Abstract
Aims
This study aimed to compare the effects of the combination of rivaroxaban and aspirin with aspirin alone on health-related quality of life in the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial.
Methods and results
Health-related quality of life assessed using the EQ-5D-3L. The treatment effects on health utility and EQ visual analogue scale (EQ VAS) scores were compared between rivaroxaban plus aspirin and aspirin alone in terms of adjusted mean difference in change from baseline and odds ratio of having deterioration events. Nine thousand forty-nine (98.9%) and 6916 (75.5%) completed the EQ-5D-3L at baseline and at final visit, respectively. Nine thousand twenty-eight (98.9%) and 6887 (76.3%) completed the EQ-5D-3L at baseline and final visit, respectively. Mean (standard deviation) health utility and EQ VAS scores at baseline were 0.871 (0.141) and 76.0 (15.3), respectively, for the rivaroxaban plus aspirin group, compared with 0.873 (0.139) and 75.8 (15.1) for the aspirin group. Adjusted mean difference in change from baseline utility was −0.002 [95% confidence interval (CI), −0.006, 0.002, P = 0.30] between the combination therapy group and the aspirin group. The odds ratio (95% CI) of experiencing deterioration in health utility was 1.01 (95% CI, 0.93, 1.10, P = 0.81) between the two groups. Adjusted mean difference in change from baseline EQ VAS was 0.02 (95% CI, −0.43, 0.47, P = 0.93) between the two groups.
Conclusion
This analysis of the COMPASS trial demonstrated that the quality of life of patients was similar between the rivaroxaban plus aspirin group and the aspirin alone group.
Registration
Trial registration number: ClinicalTrials.gov number (NCT01776424). Trial protocol and statistical analysis plan: https://www.nejm.org/doi/full/10.1056/NEJMoa1709118#APPNEJMoa1709118PRO.
Keywords: Health-related quality of life, EQ-5D, Cardiovascular disease, Patient-reported outcomes, Anticoagulation
Introduction
The Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial demonstrated that in patients with chronic stable coronary or peripheral artery disease, the combination of rivaroxaban 2.5 mg twice daily and aspirin was more effective than aspirin alone in preventing major adverse cardiovascular events (MACEs) and mortality but with more major bleeding events, whereas rivaroxaban alone was not significantly effective in preventing MACE compared with aspirin.1
Both ischaemic and bleeding events can result in a range of neurological symptoms and severe pain. Survivors may experience physical function limitations such as walking and carrying out daily activities, emotional changes such as depression or anxiety, and social isolation, leading to a significant reduction in quality of life.2–6 It is therefore important to determine the net effect of anticoagulant and antiplatelet treatments on health-related quality of life (HRQoL), an important patient-reported outcome that measures the overall impact of treatment from the perspective of patients. The EQ-5D is a HRQoL instrument measuring five dimensions of health, namely, mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, that have been commonly affected by ischaemic and bleeding events.7,8 A few systematic literature reviews have reported that the EQ-5D was one of the most frequently used instruments in measuring the HRQoL of patients with stroke,9,10 myocardial infarction (MI),11 and bleeding events.6,12 Using the data from the COMPASS trial,13 we aimed to (i) compare the effect of the combination of rivaroxaban and aspirin compared with aspirin alone on HRQoL measured using the EQ-5D and (ii) estimate EQ-5D-derived health utilities associated with MACE including stroke, MI, heart failure, and major bleeding.
Methods
Study design and participants
The COMPASS trial was a double-blind, double-dummy, factorial randomized trial conducted at 602 centres in 33 countries. The design has been previously published.13 Patients were eligible if they met the criteria for coronary artery disease, peripheral artery disease, or both. Patients with coronary artery disease who were younger than 65 years of age were also required to have documentation of atherosclerosis involving at least two vascular beds or to have at least two additional risk factors. Patients with stroke within the previous month or a history of lacunar stroke, severe heart failure, or advanced kidney disease, and those treated with antiplatelet agents other than aspirin or anticoagulation were excluded. The trial was done in accordance with the International Council for Harmonization Good Clinical Practice Guidelines and the principles of the Declaration of Helsinki 2013. Institutional review boards and relevant health authorities approved the protocol. All patients provided written, informed consent.
Randomization and masking
After the 28-day run-in period, eligible patients were randomly assigned in a 1:1:1 ratio to one of three treatment groups: rivaroxaban plus aspirin, rivaroxaban alone, or aspirin alone. The randomization scheme was stratified by centre and the use of proton-pump inhibitor therapy at the time of randomization. Patients who were eligible for the proton-pump inhibitor randomization were also randomly assigned in a 1:1 ratio to receive pantoprazole (40 mg once daily) or matched placebo. Results of this arm of the trial are not reported.
Procedures
In the rivaroxaban plus aspirin group, rivaroxaban was administered at 2.5 mg twice per day and aspirin was administered at 100 mg once a day. In the rivaroxaban alone group, rivaroxaban was administered at 5 mg twice per day with an aspirin-matched placebo administered once per day. In the aspirin group, aspirin was administered at 100 mg once per day with a rivaroxaban matched placebo administered twice per day.
Patients were followed up at 1 month and at 6 months after randomization and at 6-month intervals thereafter to collect information on treatment adherence, treatment interruption, outcomes, and adverse events. Patients were to remain in follow-up until the minimum number of primary outcome events (n = 2200) was reached (after which the final visit occurred), irrespective of whether they were still taking study treatments or whether they had experienced an outcome.
Health-related quality of life was assessed using the three-level version of the EQ-5D (EQ-5D-3L), a widely recommended, generic preference-based instrument. The EQ-5D-3L consists of five questions measuring problems in the dimensions of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each question has three response levels reflecting no, some, and extreme problems. Responses to the five EQ-5D-3L questions can be converted to a health utility index anchored at 1 for full health and 0 for dead using a pre-developed value set based on societal preferences.14 In addition, the EQ-5D-3L has a visual analogue scale (EQ VAS), which measures self-reported general health from 0 for the worst imaginable health to 100 for the best imaginable health. The EQ-5D has been validated15,16 and widely used to assess the quality of life of patients with cardiovascular diseases.17–19 The EQ-5D-5L was later developed by expanding the three-level response options of the EQ-5D to five-level response options. When the COMPASS trial was designed, the EQ-5D-5L was relatively new and lacked psychometric evidence and preference-based scoring algorithm. Therefore, the EQ-5D-3L was included in the trial. The EQ-5D-3L was administered at baseline, at 2 years, and at final visit. If there was an outcome event (e.g. stroke, MI, and bleeding), the EQ-5D was also collected at the next study clinic visit after the event.
In addition, the Standardized Assessment of Global Activities in the Elderly (SAGE) and the International Physical Activity Questionnaire (IPAQ) were also administered in the trial. The SAGE is a 15-item instrument that measures functional status in patients with vascular disease. The SAGE has three domains: activities of daily living (ADL) (i.e. daily self-care activities, including bathing, dressing, and eating); instrumental ADL (IADL) (i.e. activities not necessary for basic function, but which allow an individual to live independently in the community, including housework, managing finances, and shopping); and cognition.20 Difficulties in ADL, IADL, and cognition are measured as mild (1 point), moderate (2 points), or severe (3 points). Higher scores correspond to greater functional impairments with a maximum score of 45. The IPAQ also contains 15 items measuring physical activity levels in four domains: leisure time physical activity; domestic and gardening (yard) activities; work-related physical activity; and transport-related physical activity. It measures metabolic equivalent of task (MET) minutes per week.21
Outcomes
The primary efficacy outcome of COMPASS was the composite of cardiovascular death, non-fatal stroke, or MI. The main safety outcome was major bleeding defined using a modification of the International Society on Thrombosis and Haemostasis (ISTH) criteria including fatal bleeding, symptomatic bleeding into a critical organ, bleeding into a surgical site requiring reoperation, and bleeding that led to presentation at an acute care facility or hospitalization. Secondary efficacy outcomes included the composite of ischaemic stroke, MI, acute limb ischaemia, or death from coronary heart disease; the composite of ischaemic stroke, MI, acute limb ischaemia, or cardiovascular death; and death from any cause. Health-related quality of life was assessed using the EQ-5D-3L, a pre-specified tertiary efficacy outcome for the trial.13
Statistical analysis
The analyses on HRQoL followed the statistical analysis plan for the COMPASS trial.13 The details on the statistical methods for the primary and key secondary outcomes have previously been published.1
The primary analyses on HRQoL were based on a data set that consisted of all randomized patients who completed the EQ-5D-3L at least at baseline. Participants in the rivaroxaban plus aspirin and aspirin alone arms were included in this analysis, as this was the comparison with statistically significant positive effect on the primary endpoint. The rivaroxaban alone arm did not show a statistically significant positive effect on the primary endpoint and was excluded from this analysis. All EQ-5D-3L data were analysed at baseline and at final visit. Patients who died were excluded from the primary analyses according to recent recommendations by an international panel.22 We conducted a sensitivity analysis in which the utility at the time of death was assigned a value of 0 and included in the comparison. For comparison purposes, we also calculated the change from baseline for SAGE and IPAQ total scores. Statistical analyses were conducted in R (version 4.2.1). All tests of statistical significance were two tailed. A P-value of <0.05 was considered significant.
The five responses to the EQ-5D-3L were converted to a health utility score using the scoring function developed in the USA.23 Mean health utility was calculated for baseline and final visit. To assess the treatment effect on HRQoL outcomes, linear regression models were fitted using the change from baseline health utility score at final visit as the response variable. Each model included treatment and time at final visit, adjusting for baseline EQ-5D-3L utility score and the stratification factor of proton-pump inhibitor use. The same model was also fitted with the EQ VAS as the response variable. The treatment effect was estimated using the adjusted mean difference in change from baseline between the rivaroxaban plus aspirin group and the aspirin group. We conducted subgroup analyses on baseline patient characteristics. In a sensitivity analysis, we used multiple imputation by chained equations (MICE) to impute the missing EQ-5D health utility and EQ VAS at final visit. Covariates in the MICE included treatment, proton-pump inhibitor use, baseline value, primary endpoint, and major bleeding. We then ran the same linear regression models to estimate adjusted mean difference in change from baseline between the two groups. In addition, we also compared the distribution of change from baseline health utility over the time of final visit between the two groups.
A deterioration event for the EQ-5D-3L was defined as a decrease in health utility from baseline that exceeds 0.089, a previously published minimum important difference for patients with acute MI.24 The deterioration threshold for EQ VAS (score range 0–100) was set at 10.25 Among those who completed the EQ-5D-3L both at baseline and at final visit, the percentage of patients who had deterioration events at final visit was calculated for each group. Logistic regression models including treatment, adjusting for baseline score and the stratification factor proton-pump inhibitor use, were performed to estimate the odds ratio of having deterioration at final visit for the rivaroxaban and aspirin group compared with the aspirin group.
For patients who completed both a baseline and final EQ-5D-3L, we also calculated the percentages of patients whose EQ health state at final visit was better, worse, with mixed changes, or the same compared with baseline.26 An EQ-5D-3L health state is deemed to be ‘better’ if it is better on at least one dimension and no worse on any other dimension, ‘worse’ if it is worse on at least one dimension and no better on any other dimension, ‘mixed changes’ if it is better in at least one dimension but worse in at least another, or the same in all five dimensions at final visit.26
According to the trial protocol, patients who developed a MACE were followed up with clinic visit. Therefore, we estimated the EQ-5D-derived health utilities using the subgroup of patients who developed a MACE and completed the EQ-5D-3L within 3 months after the event. Descriptive statistics (i.e. mean, median, and interquartile ranges) were presented for each MACE.
Results
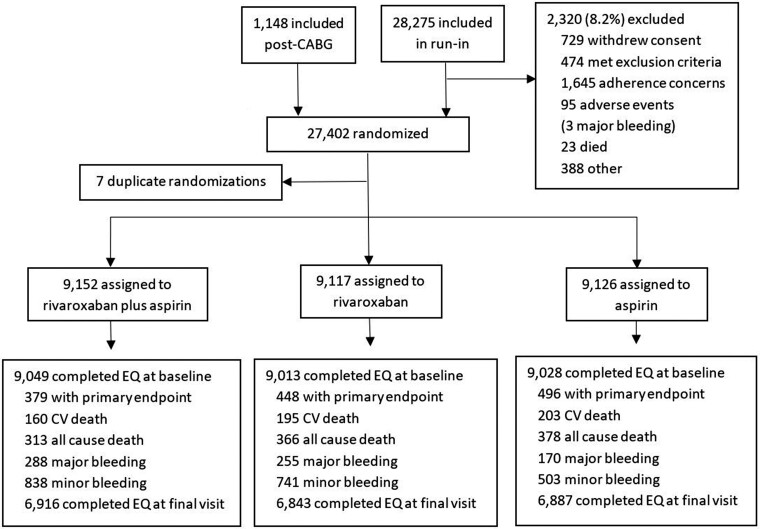
As shown in Figure 1, between March 2013 and May 2016, 27 395 patients were randomly assigned to rivaroxaban (2.5 mg twice daily) plus aspirin (n = 9152) (100 mg once daily), rivaroxaban (5 mg twice daily) (n = 9117), or aspirin (100 mg once daily) (n = 9126). In February 2017, the COMPASS Data Safety Monitoring Board recommended early discontinuation of the rivaroxaban vs. aspirin arm of the COMPASS trial because of the benefit on the primary endpoint. The last treatment end date was 18 June 2017, which was used as the cut-off date for our analyses. The median duration of treatment was 22.2 months (interquartile range, 14.5, 29.54). There were 313 (3.4%) patients who died from any cause in the rivaroxaban plus aspirin group and 378 (4.1%) in the aspirin alone group (Figure 1).
Figure 1.
COMPASS trial flow diagram.
There were 9049 (98.9%) and 9028 (98.9%) patients who completed the EQ-5D-3L at baseline in the rivaroxaban plus aspirin group and the aspirin group, respectively. Table 1 shows the demographic characteristics of these patients. Mean [standard deviation (SD)] health utility at baseline was 0.871 (0.141) in the rivaroxaban plus aspirin group compared with 0.873 (0.139) in the aspirin group. Six thousand nine hundred sixteen (76.4%) patients in the rivaroxaban plus aspirin group and 6887 (76.3%) patients in the aspirin group completed the EQ-5D also at final visit. The mean health utility at final visit was 0.874 (0.147) in the combination treatment group and 0.877 (0.147) in the aspirin group (Table 2). The adjusted mean difference in change from baseline utility was −0.002 (95% CI, −0.006, 0.002, P = 0.30) between the rivaroxaban plus aspirin group and the aspirin group. The subgroup analyses on baseline patient characteristics showed similar treatment effects (see Supplementary material online, Figure S1). The mean SAGE scores at final visit were 2.83 (4.15) compared with 2.97 (4.32) at baseline for the rivaroxaban plus aspirin group and 2.80 (4.20) and 3.00 (4.31) for the aspirin alone group (Table 3). The adjusted difference in change from baseline between the two groups was 0.04 (95% CI, −0.07, 0.15). For IPAQ, the MET minutes/week decreased at final visit compared with baseline in both groups. The adjusted difference in change from baseline between the two groups was 59.8 (95% CI, −214.5, 334.2) (Table 3).
Table 1.
Characteristics of patients with EQ-5D-3L at baseline
| Rivaroxaban plus aspirin | Aspirin alone | |
|---|---|---|
| n = 9049 | n = 9028 | |
| Age, years (SD) | 68.3 (7.92) | 68.2 (7.96) |
| Female sex, no. (%) | 2027 (22.4) | 1966 (21.8) |
| Body mass index | 28.3 (4.77) | 28.4 (4.72) |
| Blood pressure, mm Hg | ||
| Systolic | 135.8 (17.46) | 135.8 (17.50) |
| Diastolic | 77.7 (9.94) | 77.8 (9.93) |
| Cholesterol, mmol/L | 4.2 (1.08) | 4.2 (1.06) |
| Tobacco use, no. (%) | 1928 (21.3) | 1951 (21.6) |
| Hypertension, no. (%) | 6825 (75.4) | 6797 (75.3) |
| Diabetes, no. (%) | 3395 (37.5) | 3434 (38) |
| Previous stroke, no. (%) | 343 (3.8) | 331 (3.7) |
| Previous myocardial infarction, no. (%) | 5604 (61.9) | 5663 (62.7) |
| Heart failure, no. (%) | 1938 (21.4) | 1953 (21.6) |
| Carotid artery disease, no. (%) | 8214 (90.8) | 8165 (90.4) |
| Peripheral arterial disease, no. (%) | 2468 (27.3) | 2486 (27.5) |
| eGFR | ||
| <30 mL/min | 76 (0.8) | 84 (0.9) |
| 30 to <60 mL/min | 1952 (21.6) | 2011 (22.3) |
| ≥60 mL/min | 7018 (77.6) | 6933 (76.8) |
| Race, no. (%) | ||
| White | 5622 (62.1) | 5621 (62.3) |
| Black | 75 (0.8) | 91 (1) |
| Asian | 1426 (15.8) | 1385 (15.3) |
| Other | 1926 (21.3) | 1931 (21.4) |
| Region, no. (%) | ||
| North America | 1268 (14) | 1275 (14.1) |
| South America | 2030 (22.4) | 2029 (22.5) |
| Western Europe, Israel, Australia, or South Africa | 2846 (31.5) | 2840 (31.5) |
| East Europe | 1596 (17.6) | 1591 (17.6) |
| Asia-Pacific | 1309 (14.5) | 1293 (14.3) |
| Medication, no. (%) | ||
| ACE inhibitor or ARB | 6412 (70.9) | 6394 (70.8) |
| Calcium-channel blocker | 2390 (26.4) | 2462 (27.3) |
| Diuretic | 2677 (29.6) | 2701 (29.9) |
| Beta-blocker | 6306 (69.7) | 6309 (69.9) |
| Lipid-lowering agent | 8149 (90.1) | 8066 (89.3) |
| NSAID | 527 (5.8) | 470 (5.2) |
| Non-trial PPI | 3231 (35.7) | 3224 (35.7) |
There were no significant differences between the two groups.
ACE, Angiotensin-Converting Enzyme; ARB, Angiotensin Receptor Blocker; eGFR, Estimated Glomerular Filtration Rate; NSAID, Nonsteroidal Anti-Inflammatory Drug; PPI, Proton Pump Inhibitor; SD, standard deviation.
Table 2.
Changes in health utility and EQ visual analogue scale scores
| Rivaroxaban plus aspirin | Aspirin alone | |
|---|---|---|
| Mean (SD) health utility at baseline | n = 9049 | n = 9028 |
| 0.871 (0.141) | 0.873 (0.139) | |
| Mean (SD) health utility at final visit | n = 6916 | n = 6887 |
| 0.874 (0.147) | 0.877 (0.147) | |
| Adjusted difference in change from baseline (95% CI) | −0.002 (−0.006, 0.002) | |
| Patients with utility deterioration (≥MID 0.089) at final visit | n = 1306 | n = 1297 |
| Odds ratio (95% CI) vs. aspirin alone | 1.01 (0.93, 1.10) | |
| Mean (SD) EQ VAS at baseline | n = 9052 | n = 9030 |
| 76.0 (15.3) | 75.8 (15.1) | |
| Mean (SD) EQ VAS at final visit | n = 6914 | n = 6884 |
| 76.1 (15.2) | 75.9 (15.3) | |
| Adjusted difference in change from baseline (95% CI) | 0.02 (−0.43, 0.47) | |
| Patients with VAS deterioration (≥MID 10) at final visit | n = 1930 | n = 1853 |
| Odds ratio (95% CI) vs. aspirin alone | 1.04 (0.96, 1.13) | |
CI, confidence interval; MID, minimal important difference; SD, standard deviation; VAS, visual analogue scale.
Table 3.
Changes in the Standardized Assessment of Global Activities in the Elderly and International Physical Activity Questionnaire score
| Rivaroxaban plus aspirin | Aspirin alone | |
|---|---|---|
| Mean (SD) SAGE scores at baseline | n = 9072 | n = 9092 |
| 2.97 (4.32) | 3.00 (4.31) | |
| Mean (SD) SAGE scores at final visit | n = 8277 | n = 8343 |
| 2.83 (4.15) | 2.80 (4.20) | |
| Adjusted difference in change from baseline (95% CI) | 0.04 (−0.07, 0.15) | |
| IPAQ | ||
| Mean (SD) IPAQ Total Physical Activity Scores at baseline, MET minutes/week | n = 9073 | n = 9079 |
| 5399.8 (8324.9) | 5279.8 (8317.6) | |
| Mean (SD) IPAQ Total Physical Activity Scores at final visit, MET minutes/week | n = 3950 | n = 3942 |
| 4478.5 (6936.1) | 4408.9 (6872.9) | |
| Adjusted difference in change from baseline (95% CI) | 59.8 (−214.5, 334.2) | |
CI, confidence interval; IPAQ, International Physical Activity Questionnaire; MET, metabolic equivalent of task; SAGE, Standardized Assessment of Global Activities in the Elderly; SD, standard deviation.
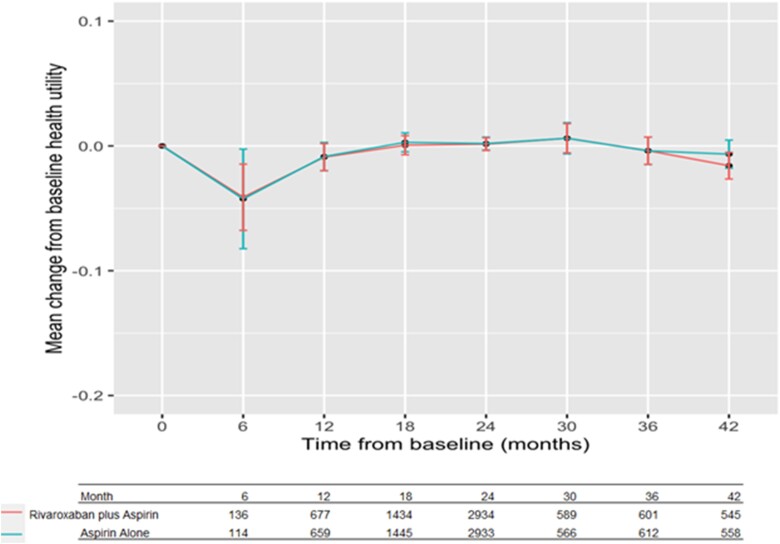
In the sensitivity analysis of including patients who died, the adjusted mean difference was 0.005 (95% CI, −0.001, 0.012) (see Supplementary material online, Table S1). With the multiple imputation, the adjusted mean difference was 0.002 (95% CI, −0.002, 0.006) (see Supplementary material online, Table S2). One thousand three hundred six (14.4%) patients reported utility deterioration (decrease ≥ 0.089) in the rivaroxaban plus aspirin group vs. 1297 (14.4%) in the aspirin group. The odds ratio (95% CI) of experiencing deterioration in health utility was 1.01 (95% CI, 0.93, 1.10, P = 0.81) in the combination therapy group compared with the aspirin group (Table 2). The distribution of mean changes from baseline over the time of final visit is shown in Figure 2. Most of the final visits occurred between 18 and 24 months from baseline, and the distribution of mean utilities was similar between the two groups. The mean utility was the lowest when the final visit occurred within first 6 months, a decrease by 0.04 from baseline in both groups. The decrease was larger in the sensitivity analyses by including patients who died and were assigned a utility value of 0 at time of death in both groups but more in the aspirin alone group (see Supplementary material online, Figure S2).
Figure 2.
Mean change from baseline utility at final visit.
For the EQ VAS, the baseline score was 76.0 (15.3) in the rivaroxaban plus aspirin group compared with 75.8 (15.1) in the aspirin group. The EQ VAS scores at final visit were 76.1 (15.2) in the rivaroxaban plus aspirin group vs. 75.9 (15.3) in the aspirin group (Table 2). Adjusted mean difference in change from baseline EQ VAS was 0.02 (95% CI, −0.43, 0.47, P = 0.93) between the rivaroxaban plus aspirin group and the aspirin group (Table 2). With the multiple imputation, the adjusted mean difference was 0.03 (95% CI, −0.48, 0.48) (see Supplementary material online, Table S2). The odds ratio for deterioration in EQ VAS (decrease ≥ 10) was 1.04 (95% CI, 0.96, 1.13, P = 0.34) (Table 2).
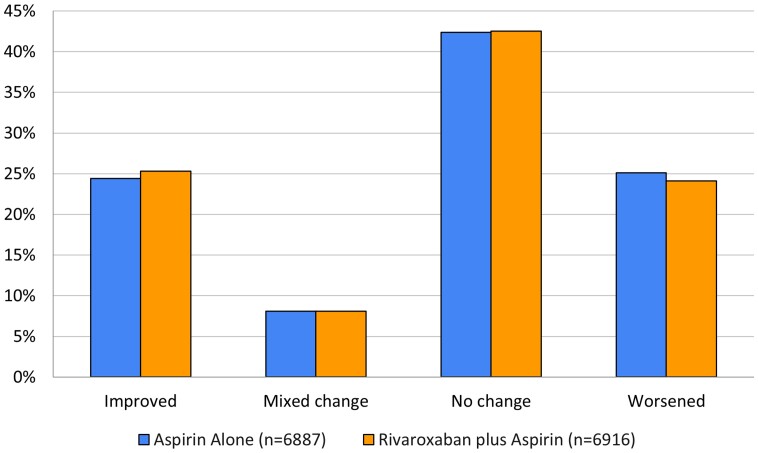
Proportions of patients in each of the three response options of the EQ-5D at baseline and at final visit are shown in Supplementary material online, Table S3. As shown in Figure 3, there was no difference in health status between the two treatment groups. Compared with baseline, 24.4% vs. 25.3% of patients reported that their health states were improved, 42.4% vs. 42.5% no change, and 25.1% vs. 24.1% worsening in the combination group and the aspirin group, respectively. In both groups, there were 8.1% of patients with mixed change. The change in response to each EQ-5D-3L domain was also similar between the two treatment arms (see Supplementary material online, Table S4).
Figure 3.
Change in EQ health status updated.
Table 4 shows health utilities estimated among the patients who had major cardiovascular events and completed the EQ-5D within 3 months of episode. Mean (SD) health utilities were 0.724 (0.253) for stroke, 0.825 (0.183) for MI, 0.760 (0.214) for heart failure, 0.777 (0.169) for venous thromboembolism, and 0.824 (0.175) for major bleeding. The health utilities of MACEs in the rivaroxaban plus aspirin group were higher than those in the aspirin alone group except for MI and major bleeding.
Table 4.
EQ-5D-3L-derived health utilities for major adverse cardiovascular events
| Rivaroxaban plus aspirin | Aspirin alone | Total | |
|---|---|---|---|
| Stroke | |||
| n, with EQ-5D available within 3 months after the event | 31 | 53 | 84 |
| Mean (SD) | 0.744 (0.209) | 0.712 (0.276) | 0.724 (0.253) |
| Median (Q1, Q3) | 0.794 (0.689, 0.849) | 0.810 (0.597, 0.854) | 0.800 (0.597, 0.854) |
| P value* | 0.948 | ||
| Myocardial infarction | |||
| n, with EQ-5D available within 3 months after the event | 67 | 80 | 147 |
| Mean (SD) | 0.816 (0.185) | 0.831 (0.182) | 0.825 (0.183) |
| Median (Q1, Q3) | 0.827 (0.761, 1.000) | 0.844 (0.778, 1.000) | 0.843 (0.770, 1.000) |
| P value* | 0.609 | ||
| Heart failure | |||
| n, with EQ-5D available within 3 months after the event | 77 | 98 | 175 |
| Mean (SD) | 0.764 (0.220) | 0.756 (0.21) | 0.760 (0.214) |
| Median (Q1, Q3) | 0.808 (0.708, 0.860) | 0.810 (0.721, 0.844) | 0.810 (0.708, 0.854) |
| P value* | 0.995 | ||
| Venous thromboembolism | |||
| n, with EQ-5D available within 3 months after the event | 5 | 16 | 21 |
| Mean (SD) | 0.839 (0.150) | 0.757 (0.175) | 0.777 (0.169) |
| Median (Q1, Q3) | 0.778 (0.708, 1.000) | 0.810 (0.703, 0.827) | 0.810 (0.708, 0.827) |
| P value* | 0.617 | ||
| Major bleeding | |||
| n, with EQ-5D available within 3 months after the event | 135 | 73 | 208 |
| Mean (SD) | 0.817 (0.185) | 0.836 (0.154) | 0.824 (0.175) |
| Median (Q1, Q3) | 0.827 (0.778, 1.000) | 0.827 (0.781, 1.000) | 0.827 (0.778, 1.000) |
| P value* | 0.944 |
SD, standard deviation.
*Mann–Whitney U test.
Discussion
Antithrombotic therapies are effective in preventing major cardiovascular events in patients with chronic cardiovascular disease and also increase the risk of bleeding. The COMPASS demonstrated that the combination of rivaroxaban and aspirin was more effective than aspirin alone at preventing MI, stroke, and cardiovascular death (relative risk reduction 0.67, 95% CI, 0.66, 0.86; P < 0.001), but major bleeding events occurred in more patients in the rivaroxaban plus aspirin group. All-cause mortality was lower with combination treatment.1 Our analyses from the COMPASS trial found that quality of life, an important patient-reported outcome that measures the overall impact of treatment, was similar between the two treatment groups. Patients on rivaroxaban plus aspirin had similar quality of life as those on aspirin alone in terms of health status, health utility index, and VAS as measured using the EQ-5D.
Patients on rivaroxaban plus aspirin had less stroke, MI, acute limb ischaemia, and related mortality than those on aspirin alone in the COMPASS trial.1 The COMPASS trial defined major bleeding using a modification of the ISTH criteria that included bleeding that led to presentation to an acute care facility with or without an overnight stay. Some of these events did not meet the criteria for major bleeding as defined by the conventional ISTH criteria, and their inclusion as major bleeds resulted in a one-third increase in the reported incidence of major bleeding.1 The categorization of these less serious events as major bleeding was demonstrated by mean utility of 0.824 for major bleeding, compared with 0.724 for stroke and 0.825 for MI in the COMPASS trial. Compared with aspirin alone, the combination arm had 59 less stroke, 27 less MI, and 118 more major bleeding events.1 As a result, the overall quality of life that took into account both the number and impact of these events was similar between the two arms, if the goal was to recover to the baseline level.
The quality of life at baseline and at final visit of COMPASS trial participants as measured by the EQ-5D and EQ VAS appears higher than previously reported for patients with cardiovascular disease.17,18 Dyer et al.18 found that mean EQ-5D utility scores ranged from 0.45 to 0.88 and EQ VAS scores from 45 to 82 for patients with cardiovascular disease [International Classification of Diseases (ICD) codes I20–I25], and EQ-5D utility scores ranged from 0.33 to 0.78 and mean VAS scores from 49 to 71 for those with peripheral vascular diseases (ICD code I73).18 A systematic literature review of HRQoL studies for cardiovascular diseases in European countries found that mean EQ-5D utility scores ranged from 0.61 to 0.88 and EQ VAS from 37.5 to 74.5 among patients before cardiac procedure or surgery.17 The mean EQ-5D utility scores ranged from 0.66 to 0.95 and EQ VAS from 50 to 89 after cardiac procedure or surgery.17 However, the COMPASS trial enrolled stable patients and only a small proportion (4.7% in the rivaroxaban plus aspirin group and 5.9% in the aspirin alone group) had clinical events that decreased their quality of life during the trial. This may explain why, at the group level, mean EQ-5D utility or EQ VAS scores for the COMPASS participants were relatively high compared with those reported in previously published studies.
Prevention of MACE among patients with stable coronary artery disease and peripheral artery disease needs to balance the benefit of preventing major cardiovascular events and the increased risk of bleeding. The COMPASS trial reported net-clinical-benefit outcome that combined cardiovascular death, stroke, MI, fatal bleeding, and symptomatic bleeding into a critical organ, which was reduced with rivaroxaban plus aspirin than with aspirin alone (hazard ratio, 0.80; 95% CI, 0.70, 0.91; P < 0.001).1 Although this metric provides a useful summary of key clinically relevant events, it does not incorporate the overall impact of these events on quality of life as reported by the patients. Assessment of how patients feel is even more important given the improved survival of patients treated with the combination of rivaroxaban and aspirin in the COMPASS trial.27 A number of disease-specific instruments have been developed to measure the quality of life of patients with cardiovascular disease.28 Some reports have suggested that disease-specific instruments have better psychometric properties than generic instruments,29 while others showed that they performed equally well.30 A recent systematic literature review identified eight commonly used cardiovascular-specific instruments.31 These instruments vary noticeably in terms of the number of health dimensions and items (range 13–70) and the quality of evidence on psychometric properties.31 The EQ-5D measures five health dimensions important for daily life as well as an overall self-assessment of health for this patient population.8,32 Some of these health domains included in the EQ-5D are also included in the SAGE and IPAQ, which were designed to focus on the measurement of physical activities. There are methodological and practical advantages of using the EQ-5D in clinical research. The EQ-5D contains only five questions, fewer than SAGE or IPAQ, which includes 15 items to obtain more comprehensive assessment on physical activities. Both SAGE and IPAQ were administered before the EQ-5D. However, there was a higher proportion of missing data for IPAQ (∼57%) than the EQ-5D. As a generic, preference-based instrument, the EQ-5D can also generate a health utility index that can be compared across treatments and across diseases and is suitable for use in cost-effectiveness analysis.6,8,10 The future clinical studies should consider the content, length, wording, and formatting of existing instruments to choose the combination that could minimize the burden on patients while meeting the measurement needs.
In our analyses, the treatment effects on quality of life between rivaroxaban plus aspirin and aspirin alone were estimates at the group level. It should be noted that quality of life varied with patient characteristics, disease severity, and treatments.33 Therefore, cautions should be exercised when interpreting and applying the finding in making treatment decisions for individual patients.
This study has several limitations. First, ∼24% of patients in each group did not complete the EQ-5D at final visit. There were significant differences in some characteristics between those with and without final EQ-5D data within group. For instance, patients without final EQ-5D data tended to be older, less likely to have heart failure, more likely to have peripheral artery disease and to use lipid-lowering agent compared with those with completed final EQ-5D (see Supplementary material online, Table S5). Nevertheless, the characteristics of patients without a final EQ-5D in the combination group were similar to those of patients without final EQ-5D in the aspirin group. The sensitivity analysis with multiple imputations showed similar treatment effect estimates between the two groups. Second, the trial was terminated early, which caused variation in the time of final visit. The quality of life was similar between the two groups across the times of final visit. Third, the trial was originally designed to collect quality of life only at 2 years after baseline for every patient participant. This two-year interval is considered too long to capture the trajectory of quality of life of patients over time. For example, if the risk of developing a clinical event was higher within 12 months of treatment (see Figure 2), it would be important to measure quality of life more frequently in the first year (e.g. at 3, 6, and 12 months).
Conclusion
This analysis of the COMPASS trial demonstrated that the overall impact on quality of life of patients was similar between the rivaroxaban plus aspirin group and the aspirin alone group. This evidence is important for both physicians and patients when considering the use of the combination of rivaroxaban and aspirin for long-term cardiovascular prevention.
Supplementary Material
Acknowledgements
We would like to express our deepest gratitude to Dr Stuart Connolly who passed away on June 2, 2024. Dr. Connolly's invaluable contributions to the field of cardiac electrophysiology have greatly influenced our work.
Contributor Information
Feng Xie, Department of Health Research Methods, Evidence, and Impact, Faculty of Health Sciences, McMaster University, 1280 Main Street West, Hamilton, Ontario, Canada L8S 4L8; Centre for Health Economics and Policy Analysis, Faculty of Health Sciences, McMaster University, Hamilton, Ontario, Canada L8S 4L8.
Jiajun Yan, Department of Health Research Methods, Evidence, and Impact, Faculty of Health Sciences, McMaster University, 1280 Main Street West, Hamilton, Ontario, Canada L8S 4L8.
John Eikelboom, Population Health Research Institute, McMaster University, Hamilton Health Sciences, Hamilton, Ontario, Canada L8L 2X2.
Sonia Anand, Population Health Research Institute, McMaster University, Hamilton Health Sciences, Hamilton, Ontario, Canada L8L 2X2.
Eva Muehlhofer, Research and Development, Pharmaceuticals TA Thrombosis, Bayer AG, Wuppertal, 42113, Germany.
Eleanor Pullenayegum, Child Health Evaluative Sciences, Hospital for Sick Children, Toronto, Ontario, Canada M5G 1X8.
Yang Wang, Department of Health Research Methods, Evidence, and Impact, Faculty of Health Sciences, McMaster University, 1280 Main Street West, Hamilton, Ontario, Canada L8S 4L8.
Alvaro Avezum, Instituto Dante Pazzanese de Cardiologia, Sao Paulo, 04012-180, Brazil.
Deepak L Bhatt, Mount Sinai Heart, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Salim Yusuf, Population Health Research Institute, McMaster University, Hamilton Health Sciences, Hamilton, Ontario, Canada L8L 2X2.
Jackie Bosch, Population Health Research Institute, McMaster University, Hamilton Health Sciences, Hamilton, Ontario, Canada L8L 2X2; School of Rehabilitation Science, Faculty of Health Sciences, McMaster University, Hamilton, Ontario, Canada L8S 1C7.
Data availability
All the data analysed in this study came from the COMPASS trial. The data that support the findings of these analyses are available from the corresponding author upon reasonable request.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Authors' contributions
Concept and design: F.X., J.Y., J.E., E.P., and J.B. Analysis and interpretation of data: F.X., J.Y., J.E., S.A., E.M., E.P., Y.W., A.A., D.B., S.Y., and J.B. Drafting of the manuscript: F.X. Critical revision of the paper for important intellectual content: F.X., J.Y., J.E., S.A., E.M., E.P., Y.W., A.A., D.B., S.Y., and J.B. Statistical analysis: F.X., J.Y., E.P., and Y.W.
Funding
The COMPASS trial was sponsored by Bayer AG. The analysis of COMPASS trial presented in this paper was sponsored by the EuroQol Research Foundation (#137-2020RA). All sponsors have no role in the conduct, analysis, and interpretation of the present analysis.
References
- 1. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Widimsky P, Hori M, Avezum A, Piegas LS, Branch KRH, Probstfield J, Bhatt DL, Zhu J, Liang Y, Maggioni AP, Lopez-Jaramillo P, O'Donnell M, Kakkar AK, Fox KAA, Parkhomenko AN, Ertl G, Störk S, Keltai M, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim JH, Tonkin AM, Lewis BS, Felix C, Yusoff K, Steg PG, Metsarinne KP, Cook Bruns N, Misselwitz F, Chen E, Leong D, Yusuf S; COMPASS Investigators . Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 2. van Mierlo ML, Schroder C, van Heugten CM, Post MWM, de Kort PLM, Visser-Meily JMA. The influence of psychological factors on health-related quality of life after stroke: a systematic review. Int J Stroke 2014;9:341–348. [DOI] [PubMed] [Google Scholar]
- 3. Mollon L, Bhattacharjee S. Health related quality of life among myocardial infarction survivors in the United States: a propensity score matched analysis. Health Qual Life Outcomes 2017;15:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Payton H, Soundy A. The experience of post-stroke pain and the impact on quality of life: an integrative review. Behav Sci (Basel) 2020;10:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corrigan AE, Carter B, Smith A, Pennington A, Hewitt J. MORe PREcISE: a multicentre prospective study of patient reported outcome measures in stroke morbidity: a cross sectional study. BMC Neurol 2022;22:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doble B, Pufulete M, Harris JM, Johnson T, Lasserson D, Reeves BC, Wordsworth S. Health-related quality of life impact of minor and major bleeding events during dual antiplatelet therapy: a systematic literature review and patient preference elicitation study. Health Qual Life Outcomes 2018;16:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Golomb BA, Vickrey BG, Hays RD. A review of health-related quality-of-life measures in stroke. Pharmacoeconomics 2001;19:155–185. [DOI] [PubMed] [Google Scholar]
- 8. Betts MB, Rane P, Bergrath E, Chitnis M, Bhutani MK, Gulea C, Qian Y, Villa G. Utility value estimates in cardiovascular disease and the effect of changing elicitation methods: a systematic literature review. Health Qual Life Outcomes 2020;18:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cameron LJ, Wales K, Casey A, Pike S, Jolliffe L, Schneider EJ, Christie LJ, Ratcliffe J, Lannin NA. Self-reported quality of life following stroke: a systematic review of instruments with a focus on their psychometric properties. Qual Life Res 2022;31:329–342. [DOI] [PubMed] [Google Scholar]
- 10. Joundi RA, Adekanye J, Leung AA, Ronksley P, Smith EE, Rebchuk AD, Field TS, Hill MD, Wilton SB, Bresee LC. Health state utility values in people with stroke: a systematic review and meta-analysis. J Am Heart Assoc 2022;11:e024296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu C, Tran PM, Leifheit EC, Spatz ES, Dreyer RP, Nyhan K, Wang SY, Lichtman JH. Association of marital/partner status and patient-reported outcomes following myocardial infarction: a systematic review and meta-analysis. Eur Heart J Open 2023;3:oead018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Res LCS, Lubberts B, Shah SH, DiGiovanni CW. Health-related quality of life after adverse bleeding events associated with antithrombotic drug therapy—a systematic review. Hellenic J Cardiol 2019;60:3–10. [DOI] [PubMed] [Google Scholar]
- 13. Bosch J, Eikelboom JW, Connolly SJ, Bruns NC, Lanius V, Yuan F, Misselwitz F, Chen E, Diaz R, Alings M, Lonn EM, Widimsky P, Hori M, Avezum A, Piegas LS, Bhatt DL, Branch KRH, Probstfield JL, Liang Y, Liu L, Zhu J, Maggioni AP, Lopez-Jaramillo P, O'Donnell M, Fox KAA, Kakkar A, Parkhomenko AN, Ertl G, Störk S, Keltai K, Keltai M, Ryden L, Dagenais GR, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim JH, Ha JW, Tonkin AM, Varigos JD, Lewis BS, Felix C, Yusoff K, Steg PG, Aboyans V, Metsarinne KP, Anand SS, Hart RG, Lamy A, Moayyedi P, Leong DP, Sharma M, Yusuf S. Rationale, design and baseline characteristics of participants in the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS). Trial. Can J Cardiol 2017;33:1027–1035. [DOI] [PubMed] [Google Scholar]
- 14. Devlin NJ, Brooks R. EQ-5D and the EuroQol group: past, present and future. Appl Health Econ Health Policy 2017;15:127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldsmith KA, Dyer MT, Schofield PM, Buxton MJ, Sharples LD. Relationship between the EQ-5D index and measures of clinical outcomes in selected studies of cardiovascular interventions. Health Qual Life Outcomes 2009;7:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nowels D, McGloin J, Westfall JM, Holcomb S. Validation of the EQ-5D quality of life instrument in patients after myocardial infarction. Qual Life Res 2005;14:95–105. [DOI] [PubMed] [Google Scholar]
- 17. Batog P, Rencz F, Pentek M, Gulácsi L, Filipiak KJ, Prevolnik Rupel V, Simon J, Brodszky V, Baji P, Závada J, Petrova G, Rotar A, Golicki D. EQ-5D studies in cardiovascular diseases in eight central and eastern European countries: a systematic review of the literature. Kardiol Pol 2018;76:860–870. [DOI] [PubMed] [Google Scholar]
- 18. Dyer MT, Goldsmith KA, Sharples LS, Buxton MJ. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health Qual Life Outcomes 2010;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou T, Guan H, Wang L, Zhang Y, Rui M, Ma A. Health-Related quality of life in patients with different diseases measured with the EQ-5D-5L: a systematic review. Front Public Health 2021;9:675523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spence J, Bosch J, Chongsi E, Lee SF, Thabane L, Mendoza P, Belley-Côté E, Whitlock R, Brady K, McIntyre WF, Lamy A, Devereaux PJ. Standardized Assessment of Global activities in the Elderly scale in adult cardiac surgery patients. Br J Anaesth 2021;127:539–546. [DOI] [PubMed] [Google Scholar]
- 21. Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–1395. [DOI] [PubMed] [Google Scholar]
- 22. Coens C, Pe M, Dueck AC, Sloan J, Basch E, Calvert M, Campbell A, Cleeland C, Cocks K, Collette L, Devlin N, Dorme L, Flechtner HH, Gotay C, Griebsch I, Groenvold M, King M, Kluetz PG, Koller M, Malone DC, Martinelli F, Mitchell SA, Musoro JZ, O'Connor D, Oliver K, Piault-Louis E, Piccart M, Quinten C, Reijneveld JC, Schürmann C, Smith AW, Soltys KM, Taphoorn MJB, Velikova G, Bottomley A; Setting International Standards in Analyzing Patient-Reported Outcomes and Quality of Life Endpoints Data Consortium . International standards for the analysis of quality-of-life and patient-reported outcome endpoints in cancer randomised controlled trials: recommendations of the SISAQOL consortium. Lancet Oncol 2020;21:e83–e96. [DOI] [PubMed] [Google Scholar]
- 23. Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care 2005;43:203–220. [DOI] [PubMed] [Google Scholar]
- 24. Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res 2005;14:1523–1532. [DOI] [PubMed] [Google Scholar]
- 25. Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Devlin NJ, Parkin D, Browne J. Patient-reported outcome measures in the NHS: new methods for analysing and reporting EQ-5D data. Health Econ 2010;19:886–905. [DOI] [PubMed] [Google Scholar]
- 27. Cepeda-Valery B, Cheong AP, Lee A, Yan BP. Measuring health related quality of life in coronary heart disease: the importance of feeling well. Int J Cardiol 2011;149:4–9. [DOI] [PubMed] [Google Scholar]
- 28. Thompson DR, Yu C-M. Quality of life in patients with coronary heart disease-I: assessment tools. Health Qual Life Outcomes 2003;1:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wolinsky FD, Wyrwich KW, Nienaber NA, Tierney WM. Generic versus disease-specific health status measures. An example using coronary artery disease and congestive heart failure patients. Eval Health Prof 1998;21:216–243. [DOI] [PubMed] [Google Scholar]
- 30. Garster NC, Palta M, Sweitzer NK, Kaplan RM, Fryback DG. Measuring health-related quality of life in population-based studies of coronary heart disease: comparing six generic indexes and a disease-specific proxy score. Qual Life Res 2009;18:1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li X, Li R, Li M, Yao L, Van Spall H, Zhao K, Chen Y, Xiao F, Fu Q, Xie F. A systematic review and quality assessment of cardiovascular disease-specific health-related quality-of-life instruments part I: instrument development and content validity. Value Health 2024;27:1130–1148. [DOI] [PubMed] [Google Scholar]
- 32. Geyh S, Cieza A, Kollerits B, Grimby G, Stucki G. Content comparison of health-related quality of life measures used in stroke based on the international classification of functioning, disability and health (ICF): a systematic review. Qual Life Res 2007;16:833–851. [DOI] [PubMed] [Google Scholar]
- 33. Dou L, Mao Z, Fu Q, Chen G, Li S. Health-related quality of life and its influencing factors in patients with coronary heart disease in China. Patient Prefer Adherence 2022;16:781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data analysed in this study came from the COMPASS trial. The data that support the findings of these analyses are available from the corresponding author upon reasonable request.