SE, Ben-Shlomo Y, Lees AJ (2002) The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 125(4):861–870 [DOI] [PubMed] [Google Scholar]
- 2.Adler CH, Beach TG, Hentz JG, Shill HS, Caviness JN, Driver-Dunckley E et al (2014) Low clinical diagnostic accuracy of early vs advanced Parkinson disease. Neurology 83(5):406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G (2016) Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology 86(6):566–576 [DOI] [PubMed] [Google Scholar]
- 4.Virameteekul S, Revesz T, Jaunmuktane Z, Warner TT, De Pablo-Fernández E (2023) Clinical diagnostic accuracy of Parkinson’s disease: where do we stand? Mov Disord 38(4):558–566 [DOI] [PubMed] [Google Scholar]
- 5.Groveman BR, Orrù CD, Hughson AG, Raymond LD, Zanusso G et al (2018) Rapid and ultra-sensitive quantitation of disease-associated a-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol Commun 6(1):7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srivastava A, Alam P, Caughey B (2022) RT-QuIC and related assays for detecting and quantifying prion-like pathological seeds of a-synuclein. Biomolecules 12(4):576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoo D, Bang JI, Ahn C, Nyaga VN, Kim YE, Kang MJ et al (2002) Diagnostic value of a-synuclein seeding amplification assays in a-synucleinopathies: a systematic review and meta-analysis. Parkinsonism Relat Disord 104:99–109 [DOI] [PubMed] [Google Scholar]
- 8.Fairfoul G, McGuire LI, Pal S, Ironside JW, Neumann J, Christie S et al (2016) Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol 3(10):812–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang UJ, Boehme AK, Fairfoul G, Shahnawaz M, Ma TC, Hutten SJ et al (2019) Comparative study of cerebrospinal fluid a-synuclein seeding aggregation assays for diagnosis of Parkinson’s disease. Mov Disord 34(4):536–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orrù CD, Ma TC, Hughson AG, Groveman BR, Srivastava A, Galasko D et al (2021) A rapid a-synuclein seed assay of Parkinson’s disease CSF panel shows high diagnostic accuracy. Ann Clin Transl Neurol 8(2):374–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo MJ, Orru CD, Concha-Marambio L, Giaisi S, Groveman BR, Farris CM et al (2021) High diagnostic performance of independent alpha-synuclein seed amplification assays for detection of early Parkinson’s disease. Acta Neuropathol Commun 9(1):179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bräuer S, Rossi M, Sajapin J, Henle T, Gasser T, Parchi P et al (2023) Kinetic parameters of alpha-synuclein seed amplification assay correlate with cognitive impairment in patients with Lewy body disorders. Acta Neuropathol Commun 11(1):162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Concha-Marambio L, Pritzkow S, Shahnawaz M, Farris CM, Soto C (2023) Seed amplification assay for the detection of pathologic alpha-synuclein aggregates in cerebrospinal fluid. Nat Protoc 18(4):1179–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Concha-Marambio L, Weber S, Farris CM, Dakna M, Lang E, Wicke T et al (2023) Accurate detection of α-synuclein seeds in cerebrospinal fluid from isolated rapid eye movement sleep behavior disorder and patients with Parkinson’s disease in the de novo Parkinson (DeNoPa) cohort. Mov Disord 38(4):567–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oftedal L, Maple-Grødem J, Tysnes OB, Alves G, Lange J (2023) Seed amplification assay as a diagnostic tool in newly-diagnosed Parkinson’s disease. J Parkinsons Dis 13(5):841–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siderowf A, Concha-Marambio L, Lafontant DE, Farris CM, Ma Y, Urenia PA et al (2023) Parkinson’s progression markers Initiative. Assessment of heterogeneity among participants in the Parkinson’s progression markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol 22(5):407–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brockmann K, Lerche S, Baiardi S, Rossi M, Wurster I, Quadalti C et al (2024) CSF a-synuclein seed amplification kinetic profiles are associated with cognitive decline in Parkinson’s disease. NPJ Parkinsons Dis 10(1):24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stefani A, Iranzo A, Holzknecht E, Perra D, Bongianni M, Gaig C et al (2021) Alpha-synuclein seeds in olfactory mucosa of patients with isolated REM sleep behaviour disorder. Brain 144(4):1118–1126 [DOI] [PubMed] [Google Scholar]
- 19.Bargar C, De Luca CMG, Devigili G, Elia AE, Cilia R, Portaleone SM et al (2021) Discrimination of MSA-P and MSA-C by RT-QuIC analysis of olfactory mucosa: the first assessment of assay reproducibility between two specialized laboratories. Mol Neurodegener 16(1):82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manne S, Kondru N, Jin H, Serrano GE, Anantharam V, Kanthasamy A et al (2020) Blinded RT-QuIC analysis of a-synuclein biomarker in skin tissue from Parkinson’s disease patients. Mov Disord 35(12):2230–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Becker K, Donadio V, Siedlak S, Yuan J, Rezaee M et al (2020) Skin a-synuclein aggregation seeding activity as a novel biomarker for Parkinson disease. JAMA Neurol 78(1):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bargar C, Wang W, Gunzler SA, LeFevre A, Wang Z, Lerner AJ et al (2019) Streamlined alpha-synuclein RT-QuIC assay for various biospecimens in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol Commun 9(1):62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuzkina A, Bargar C, Schmitt D, Rößle J, Wang W, Schubert AL et al (2021) Diagnostic value of skin RT-QuIC in Parkinson’s disease: a two-laboratory study. NPJ Parkinsons Dis 7(1):99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manne S, Kondru N, Jin H, Anantharam V, Huang X, Kanthasamy A, Kanthasamy AG (2020) a-Synuclein real-time quaking-induced conversion in the submandibular glands of Parkinson’s disease patients. Mov Disord 35(2):268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vascellari S, Orrù CD, Groveman BR, Parveen S, Fenu G, Pisano G et al (2023) a-Synuclein seeding activity in duodenum biopsies from Parkinson’s disease patients. PLoS Pathog 19(6):e1011456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luan M, Sun Y, Chen J, Jiang Y, Li F, Wei L et al (2022) Diagnostic Value of Salivary Real-Time Quaking-Induced Conversion in Parkinson’s Disease and multiple system atrophy. Mov Disord 37(5):1059–1063 [DOI] [PubMed] [Google Scholar]
- 27.Vivacqua G, Mason M, De Bartolo MI, Węgrzynowicz M, Calò L, Belvisi D et al (2023) Salivary a-synuclein RT-QuIC correlates with disease severity in de novo Parkinson’s disease. Mov Disord 38(1):153–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kluge A, Bunk J, Schaeffer E, Drobny A, Xiang W, Knacke H et al (2022) Detection of neuron-derived pathological a-synuclein in blood. Brain 145(9):3058–3071 [DOI] [PubMed] [Google Scholar]
- 29.Kluge A, Schaeffer E, Bunk J, Sommerauer M, Röttgen S, Schulte C et al (2024) Detecting misfolded a-synuclein in blood years before the diagnosis of Parkinson’s disease. Mov Disord 39(8):1289-1299 [DOI] [PubMed]
- 30.Okuzumi A, Hatano T, Matsumoto G, Nojiri S, Ueno SI, Imamichi-Tatano Y et al (2023) Propagative a-synuclein seeds as serum biomarkers for synucleinopathies. Nat Med 29(6):1448–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaeffer E, Kluge A, Schulte C, Deuschle C, Bunk J, Welzel J et al (2024) Association of misfolded a-synuclein derived from neuronal exosomes in blood with Parkinson’s disease diagnosis and duration. J Parkinsons Dis 14(4):667–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daniel SE, Lees AJ (1993) Parkinson’s disease society brain bank, London: overview and research. J Neural Transm Suppl 39:165–172 [PubMed] [Google Scholar]
- 33.Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56(1):33–39 [DOI] [PubMed] [Google Scholar]
- 34.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30(12):1591–1601 [DOI] [PubMed] [Google Scholar]
- 35.Becker K, Wang X, Vander Stel K, Chu Y, Kordower J, Ma J (2018) Detecting alpha synuclein seeding activity in formaldehyde-fixed MSA patient tissue by PMCA. Mol Neurobiol 55(11):8728–8737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volpicelli-Daley LA, Luk KC, Lee VM (2014) Addition of exogenous α-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous a-synuclein to Lewy body and Lewy neurite-like aggregates. Nat Protoc 9(9):2135–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajian-Tilaki K (2013) Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Casp J Intern Med 4(2):627–635 [PMC free article] [PubMed] [Google Scholar]
- 38.Cardoso F, Goetz CG, Mestre TA, Sampaio C, Adler CH, Berg D et al (2024) A statement of the MDS on biological definition, staging, and classification of Parkinson’s disease. Mov Disord 39(2):259–266 [DOI] [PubMed] [Google Scholar]
- 39.Haehner A, Hummel T, Reichmann H (2011) Olfactory loss in Parkinson’s disease. Parkinsons Dis 2011:450939 [DOI] [PMC free article] [PubMed]
- 40.Majbour N, Aasly J, Abdi I, Ghanem S, Erskine D, van de Berg W et al (2022) Disease-associated a-synuclein aggregates as biomarkers of Parkinson disease clinical stage. Neurology 99(21):e2417–e2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are included in tables and supplemental materials.