Abstract
Background:
Rotavirus vaccine impact on rotavirus hospitalizations is not well-documented globally. We performed a systematic review to estimate the number of rotavirus hospitalizations that 1) occur annually, 2) are currently prevented by rotavirus vaccines, and 3) could be prevented with improved vaccine coverage and universal vaccine introduction.
Methods:
We systematically reviewed articles indexed in the PubMed database published from 1 January 2000 to 31 December 2019. We included all primary peer-reviewed studies with rotavirus hospitalization rates for children <5 years that reported data prior to vaccine introduction, utilized at least one continuous year of data collection, and collected hospitalization data after 2000 using active surveillance. We grouped pre-vaccine country estimates by childhood mortality strata and calculated the median rate among each group. We then assigned the mortality stratum-specific hospitalization rates to each country and calculated the number of rotavirus hospitalizations by country, mortality strata, and WHO region.
Results:
Our search strategy identified 4,590 manuscripts, of which 32 were included in the final dataset. In 2019, an estimated 1,760,113 (Interquartile range [IQR]: 1,422,645–2,925,372) rotavirus hospitalizations occurred globally, with 524,871 (IQR: 415,987–814,835) prevented by rotavirus vaccination. With universal introduction of rotavirus vaccines and increased vaccine coverage, we estimate that an additional 751,609 (IQR:607,671–1,318,807) rotavirus hospitalizations can be prevented annually.
Conclusions:
This analysis highlights the continued burden of rotavirus hospitalizations among children <5 years. A large, preventable proportion of this burden could be eliminated by expanding introductions to new countries and increasing rotavirus vaccine coverage to levels seen with other childhood vaccinations.
Keywords: rotavirus, diarrhea, vaccine, global, impact, epidemiology
Summary:
This study provides the first global hospitalization estimate for rotavirus using population-based data available in published literature. We estimate the burden of rotavirus hospitalizations in the absence of vaccine, with current vaccine coverage, and those potentially prevented with increased coverage.
Introduction
Rotavirus is the leading cause of acute gastroenteritis among children globally, and prior to the introduction of rotavirus vaccines to the global market, rotavirus caused an estimated 528,000 deaths every year among children <5 years of age (1). In 2006, the first two rotavirus vaccines became available and the World Health Organization (WHO) recommended their routine use in North America, South America, Australia, and Europe (2). Following the release of additional vaccine efficacy data from countries in Africa and Asia in 2009, WHO updated its recommendation and now universally recommends rotavirus vaccine for all children (3). Today, more than 100 countries have implemented a rotavirus vaccine into their national immunization plan (4).
Since their licensure, rotavirus vaccines have continued to demonstrate substantial reduction on the burden of rotavirus disease globally (5). When determining global impact of rotavirus vaccine introduction, studies often examine reductions in mortality, as this outcome is commonly estimated and remains a key concern for vaccine decision making. However, with the introduction of oral rehydration salts and advances in medical care, overall and rotavirus-associated diarrhea mortality rates continue to decline, even in the absence of rotavirus vaccine (6). An often-overlooked metric of vaccine impact is the number of hospitalizations prevented due to rotavirus vaccine introduction, which can be used to inform decisions pertaining to the cost-effectiveness of implementing rotavirus vaccine into a country’s national immunization plan. Unfortunately, the incidence of rotavirus hospitalizations is not calculated as frequently as mortality, limiting the availability of global rotavirus hospitalization estimates.
Published estimates of the number of rotavirus hospitalizations occurring globally are scarce. In 2003, Parashar et al. published the first global estimate and predicted that approximately 2 million hospitalizations occurred annually from rotavirus gastroenteritis among children <5 years of age worldwide (7). To develop this estimate, the authors used published rotavirus hospitalization rates and population attributable fractions to calculate rotavirus hospitalizations among developed and developing countries independently, and then combined both to obtain the global burden. However, this review was based on sparse data, particularly for developing countries which used the population attributable fraction of diarrhea requiring hospitalization from a single study done in one country. More recently, Troeger et al. re-calculated this burden accounting for rotavirus vaccination, and estimated 1,537,000 rotavirus hospitalizations occurred globally in 2016 (8). However, this hospitalization estimate was calculated entirely using the population attributable fraction method, in which the global diarrheal hospitalization burden (9) was multiplied by the modelled fraction of severe all-cause diarrhea attributable to rotavirus. We believe that utilizing population-based rotavirus hospitalization rates (e.g. hospitalizations per 10,000 children <5 years of age) based on laboratory testing of gastroenteritis hospitalizations for rotavirus may offer a more direct approach to calculate this burden, as these data are locally generated. While several studies have used population-based rotavirus hospitalization rates to estimate the number of rotavirus hospitalizations before and after vaccine introduction in the United States (10), Latin America (11), Asia (12), and Africa (13), a global estimate has never been achieved. We performed a systematic review to estimate the number of hospitalizations that occurred globally due to rotavirus disease in 2019, the number that are currently prevented by rotavirus vaccines, and the number that could be prevented with improved vaccine coverage and further national introductions.
Methods
Literature Review
We systematically reviewed articles indexed in the PubMed database from 1 January 2000 to 31 December 2019 to calculate the median incidence of hospitalizations attributed to rotavirus disease and the global impact of rotavirus vaccine on rotavirus hospitalizations. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (14), we searched article titles and abstracts using the search terms (‘Rotavirus’ OR ‘Diarrhea’) AND (‘burden’ OR ‘hospitali*’ OR ‘incidence’ OR ‘epidemiology’ OR ‘surveillance’) AND (‘child*’). We did not restrict the search by language; texts not published in English were sent to fluent speakers with a brief description of the systematic review and desired data. Additional citations were obtained from references in articles selected for full text review.
Inclusion/Exclusion Criteria
We included all primary peer-reviewed studies that contained an annual rotavirus hospitalization rate prior to rotavirus vaccine introduction for children <5 years. Additionally, to be included articles must have contained at least one continuous year of data collection and collected hospitalization data after 2000. To ensure we did not include studies that may have systematically under or overestimated the rotavirus burden we abstracted rates exclusively from studies that used active surveillance to identify laboratory-confirmed rotavirus hospitalizations. We excluded studies that included nosocomial infections, focused on special populations (e.g. among children at military bases), studied non-human rotavirus, or used hospital discharge codes to calculate rates. Studies occurring in non-WHO member countries were excluded (2,504,280 children of the 2020 global population of children <5 (0.4%)) due to a lack of data on vaccine coverage, childhood mortality rates, and <5 populations.
Data Abstraction
We abstracted information from articles using a standardized collection form in Microsoft Excel. Variables included country, pre-vaccine surveillance dates, age of population, pre-vaccine hospitalization rate and 95% confidence intervals, study population, and method of data collection. Articles were independently reviewed by BDH and TC for inclusion and abstraction of data, with discrepancies resolved by consensus discussion with JET and UDP. Our outcome of interest was the reported mean hospitalization rate among pre-vaccine years for children <5 years of age. If multiple years of pre-vaccine data were included without an aggregated mean, a mean pre-vaccine rotavirus hospitalization rate was calculated using annual estimates.
Study Parameters
We obtained population and childhood mortality estimates from the most recent United Nations World Populations Prospects report (15). Due to the absence of annual population data, the 2020 population was used as a proxy of 2019 population data, which estimated a total 675,366,379 children <5 years of age in 2020. Rotavirus has demonstrated variable incidence and mortality among high- and low-income groups (8), and VE performance across mortality strata (16). We therefore stratified our analyses by childhood mortality groups and WHO region. All countries with childhood mortality rates in the lowest quartile were considered low mortality, those in the second lowest quartile were considered medium mortality, and countries in the third and fourth quartiles were considered high mortality in our analysis, aligning with previous methodology (16). We obtained 2018 vaccine coverage estimates from the 2019 WHO/UNICEF Estimates of National Immunization Coverage (WUENIC) report (17). This report provides vaccine coverage estimates for all 194 WHO member countries for vaccines included in their national immunization plan, including rotavirus and diphtheria, tetanus, and pertussis (DTP) vaccines. DTP vaccine is given in a three-dose series on the same schedule as rotavirus vaccines. Despite similar schedules, DTP has demonstrated higher coverage than rotavirus vaccines (18), and therefore was used as a potential benchmark of reasonably attainable rotavirus vaccine coverage in our analyses. At the beginning of 2019 the two-dose Rotarix vaccine was the most used formulation globally, with exclusive use in 74 countries and concurrent use with RotaTeq in 9 additional countries (19).Thus, we used second dose DTP coverage (DTP2; calculated as the average between first and third DTP dose estimates) as our comparison for reasonably attainable rotavirus vaccine coverage across all countries. For countries in which DTP coverage was unknown, the median DTP2 coverage of the relevant mortality strata was applied.
Data Analysis
For rotavirus hospitalization rates we grouped pre-vaccine estimates from our literature review by mortality strata and calculated the median rotavirus hospitalization rate and interquartile range (IQR) for each mortality strata. We assigned the mortality stratum-specific median hospitalization rate to each country in that stratum. We then calculated the burden of rotavirus hospitalizations that would occur in the absence of rotavirus vaccine in 2019 using the equation
where the Hospitalization Rate was the calculated stratum-specific hospitalization rate for children <5 years prior to vaccine introduction. Next, we calculated the estimated number of prevented hospitalizations for 2019 given current vaccine introduction status. For this, we used the equation
in which Rotavirus Vaccine Coverage was the WUENIC estimate, and VE was the most recently calculated vaccine effectiveness estimate against laboratory-confirmed rotavirus, at 83%, 67%, and 58% in countries with low, medium, and high childhood mortality rates, respectively (16). We used VE estimates for Rotarix as it is the most widely used rotavirus vaccine globally. Using two dose DTP coverage estimates we calculated the potential impact if rotavirus vaccine if it was introduced in all WHO member countries and coverage matched DTP2 coverage, using the following equation
where Optimum Vaccine Coverage was the DTP2 estimate if rotavirus coverage was lower. For each of the three scenarios we calculated estimates by country, mortality strata, and WHO region.
Role of the funding source
There was no funding source for this study.
Results
Our search strategy identified 4,590 manuscripts, of which 390 were selected for full text review and 32 were included in the final dataset (20–51) (Figure 1). Among these we identified 39 rotavirus hospitalization rates: 18 (46%) estimates from low mortality countries, 9 (23%) estimates from medium mortality countries, and 12 (31%) estimates from high mortality countries. By WHO region, we identified 4 (10%) estimates from AFR, 4 (10%) from AMR, 3 (8%) from EMR, 16 (41%) from EUR, 4 (10%) from SEAR, and 8 (21%) from WPR. As of December 2019, 35 (74%) AFR, 20 (57%) AMR, 14 (67%) EMR, 20 (53%) EUR, 1 (9%) SEAR, and 8 (30%) WPR WHO-member countries had introduced rotavirus vaccine (Figure 2). A summary of included publications is included in Table 1.
Figure 1.
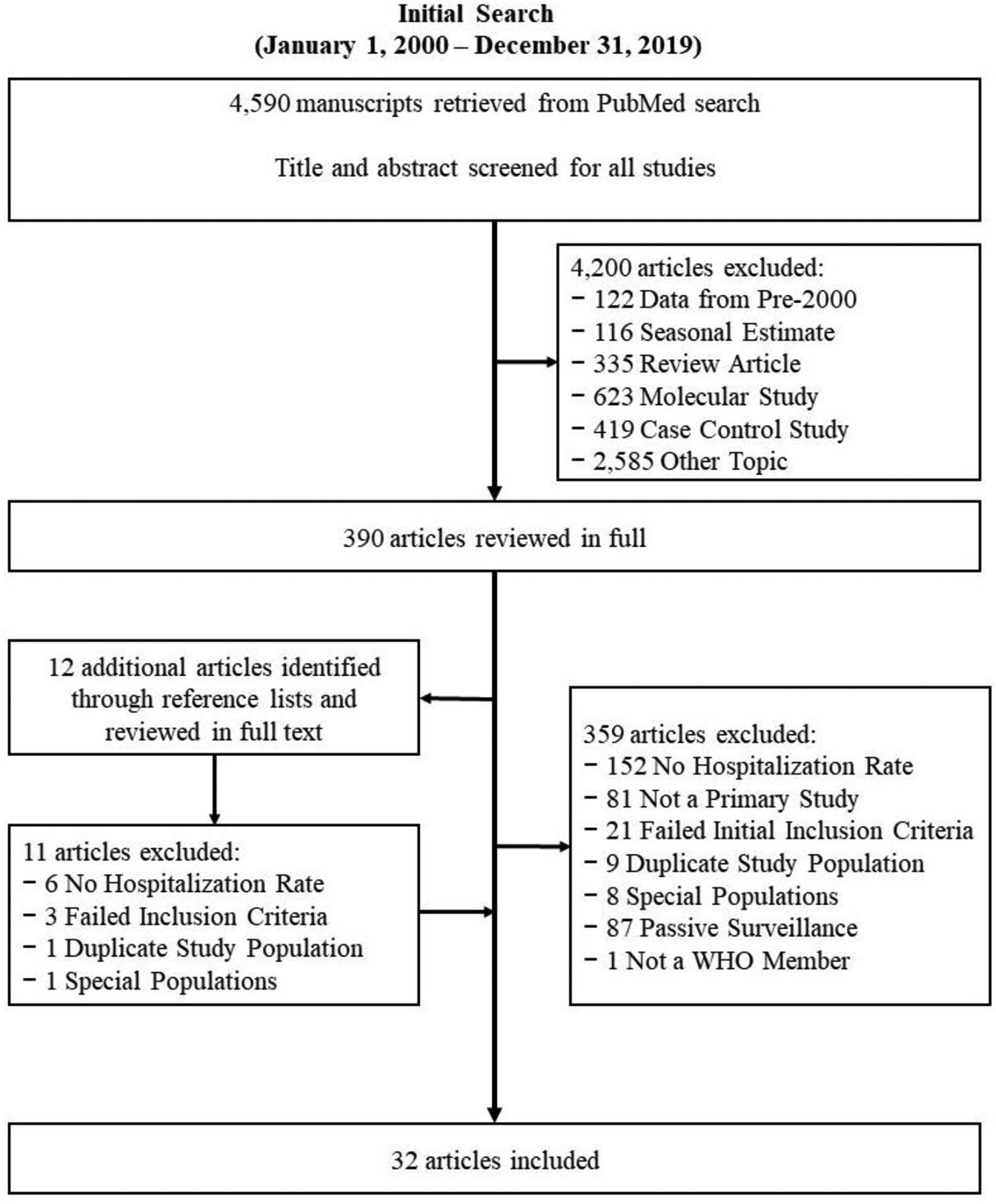
Prisma Article Inclusion Diagram
Figure 2.
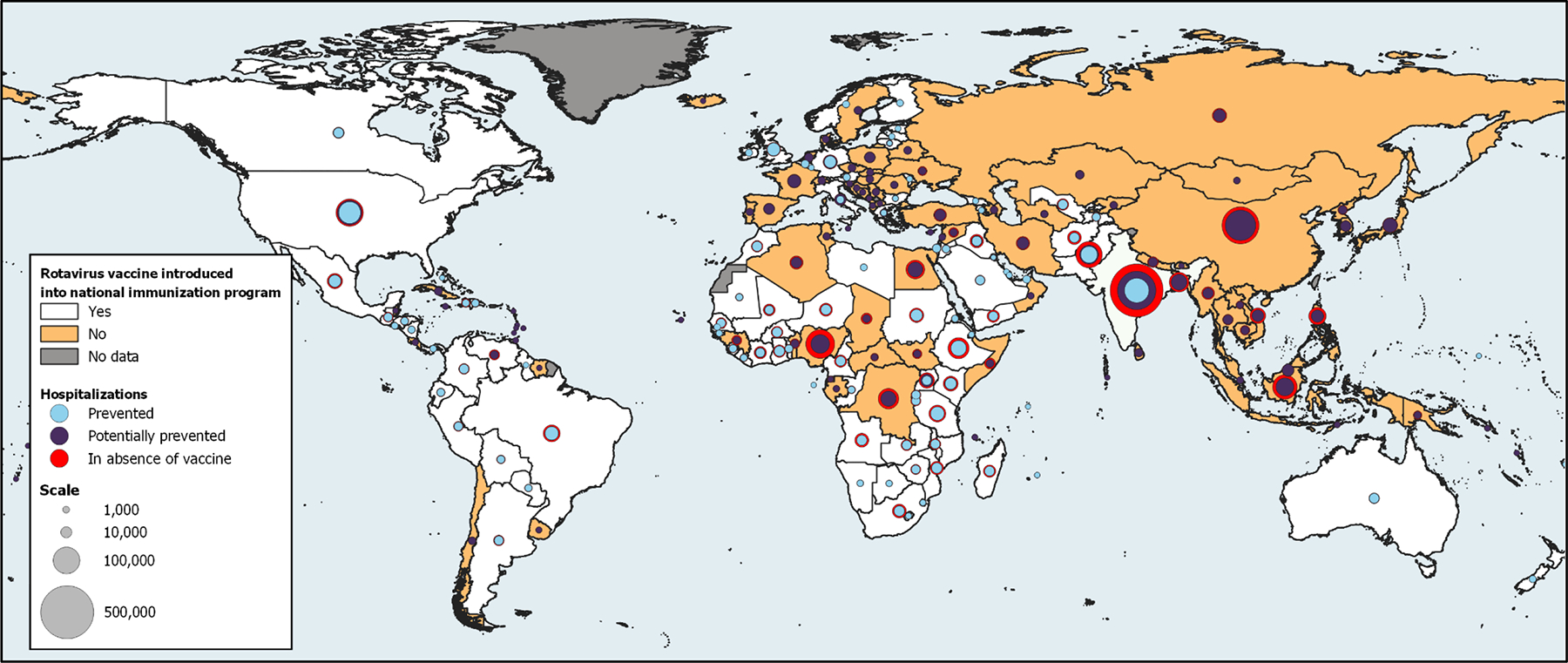
The projected number of rotavirus hospitalizations in the absence of vaccination (red circle), the estimated number of rotavirus hospitalizations prevented by vaccine introduction (blue circle), and the potential additional hospitalizations that could be prevented by increased vaccine introduction and higher vaccine coverage (purple circle), by WHO member countries in 2019.
Table 1.
Summary characteristics of included studies from systematic review of rotavirus hospitalization rates published from 1 January 2000 – 31 December 2019
| Authors | Country | Surveillance Start Date | Surveillance End Date | Mean Annual Incidence per 10,000 children <5 | Mortality Strata | WHO Region | Study Catchment Area |
|---|---|---|---|---|---|---|---|
| Asada, K, et al. (20) | Japan | 01/11/2007 | 31/10/2011 | 42 | Low | WPRO | 2 hospitals located in Tsue City, Mie Prefecture |
| Bruijning-Verhagen, P, et al. (23) | Netherlands | 01/12/2005 | 30/11/2010 | 51 | Low | EURO | 4 hospitals throughout the Netherlands |
| Hung, LC, et al. (28) | Malaysia | 01/02/2001 | 30/04/2003 | 27 | Low | WPRO | 2 government hospitals, located in Kuala Lumpur and Kuchinh |
| Kim, JS, et al. (31) | Republic of Korea | 01/07/2002 | 30/06/2004 | 116 | Low | WPRO | 3 hospitals in Jeongeub city |
| McAuliffe, GN, et al. (34) | New Zealand | 01/01/2009 | 31/12/2013 | 25.8 | Low | WPRO | 3 hospitals in Auckland region |
| Muhsen, K, et al. (35) | Israel | 01/01/2008 | 31/12/2010 | 56 | Low | EURO | 3 hospitals in northern Israel |
| Muhsen, K, et al. (36) | Israel | 01/01/2008 | 31/12/2010 | 55 | Low | EURO | 3 hospitals in northern Israel |
| Panatto, D, et al. (40) | Italy | 01/01/2006 | 31/12/2006 | 55 | Low | EURO | 1 children’s hospital in Genoa |
| Rendi-Wagner, P, et al. (41) | Austria | 01/01/1997 | 31/12/2003 | 76.6 | Low | EURO | 10 hospitals throughout Austria |
| Rinder, M, et al. (42) | Sweden | 15/10/2007 | 14/10/2008 | 38.8 | Low | EURO | 4 hospitals throughout Sweden |
| Trimis, G, et al. (46) | Greece | 01/09/2006 | 31/08/2007 | 16.33 | Low | EURO | 1 children’s hospital in Attica prefecture |
| Van Damme, P, et al. (47) | Belgium | 01/10/2004 | 30/09/2005 | 99 | Low | EURO | ≥1 hospital in study area, participating in REVEAL study |
| Van Damme, P, et al. (47) | France | 01/10/2004 | 30/09/2005 | 87 | Low | EURO | ≥1 hospital in study area, participating in REVEAL study |
| Van Damme, P, et al. (47) | Germany | 01/10/2004 | 30/09/2005 | 50 | Low | EURO | ≥1 hospital in study area, participating in REVEAL study |
| Van Damme, P, et al. (47) | Italy | 01/10/2004 | 30/09/2005 | 52 | Low | EURO | ≥1 hospital in study area, participating in REVEAL study |
| Van Damme, P, et al. (47) | Spain | 01/10/2004 | 30/09/2005 | 65 | Low | EURO | ≥1 hospital in study area, participating in REVEAL study |
| Van Damme, P, et al. (47) | Sweden | 01/10/2004 | 30/09/2005 | 77 | Low | EURO | ≥1 hospital in study area, participating in REVEAL study |
| Van Damme, P, et al. (47) | United Kingdom | 01/10/2004 | 30/09/2005 | 29 | Low | EURO | >1 hospital in study area, participating in REVEAL study |
| Caceres, DC, et al. (24) | Colombia | 01/12/2003 | 30/11/2004 | 160 | Medium | AMRO | 3 children’s referral hospitals in Barranquilla, Cali, and Bogota |
| Hacimustafaoglu, M, et al. (27) | Turkey | 01/01/2007 | 31/12/2007 | 29.3 | Medium | EURO | 4 hospitals throughout Bursa region |
| Jit, M, et al. (29) | Armenia | 01/07/2009 | 30/06/2010 | 65 | Medium | EURO | 2 hospitals in Yerevan |
| Lou, JT, et al. (32) | China | 01/01/2007 | 31/12/2008 | 21 | Medium | WPRO | 1 hospital in Hangzhou district |
| Soltani, M, et al. (44) | Tunisia | 01/04/2009 | 31/03/2011 | 1.1 | Medium | EMRO | 11 hospitals throughout Tunisia |
| Tharmaphornpilas, P, et al. (45) | Thailand | 01/09/2012 | 31/10/2014 | 220 | Medium | SEARO | 12 public hospitals located in Sukhothai and Phetchabun provinces |
| Yen, C, et al. (49) | El Salvador | 01/01/2006 | 31/12/2006 | 22.5 | Medium | AMRO | 7 hospitals throughout El Salvador |
| Zhang, J, et al. (51) | China | 01/07/2012 | 30/06/2013 | 4.4 | Medium | WPRO | 6 medical institutions throughout Beijing municipality and Gansu Province |
| Zhang, J, et al. (51) | China | 01/07/2012 | 30/06/2013 | 23.8 | Medium | WPRO | 6 medical institutions throughout Beijing municipality and Gansu Province |
| Bahl, R, et al. (21) | India | 01/08/2000 | 31/07/2001 | 33.7 | High | SEARO | 6 hospitals located in south New Delhi |
| Benhafid, M, et al. (22) | Morocco | 01/06/2006 | 31/05/2010 | 62.5 | High | EMRO | 4 children’s hospitals throughout Morocco |
| Carlos, CC, et al. (25) | Philippines | 01/01/2005 | 31/12/2006 | 28.1 | High | WPRO | 4 hospitals in Muntilupa City |
| Cortes, J, et al. (26) | Guatemala | 01/10/2007 | 30/09/2009 | 36 | High | AMRO | 1 regional hospital in Cuilapa, Santa Rosa District |
| Khagayi, S, et al. (30) | Kenya | 01/01/2010 | 31/12/2011 | 50.1 | High | AFRO | 1 district hospital and 1 health center in Nyanza Province, western Kenya |
| Mapaseka, SL, et al. (33) | South Africa | 01/01/2003 | 31/12/2005 | 37.9 | High | AFRO | 2 hospitals, located in Gauteng Province and North West Province |
| Mustafa, A, et al. (37) | Sudan | 01/06/2009 | 31/05/2011 | 32.79 | High | EMRO | 8 hospitals throughout Sudan |
| Nokes, DJ, et al. (38) | Kenya | 01/01/2002 | 31/12/2004 | 47.8 | High | AFRO | 1 district hospital in Kilifi District, eastern Kenya |
| Omore, R, et al. (39) | Kenya | 01/01/2010 | 31/12/2013 | 27.456 | High | AFRO | 1 county referral hospital, Siaya county |
| Salinas, B, et al. (43) | Venezuela | 01/01/1998 | 31/12/2002 | 27.78 | High | AMRO | 1 hospital in Carabobo state |
| Wangchuk, S, et al. (48) | Bhutan | 01/01/2010 | 31/12/2012 | 24 | High | SEARO | 1 national referral hospital in Thimphu |
| Zaman, K, et al. (50) | Bangladesh | 01/01/2000 | 31/12/2006 | 164 | High | SEARO | 1 hospital and 1 treatment center in Matlab |
Using pre-vaccine rotavirus hospitalization rates stratified by mortality stratum, we estimate that the median rotavirus hospitalization rate was 53.5 (IQR: 38.8–76.6) per 10,000 children <5 years of age in low mortality countries, 23.8 (IQR: 21.0–65.9) in medium mortality countries, and 34.9 (IQR: 27.9–49.0) in high mortality countries (Table 2). Using these rates, we project that without any rotavirus vaccine introduction, a median of 2,284,985 (IQR: 1,838,632–3,740,207) children <5 years of age globally would have been hospitalized for rotavirus in 2019. The majority (68%) of these hospitalizations would have impacted children in high childhood mortality countries due to a larger underlying population (Figure 3). Accounting for current vaccine coverage, we estimate 524,871 (IQR: 415,987–814,835) or 23% of the globally projected hospitalizations were prevented in 2019, and a residual 1,760,113 (IQR: 1,422,645–2,5,372) or 77% of these hospitalizations occurred (Table 3). Additionally, we estimate that with universal introduction of rotavirus vaccines globally and with vaccine coverage levels equivalent to DTP, an additional 751,609 (IQR:607,671–1,318,807) or 42% of the currently remaining burden could be prevented (Table 3).
Table 2.
The projected number of rotavirus hospitalizations that would have occurred among children <5 years of age in 2019 in the absence of any use of rotavirus vaccine, by mortality stratum and overall.
| Mortality Strata | Median RV hospitalization rate per 10,000 children <5 (IQR) | # of estimates | <5 population in 2019* | Projected rotavirus hospitalizations in the absence of a rotavirus vaccine |
|---|---|---|---|---|
| Low | 53.5 (38.8–76.6) | 18 | 61,117,438 | 326,978 (237,136–468,160) |
| Medium | 23.8 (21.0–65.0) | 9 | 165,295,305 | 393,403 (347,120–1,074,419) |
| High | 34.9 (27.9–49.0) | 12 | 448,953,639 | 1,564,603 (1,254,376–2,197,628) |
| Total | 39 | 675,366,379 | 2,284,985 (1,838,632–3,740,207) |
Expected rotavirus hospitalizations= median RV (global or regional) * <5 population.
Due to the absence of annual <5 population estimates, data from 2020 was used in place of 2019 population data.
Figure 3.
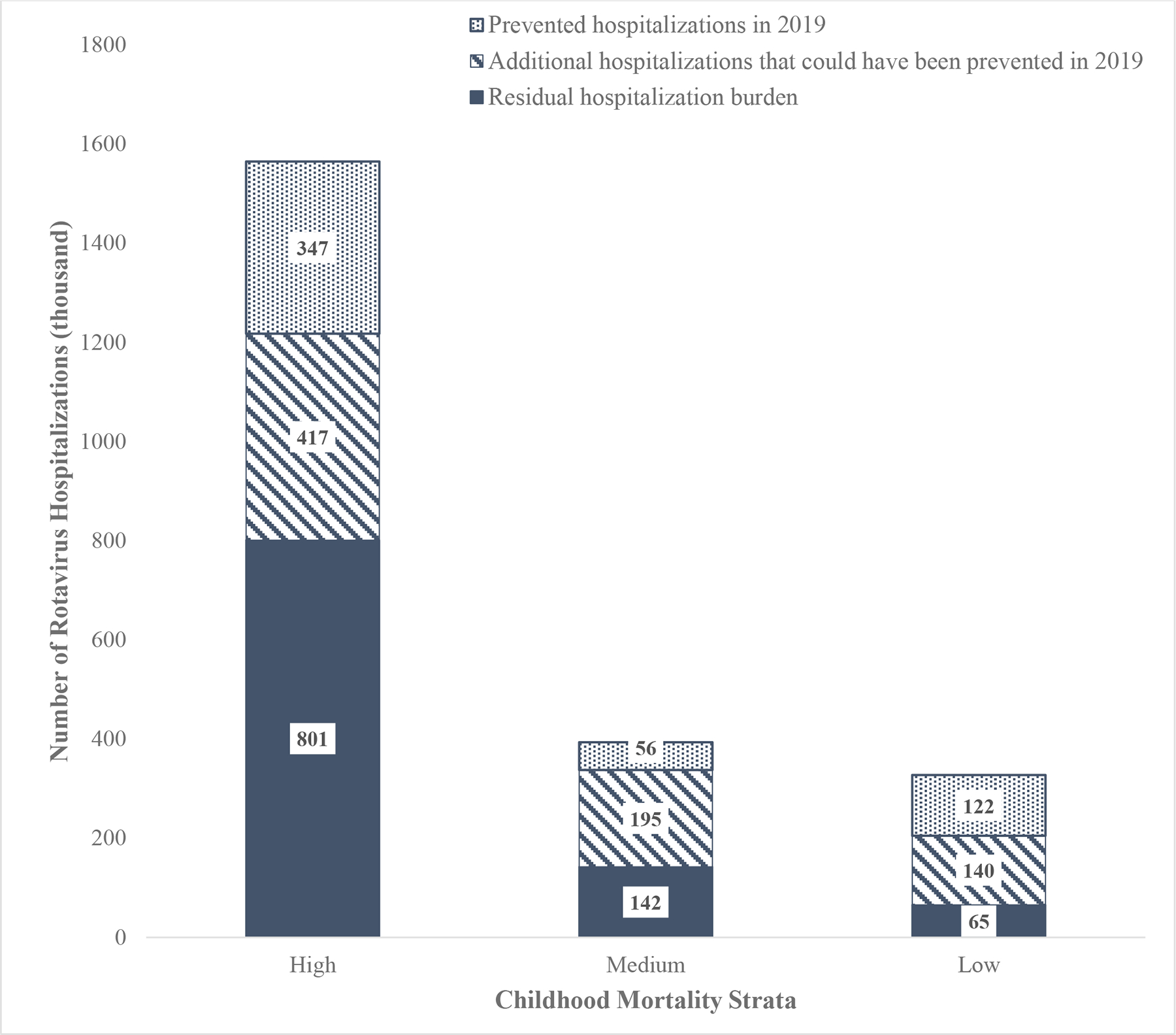
The estimated number of rotavirus hospitalizations prevented by rotavirus vaccine, the estimated number of additional hospitalizations that could be prevented through improved vaccine coverage and global introduction, and the number of residual rotavirus hospitalizations that would remain due to vaccine coverage and vaccine effectiveness limitations, by mortality strata in 2019.
Table 3.
The estimated number of rotavirus hospitalizations that occurred in 2019, the estimated number of hospitalizations that were prevented in 2019 by use of rotavirus vaccine, and the projected number of hospitalizations that can be additionally prevented with universal rotavirus vaccine introduction and vaccine coverage rates similar to DTP2, by mortality strata and WHO region.
| Estimated Number of Rotavirus Hospitalizations in 2019 (IQR; %) | Estimated number of rotavirus hospitalizations prevented in 2019 (IQR; %)* | Projected number of additional rotavirus hospitalizations that could be prevented with global introduction and higher vaccination rates (IQR; %)** | |
|---|---|---|---|
| Global Total | 1,760,113 (1,422,645–2,925,372; 100.0) | 524,871 (415,987–814,835; 100.0) | 751,609 (607,671–1,318,807; 100.0) |
| Mortality Strata | |||
| Low | 205,013 (148,682–293,532; 11.6) | 121,966 (88,454–174,627; 23.2) | 139,527 (101,190–199,771; 18.6) |
| Medium | 337,398 (297,704–921,466; 19.2) | 56,005 (49,416–152,954; 10.7) | 195,485 (172,487–533,888; 26.0) |
| High | 1,217,702 (976,258–1,710,374; 69.2) | 346,901 (278,118–487,254; 66.1) | 416,597 (333,995–585,148; 55.4) |
| WHO Region | |||
| AFR | 433,194 (347,306–608,542; 24.6) | 169,066 (135,553–237,614; 32.2) | 110,937 (88,941–155,824; 14.8) |
| AMR | 118,279 (94,883–229,030; 6.7) | 133,875 (105,860–254,826; 25.5) | 35,152 (26,969–59,512; 4.7) |
| EMR | 203,793 (165,516–321,911; 11.6) | 80,526 (64,872–121,071; 15.3) | 61,724 (50,743–107,390; 8.2) |
| EUR | 169,754 (132,433–309,540; 9.6) | 50,894 (37,721–73,989; 9.7) | 110,610 (85,635–199,587; 14.7) |
| SEAR | 501,690 (403,231–721,376; 28.5) | 82,687 (66,292–116,141; 15.8) | 224,645 (180,772–326,541; 29.9) |
| WPR | 333,403 (279,276–734,972; 18.9) | 7,822 (5,690–11,194; 1.5) | 208,541 (174,611–469,952; 27.7) |
Estimated number of hospitalizations prevented in 2019 = rotavirus vaccine VE * Expected number of hospitalizations (regional or global estimate without rotavirus vaccine) * 2018 rotavirus vaccine coverage. (limited to countries with vaccine introduction)
Estimated number of hospitalizations with global introduction and higher vaccination rates = rotavirus vaccine VE * expected number of hospitalizations (regional or global estimate without rotavirus vaccine) * 2018 DTP2 coverage – hospitalizations currently prevented. (assuming rotavirus vaccination coverage equal to that achieved by DTP2. If DTP2 coverage unknown, median of relevant mortality strata applied)
With global rotavirus vaccine introduction and coverage equal to DTP, we estimate that 1,276,476 or 56% of the total pre-vaccine projected rotavirus hospitalizations are preventable. Current rotavirus vaccination coverage and introductions addresses less than half (41 %) of the preventable burden. With current vaccine introductions and coverage levels, low mortality countries are currently preventing 37% the projected rotavirus hospitalizations that would have occurred in the absence of rotavirus vaccine, compared to 14% in medium mortality countries, and 22% in high mortality countries. With improved vaccine coverage and universal introductions globally, 80%, 64%, and 49% of the projected burden could be prevented in low, medium, and high mortality countries, respectively (Table 2 and 3).
In 2019, SEAR (501,690 (IQR:403,231–721,376)) and AFR (433,194 (IQR: 347,306–608,542)) had the highest rotavirus hospitalization burden, representing a respective 29% and 25% of the residual burden (Table 3; Figure 4). Due to the baseline burden, the number of countries who have introduced rotavirus vaccine, and their current vaccine coverage, AFR (169,066 (IQR: 135,553–237,614)) and AMR (133,875 (IQR: 105,860–254,826)) are currently preventing the highest number of rotavirus hospitalizations, contributing 32% and 26% of global prevented hospitalizations, respectively. With universal introduction and improved vaccine coverage, SEAR (224,645 (IQR: 180,772–326,541)) and WPR (208,541 (IQR:174,611–469,952)) would see the greatest additional reduction in residual rotavirus hospitalizations, accounting for 58% of the additional 751,609 hospitalizations that could be prevented globally. The number of rotavirus hospitalizations in the absence of rotavirus vaccine in 2019, those currently prevented, and those potentially prevented with increased introductions and coverage are presented in Figure 2 by country.
Figure 4.
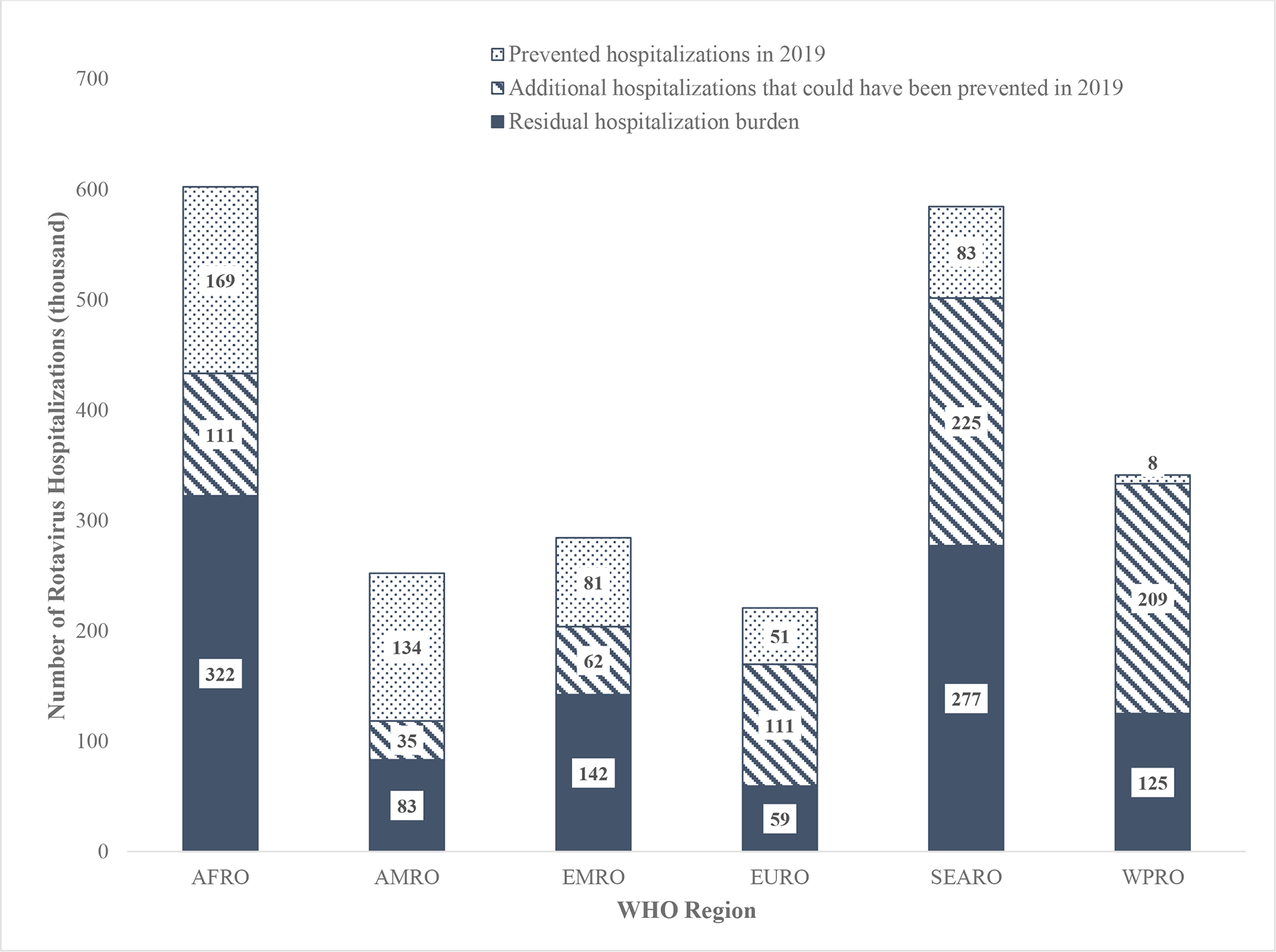
The estimated number of rotavirus hospitalizations prevented by rotavirus vaccine, the estimated number of additional hospitalizations that could be prevented through improved vaccine coverage and global introduction, and the number of residual rotavirus hospitalizations that would remain due to vaccine coverage and vaccine effectiveness limitations, by mortality strata in 2019.
Discussion
This analysis demonstrates the significant burden of rotavirus disease globally in children <5 years of age, with an estimated 1.76 million hospitalizations attributed to rotavirus in 2019. We identified the highest childhood rotavirus hospitalization rates were among countries in the low child mortality stratum, which may reflect differences in access to care or healthcare-seeking behaviors between groups. We estimate that current rotavirus vaccine introductions and coverage levels prevented >500,000 hospitalizations in 2019, with nearly 60% of the prevented hospitalizations coming from American and African regions where rotavirus vaccines are widely used. With universal rotavirus vaccine introduction and improved vaccine coverage, an additional 750,000 rotavirus hospitalizations could have been prevented in 2019, with Asian regions accounting for nearly 58% of the additional prevented hospitalizations.
Our estimate of 2,284,985 hospitalizations that would occur in the absence of rotavirus vaccine is relatively close to the prevalence estimated by Parashar et al. (7) in 2003 of 2,143,000 prior to rotavirus vaccine licensure. According to United Nations World Populations Prospects report (15) the <5 population grew by approximately 10% between these two estimates. Still, the methods used in this early estimation are not completely dissimilar to ours, as they utilized published hospitalization rates to calculate the hospitalization burden among developed countries (albeit estimates for developing countries were achieved using proportion attributable fractions). Furthermore, the estimate by Troeger et al. (8) of 1,537,000 in 2016 accounting for the impact of rotavirus vaccine is also similar to the residual 1,760,113 rotavirus hospitalizations we estimate would occur in 2019 with current vaccine coverage despite disparate study designs. Specifically, their estimate was developed top-down using a global all-cause diarrheal estimate, then multiplying this estimate by the attributable fraction of severe diarrhea due to rotavirus. Our methodology represents a bottom-up approach in which country-level estimates generated through sentinel surveillance platforms were pooled by region then combined to produce our global estimates. The similar range of estimates across all three studies despite different methodologies is reassuring and provides confidence in our burden estimates.
While estimated diarrhea mortality has declined substantially from nearly 1.9 million in 2000 (52) to 0.5 million in 2015 (53), severe morbidity from rotavirus diarrhea does not appear to have substantially declined over this period. This finding is consistent with studies that showed that while diarrhea mortality declined substantially between 1980–2000, morbidity from diarrheal illness in young children did not change appreciably (54). It is possible that measures to improve treatment of diarrheal illness such as ORS might disproportionately affect mortality from diarrhea compared with severe morbidity. While our current analysis describes the impact of vaccination on hospitalizations, it should be noted that various levels of severity may result in hospitalization depending on local context (e.g. affordability, access to care, transportation, etc.). Collectively, these findings indicate that vaccination may play a role in prevention of hospitalization due to rotavirus diarrhea.
To date, rotavirus vaccines have made a substantial impact on childhood morbidity and mortality from rotavirus diarrhea in countries where they have been introduced. A recent review of published manuscripts using data from 49 countries estimated that these vaccines have resulted in a global median 59% relative reduction in rotavirus hospitalizations, 36% reduction in AGE hospitalizations, and 36% reduction in AGE mortality after introduction (5). Furthermore, findings from the Global Rotavirus Surveillance Network, which operates in over 80 countries, estimate that the proportion of children hospitalized with rotavirus dropped 40% among sentinel hospitals from 2008–2016 where rotavirus vaccine had been introduced (55). Our review quantifies the absolute impact of vaccines in reducing the burden of rotavirus diarrhea hospitalizations.
This study has several limitations. Availability of population-based rates can lead to unequal representation of some regions. For example, publications from AFR and SEAR countries each represent only 10% of the articles included in this review, despite the large disease burdens and large populations in these regions. This disparity is likely due to the difficulty of conducting active, population-based surveillance including capturing all hospitalizations as well as the availability of census data within catchment areas. We also generalized rates across mortality strata based on available data. Further studies that generate population-based rates could be used to verify the accuracy of these estimates. Additionally, we used Rotarix-specific vaccine effectiveness against laboratory-confirmed rotavirus to estimate prevented hospitalizations in our analysis. This generalization was made for currently licensed rotavirus vaccines which are all live, attenuated oral vaccines, whereas this may not be applicable for other types of upcoming (e.g. parenterally administered or subunit) vaccines that are currently in testing (19). Rotarix is also the only licensed 2-dose rotavirus vaccine; all other rotavirus vaccines require 3 doses for a child to be fully vaccinated. Lastly, the WUENIC estimates used in this analysis are based on national-level reports submitted by WHO member countries and any supplemental published or grey literature. As such, availability of estimates for each country may impact the accuracy of estimates compared to the true coverage. Still, WUENIC estimates represent the most thorough and uniform estimation for global vaccine coverage available. Additionally, these estimates only provide data for countries with full introductions of globally available vaccines into national immunization programs. Therefore, vaccine use in countries with nationally licensed vaccines such as Rotavin and Lanzhou Lamb Vaccine, as well as subnational or private market coverage were not captured in this analysis. Our analyses have included the number of rotavirus hospitalizations in these countries in absence of vaccine, but not the amount prevented by nationally licensed or private market vaccines.
This analysis highlights the large number of rotavirus hospitalizations among children <5 years of age currently prevented by rotavirus vaccines; however, a substantial burden of preventable rotavirus hospitalizations still exists. To continue to reduce the number of rotavirus hospitalizations occurring in children globally, efforts should be directed at facilitating new introductions of rotavirus vaccines, particularly in the Asian region which is lagging, and renewed efforts to increase vaccine coverage. Our data also indicate that even with universal vaccine use at high coverage levels, a substantial proportion of rotavirus hospitalization will remain unprevented by direct effects of vaccination, primarily because of the relatively modest efficacy of these vaccines in low-income countries. Indirect (herd) immunity may result in additional reductions in rotavirus burden (56) although these were not measured in the current study. Other efforts to address this challenge, including development of parenterally administered vaccines that can overcome the barriers to efficacy of the current orally administered vaccines in low-income countries, may further enhance the impact of rotavirus vaccination worldwide.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000–2013. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;62 Suppl 2:S96–s105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Rotavirus Vaccines. Weekly Epidemiological Record. 2007(82):285–95. [PubMed] [Google Scholar]
- 3.World Health Organization. Rotavirus vaccines: an update. Weekly Epidemiological Record= Relevé épidémiologique hebdomadaire. 2009;84(51–52):533–7. [PubMed] [Google Scholar]
- 4.Hallowell BD, Tate J, Parashar U. An overview of rotavirus vaccination programs in developing countries. Expert Rev Vaccines. 2020;19(6):529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnett E, Parashar UD, Tate JE. Global impact of rotavirus vaccination on diarrhea hospitalizations and deaths among children <5 years old: 2006–2019. J Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munos MK, Walker CL, Black RE. The effect of oral rehydration solution and recommended home fluids on diarrhoea mortality. Int J Epidemiol. 2010;39 Suppl 1:i75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerging infectious diseases. 2003;9(5):565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, et al. Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea Among Children Younger Than 5 Years. JAMA Pediatr. 2018;172(10):958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (London, England). 2017;390(10100):1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pindyck T, Tate JE, Parashar UD. A decade of experience with rotavirus vaccination in the United States - vaccine uptake, effectiveness, and impact. Expert Rev Vaccines. 2018;17(7):593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavers T, De Oliveira LH, Parashar UD, Tate JE. Post-licensure experience with rotavirus vaccination in Latin America and the Caribbean: a systematic review and meta-analysis. Expert Rev Vaccines. 2018;17(11):1037–51. [DOI] [PubMed] [Google Scholar]
- 12.Burnett E, Tate JE, Kirkwood CD, Nelson EAS, Santosham M, Steele AD, et al. Estimated impact of rotavirus vaccine on hospitalizations and deaths from rotavirus diarrhea among children <5 in Asia. Expert Rev Vaccines. 2018;17(5):453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah MP, Tate JE, Mwenda JM, Steele AD, Parashar UD. Estimated reductions in hospitalizations and deaths from childhood diarrhea following implementation of rotavirus vaccination in Africa. Expert Rev Vaccines. 2017;16(10):987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nations United. Department of Economic and Social Affairs PD. World Population Prospects: The 2019 Revision. Online Edition; 2019. [Google Scholar]
- 16.Burnett E, Parashar UD, Tate JE. Real-world effectiveness of rotavirus vaccines, 2006–19: a literature review and meta-analysis. Lancet Glob Health. 2020;8(9):e1195–e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. WHO-UNICEF estimates of rotavirus vaccine coverage 2019. [Available from: https://www.who.int/immunization/monitoring_surveillance/routine/coverage/en/index4.html. [Google Scholar]
- 18.Sederdahl BK, Yi J, Jerris R, Gillespie SE, Westblade L, Kraft CS, et al. The Residual Vaccine-preventable Burden of Rotavirus Disease. Pediatr Infect Dis J. 2017;36(8):780–1. [DOI] [PubMed] [Google Scholar]
- 19.Burke RM, Tate JE, Kirkwood CD, Steele AD, Parashar UD. Current and new rotavirus vaccines. Curr Opin Infect Dis. 2019;32(5):435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asada K, Kamiya H, Suga S, Nagao M, Ichimi R, Fujisawa T, et al. Rotavirus vaccine and health-care utilization for rotavirus gastroenteritis in Tsu City, Japan. Western Pac Surveill Response J. 2016;7(4):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bahl R, Ray P, Subodh S, Shambharkar P, Saxena M, Parashar U, et al. Incidence of severe rotavirus diarrhea in New Delhi, India, and G and P types of the infecting rotavirus strains. The Journal of infectious diseases. 2005;192 Suppl 1:S114–9. [DOI] [PubMed] [Google Scholar]
- 22.Benhafid M, Rguig A, Trivedi T, Elqazoui M, Teleb N, Mouane N, et al. Monitoring of rotavirus vaccination in Morocco: establishing the baseline burden of rotavirus disease. Vaccine. 2012;30(46):6515–20. [DOI] [PubMed] [Google Scholar]
- 23.Bruijning-Verhagen P, Sankatsing V, Kunst A, van den Born C, Bleeker E, Thijsen S, et al. Rotavirus-related hospitalizations are responsible for high seasonal peaks in all-cause pediatric hospitalizations. Pediatr Infect Dis J. 2012;31(12):e244–9. [DOI] [PubMed] [Google Scholar]
- 24.Caceres DC, Pelaez D, Sierra N, Estrada E, Sanchez L. [Burden of rotavirus-related disease among children under five, Colombia, 2004]. Rev Panam Salud Publica. 2006;20(1):9–21. [DOI] [PubMed] [Google Scholar]
- 25.Carlos CC, Inobaya MT, Bresee JS, Lagrada ML, Olorosa AM, Kirkwood CD, et al. The burden of hospitalizations and clinic visits for rotavirus disease in children aged <5 years in the Philippines. J Infect Dis. 2009;200 Suppl 1:S174–81. [DOI] [PubMed] [Google Scholar]
- 26.Cortes J, Arvelo W, Lopez B, Reyes L, Kerin T, Gautam R, et al. Rotavirus disease burden among children <5 years of age--Santa Rosa, Guatemala, 2007–2009. Trop Med Int Health. 2012;17(2):254–9. [DOI] [PubMed] [Google Scholar]
- 27.Hacimustafaoglu M, Celebi S, Agin M, Ozkaya G. Rotavirus epidemiology of children in Bursa, Turkey: a multi-centered hospital-based descriptive study. Turk J Pediatr. 2011;53(6):604–13. [PubMed] [Google Scholar]
- 28.Hung LC, Wong SL, Chan LG, Rosli R, Ng AN, Bresee JS. Epidemiology and strain characterization of rotavirus diarrhea in Malaysia. Int J Infect Dis. 2006;10(6):470–4. [DOI] [PubMed] [Google Scholar]
- 29.Jit M, Yuzbashyan R, Sahakyan G, Avagyan T, Mosina L. The cost-effectiveness of rotavirus vaccination in Armenia. Vaccine. 2011;29(48):9104–11. [DOI] [PubMed] [Google Scholar]
- 30.Khagayi S, Burton DC, Onkoba R, Ochieng B, Ismail A, Mutonga D, et al. High burden of rotavirus gastroenteritis in young children in rural western Kenya, 2010–2011. Pediatr Infect Dis J. 2014;33 Suppl 1:S34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JS, Kang JO, Cho SC, Jang YT, Min SA, Park TH, et al. Epidemiological profile of rotavirus infection in the Republic of Korea: results from prospective surveillance in the Jeongeub District, 1 July 2002 through 30 June 2004. J Infect Dis. 2005;192 Suppl 1:S49–56. [DOI] [PubMed] [Google Scholar]
- 32.Lou JT, Xu XJ, Wu YD, Tao R, Tong MQ. Epidemiology and burden of rotavirus infection among children in Hangzhou, China. J Clin Virol. 2011;50(1):84–7. [DOI] [PubMed] [Google Scholar]
- 33.Mapaseka SL, Dewar JB, van der Merwe L, Geyer A, Tumbo J, Zweygarth M, et al. Prospective hospital-based surveillance to estimate rotavirus disease burden in the Gauteng and North West Province of South Africa during 2003–2005. J Infect Dis. 2010;202 Suppl:S131–8. [DOI] [PubMed] [Google Scholar]
- 34.McAuliffe GN, Taylor SL, Drinkovic D, Roberts SA, Wilson EM, Best EJ. Rotavirus Infection in the Auckland Region After the Implementation of Universal Infant Rotavirus Vaccination: Impact on Hospitalizations and Laboratory Implications. The Pediatric infectious disease journal. 2018;37(1):e1–e5. [DOI] [PubMed] [Google Scholar]
- 35.Muhsen K, Kassem E, Rubenstein U, Goren S, Ephros M, Cohen D, et al. Incidence of rotavirus gastroenteritis hospitalizations and genotypes, before and five years after introducing universal immunization in Israel. Vaccine. 2016;34(48):5916–22. [DOI] [PubMed] [Google Scholar]
- 36.Muhsen K, Kassem E, Rubenstein U, Goren S, Ephros M, Shulman LM, et al. No evidence of an increase in the incidence of norovirus gastroenteritis hospitalizations in young children after the introduction of universal rotavirus immunization in Israel. Hum Vaccin Immunother. 2019;15(6):1284–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mustafa A, Makki A, Siddig O, Haithami S, Teleb N, Trivedi T, et al. Baseline burden of rotavirus disease in Sudan to monitor the impact of vaccination. Pediatr Infect Dis J. 2014;33 Suppl 1:S23–7. [DOI] [PubMed] [Google Scholar]
- 38.Nokes DJ, Abwao J, Pamba A, Peenze I, Dewar J, Maghenda JK, et al. Incidence and clinical characteristics of group A rotavirus infections among children admitted to hospital in Kilifi, Kenya. PLoS Med. 2008;5(7):e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Omore R, Khagayi S, Ogwel B, Onkoba R, Ochieng JB, Juma J, et al. Rates of hospitalization and death for all-cause and rotavirus acute gastroenteritis before rotavirus vaccine introduction in Kenya, 2010–2013. BMC Infect Dis. 2019;19(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panatto D, Amicizia D, Giacchino R, Tacchella A, Natalizia AR, Melioli G, et al. Burden of rotavirus infections in Liguria, Northern Italy: hospitalisations and potential savings by vaccination. Eur J Clin Microbiol Infect Dis. 2011;30(8):957–64. [DOI] [PubMed] [Google Scholar]
- 41.Rendi-Wagner P, Kundi M, Mikolasek A, Mutz I, Zwiauer K, Wiedermann U, et al. Active hospital-based surveillance of rotavirus diarrhea in Austrian children, period 1997 to 2003. Wien Klin Wochenschr. 2006;118(9–10):280–5. [DOI] [PubMed] [Google Scholar]
- 42.Rinder M, Tran AN, Bennet R, Brytting M, Cassel T, Eriksson M, et al. Burden of severe rotavirus disease leading to hospitalization assessed in a prospective cohort study in Sweden. Scand J Infect Dis. 2014;46(4):294–302. [DOI] [PubMed] [Google Scholar]
- 43.Salinas B, Gonzalez G, Gonzalez R, Escalona M, Materan M, Schael IP. Epidemiologic and clinical characteristics of rotavirus disease during five years of surveillance in Venezuela. Pediatr Infect Dis J. 2004;23(10 Suppl):S161–7. [DOI] [PubMed] [Google Scholar]
- 44.Soltani M, Bouanene I, Trabelsi A, Harbi A, Hachicha M, Amri F, et al. [Epidemiology of rotavirus gastroenteritis among children under 5 years of age in Tunisia - results of sentinel hospital surveillance 2009 to 2011]. Rev Epidemiol Sante Publique. 2012;60(6):473–80. [DOI] [PubMed] [Google Scholar]
- 45.Tharmaphornpilas P, Jiamsiri S, Boonchaiya S, Rochanathimoke O, Thinyounyong W, Tuntiwitayapun S, et al. Evaluating the first introduction of rotavirus vaccine in Thailand: Moving from evidence to policy. Vaccine. 2017;35(5):796–801. [DOI] [PubMed] [Google Scholar]
- 46.Trimis G, Koutsoumbari I, Kottaridi C, Palaiologou N, Assimakopoulou E, Spathis A, et al. Hospital-based surveillance of rotavirus gastroenteritis in the era of limited vaccine uptake through the private sector. Vaccine. 2011;29(43):7292–5. [DOI] [PubMed] [Google Scholar]
- 47.Van Damme P, Giaquinto C, Huet F, Gothefors L, Maxwell M, Van der Wielen M. Multicenter prospective study of the burden of rotavirus acute gastroenteritis in Europe, 2004–2005: the REVEAL study. The Journal of infectious diseases. 2007;195 Suppl 1:S4–s16. [DOI] [PubMed] [Google Scholar]
- 48.Wangchuk S, Dorji T, Tsheten, Tshering K, Zangmo S, Pem Tshering K, et al. A Prospective Hospital-based Surveillance to Estimate Rotavirus Disease Burden in Bhutanese Children under 5 Years of Age. Trop Med Health. 2015;43(1):63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yen C, Armero Guardado JA, Alberto P, Rodriguez Araujo DS, Mena C, Cuellar E, et al. Decline in rotavirus hospitalizations and health care visits for childhood diarrhea following rotavirus vaccination in El Salvador. Pediatr Infect Dis J. 2011;30(1 Suppl):S6–S10. [DOI] [PubMed] [Google Scholar]
- 50.Zaman K, Yunus M, Faruque AS, El Arifeen S, Hossain I, Azim T, et al. Surveillance of rotavirus in a rural diarrhoea treatment centre in Bangladesh, 2000–2006. Vaccine. 2009;27 Suppl 5:F31–4. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J, Liu H, Jia L, Payne DC, Hall AJ, Xu Z, et al. Active, population-based surveillance for rotavirus gastroenteritis in Chinese children: Beijing Municipality and Gansu Province, China. Pediatr Infect Dis J. 2015;34(1):40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bryce J, Boschi-Pinto C, Shibuya K, Black RE, Group WHOCHER. WHO estimates of the causes of death in children. Lancet (London, England). 2005;365(9465):1147–52. [DOI] [PubMed] [Google Scholar]
- 53.Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet (London, England). 2016;388(10063):3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boschi-Pinto C, Lanata CF, Mendoza W, Habte D. Diarrheal Diseases. In: Jamison DT, Feachem RG, Makgoba MW, Bos ER, Baingana FK, Hofman KJ, et al. , editors. Disease and Mortality in Sub-Saharan Africa. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; [PubMed] [Google Scholar]; Copyright © 2006, The International Bank for Reconstruction and Development/The World Bank.; 2006.
- 55.Aliabadi N, Antoni S, Mwenda JM, Weldegebriel G, Biey JNM, Cheikh D, et al. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008–16: findings from the Global Rotavirus Surveillance Network. Lancet Glob Health. 2019;7(7):e893–e903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pollard SL, Malpica-Llanos T, Friberg IK, Fischer-Walker C, Ashraf S, Walker N. Estimating the herd immunity effect of rotavirus vaccine. Vaccine. 2015;33(32):3795–800. [DOI] [PubMed] [Google Scholar]


