Abstract
Nasal immunoglobulin A provides an initial defense against inhaled respiratory pathogens. However, it is not known whether the nasal-associated lymphoid tissues (NALT) are able to mount an effective long-lasting pathogen-specific immune response, nor is it known whether functional differences exist between the organized NALT (O-NALT) and the diffuse NALT lining the nasal passages (D-NALT). Here we show that although both the O-NALT and the D-NALT are capable of producing virus-specific antibody in response to influenza virus infection, the frequency of specific antibody-forming cells in the D-NALT is much greater than the frequency observed in the O-NALT. Furthermore, we show that the D-NALT but not the O-NALT is the site of long-term virus-specific humoral immunity which lasts for the life of the animal. These results indicate that the D-NALT is not only the major effector site of the NALT but also the site of local long-term specific antibody production.
The upper respiratory tract is an important site for host defense against invading pathogens, since it is the site at which inhaled antigens first come into contact with the immune system (9). Respiratory viruses such as influenza virus affect primarily the upper and lower respiratory tracts, and viremia does not normally occur. Intranasal immunization can elicit antigen-specific immune responses in both the mucosal and systemic compartments following administration of pathogens and even nonreplicating protein antigens (4, 16, 17). Furthermore, intranasal immunization is an effective means of evoking not only local immunity in the respiratory tract but also immunity at distal mucosal sites (10, 15).
The nasal-associated lymphoid tissues (NALT) in the mouse are composed of a pair of organized lymphoid aggregates (O-NALT) located on the palate at the entrance to the nasopharyngeal duct and the less well organized diffuse lymphoid tissue lining the nasal passages (D-NALT) (9). These nasal tissues appear to be functionally equivalent to the Waldeyer's ring of tonsils and adenoids in the human and are most likely responsible for the local immune responses generated following intranasal immunization in the mouse (14). An indication of the importance of the nasopharyngeal lymphoid tissue in humans is the diminished poliovirus-specific antibody levels in nasal secretions from children following tonsillectomy (11). In humans resistance to infection with a cold-adapted vaccine influenza virus has been correlated with antihemagglutinin (anti-HA) immunoglobulin A (IgA) in nasal washes (3). In the mouse model, following intranasal infection, local antibody-forming cell (AFC) production in the NALT of BALB/c mice parallels detection of influenza virus-specific antibodies in the nasal wash and correlates with virus clearance from the nose (14). Furthermore, nasal IgA has been shown to directly mediate local anti-influenza virus immunity in the mouse model, confirming the importance of IgA in protection against virus infection in the upper respiratory tract (12). Specific AFCs secreting antibody to protein antigens can also be detected in the O-NALT of BALB/c mice after repeated intranasal immunization (18).
Previous work has shown that in BALB/c mice both the O-NALT and the D-NALT are composed of roughly similar ratios of T to B cells (2). The majority of T cells express the αβ T-cell receptor (αβTCR), with few γδTCR+ T cells (1). Studies to date suggest that the O-NALT is rich in unswitched, naive B cells and naive T cells, suggesting that it is a mucosal inductive site, whereas the D-NALT may function as an effector site. It has also been shown that in the O-NALT CD4+ T cells are mainly of the TH0 type and that in the D-NALT the CD4+ T cells are predominantly of the TH2 type (6, 18).
It is presently unknown whether either the O-NALT or the D-NALT is able to generate long-lasting humoral immunity to pathogens. Such information would be of great benefit to assess the local effectiveness of delivering potential vaccine candidates by the intranasal route. We have examined the longevity of influenza virus-specific antibody responses in the O-NALT and the D-NALT in the mouse following intranasal influenza virus infection. We show that virus-specific AFCs are generated in both the O-NALT and the D-NALT following exposure to virus. However, the frequency of AFCs was much greater in the D-NALT than in the O-NALT over the course of a primary infection with influenza virus, with a higher number of AFCs continuing to secrete antibody for a longer time period. Moreover, long-term virus-specific antibody was still detectable in the D-NALT 18 months after primary infection, whereas no AFCs were detectable in the O-NALT after approximately 5 months postinfection. These results show that the D-NALT is the major B-cell effector site of the nasal tissues and indicate that local long-term virus-specific antibody generated to influenza virus resides in the AFCs lining the nasal passages.
Experimental procedures.
Inbred female C57BL/6 mice were obtained from the Institute of Animal Health, Compton, Berkshire, United Kingdom. All mice were held under specific-pathogen-free conditions and were used at 8 to 12 weeks of age. The HKx31 (H3N2) strain of influenza A virus was grown in the allantoic cavities of 10-day-old embryonated eggs and stored at −70°C until use. Mice were anesthetized by intraperitoneal injection of 2,2,2-tribromoethanol (Avertin) and then infected intranasally with 30 μl of phosphate-buffered saline containing 5 × 105 50% egg infectious doses of virus. Following infection mice were kept in filter-top cages until use. No seroconversion was ever observed in sentinel mice stored in open-top cages placed beside experimental cages. At certain time points postinfection, mice were sacrificed for collection of NALT, cervical lymph nodes, lungs, and bone marrow. In all experiments NALT cells from four or five mice were pooled for each time point, and the entire experimental time course was repeated three times. Cell suspensions were prepared from lymph nodes by gently pressing between frosted slides followed by filtration through gauze. The O-NALT and D-NALT cells were extracted by a previously described method (2). Lymphocytes were extracted from the lungs by mincing the tissue followed by incubation with 4 mg of collagenase A (Boehringer-Mannheim, Indianapolis, Ind.) per ml for 30 min and were recovered from the interface of 75 and 40% Percoll gradients.
Monoclonal anti-mouse antibodies used for flow cytometry were the following: anti-B220–phycoerythrin, anti-γδTCR–phycoerythrin, and anti-TCRβ–fluorescein isothiocyanate (PharMingen, San Diego, Calif.) and anti-CD8α–phycoerythrin and anti-CD4–fluorescein isothiocyanate (Sigma Immuno Chemicals, St. Louis, Mo.). Samples were analyzed on a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, Calif.) and analyzed using WinMDI Version 2.8 (The Scripps Research Institute).
The presence of influenza virus was assessed by inoculation of homogenized tissue into the allantoic cavities of 10-day-old embryonated hen eggs followed by HA assays using chicken red blood cells.
ELISPOT and enzyme-linked immunosorbent assays were carried out as described previously (7). Purified influenza virus for coating plates to enumerate virus-specific AFCs and assess serum influenza virus titers was obtained from SPAFAS Laboratory (Preston, Conn.). Plaques were detected with goat anti-mouse Ig isotype-specific reagents conjugated to alkaline phosphatase (Southern Biotechnology Associates, Birmingham, Ala.).
Phenotype and cellularity of the NALT after influenza virus infection.
Both the O-NALT and the D-NALT cell populations isolated from uninfected C57BL/6 mice are largely (70 to 76%) composed of B lymphoid cells (Table 1). This is in contrast to the case for BALB/c mice, where the percentage of B cells is much lower (45 to 50%) (2). In naive C57BL/6 mice fewer than 2% of the T cells in the D-NALT and O-NALT were γδTCR+, and these percentages did not change significantly after infection (results not shown).
TABLE 1.
Phenotypes and cell recoveries of C57BL/6 O-NALT and D-NALT preparations isolated at certain time points postinfection with influenza virus
| Tissue | Day | %a with phenotype:
|
Total cell recovery (104)a/mouse | ||
|---|---|---|---|---|---|
| CD4 | CD8 | B220 | |||
| O-NALT | 0 | 5.1 ± 1.4 | 6.3 ± 1.7 | 75.7 ± 3.9 | 3.1 ± 0.6 |
| 3 | 6.0 ± 0.7 | 8.7 ± 0.5 | 68.9 ± 2.6 | 6.8 ± 2.8 | |
| 5 | 7.7 ± 0.8 | 11.0 ± 0.7 | 71.0 ± 0.6 | 16.2 ± 4.5 | |
| 7 | 7.1 ± 0.9 | 10.1 ± 1.9 | 66.8 ± 3.9 | 6.0 ± 1.6 | |
| 9 | 6.6 ± 3.2 | 10.4 ± 3.0 | 76.0 ± 1.4 | 14.8 ± 4.2 | |
| 12 | 3.8 ± 0.1 | 8.2 ± 1.2 | 72.9 ± 4.9 | 9.7 ± 0.2 | |
| 15 | 7.0 ± 3.0 | 8.0 ± 2.8 | 76.5 ± 4.8 | 9.9 ± 4.5 | |
| 34+b | 6.6 ± 1.6 | 8.7 ± 1.4 | 74.2 ± 6.4 | 3.2 ± 1.6 | |
| D-NALT | 0 | 2.3 ± 0.7 | 2.2 ± 0.9 | 70.6 ± 3.9 | 19.6 ± 4.8 |
| 3 | 2.2 ± 0.7 | 4.3 ± 1.1 | 61.2 ± 0.4 | 54.8 ± 6.7 | |
| 5 | 2.7 ± 1.4 | 6.9 ± 0.4 | 48.5 ± 3.5 | 116 ± 17.8 | |
| 7 | 3.5 ± 1.2 | 7.0 ± 1.4 | 49.9 ± 5.1 | 38.3 ± 13.8 | |
| 9 | 10.7 ± 2.9 | 22.7 ± 2.0 | 48.1 ± 3.9 | 38.5 ± 11.6 | |
| 12 | 8.2 ± 2.0 | 19.8 ± 4.1 | 53.8 ± 1.1 | 39.5 ± 17.7 | |
| 15 | 7.4 ± 0.1 | 15.3 ± 0.7 | 57.1 ± 14.6 | 55.8 ± 38.5 | |
| 34+b | 5.0 ± 1.5 | 8.6 ± 0.7 | 64.2 ± 3.8 | 30.3 ± 1.2 | |
Mean ± standard deviation from several repeat experiments, each consisting of four or five mice per time point.
Data obtained from time points after and including day 34.
An increase in the cellularity of the O-NALT was observed following influenza virus infection from day 5 to 12 postinfection. During this time there was a small increase in the percentage of T cells and a concomitant decrease in the B-cell population (Table 1). The O-NALT B-cell population declined from 76 to 67% by day 7 postinfection but returned to naive percentages by 9 to 12 days postinfection. The O-NALT CD8+ T cells increased from approximately 6% in naive mice to 10 to 11% at 1 week postinfection. The percentage of CD4+ T cells in the O-NALT, however, did not change significantly over the course of the infection. The numbers of B cells in the D-NALT of naive mice increased by fourfold up to day 5 postinfection despite showing a decrease from 71 to 48% by 5 to 9 days postinfection. This is perhaps a reflection of the more dramatic increases in the T-cell populations. D-NALT CD4+ T cells increased from 2% in naive mice to up to 10%, and CD8+ T cells increased from 2 to 3% in uninfected mice to 22 to 23% by 9 days postinfection (Table 1). Interestingly, there was a decrease in cell recoveries in the O-NALT at around day 7 and in the D-NALT at days 7 to 9 postinfection, followed by a later increase in numbers. This was observed in all time course experiments. These data may signify different rounds of cell migration, differentiation, and expansion within these tissues and subsequent cell migration from O-NALT to D-NALT, where effector function appears to be taking place. After 34 days postinfection, both the D-NALT and the O-NALT phenotypes and cell recoveries remained at stable configurations for the lives of the animals. It is interesting that the percentages of B and T cells in the NALT may differ considerably between different mouse strains. In both the O-NALT and the D-NALT of BALB/c mice, there is a greater frequency of T cells with fewer B cells than in the O- and D-NALT of C57BL/6 mice (reference 14 and our unpublished data). Other, smaller populations of cells can also be found in the lymphocyte gate during analyses of fluorescence-activated cell sorter data. For example, 12 to 14% of the total D-NALT cells on day 9 after infection were found to be Gr-1+ neutrophils, and small numbers of CD11b macrophages and DX5+ NK cells were also found at this time.
Localized antibody production within the NALT after influenza virus infection.
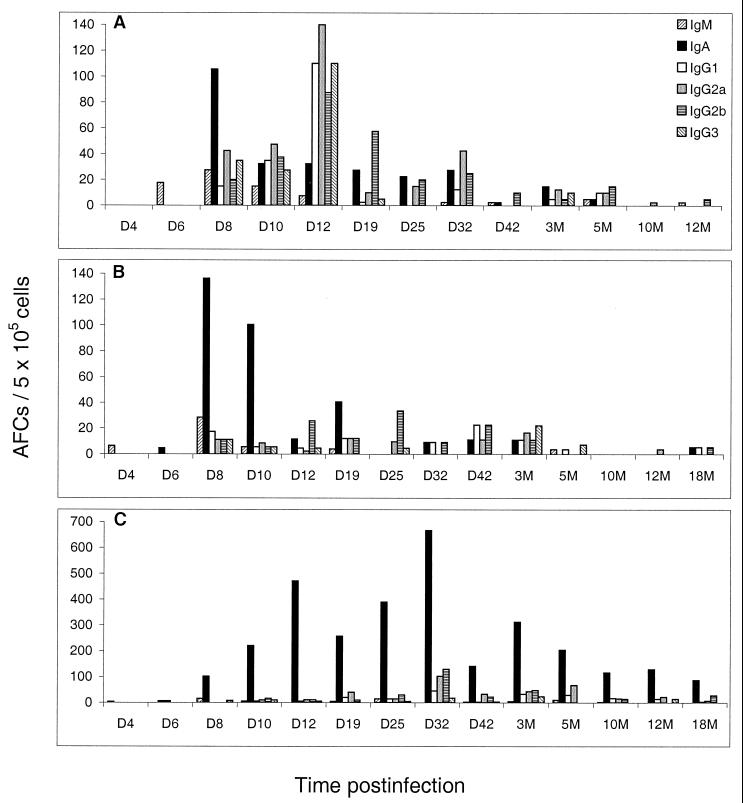
We used the ELISPOT assay to determine the isotypes and longevities of virus-specific antibody responses in the nasal tissues of C57BL/6 mice. In the O-NALT there was a dominant IgA response early after infection, peaking at 8 days postinfection. A total of 214 AFCs/5 × 105 cells were detected, of which around 60% were of the IgA isotype. By 12 days postinfection the response was declining, and only negligible numbers of virus-specific cells were still detectable by 5 months postinfection (Fig. 1B), after which virus-specific AFCs could no longer be detected in the O-NALT. The cervical lymph nodes which drain the NALT area generated IgA and IgG virus-specific AFC responses that were also maintained for approximately 5 months postinfection (Fig. 1A). Similarly, virus-specific AFC responses in the lung, first observed by Jones and Ada (8), also lasted for approximately 5 months postinfection (Fig. 2A).
FIG. 1.
Virus-specific antibody responses in the D-NALT are maintained for at least 18 months after primary intranasal infection with influenza virus. Mice were sacrificed at certain time points postinfection, and virus-specific AFCs in the cervical lymph nodes (A), O-NALT (B), and D-NALT (C) were enumerated by the ELISPOT assay. Cells were pooled from four or five mice per time point. Each panel is representative of one experimental time course. Each time course was carried out three times independently with similar results. D, day; M, months.
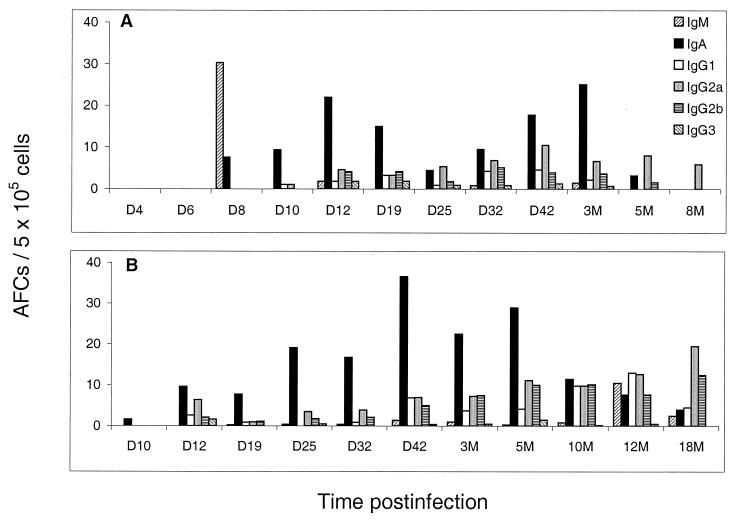
FIG. 2.
Virus-specific AFC frequencies in the lung (A) and bone marrow (B) after primary intranasal infection with influenza virus. Mice were sacrificed at certain time points postinfection, and virus-specific AFCs in the lung and bone marrow were enumerated by the ELISPOT assay. Cells were pooled from four or five mice per time point. Each panel is representative of one experimental time course. Each time course was carried out three times independently with similar results. D, day; M, months.
In contrast to the case for the O-NALT, much greater numbers of virus-specific AFCs were observed in the D-NALT after influenza virus infection. The response peaked later than that in the O-NALT, at around 32 days postinfection, when a total of 962 AFCs/5 × 105 cells were detected, with almost 70% of these being of the IgA isotype (Fig. 1C). High frequencies of virus-specific AFCs could still be detected at 18 months after influenza virus infection, with numbers around 125 total virus-specific AFCs/5 × 105 cells. These data show that the lymphoid cells of the D-NALT, which are the first immune cells to encounter virus following intranasal infection, include specific AFCs in locally significant numbers which last for the lifetime of the animal following a single exposure to influenza virus. The D-NALT is therefore the only mucosal tissue of which we are aware that can maintain such long-lasting antibody responses following a single exposure to influenza virus infection. Although the nasal area has previously been noted to be important for local protection against influenza virus (12), the present study details the site and mechanisms that are involved in local B-cell immunity in the nose, confirming the potential importance of the nasal lymphoid tissue during reexposure to virus. In this study we examined the response of the nasal tissues to influenza virus in a dose which would ensure tracheal and pulmonary infection, with the aim of analyzing the contribution of the nasal tissues to the overall immune response and elucidating whether the NALT is capable of maintaining long-term local immunity. Thus, from our data we cannot speculate whether the AFCs are actually being induced in the nasal tissues or are recruited in from elsewhere shortly after infection. It is unlikely that these long-term antibody producers are seeding from the bone marrow, the only other identified site of long-term antibody producers, as the bone marrow anti-influenza virus response cannot be observed until 12 to 14 days after infection (Fig. 2B), whereas the D-NALT response can be observed from day 8 (Fig. 1C). Some indication may be found in a study by Tamura et al. (14), where a very small volume of influenza virus was administered intranasally, thereby restricting the virus solely to the nasal area. Their results showed far fewer virus-specific AFCs and a much shorter response in the D-NALT following influenza virus infection, suggesting perhaps that although the NALT may be capable of generating AFCs to some extent and a peak of virus-specific AFCs is observed, recruitment of precursors from elsewhere for long-term maintenance of responses is required.
Our previous studies showed that the serum antibody in mice long after a single exposure to influenza virus infection is generated from terminally differentiated plasma cells located in the bone marrow (7), and this was later confirmed by others with other virus systems (13). In the present study influenza virus-specific AFC numbers in the bone marrow were maintained with similar frequencies from 3 months (42 AFCs/5 × 105 cells) up to 18 months (43 AFCs/5 × 105 cells) postinfection with influenza virus (Fig. 2B). Clearly, therefore, bone marrow responses are of great importance in the protection of the animal on reexposure due to the much greater size of the bone marrow compartment. However, it is likely that due to the highly localized nature of the D-NALT response to the site of initial contact with inhaled antigen and the large number of virus-specific AFCs located there, this mucosal site is of significant importance.
Virus is cleared more slowly from the D-NALT than from the lung and O-NALT.
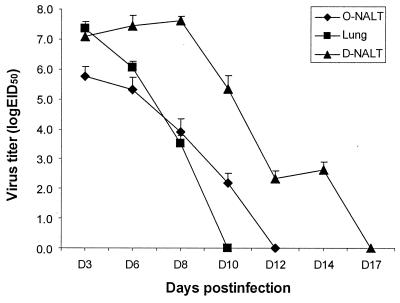
Interestingly, we found that unlike the case for the lung, where influenza virus is normally cleared by 10 days postinfection and viral RNA sequences are undetectable by PCR 14 days postinfection with influenza virus (5), the virus is not cleared from the D-NALT until approximately 14 to 17 days after infection (Fig. 3). This may be a reflection of the late influx of CD8+ T cells into the D-NALT between days 9 and 15 (Table 1). In contrast, influenza virus is cleared from the O-NALT at around 12 days postinfection. Another study (14), which measured influenza virus in the nasal washes, showed virus completely cleared by day 11 postinfection. By analyzing the nasal tissues themselves, however, we were able to determine a more accurate measure of the virus titer.
FIG. 3.
Influenza virus is cleared later in the D-NALT than in the O-NALT or the lung. Influenza virus titers were determined by inoculation into the allantoic fluid cavities of 10-day-old embryonated hen eggs. HA activity was assayed 72 h later. Assays were performed in triplicate with three mice per time point, and results are the means and standard deviations from three independent experiments. EID50, 50% egg infective dose; D, day.
In summary, we have shown that a long-lasting, specific effector antibody response occurs in the D-NALT but not in the O-NALT following a single exposure to influenza virus. The predominant isotype produced is IgA. The specific antibody generated by this mucosal site is likely to be important in the protection against further infection at the initial site of contact with an inhaled antigen.
REFERENCES
- 1.Asanuma H, Inaba Y, Aizawa C, Kurata T, Tamura S. Characterisation of mouse nasal lymphocytes isolated by enzymatic extraction with collagenase. J Immunol Methods. 1995;187:41–51. doi: 10.1016/0022-1759(95)00165-7. [DOI] [PubMed] [Google Scholar]
- 2.Asanuma H, Thompson A, Iwasaki T, Sato Y, Inaba Y, Aizawa C, Kurata T, Tamura S. Isolation and characterisation of mouse nasal-associated lymphoid tissue. J Immunol Methods. 1997;202:123–131. doi: 10.1016/s0022-1759(96)00243-8. [DOI] [PubMed] [Google Scholar]
- 3.Clements L M, O'Donnell S, Levine M M, Chanock R M, Murphy B R. Dose response of A/Alaska/6/77 (H3N2) cold-adapted reassortant vaccine virus in adult volunteers: role of local antibody in resistance to infection with vaccine virus. Infect Immun. 1983;40:1044–1051. doi: 10.1128/iai.40.3.1044-1051.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clements L M, Murphy B R. Development and persistence of local and systemic antibody responses in adults given live attenuated or inactivated influenza A virus vaccine. J Clin Microbiol. 1986;23:66–72. doi: 10.1128/jcm.23.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eichelberger M C, Wang M, Allan W, Webster R G, Doherty P C. Influenza virus RNA in the lung and lymphoid tissue of immunologically intact and CD4-depleted mice. J Gen Virol. 1991;72:1695–1698. doi: 10.1099/0022-1317-72-7-1695. [DOI] [PubMed] [Google Scholar]
- 6.Hiroi T, Iwatani K, Iijima H, Kodama S, Yanagita M, Kiyono H. Nasal immune system: distinctive Th0 and Th1/Th2 type environments in murine nasal-associated lymphoid tissues and nasal passage, respectively. Eur J Immunol. 1998;28:3346–3353. doi: 10.1002/(SICI)1521-4141(199810)28:10<3346::AID-IMMU3346>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 7.Hyland L, Sangster M, Sealy R, Coleclough C. Respiratory virus infection of mice provokes a permanent humoral immune response. J Virol. 1994;68:6083–6086. doi: 10.1128/jvi.68.9.6083-6086.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones P D, Ada G L. Influenza virus-specific antibody-secreting cells in the murine lung during primary influenza virus infection. J Virol. 1986;60:614–619. doi: 10.1128/jvi.60.2.614-619.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuper C F, Koornstra P J, Hameleers D M H, Biewenga J, Spit B J, Duijvestijn A M, van Breda Vriesman P J C, Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 10.Langermann S, Palaszynski S, Sadziene A, Stover C V, Koenig S. Systemic and mucosal immunity induced by BCG vector expressing outer-surface protein A of Borrelia burgdorferi. Nature. 1994;372:552–555. doi: 10.1038/372552a0. [DOI] [PubMed] [Google Scholar]
- 11.Ogra P L. Effect of tonsillectomy and adenoidectomy on nasopharyngeal antibody response to poliovirus. N Engl J Med. 1971;284:59–64. doi: 10.1056/NEJM197101142840201. [DOI] [PubMed] [Google Scholar]
- 12.Renegar K B, Small P A. Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J Virol. 1991;65:2146–2148. doi: 10.1128/jvi.65.4.2146-2148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slifka M, Ahmed R. Long-term antibody production is sustained by antibody-secreting cells in the bone marrow following acute viral infection. Ann N Y Acad Sci. 1996;797:166–176. doi: 10.1111/j.1749-6632.1996.tb52958.x. [DOI] [PubMed] [Google Scholar]
- 14.Tamura S, Iwasaki T, Thompson A, Asanuma H, Chen Z, Suzuki Y, Aizawa C, Kurata T. Antibody-forming cells in the nasal-associated lymphoid tissue during primary influenza virus infection. J Gen Virol. 1998;79:291–299. doi: 10.1099/0022-1317-79-2-291. [DOI] [PubMed] [Google Scholar]
- 15.Vadolas J, Davies J K, Wright P J, Strugnell R A. Intranasal immunization with liposomes induces strong mucosal immune responses in mice. Eur J Immunol. 1995;25:969–975. doi: 10.1002/eji.1830250417. [DOI] [PubMed] [Google Scholar]
- 16.Waldo F B. Nasal immunization with tetanus toxoid increases the subsequent systemic dimeric IgA1 antibody response to intramuscular immunization. J Clin Lab Immunol. 1991;43:125–129. [PubMed] [Google Scholar]
- 17.Wu H Y, Russell M W. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect Immun. 1993;61:314–322. doi: 10.1128/iai.61.1.314-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu H Y, Nikolova E B, Beagley K W, Russell M W. Induction of antibody-secreting cells and T-helper and memory cells in murine nasal lymphoid tissue. Immunology. 1996;88:493–500. doi: 10.1046/j.1365-2567.1996.d01-690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]