Abstract
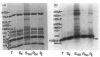
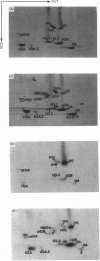
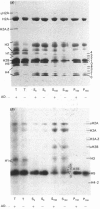
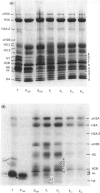
The incorporation of newly synthesized histones among various chromatin fraction isolated from non-replicating cell-cycle-phase-Go chicken immature erythrocytes was investigated. We find that newly synthesized erythroid-specific histone Hl variant H5, is incorporated randomly into chromatin. In contrast, newly synthesized nucleosomal histones H2A, H2A.Z, H2B, H3.3, and H4 are preferentially found in a fraction that is highly enriched in active/competent gene chromatin fragments and depleted in repressed gene chromatin. Moreover, ubiquitinated species of histones H2A and H2B and hyperacetylated species of H4 and H2B, which are complexed to active DNA, are labelled. These observations provide evidence that newly synthesized histones preferentially exchange with the nucleosomal histones of transcriptionally active/component chromatin domains. The results of this study suggest that nucleosomes of active chromatin may be inherently less stable than bulk nucleosomes in vivo and have implications for chromatin remodelling.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affolter M., Côté J., Renaud J., Ruiz-Carrillo A. Regulation of histone and beta A-globin gene expression during differentiation of chicken erythroid cells. Mol Cell Biol. 1987 Oct;7(10):3663–3672. doi: 10.1128/mcb.7.10.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer B. W., Rhodes D. Eukaryotic RNA polymerase II binds to nucleosome cores from transcribed genes. Nature. 1983 Feb 10;301(5900):482–488. doi: 10.1038/301482a0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Wu R. S., Panusz H. T., Muneses C. Kinetics of accumulation and depletion of soluble newly synthesized histone in the reciprocal regulation of histone and DNA synthesis. Biochemistry. 1988 Aug 23;27(17):6542–6550. doi: 10.1021/bi00417a052. [DOI] [PubMed] [Google Scholar]
- Delcuve G. P., Davie J. R. Chromatin structure of erythroid-specific genes of immature and mature chicken erythrocytes. Biochem J. 1989 Oct 1;263(1):179–186. doi: 10.1042/bj2630179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton K. S., Dhar V., Brown E. H., Iqbal M. A., Stuart S., Didamo V. T., Schildkraut C. L. Replication program of active and inactive multigene families in mammalian cells. Mol Cell Biol. 1988 May;8(5):2149–2158. doi: 10.1128/mcb.8.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbes T. R., Thorne A. W., Crane-Robinson C. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 1988 May;7(5):1395–1402. doi: 10.1002/j.1460-2075.1988.tb02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendzel M. J., Davie J. R. Distribution of methylated histones and histone methyltransferases in chicken erythrocyte chromatin. J Biol Chem. 1989 Nov 15;264(32):19208–19214. [PubMed] [Google Scholar]
- Ip Y. T., Jackson V., Meier J., Chalkley R. The separation of transcriptionally engaged genes. J Biol Chem. 1988 Oct 5;263(28):14044–14052. [PubMed] [Google Scholar]
- Jackson V., Chalkley R. Histone synthesis and deposition in the G1 and S phases of hepatoma tissue culture cells. Biochemistry. 1985 Nov 19;24(24):6921–6930. doi: 10.1021/bi00345a026. [DOI] [PubMed] [Google Scholar]
- Jackson V. In vivo studies on the dynamics of histone-DNA interaction: evidence for nucleosome dissolution during replication and transcription and a low level of dissolution independent of both. Biochemistry. 1990 Jan 23;29(3):719–731. doi: 10.1021/bi00455a019. [DOI] [PubMed] [Google Scholar]
- Lin R., Leone J. W., Cook R. G., Allis C. D. Antibodies specific to acetylated histones document the existence of deposition- and transcription-related histone acetylation in Tetrahymena. J Cell Biol. 1989 May;108(5):1577–1588. doi: 10.1083/jcb.108.5.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loidl P. Towards an understanding of the biological function of histone acetylation. FEBS Lett. 1988 Jan 25;227(2):91–95. doi: 10.1016/0014-5793(88)80874-3. [DOI] [PubMed] [Google Scholar]
- Louters L., Chalkley R. Exchange of histones H1, H2A, and H2B in vivo. Biochemistry. 1985 Jun 18;24(13):3080–3085. doi: 10.1021/bi00334a002. [DOI] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Nacheva G. A., Guschin D. Y., Preobrazhenskaya O. V., Karpov V. L., Ebralidse K. K., Mirzabekov A. D. Change in the pattern of histone binding to DNA upon transcriptional activation. Cell. 1989 Jul 14;58(1):27–36. doi: 10.1016/0092-8674(89)90399-1. [DOI] [PubMed] [Google Scholar]
- Nickel B. E., Allis C. D., Davie J. R. Ubiquitinated histone H2B is preferentially located in transcriptionally active chromatin. Biochemistry. 1989 Feb 7;28(3):958–963. doi: 10.1021/bi00429a006. [DOI] [PubMed] [Google Scholar]
- Nickel B. E., Roth S. Y., Cook R. G., Allis C. D., Davie J. R. Changes in the histone H2A variant H2A.Z and polyubiquitinated histone species in developing trout testis. Biochemistry. 1987 Jul 14;26(14):4417–4421. doi: 10.1021/bi00388a034. [DOI] [PubMed] [Google Scholar]
- Ridsdale J. A., Davie J. R. Chicken erythrocyte polynucleosomes which are soluble at physiological ionic strength and contain linker histones are highly enriched in beta-globin gene sequences. Nucleic Acids Res. 1987 Feb 11;15(3):1081–1096. doi: 10.1093/nar/15.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridsdale J. A., Hendzel M. J., Delcuve G. P., Davie J. R. Histone acetylation alters the capacity of the H1 histones to condense transcriptionally active/competent chromatin. J Biol Chem. 1990 Mar 25;265(9):5150–5156. [PubMed] [Google Scholar]
- Ridsdale J. A., Rattner J. B., Davie J. R. Erythroid-specific gene chromatin has an altered association with linker histones. Nucleic Acids Res. 1988 Jul 11;16(13):5915–5926. doi: 10.1093/nar/16.13.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariban E., Wu R. S., Erickson L. C., Bonner W. M. Interrelationships of protein and DNA syntheses during replication of mammalian cells. Mol Cell Biol. 1985 Jun;5(6):1279–1286. doi: 10.1128/mcb.5.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale R. L. In vivo assembly of newly synthesized histones. Biochemistry. 1981 Oct 27;20(22):6432–6437. doi: 10.1021/bi00525a023. [DOI] [PubMed] [Google Scholar]
- Waithe W. I., Renaud J., Nadeau P., Pallotta D. Histone synthesis by lymphocytes in G0 and G1. Biochemistry. 1983 Apr 12;22(8):1778–1783. doi: 10.1021/bi00277a006. [DOI] [PubMed] [Google Scholar]
- Williams A. F. DNA synthesis in purified populations of avian erythroid cells. J Cell Sci. 1972 Jan;10(1):27–46. doi: 10.1242/jcs.10.1.27. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Panusz H. T., Hatch C. L., Bonner W. M. Histones and their modifications. CRC Crit Rev Biochem. 1986;20(2):201–263. doi: 10.3109/10409238609083735. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Perry L. J., Bonner W. M. Fate of newly synthesized histones in G1 and G0 cells. FEBS Lett. 1983 Oct 3;162(1):161–166. doi: 10.1016/0014-5793(83)81070-9. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Tsai S., Bonner W. M. Changes in histone H3 composition and synthesis pattern during lymphocyte activation. Biochemistry. 1983 Aug 2;22(16):3868–3873. doi: 10.1021/bi00285a023. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Tsai S., Bonner W. M. Patterns of histone variant synthesis can distinguish G0 from G1 cells. Cell. 1982 Dec;31(2 Pt 1):367–374. doi: 10.1016/0092-8674(82)90130-1. [DOI] [PubMed] [Google Scholar]
- Zhang D. E., Nelson D. A. Histone acetylation in chicken erythrocytes. Rates of acetylation and evidence that histones in both active and potentially active chromatin are rapidly modified. Biochem J. 1988 Feb 15;250(1):233–240. doi: 10.1042/bj2500233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Nelson D. A. Histone acetylation in chicken erythrocytes. Estimation of the percentage of sites actively modified. Biochem J. 1986 Dec 15;240(3):857–862. doi: 10.1042/bj2400857. [DOI] [PMC free article] [PubMed] [Google Scholar]