Abstract
Introduction
Bisphenol A (BPA) is a common contaminant widely used in many industrial sectors. Because of its wide use and dispersion, it can be accumulated in living human bodies through both oral assumption and nondietary routes. BPA exhibits hormone-like properties, falling under the class of endocrine disruptors; therefore, it can alter relevant physiological functions. In particular, in women, it can affect folliculogenesis and therefore reproduction, contributing not only to infertility, but also to endometriosis and premature puberty.
Methods
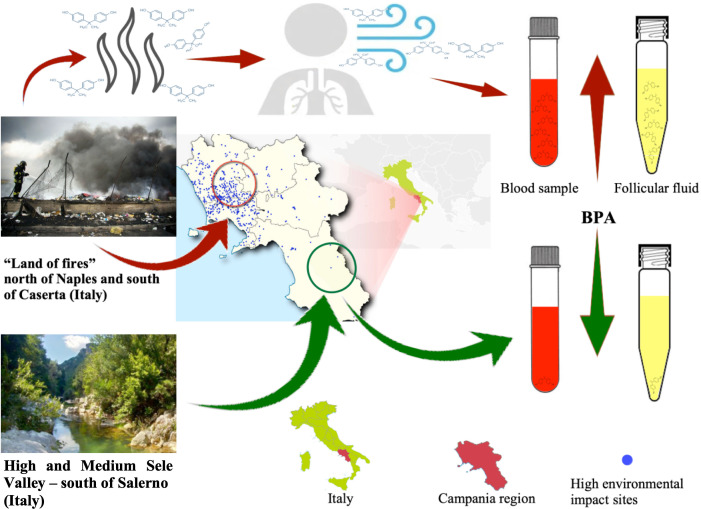
We conducted a multicenter study on 91 women undergoing a first in vitro fertilization (IVF) treatment in the Campania region (Southern Italy). We investigated the presence and concentration of BPA in serum and follicular fluids to assess the effects of airborne BPA contamination. The analysis was conducted on 32 women living in a low environmental impact (LEI) area, from the Sele Valley River and Cilento region, and 59 women living in a high environmental impact (HEI) area, the so-called “Land of Fires”, a highly contaminated territory widely exposed to illegal waste practices.
Results
A higher average BPA content in both blood serum and follicular fluid was revealed in the HEI group when compared with the LEI group. In addition, we revealed higher average BPA content in blood serum than in folliclular fluid in the HEI area, with opposite average content in the two fluids in the LEI zone. In addition, our results also showed a lack of correlation between BPA content in follicular and serum fluids both in the overall population and in the HEI and LEI groups, with peculiar trends in different subsets of women.
Conclusion
From our results, we revealed a heterogeneity in the distribution of BPA content between serum and follicular fluid. Further studies are needed to unravel the bioaccumulation mechanisms of BPA in highly polluted and nonpolluted areas.
Keywords: BPA, airborne bisphenol, pollution, follicular fluid, land of fires, environmental health, ART
Graphical Abstract
Highlights
Higher BPA levels are found in the blood and follicular fluid of women living in a polluted area
Airborne bisphenol can strongly impact BPA accumulation in bodily fluids
BPA bioaccumulation in blood serum and follicular fluid shows no correlation
Introduction
Pollutants consist of various chemical substances and metabolites that may affect human health at different extents (1). Plastic derivatives in the environment are assumed to be increasing at an annual rate of 5% (2, 3). The development of recycling techniques for polymeric materials in recent years has not only contributed to an increase in their disposal, but also caused a considerable environmental impact involving the atmosphere (4, 5). Large quantities of plastics are disposed daily in landfills or burnt accidentally or intentionally, resulting in the release of volatile by-products into the atmosphere (2, 6, 7). Specifically, there is increasing attention on the levels of plastic derivatives in the air we breathe, probably due to the increasing knowledge on their possible impact on human health.
Bisphenol A (BPA), with a chemical formula of 4,4-dihydroxy-2,2-diphenylpropane, is a widely used substance, found as a raw material in the production of polycarbonate plastics and epoxy resins, key elements in varied industrial productions, such as food and beverage packaging, thermal paper (8), dentistry, and water pipes (9). Human beings can be exposed to BPA by food assumption and by non-dietary routes (10). Several authors speculate that oral exposure by food ingestion is the main source of BPA in humans in all age groups when not professionally exposed to this substance (11). The United States Environmental Protection Agency (EPA) has established a dose for humans (RfD) of 50 mg BPA/kg BW/day, based on toxicological studies (USEPA = United States Environmental Protection Agency, 2010), and the European Community has also adopted a similar metric. It is important to emphasize that the tolerable daily intake (TDI) usually considered assumes that oral intake is the main primary route of exposure. Dermal exposure and inhalation are not included, so the current TDI seems to be inadequate to suitably measure the real impact of BPA on human health. The environmental BPA pollution is therefore not adequately taken into consideration, although BPA concentrations in the environment have been extensively studied in aquatic and soil environments (12, 13), whereas studies on its presence in the air are still limited in number (14–16). Interestingly, recent studies highlight that BPA accumulated in the atmosphere can reach high concentration and can affect human health (17). Indeed, it is known that it is persistent in the environment due to continuous emissions (18) that allow its migration also in food (19), in the air (20, 21), in the skin (22), and in the blood (21). Measures of BPA bioaccumulation in the human body indicates that BPA is ubiquitous, and that it can be found in serum, amniotic fluid, urine, umbilical cord, blood, and placental tissues (23). Animal studies have shown that BPA exposure negatively affects the reproductive system (24). It is interesting to note that BPA was initially developed as a synthetic estrogen, but it was later proven to act as an endocrine disruptor. Indeed, it alters the endocrine system through binding to physiological receptors, such as estrogen receptors (ER) (ER1 and ER2), membrane-bound ERs, androgen receptors, peroxisome proliferator-activated receptor gamma, and thyroid hormone receptors (25). However, the genes activated by BPA differ from those triggered by estradiol (26).
Concerns about the effects of BPA exposure on reproductive health have led to numerous in vitro, animal, and human studies. BPA affects gametes by interfering with meiosis, folliculogenesis, and steroidogenesis. It also affects endometrial proliferation and endometrial receptivity (27, 28). The effects of BPA on human oocytes were also demonstrated (29). A significant increase in meiotic abnormalities due to casual exposure to BPA is the main indicator of adverse effects on oocyte development (30). Massive concentrations of BPA cause alterations in murine oocyte development, presumably because of oxidative stress, inducing increased meiotic arrest in germinal vesicles (GVs) or in metaphase I stages (31, 32).
Despite these lines of evidence, very little is known about the role of BPA as a reproductive toxicant in humans. One epidemiological study failed to prove an effect on human fertility (33); however, after controlled ovarian stimulation (COS), the level of urinary BPA appears to be inversely correlated with the number of oocytes retrieved. Indeed, as urinary BPA increases, the number of recovered oocytes decreases, on average by 12%, and the ovulatory peak of serum estradiol is reduced (34). In addition, urinary BPA appears inversely correlated with the number of mature oocytes after COS (35). Antral follicle count is a marker of ovarian reserve, a good predictor of ovarian response to COS, and it reduces significantly with increased urinary BPA (36). With regard to oocyte maturation (37), a negative dose–response correlation was observed between the rate of maturation of human oocytes in vitro, the percentage of bipolar spindles in the embryos, and the concentration of BPA added to the culture medium (38). Finally, a higher probability of embryo implantation failure was found in the presence of higher urinary BPA concentrations (39). However, it is not known whether these failures were of embryonic and/or endometrial origin.
Plasmatic components influence the production of follicular fluid (FF), which, in turn, is crucial for the development and vitality of oocytes (40, 41). Studies have shown that medical devices containing plastic can be a source of exposure to BPA in intensive care units (21, 42, 43). It is essential to assess chemical contaminants in assisted reproductive techniques to avoid exposure of gametes and embryos, e.g., BPA present in the needle tube for oocyte aspiration, in the FF collection tube, in Petri dishes for oocyte identification, and in the kit (syringe and catheter) for embryo or blastocyst transfer.
A study was conducted on 117 women with an average age of 34 years who underwent an assisted reproductive technology (ART) treatment. Blood serum showed increased levels of BPA correlating with an increased FF during oocyte pick-up (44). A study on 146 couples undergoing in vitro fertilization (IVF) found that BPA concentrations in various bodily fluids did not significantly impact reproductive parameters (45). In a cross-sectional study of 90 women undergoing IVF, higher BPA levels in FF were associated with more GV, while lower serum BPA concentrations were linked to more mature oocytes (29). A multicenter observational study involving 122 women undergoing PMA cycles found no association between BPA levels, oocyte quality, and pregnancy rate (46).
Despite the wide attention in assessing possible contaminations from BPA in blood serum and in FF in ART, only few human studies have focused on measuring BPA in fluids in association with different environmental conditions and pollution (44, 47–49).
Regardless of the large amount of effort exerted to improve environment quality, environmental pollution remains a significant problem and represents a relevant health risk on reproductive health. Heavy metals, polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls, dioxins, pesticides, and ultra-fine particles severely impair the whole human defense system mechanisms. For example, the correlation between heavy metals and oxidative DNA damage has been widely discussed (50). Indeed, some heavy metals can change the properties of the sperm nuclear basic proteins, which can change their canonical ratios and their protective role on DNA, being involved in oxidative DNA damage (51), as demonstrated in areas with a high environmental impact. It has also been shown that there is a lower seminal antioxidant activity in spermatozoa of residents in polluted areas (52). The phenomenon is so significant that reproductive system components have been considered important biosensors of environmental pollution (53). This is because, in areas with greater environmental impact, the altered environmental conditions, together with direct and indirect short- and long-term effects, and also due to other adverse events, such as viral infections, could cause a deterioration in sperm quality with important consequences on male fertility (54–56).
Environmental pollution also has a strong impact on the female reproductive domain. In this regard, it was recently shown that kallikrein bound to serine peptidase 3 could be an early biomarker of environmental exposure on the immune and reproductive systems of young women (57).
In this paper, we report the results of a specific research on women in the context of the EcoFoodFertility research project (www.ecofoodfertility.it). We evaluated BPA levels in blood and FF in a population of women, permanently living in two areas with different environmental impacts in the Campania Region (Southern Italy), who underwent an ART cycle for the first time. The two residential areas from which the recruited women were selected are called the “Land of Fires”, straddling the provinces of Naples and Caserta, an area territorially known for the illicit disposal and incineration of toxic and urban waste. These illegal practices have significant consequences on the health of the local population (58, 59), and the area is therefore classified, according to a mathematical model developed by a multidisciplinary team, as a high environmental impact (HEI) area (60). The second area is the Sele Valley, a low environmental impact (LEI) area located in the province of Salerno. More specifically, there are regions in the world where the practice of burning plastic waste is prevalent, such as certain areas in India where the highest levels of bisphenol in the air have been measured. A positive correlation between BPA and 1,3,5-triphenylbenzene, a tracer for plastic burning, has been identified in these regions. This correlation highlights that the open burning of plastic waste is a significant source of BPA emissions into the atmosphere (6, 7). Consequently, it is highly plausible that the widespread and illicit incineration of waste containing amounts of plastics, so pervasive in the “Land of Fires”, may represent a substantial source of human contamination via inhalation.
Our study reveals significant differences in the bioaccumulation of BPA in blood serum and FF, which is higher, on average, in women living in an HEI area than in those living in an LEI area. In addition, we revealed peculiar trends showing a heterogeneous level of BPA in different subsets of women and therefore the need for further studies to unravel the mechanisms of the bioaccumulation of BPA in the bodily fluids of women in highly polluted and nonpolluted areas.
Materials and methods
Cohort selection
The present work is part of the EcoFoodFertility research project (www.ecofoodfertility.it, approved by the Ethical Committee of the Local Health Authority Campania Sud-Salerno, Committee code n. 43 of 30 June 2015).
We selected 91 women with normal ovarian reserve, aged between 26 and 47 years [34 (mean) ± 4 (SD)] with a history of infertility ranging from 26 to 39 months, who underwent a cycle of ART treatment for the first time, to evaluate BPA levels in blood and FF.
The women were from two residential areas in the Campania Region (Southern Italy), where they permanently lived (at least 5 years): the “Land of Fires” straddling the provinces of Naples and Caserta, an area territorially known for the illicit disposal and incineration of toxic and urban waste, classified, according to a mathematical model developed by a multidisciplinary team, as an HEI area (60). The second area is the Sele Valley, an LEI area located in the province of Salerno.
The women were divided into two groups according to living area. The LEI group consisted of 32 women ( Supplementary Table 1 , numbered from 1 to 32) selected from the LEI, with normal ovarian reserve inferred by the anti-Müllerian (AMH=3 ± 2 ng/ml) (61–63), and the HEI group is composed of 59 women ( Supplementary Table 1 , numbered from 33 to 91) living in the HEI area, with normal ovarian reserve (AMH=2 ± 2 ng/mL).
Metadata and variable description
Metadata concerning the participants were collected through a “clinical-anamnestic form” in which they reported about their health conditions, as well as use and/or abuse of alcohol, smoking, and, in some cases, previous pregnancies. Participants have no other severe chronic diseases beyond those, if any, associated with gynecological aspects. They have been living in their respective selected areas for at least 5 years; they are not professionally exposed to risk factors and have not taken contraceptive pills for at least 2 years. They deny having used drugs over the 12 months before serum collection and oocyte pick-up. Participants were asked about age at menarche and whether they had experienced previous spontaneous and/or voluntary abortions. All participants have a regular menstruation cycle; the cycle length varies between 28 and 30 days, regular for length, rhythm, and volume. Moreover, body mass index (BMI), waist–height ratio (WHR), and the Ferriman–Gallwey (FG) score for hirsutism were also determined. BMI classes were defined according to the World Health Organization (WHO) obesity levels.
Health conditions are described in terms of ovulation regularity, ovarian reserve (OR), possible endometriosis, and/or polycystic ovary syndrome (PCOS). FG score values range from 1 to 4 according to Ferriman and Gallwey (64, 65).
Smoking attitudes are indicated with “yes/no/p.h.” (previous history of smoking) values. No previous pregnancy (nulliparous) variables are indicated with “yes/no” values.
The fertilization rate (FR) indicates possible events of previous assisted reproductive technologies (e.g., IUI or IVF) or miscarriages (spontaneous or voluntary); classes were defined as follows: 0 for no previous events; 1 for one event of assisted fertilization or spontaneous miscarriage; 2 for at least two events of assisted fertilization or spontaneous miscarriage.
Table 1 summarizes the categorical variables, their classes, and the population distributions. Numerical variables' summary statistics are indicated in Table 2 .
Table 1.
Metadata description, variable classes, and sample size (total and distribution per zone).
| Variable | Class | Total | Size per zone | |
|---|---|---|---|---|
| HEI | LEI | |||
| Population | 91 | 59 | 32 | |
| Age_Groups | 26 ≤ x ≤ 30 | 21 | 13 | 8 |
| 31 ≤ x ≤ 34 | 41 | 25 | 16 | |
| 35 ≤ x ≤ 47 | 29 | 21 | 8 | |
| BMI_Groups* | 19 ≤ x ≤ 24 | 44 | 32 | 12 |
| 25 ≤ x ≤ 30 | 41 | 21 | 20 | |
| 31 ≤ x ≤ 38 | 6 | 6 | 0 | |
| Health_Conditions | Normovulatory | 56 | 34 | 22 |
| norm_OR | 6 | 2 | 4 | |
| Oligomenorrhea | 11 | 9 | 2 | |
| poor_OR | 2 | 2 | 0 | |
| PCOS | 11 | 8 | 3 | |
| Endometriosis | 5 | 4 | 1 | |
| FG_score*** | 1 | 47 | 20 | 27 |
| 2 | 39 | 34 | 5 | |
| 3 | 5 | 5 | 0 | |
| Smoker | Yes | 10 | 8 | 2 |
| No | 65 | 40 | 25 | |
| p.h. | 16 | 11 | 5 | |
| Alcohol | Yes | 24 | 16 | 8 |
| No | 67 | 43 | 24 | |
| Nulliparous | Yes | 80 | 54 | 26 |
| No | 11 | 5 | 6 | |
| FR*** | 0 | 26 | 7 | 19 |
| 1 | 44 | 32 | 12 | |
| 2 | 21 | 20 | 1 | |
norm_OR, normal ovarian reserve.
poor_OR, reduced ovarian reserve.
p.h., previous history (of smoking).
PCOS, polycystic ovary syndrome.
Statistically significant differences by ChiSq tests in the population size between
zones per class in each variable are indicated (*p<0.05: ***p<0.001).
Table 2.
Summary statistics in total and per zone.
| Variable | Min | Total | Max | Min | HEI | Max | Min | LEI | Max |
|---|---|---|---|---|---|---|---|---|---|
| Mean(SD) | Mean(SD) | Mean(SD) | |||||||
| Seric BPA*** | 1.5 | 65(49) | 182.4 | 3.1 | 96(27) | 182.4 | 1.5 | 7(15) | 86.1 |
| Follicle BPA*** | 4.2 | 21(15) | 81.5 | 4.2 | 25(16) | 81.5 | 5.8 | 13(8) | 35.6 |
| FSH*** | 0.98 | 7(3) | 18.3 | 2.6 | 8(3) | 16.2 | 0.98 | 4(3) | 18.3 |
| E2*** | 10.2 | 54(30) | 124.2 | 10.2 | 66(30) | 124.2 | 12.5 | 33(17) | 80.3 |
| AMH | 0.02 | 3(2) | 12.4 | 0.02 | 2(2) | 12.4 | 0.09 | 3(2) | 8.49 |
| WHR** | 0.4 | 0.75(0.01) | 1 | 0.4 | 0.77(0.13) | 1 | 0.6 | 0.72(0.06) | 0.8 |
| BMI | 20.7 | 25(3) | 38 | 21 | 26(3) | 38 | 20.7 | 25(2) | 28.2 |
| Age | 26 | 34(4) | 47 | 27 | 34(4) | 45 | 26 | 33(5) | 47 |
Statistically significant differences of means between HEI and LEI zones are indicated
as **p<0.01; ***p<0.001 (Wilcoxon test, two-tailed).
Determination of hormones and BPA
To determine the concentrations of hFSH, hLH, and estradiol, an immunoassay in chemiluminescence with paramagnetic particles is used, using the Access 2 Immunoassay System by Beckman Coulter according to the manufacturer’s instructions. The values are expressed as follows: hLH and hFSH in mIU/mL, and estradiol in pg/mL.
For the quantitative measurement of circulating AMH, the ELFA (enzyme-linked fluorescent assay) technique is used, with an automatic VIDAS analyzer from BioMerieux-France, according to the manufacturer’s instructions. Values are expressed in ng/mL.
For the BPA assay, an enzyme-linked immunosorbent assay was used, and values are expressed in ng/mL (Booster Biological Technology, 3942 B Valley Ave, Pleasanton, CA 94566). For all samples, BD Vacutainer®-treated glass-reinforced USP Type III, non-siliconized, 5-mL tubes with a red cap, without additives and kept in a horizontal position, were used. The FF processed were bloodless with a negative albumin test.
Statistical analysis
The statistical significance of differences in means defined in the two regions under investigation for any of the numerical variables was evaluated by a two-tailed Wilcoxon test, while a chi-square test of independence was used to determine whether categorical variables are likely to be related or not to living area (McHugh ML. 2013).
Clustering
In order to discover groups of patients with a similar content of BPA in fluids, we performed a K-means clustering (K was set to 6, according to the corresponding figure of merit, not shown). Mean normalization and scaling were applied as a preprocessing step in order to account for variance inequalities per variable. Metadata description is schematized in the clustering per patient according to the classification of each variable.
Pearson correlation analyses were performed in order to assess the relationships between serum and follicular BPA contents and among all numerical physiological variables. This was done for the whole population (HEI+LEI) and stratified per area (HEI or LEI) and per cluster group.
Results
Cohort variables per area
Table 1 shows the data organization for the sampled population with the total distribution per variable and per area (HEI or LEI), evaluating statistical significance of differences per area by a chi-square test. The sampled population results are not uniformly distributed for BMI, FG score, and FR (risk of fertility) variables. These differences may reflect a bias in the sampling or effects of the specific environmental context.
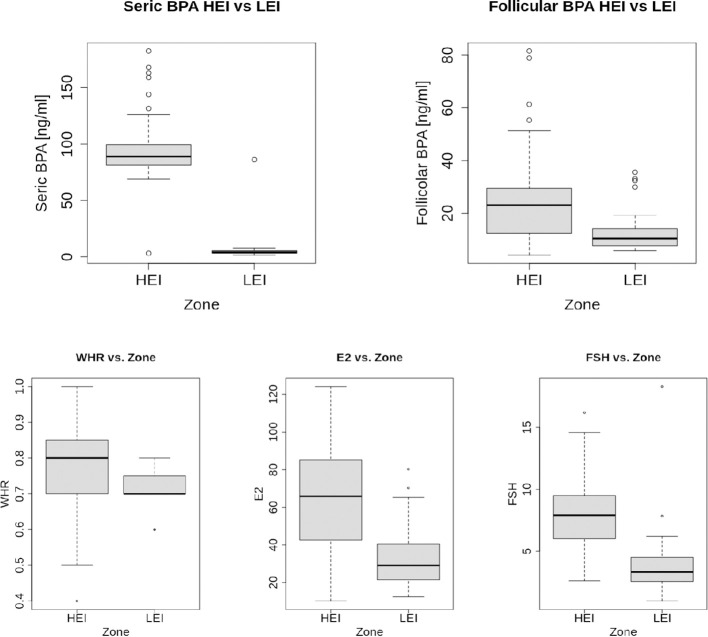
Table 2 shows summary statistics of the numerical variables representing physiological parameters together with mean and standard deviation (SD) values for age and BMI in total and per zone. Statistically significant differences in means between the HEI and the LEI populations occur in BPA, in FSH, in E2 contents (p<0.001) and in WHR (p<0.01). All these variables show higher mean values in the HEI population than in the LEI. It is noteworthy that the serum BPA content shows striking differences in the two zones ( Figure 1 ).
Figure 1.
Boxplots of numerical variables that resulted with significant differences in means per zone (two-tailed Wilcoxon test shown in Table 2 ).
Table 3 indicates summary statistics per serum and follicle BPA in total, per zone, and stratified per age and per BMI, since these two variables may be confounders in the assessment of the BPA content in the human body ( Supplementary Figure 1 ). The stratification shows that age and BMI in the two populations do not affect the statistical significance of the difference in means of the BPA content in the two areas for both tissues. In addition, average BPA values also show opposite average trends in the two areas: the mean BPA content in blood serum is significantly higher than that in the FF of people from the HEI area, while, although with lower discrepancy, FF BPA is significantly higher than blood serum content (p<0.001 by a two-tailed Wilcoxon test) in people from the LEI area. Last, no significant correlation was found among numerical variables and BPA contents in each tissue (details not shown).
Table 3.
Summary statistics of BPA in seric and follicular fluids stratified per age and BMI groups (total and per zone).
| Variable | Class | Mean(SD) | ||
|---|---|---|---|---|
| Total | HEI | LEI | ||
| Seric BPA | 26 ≤ age ≤ 30*** | 58(39) | 85(8) | 15(29) |
| 31 ≤ age ≤ 34*** | 62(50) | 98(24) | 4(24) | |
| 35 ≤ age ≤ 47*** | 74(54) | 101(37) | 4.3(1.3) | |
| Follicle BPA | 26 ≤ age ≤ 30* | 16(8) | 19(7) | 12.4(7.6) |
| 31 ≤ age ≤ 34** | 19(13) | 23(14) | 12(14) | |
| 35 ≤ age ≤ 47 | 27(20) | 30(21) | 17(11) | |
| Seric BPA | 19 ≤ BMI ≤ 24*** | 74(49) | 101(27) | 3.9(1.4) |
| 25 ≤ BMI ≤ 30*** | 50(48) | 90(30) | 8(18) | |
| 31 ≤ BMI ≤ 38 | 94(20) | 94(20) | / | |
| Follicular BPA | 19 ≤ BMI ≤ 24** | 23(18) | 26(20) | 12(8) |
| 25 ≤ BMI ≤ 30*** | 19(11) | 24(11) | 14(8) | |
| 31 ≤ BMI ≤ 38 | 20(10) | 20(10) | / | |
Statistically significant differences by the Wilcoxon test (two-tailed) between HEI
and LEI zones are indicated (*p<0.05; **p<0.01; ***p<0.001)./ indicates no people from the specific zone.
Clustering in terms of BPA content in the fluids
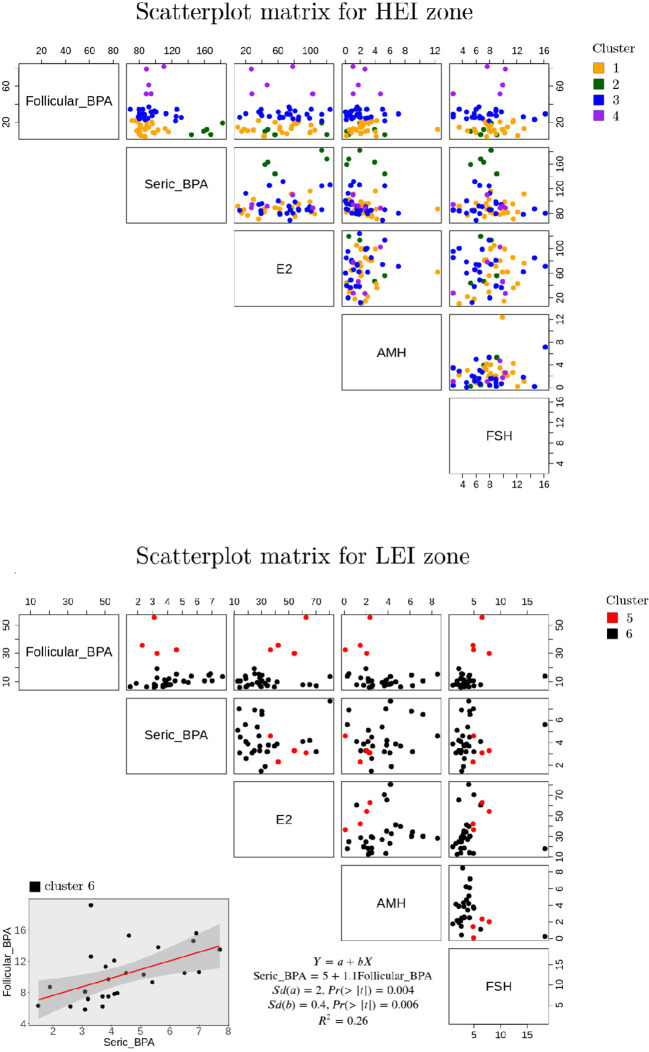
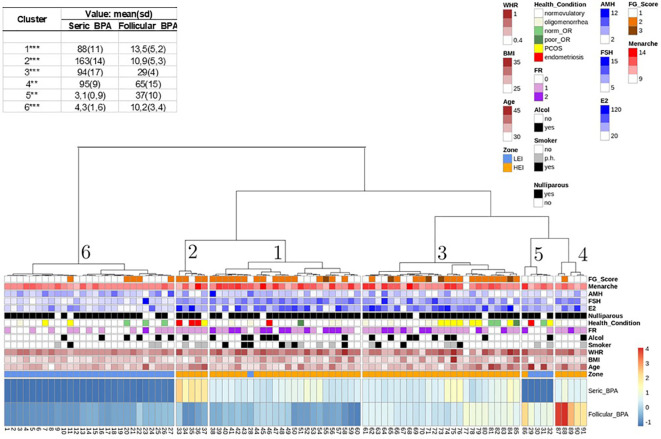
The unsupervised hierarchical clustering based on a K-means approach on PBA contents in blood serum and FF on the entire sample of 91 individuals is shown in Figure 2 (list of individuals per area, BPA levels, and distribution per cluster are also reported in Supplementary Table 1 ). Statistics on the BPA content per cluster are also summarized in Figure 3 .
Figure 2.
Scatterplot matrices of the principal parameters considered per cluster in the LEI and HEI areas.
Figure 3.
Hierarchical K-mean clustering on seric and follicular fluid BPA. All other variables and their range of variability are reported in the legend. Means and statistical significance between HEI and LEI zones are also indicated as *p<0.05:**p<0.01;***p<0.001 (two-tailed Wilcoxon test).
Cluster 6 includes the majority of individuals from the LEI area (27 out of 32 women). Cluster 5 is also composed mainly of LEI individuals; specifically, four individuals are from the LEI area and one (individual number 86) is from the HEI area. Cluster 1 (24 individuals), cluster 2 (5 individuals), cluster 3 (25 individuals), and cluster 4 (5 individuals) are all from the HEI area with the only exception of individual number 28 in cluster 1, who is reported to be from the LEI area. It is evident that the BPA compositional features of the serum for individuals 28, classified as belonging to the LEI area, and 86, classified to be from the HEI area, are atypical in comparison with people from the same area, reflecting a higher similarity to people living in the other area, respectively. This explains why these two individuals fall under two clusters composed of people from the other area. This also confirms that blood serum BPA appears to be a major indicator of the living area with only two exceptions. Interestingly, in a second verification from the medical doctor, it was revealed that individual 28 was indeed living in the HEI zone.
Cluster 6 patients are those with the lowest BPA content in both follicular and serum fluids (as shown in Figure 3 ), when compared with the other clusters from the analysis. Moreover, on average, the follicle BPA content is significantly higher than the serum content, confirming the general average trend presented for people in the LEI area ( Table 2 ). Cluster 5 includes only five individuals, four from the LEI area and 1 from the HEI area. This group is also characterized by a higher BPA follicle content than serum fluid content (as shown in Figure 3 ). However, it should be emphasized that the BPA content in the follicle in this cluster is three times higher than the average PBA content of the LEI population, indicating a peculiar PBA content in FF for this cluster of people. Interestingly, all members of this population suffered from allergies and were exposed to cortisone treatments before the pick-up (personal communication with a medical doctor).
Clusters 1, 2, 3, and 4, mainly representing people from the HEI area, show a higher BPA content in serum than in FF, with the highest value in cluster 2. Notably, this is the cluster with the lowest average BPA content in the follicle among people from the HEI area ( Figure 2 ). Interestingly, four out of five individuals in cluster 2 show pathologies in the reproductive systems with endometriosis (one has polycystic ovary). cluster 4 has the highest BPA content in FF in the overall dataset, although no peculiar trends in BPA content are revealed in serum.
The variability of the relative BPA content in the two fluids in both HEI and LEI areas revealed by the cluster analysis further confirms the lack of correlation between BPA contents in blood serum and FF, as it was revealed by the higher BPA content in blood serum when compared to FF in the HEI area, and the lower BPA content in blood serum when compared to FF in the LEI area ( Table 3 ), highlighting the peculiar bioaccumulation in the two populations as well as in the different clusters of individuals, although confirming the main average trends in the areas, i.e., a higher average BPA content in the HEI area in both blood serum and FF with respect to the LEI area ( Table 3 ).
To further investigate this heterogeneity in relative content, we also evaluated if any significant linear correlation existed between blood serum and FF BPA, and among these variables and other numerical variables, in total, in the HEI and LEI populations, and in a larger cluster (1,3,6) ( Figure 2 ). We did not detect any significant correlation among numerical variables showing significant differences in Table 2 (results not shown). BPA content in the two fluids in each cluster showed a weak linear correlation only in cluster 6 ( Figure 2 ).
Discussion
Only few experimental studies have assessed the bioaccumulation of BPA in FF. Consequently, there is limited evidence regarding the potential direct effect of BPA on ovarian sexual steroid synthesis (38, 48, 66). In vivo and in vitro studies using murine models have reported conflicting associations between higher BPA exposures and reduced steroidogenic enzyme activities in the ovarian theca (23, 67) and in granulosa cells (58, 66, 68). This discrepancy may explain the negative impact on oocyte quality. While some studies have reported null or inconsistent results (69–71), more recent scientific evidence suggests that high levels of BPA in FF can negatively impact pregnancy rates (72, 73). However, the effective dose of unconjugated BPA needed to disrupt estrogen synthesis in human populations may not align with general levels of “baseline” exposure, mainly through dietary sources. The clinical impact of this disruption remains unclear. Nonetheless, susceptible subgroups or those with unusually high exposure to BPA, as seen in HEI areas, may be considered vulnerable populations (74).
The ovarian blood–follicle barrier (BFB) likely plays an important but still poorly understood role in this process. The BFB is crucial for preventing the entry of foreign or harmful substances (toxics substances, drugs, etc.) into the ovary. It regulates the composition of FF during follicle development and filters various components based on the needs of folliculogenesis (e.g., proteins, peptides, electrolytes, ions, and sugars). This barrier is nonselective for certain drugs and/or chemotherapy. One example is doxorubicin, an anticancer drug, which can penetrate the BFB and induce ovarian failure, leading to oocyte apoptosis (75, 76).
Our multicenter study aims to monitor BPA contamination in the serum and FF of women undergoing oocyte pick-up for IVF in two different geographical areas with a different environmental impact as part of the EcoFoodFertility project (www.ecofoodfertility.it). The occurrence of different environmental sources of BPA contamination (hazardous sites near living places such as deliberate burning of plastics, exposure or use of potentially toxic agents, consumption of local food, etc.) suggests that individuals permanently residing in HEI areas are at a higher risk of BPA exposure. Our results, comparing samples from women permanently living for at least 5 years in one of two areas, a very high polluted area of the Campania region (HEI), the so-called “Land of Fires”, due to toxic plastic waste and its illegal vandalistic combustion, and an LEI area, which includes the Sele Valley River and the Cilento regions in the province of Salerno, confirm this hypothesis, with high average differences in BPA concentrations in blood serum and FF in the HEI and LEI populations.
Patients in the HEI area show notably higher BPA levels in both tissues compared to those in the LEI area. The significant differences in serum concentrations between the two groups can be explained by also considering numerous studies in mouse and human models that hypothesize epithelial damage of membrane proteases or the targeted deletion of both structural and junctional barrier genes that alter permeability (77). BPA is acknowledged as a xenoestrogen and is responsible for the permeability of the hemato-alveolar and hemato-gastric membrane directly proportional to its concentration (78). Although some studies show that exogenous proteases alone can disrupt the ovarian BFB, this does not fully explain why it also occurs in a proportion of the population living in the same area (79). The variability in BPA concentrations in FF between and within the two groups of women therefore prompts further investigation.
The additional significant finding reported here is that patients in the HEI area have a considerably higher BPA content in FF compared to participants in the LEI group, but also that the LEI group has a higher BPA content in the follicle than in the serum. In general, there is a lack of correlation between BPA contents in serum and FF, that could be interpreted as a different permeability and/or impermeability of the ovarian hemato-follicular membrane as an effect of exposure not only to BPA but also to other harmful factors. Recent studies have shown that this alteration occurred in patients with PCOS (80). The presence of a weak correlation between BPA in blood serum and FF in the LEI population in cluster 6, grouping only people from the LEI area, does not appear to be a general rule in our sampling, highlighting that lower contamination levels follow the expected trends of bioaccumulation suggested in previous similar efforts that addressed the correlation between blood serum BPA levels and FF (29).
In addition, the presence of two clusters of people in the LEI zone reveals that alterations in FF BPA levels can be due to additional factors influencing or related to health conditions, such as the reported allergies (personal communication with doctors) for people in cluster 5. Indeed, it is known that allergies are associated with BPA exposure (81, 82) and that BPA bioaccumulation is associated with immune-related diseases (83, 84).
No relevant feature emerging from the associated metadata based on the information collected from the sampled populations in our dataset was useful to consistently explain the different clusters of people in the HEI area, with the only exception of cluster 2, where the highest BPA content in blood serum in the overall group of people is associated with four out of five individuals who manifest alterations of tissues from organs of the reproductive system because of endometriosis (three out of five individuals) and PCOS (one out of five individuals) as discussed in other studies (72, 85, 86). Nevertheless, PCOS-affected women in our sample, although more frequent in the HEI area, do not appear to be significantly over-represented in this area, neither are they associated with BPA content in the two fluids, in relation with other previous lines of evidence (87).
It is evident from our analysis that further sampling and deeper molecular analyses would be necessary to check on these aspects and confirm our evidence. Indeed, our results are clear in distinguishing BPA content in fluids from people living in the two different areas, but further investigations are needed to fully unravel the bioaccumulation mechanisms of BPA in blood serum and FF and to explain the BPA absorption and consequent health damage. Nevertheless, the results of this study indicate that the higher bioaccumulation in the HEI group of women appears to be associated withare different environmental conditions of the areas under investigation and with comparative studies already carried out within the EcoFoodFertility project on the male side. Indeed, it provides further evidence that pollution in this area is not just a reproductive risk (80, 88–93). Of course, molecular analyses will be needed to investigate the effects of BPA in more detail, just as we looked for the effects of heavy metals and VOCs in human sperm (94, 95).
Conclusions
Our analyses revealed significant differences in BPA concentrations in the serum and FF of women undergoing an IVF program in geographical areas with different environmental impacts. The presence of various environmental sources of BPA pollution, particularly due to the widespread incineration of toxic and municipal waste containing a significant amount of plastic, would entail a higher risk of human contamination by high atmospheric levels of BPA and other contaminants for women residing in the HEI area. Considering the inhomogeneity of BPA concentrations in the FF between the two groups and in the same groups, and the lack of overall correlations between the different groups and subgroups, they deserve more in-depth investigations. Although many studies demonstrate that exogenous proteases (including BPA) are sufficient to deteriorate the epithelium of the BFB, this does not fully explain why this occurs at different extents in the population of the same area. The lack of correlation between BPA values between serum and FF should be interpreted as different permeability and/or impermeability of the ovarian blood–follicular membrane, which can be hypothesized to be associated with specific pathological conditions (PCOS and endometriosis). From the current study, the presence of BPA in the FF of infertile women undergoing IVF reveals a presumable negative impact on pregnancy rates as well.
Further biomonitoring studies with larger sample size and integration with additional biomarkers and metadata collections, possibly associated with more in-depth examination of the cellular and molecular assets in patients, are essential to understand the mechanisms that regulate the presence of BPA and/or its metabolites, and undertake territorial reclamation to protect public health.
Acknowledgments
The authors are thankful to all volunteers who participated in the study. Special thanks to all the members of the EcoFoodFertility project.
Funding Statement
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Committee of the Local Health Authority Campania Sud-Salerno. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SR: Conceptualization, Investigation, Methodology, Resources, Writing – original draft. MC: Data curation, Formal analysis, Software, Writing – original draft. MG: Investigation, Writing – review & editing. TG: Investigation, Writing – review & editing. FC: Investigation, Writing – review & editing. RG: Investigation, Writing – review & editing. DD: Investigation, Writing – review & editing. LSi: Investigation, Writing – review & editing. CC: Investigation, Writing – review & editing. MP: Investigation, Writing – review & editing. SI: Investigation, Writing – review & editing. MI: Investigation, Writing – review & editing. AF: Investigation, Writing – review & editing. FL: Investigation, Writing – review & editing. AE: Investigation, Writing – review & editing. CZ: Investigation, Writing – review & editing. LoSo: Investigation, Writing – review & editing. LaSo: Investigation, Writing – review & editing. IF: Investigation, Writing – review & editing. MP: Investigation, Methodology, Writing – review & editing. TN: Investigation, Writing – review & editing. RL: Data curation, Formal analysis, Writing – review & editing. AG: Investigation, Writing – review & editing. LM: Conceptualization, Data curation, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1392550/full#supplementary-material
Boxplots of numerical variables that resulted with significant differences in means, stratified per Age and BMI per zone (two tailed Wilcoxon test shown in Table 2 ).
References
- 1. Carre í J, Gatimel N, Moreau J, Parinaud J, Leandri R. Influence of air quality on the results of in vitro fertilization attempts: A retrospective study. Eur J Obstet Gynecol Reprod Biol. (2017) 210:116–22. doi: 10.1016/j.ejogrb.2016.12.012 [DOI] [PubMed] [Google Scholar]
- 2. Simoneit BRT, Medeiros PM, Didyk BM. Combustion products of plastics as indicators for refuse burning in the atmosphere. Environ Sci Technol. (2005) 39:6961–70. doi: 10.1021/es050767x [DOI] [PubMed] [Google Scholar]
- 3. Wang Z, Liu H, Liu S. Low-dose bisphenol a exposure: a seemingly instigating carcinogenic effect on breast cancer. Adv Sci. (2017) 4:1600248. doi: 10.1002/advs.201600248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fukazawa H, Watanabe M, Shiraishi F, Shiraishi H, Shiozawa T, Matsushita H, et al. Formation of chlorinated derivatives of bisphenol a in waste paper recycling plants and their estrogenic activities. J Health Sci. (2002) 48:242–9. doi: 10.1248/jhs.48.242 [DOI] [Google Scholar]
- 5. Hamad K, Kaseem M, Deri F. Recycling of waste from polymer materials: an overview of the recent works. Polym Degrad Stabil. (2013) 98:2801–12. doi: 10.1016/j.polymdegradstab.2013.09.025 [DOI] [Google Scholar]
- 6. Fu P, Kawamura K. Ubiquity of bisphenol A in the atmosphere. Environ pollut. (2010) 158:3138–43. doi: 10.1016/j.envpol.2010.06.040 [DOI] [PubMed] [Google Scholar]
- 7. Darbre PD. Overview of air pollution and endocrine disorders. Int J Gen Med. (2018) 11:191–207. doi: 10.2147/IJGM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. WHO . Toxicological and Health Aspects of Bisphenol A. Report of Joint FAO/WHO Expert Meeting. Ottawa, Canada: World Health Organization; (2011) p. 1–60. [Google Scholar]
- 9. Fujimoto VY, Kim D, vom Saal FS, Lamb JD, Taylor JA, Bloom MS. Serum unconjugated bisphenol A concentrations in women may adversely influence oocyte quality during in vitro fertilization. Fertil Steril. (2011) 95:1816–9. doi: 10.1016/j.fertnstert.2010.11.008 [DOI] [PubMed] [Google Scholar]
- 10. Geens T, Aerts D, Berthot C, Bourguignon JP, Goeyens L, Lecomte P, et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol. (2012) 50:3725–3740. doi: 10.1016/j.fct.2012.07.059 [DOI] [PubMed] [Google Scholar]
- 11. Dekant W, Völkel W. Human exposure to bisphenol A by biomonitoring: methods, results and assessment of environmental exposures. Toxicol Appl Pharmacol. (2008) 228:114–34. doi: 10.1016/j.taap.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 12. Fromme H, Küchler T, Otto T, Pilz K, Müller J, Wenzel A. Occurrence of phthalates and bisphenol A and F in the environment. Water Res. (2002) 36:1429–38. doi: 10.1016/S0043-1354(01)00367-0 [DOI] [PubMed] [Google Scholar]
- 13. Staples CA, Dome PB, Klecka GM, Oblock ST, Harris LR. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere. (1998) 36:2149–73. doi: 10.1016/S0045-6535(97)10133-3 [DOI] [PubMed] [Google Scholar]
- 14. Berkner S, Streck G, Herrmann R. Development and validation of a method for determination of trace levels of alkylphenols and bisphenol A in atmospheric samples. Chemosphere. (2004) 54:575–84. doi: 10.1016/S0045-6535(03)00759-8 [DOI] [PubMed] [Google Scholar]
- 15. Matsumoto H, Adachi S, Suzuki Y. Bisphenol a in ambient air particulates responsible for the proliferation of MCF-7 human breast cancer cells and its concentration changes over 6 months. Arch Environ Contam Toxicol. (2005) 48:459–66. doi: 10.1007/s00244-003-0243-x [DOI] [PubMed] [Google Scholar]
- 16. Graziani NS, Carreras H, Wannaz E. Atmospheric levels of BPA associated with particulate matter in an urban environment. Heliyon. (2019) 5:e01419. doi: 10.1016/j.heliyon.2019.e01419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barba M, Mazza A, Guerriero C, Di Maio M, Romeo F, Maranta P, et al. Wasting lives: The effects of toxic waste exposure on health - The case of Campania, Southern Italy. Cancer Biol Ther. (2011) 12:106–11. doi: 10.4161/cbt.12.2.16910 [DOI] [PubMed] [Google Scholar]
- 18. Oehlmann J, Schulte-Oehlmann U, Kloas W, Jagnytsch O, Lutz I, Kusk KO, et al. A critical analysis of the biological impacts of plasticizers on wildlife. Philos Trans R Soc Lond B Biol Sci. (2009) 364:2047–62. doi: 10.1098/rstb.2008.0242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bolli A, Galluzzo P, Ascenzi P, Del Pozzo G, Manco I, Vietri MT, et al. Laccase treatment impairs bisphenol A-induced cancer cell proliferation affecting estrogen receptor α-dependent rapid signals. IUBMB Life. (2008) 60:843–52. doi: 10.1002/iub.130 [DOI] [PubMed] [Google Scholar]
- 20. Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. (2008) 116:39–44. doi: 10.1289/ehp.10753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, et al. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect. (2009) 117:639–64. doi: 10.1289/ehp.0800265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, et al. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect. (2011) 119:131–7. doi: 10.1289/ehp.1002366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peretz J, Gupta RK, Singh J, Herna índez-Ochoa I, Flaws JA. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sci. (2010) 119:209–17. doi: 10.1093/toxsci/kfq319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang M, Dai X, Lu Y, Miao Y, Zhou C, Cui Z, et al. Melatonin protects oocyte quality from Bisphenol A- induced deterioration in the mouse. J Pineal Res. (2017), 62. doi: 10.1111/jpi.12396 [DOI] [PubMed] [Google Scholar]
- 25. Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. (2007) 24:199–224. doi: 10.1016/j.reprotox.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lathi RB, Liebert CA, Brookfield KF, Taylor JA, Vom Saal FS, Fujimoto VY, et al. Conjugated bisphenol A in maternal serum in relation to miscarriage risk. Fertil Steril. (2014) 102:123–8. doi: 10.1016/j.fertnstert.2014.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Caserta D, Di Segni N, Mallozzi M, Giovanale V, Mantovani A, Marci R, et al. Bisphenol A and the female reproductive tract: an overview of recent laboratory evidence and epidemiological studies. Reprod Biol Endocrinol. (2014) 12:37. doi: 10.1186/1477-7827-12-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, et al. Bisphenol a and reproductive health: update of experimental and human evidence, 2007–2013. Environ Health Perspect. (2014) 122:775–86. doi: 10.1289/ehp.1307728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poormoosavi SM, Behmanesh MA, Janati S, Najafzadehvarzi H. Level of bisphenol A in follicular fluid and serum and oocyte morphology in patients undergoing IVF treatment. J Family Reprod Health. (2019) 13:154–9. doi: 10.18502/jfrh.v13i3.2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, et al. Bisphenol A exposure causes meiotic aneuploidy in the female mouse. Curr Biol. (2003) 13:546–53. doi: 10.1016/S0960-9822(03)00189-1 [DOI] [PubMed] [Google Scholar]
- 31. Lenie S, Cortvrindt R, Eichenlaub-Ritter U, Smitz J. Continuous exposure to bisphenol A during in vitro follicular development induces meiotic abnormalities. Mutat Res. (2008) 651:71–81. doi: 10.1016/j.mrgentox.2007.10.017 [DOI] [PubMed] [Google Scholar]
- 32. Acunãa-Herna índez DG, Arreola-Mendoza L, Santacruz-Ma írquez R, Garci ía-Zepeda SP, Parra-Forero LY, Olivares-Reyes JA, et al. Bisphenol A alters oocyte maturation by prematurely closing gap junctions in the cumulus cell-oocyte complex. Toxicol Appl Pharmacol. (2018) 344:13–22. doi: 10.1016/j.taap.2018.02.011 [DOI] [PubMed] [Google Scholar]
- 33. Buck Louis GM, Peterson CM, Chen Z, Croughan M, Sundaram R, Stanford J, et al. Bisphenol A and phthalates and endometriosis: the Endometriosis: Natural History, Diagnosis and Outcomes Study. Fertil Steril. (2013) 100:162–9.e92. doi: 10.1016/j.fertnstert.2013.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, et al. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl. (2010) 33:385–93. doi: 10.1111/j.1365-2605.2009.01014.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ehrlich S, Williams PL, Missmer SA, Flaws JA, Ye X, Calafat AM, et al. Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum Reprod. (2012) 27:3583–92. doi: 10.1093/humrep/des328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Souter I, Smith KW, Dimitriadis I, Ehrlich S, Williams PL, Calafat AM, et al. The association of bisphenol-A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reprod Toxicol. (2013) 42:224–31. doi: 10.1016/j.reprotox.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Machtinger R, Combelles CM, Missmer SA, Correia KF, Williams P, Hauser R, et al. Bisphenol-A and human oocyte maturation in vitro. Hum Reprod. (2013) 28:2735–45. doi: 10.1093/humrep/det312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. (2002) 17:2839–41. doi: 10.1093/humrep/17.11.2839 [DOI] [PubMed] [Google Scholar]
- 39. Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, et al. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ Health Perspect. (2012) 120:978–83. doi: 10.1289/ehp.1104307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tan J, Zou Y, Wu XW, Tian LF, Su Q, He JX, et al. Increased SCF in follicular fluid and granulosa cells positively correlates with oocyte maturation, fertilization, and embryo quality in humans. Reprod Sci. (2017) 24:1544–50. doi: 10.1177/1933719117697125 [DOI] [PubMed] [Google Scholar]
- 41. Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril. (2015) 103:303–16. doi: 10.1016/j.fertnstert.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 42. Duty SM, Mendonca K, Hauser R, Calafat AM, Ye X, Meeker JD, et al. Potential sources of bisphenol A in the neonatal intensive care unit. Pediatrics. (2013) 131:483–489. doi: 10.1542/peds.2012-1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huygh J, Clotman K, Malarvannan G, Covaci A, Schepens T, Verbrugghe W, et al. Considerable exposure to the endocrine disrupting chemicals phthalates and bisphenol-A in intensive care unit (ICU) patients. Environ Int. (2015) 81:64–72. doi: 10.1016/j.envint.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 44. Parikh FR, Uttamchandani S, Athalye A, Sinkar P, Panpalia M, Makwana P, et al. Bisphenol a (BPA) levels in the serum of Indian women collected at the time of oocyte retrieval may predict BPA levels in their follicular fluid (FF). Fert Steril. (2018) 110:e171. doi: 10.1016/j.fertnstert.2018.07.507 [DOI] [Google Scholar]
- 45. Hyun-Ki K, Dae-Hyun K, Woochang L, Kwang-Rae K, Sail C, Junghan S, et al. Body fluid concentrations of bisphenol A and their association with in vitro fertilization outcomes. Hum Fertility. (2019) 24:199–207. doi: 10.1080/14647273.2019.1612104 [DOI] [PubMed] [Google Scholar]
- 46. Paoli D, Pallotti F, Dima AP, Albani E, Alviggi C, Causio F, et al. Phthalates and bisphenol A: presence in blood serum and follicular fluid of italian women undergoing assisted reproduction techniques. Toxic. (2020) 8:1–15. doi: 10.3390/toxics8040091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. (2009) 7:40. doi: 10.1186/1477-7827-7-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine Disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. (2011) 127:204–15. doi: 10.1016/j.jsbmb.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Krotz SP, Carson SA, Tomey C, Buster JE. Phthalates and bisphenol do not accumulate in human follicular fluid. J Assist Reprod Genet. (2012) 29:773–7. doi: 10.1007/s10815-012-9775-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Paithankar JG, Saini S, Dwivedi S, Sharma A, Chowdhuri DK. Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction. Chemosphere. (2021) 262:128350. doi: 10.1016/j.chemosphere.2020.128350 [DOI] [PubMed] [Google Scholar]
- 51. Lettieri G, D’Agostino G, Mele E, Cardito C, Esposito R, Cimmino A, et al. Discovery of the involvement in DNA oxidative damage of human sperm nuclear basic proteins of healthy young men living in polluted areas. Int J Mol Sci. (2020) 21:4198. doi: 10.3390/ijms21124198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lettieri G, Marra F, Moriello C, Prisco M, Notari T, Trifuoggi M, et al. Molecular alterations in spermatozoa of a family case living in the land of fires - a first look at possible transgenerational effects of pollutants. Int J Mol Sci. (2020) 21:6710. doi: 10.3390/ijms21186710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Longo V, Forleo A, Radogna AV, Siciliano P, Notari T, Pappalardo S, et al. A novel human biomonitoring study by semiconductor gas sensors in Exposomics: investigation of health risk in contaminated sites. Environ pollut. (2022) 304:119119. doi: 10.1016/j.envpol.2022.119119 [DOI] [PubMed] [Google Scholar]
- 54. Montano L, Donato F, Bianco PM, Lettieri G, Guglielmino A, Motta O, et al. Air pollution and COVID-19: a possible dangerous synergy for male fertility. Int J Environ Res Publ. Health. (2021) 25:6846. doi: 10.3390/ijerph18136846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Montano L, Donato F, Bianco PM, Lettieri G, Guglielmino A, Motta O, et al. Semen quality as a potential susceptibility indicator to SARS-CoV-2 insults in polluted areas. Environ Sci pollut Res. (2021) 28:37031–40. doi: 10.1007/s11356-021-14579-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Montano L, Piscopo M, Brogna C, Chiusano ML. Air pollution and COVID-19. A synergistic effect accelerating male infertility and cancer. In: Oncology and COVID 19 (2023), Taylor & Francis Group; 9781003362562. [Google Scholar]
- 57. Raimondo S, Gentile M, Esposito G, Gentile T, Ferrara I, Crescenzo C, et al. Could kallikrein-related serine peptidase 3 Be an early biomarker of environmental exposure in young women? Int. J Environ Res Publ. Health. (2021) 21:8833. doi: 10.3390/ijerph18168833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huang H, Leung LK. Bisphenol A downregulates CYP19 transcription in JEG-3 cells. Toxicol Lett. (2009) 189:248–52. doi: 10.1016/j.toxlet.2009.06.853 [DOI] [PubMed] [Google Scholar]
- 59. Alberti P. The 'land of fires': epidemiological research and public health policy during the waste crisis in Campania, Italy. Heliyon. (2022) 8:e1233. doi: 10.1016/j.heliyon.2022.e12331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pizzolante A, Nicodemo F, Pierri A, Ferro A, Pierri B, Buonerba C, et al. Development of a municipality index of environmental pressure in Campania, Italy. Future Sci OA. (2021) 7:FSO720. doi: 10.2144/fsoa-2021-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van Rooij IAJ, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, et al. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. (2002) 17:3065–71. doi: 10.1093/humrep/17.12.3065 [DOI] [PubMed] [Google Scholar]
- 62. Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. (2011) 95:170–5. doi: 10.1016/j.fertnstert.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 63. Rajbhar S, Jyoti Mishra J, Mayank Gupta M, Sharma M, Geeta Deshmukh G, Ali W. Anti-mullerian hormone: A marker of ovarian reserve and its association with polycystic ovarian syndrome. J Clin Diagn Res. (2016) 10:QC10–2. doi: 10.7860/JCDR/2016/20370.8988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. (1961) 21:1440–7. doi: 10.1210/jcem-21-11-1440 [DOI] [PubMed] [Google Scholar]
- 65. Lumezi BG, Berisha VL, Pupovci HL, Goçi A, Hajrushi AB. Grading of hirsutism based on the Ferriman-Gallwey scoring system in Kosovar women. Postepy Dermatol Alergol. (2018) 35:631–5. doi: 10.5114/ada.2018.77615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Téteau O, Carvalho AV, Papillier P, Mandon-Pépin B, Jouneaus L, Jarrier-Gaillard P, et al. Bisphenol A and bisphenol S both disrupt ovine granulosa cell steroidogenesis but through different molecular pathways. J Ovarian Res. (2023) 16:30. doi: 10.1186/s13048-023-01114-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Peretz J, Flaws JA. Bisphenol A down-regulates rate-limiting Cyp11a1 to acutely inhibit steroidogenesis in cultured mouse antral follicles. Toxicol Appl Pharmacol. (2013) 271:249–56. doi: 10.1016/j.taap.2013.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mansur A, Adir M, Yerushalmi G, Hourvitz A, Gitman H, Yung Y, et al. Does BPA alter steroid hormone synthesis in human granulosa cells in vitro ? Hum Reprod. (2016) 31:1562–9. doi: 10.1093/humrep/dew088 [DOI] [PubMed] [Google Scholar]
- 69. Zhou W, Liu H, Liao L, Han S, Liu J. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol Cell Endocrinol. (2008) 283:12–8. doi: 10.1016/j.mce.2007.10.010 [DOI] [PubMed] [Google Scholar]
- 70. Li Y, Zhang W, Liu J, Wang W, Li H, Zhu J, et al. Prepubertal bisphenol A exposure interferes with ovarian follicle development and its relevant gene expression. Reprod Toxicol. (2014) 44:33–40. doi: 10.1016/j.reprotox.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 71. Moore-Ambriz TR, Acuna-Hernandez DG, Ramos-Robles B, Sanchez-Gutierrez M, Santacruz-Marquez R, Sierra-Santoyo A, et al. Exposure to bisphenol A in young adult mice does not alter ovulation but does alter the fertilization ability of oocytes. Toxicol Appl Pharmacol. (2015) 289:507–14. doi: 10.1016/j.taap.2015.10.010 [DOI] [PubMed] [Google Scholar]
- 72. Siracusa JS, Yin L, Measel E, Liang S and Xiaozhong Yu X. Effects of bisphenol A and its analogs on reproductive health: A mini review. Reprod Toxicol. (2018) 79:96–123. doi: 10.1016/j.reprotox.2018.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Herez SH, Jawad MA, MAbbood MS. Effect of bisphenol A level in follicular fluid on ICSI outCome. J Pharm Negative Results. (2022) 13:370–6. doi: 10.47750/pnr.2022.13.04.045 [DOI] [Google Scholar]
- 74. Di Napoli I, Tagliaferri S, Sommella E, Salviati E, Porri D, Raspini B, et al. Lifestyle habits and exposure to BPA and phthalates in women of childbearing age from northern Italy: A pilot study. Int J Environ Res Public Health. (2021) 18:9710. doi: 10.3390/ijerph18189710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bar-Joseph H, Ben-Aharon I, Rizel S, Stemmer SM, Tzabari M, Shalgi R. Doxorubicin-induced apoptosis in germal vesicle (GV) oocytes. Reprod Toxicol. (2010) 30:566–72. doi: 10.1016/j.reprotox.2010.07.003 [DOI] [PubMed] [Google Scholar]
- 76. Siu MKC, Cheng Y. The blood-follicle barrier (BFB) in disease and in ovarian function. Adv Exp Med Biol. (2012) 763:186–92. doi: 10.1007/978-1-4614-4711-5_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nowak K, Jabłońska E, Ratajczak-Wrona W. Immunomodulatory effects of synthetic endocrine disrupting chemicals on the development and functions of human immune cells. Environ Int. (2019) 125:350–64. doi: 10.1016/j.envint.2019.01.078 [DOI] [PubMed] [Google Scholar]
- 78. Schleimer RP, Berdnikovs S. Etiology of epithelial barrier dysfunction in patients with type 2 inflammatory diseases. J Allergy Clin Immunol. (2017) 139:1752–61. doi: 10.1016/j.jaci.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Loffredo LF, Coden ME, Berdnikovs S. Endocrine disruptor bisphenol A (BPA) triggers systemic para-inflammation and is sufficient to induce airway allergic sensitization in mice. Nutrients. (2020) 12:343. doi: 10.3390/nu12020343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhou H, Ohno N, Terada N, Saitoh S, Naito I, Ohno S. Permselectivity of blood follicle barriers in mouse ovaries of the mifepristone-induced polycystic ovary model revealed by in vivo cryotechnique Reproduction. Reproduction. (2008) 136:599–610. doi: 10.1530/REP-08-0022 [DOI] [PubMed] [Google Scholar]
- 81. Zhou A, Chang H, Huo W, Zhang B, Hu Y, Xia W, et al. Prenatal exposure to bisphenol A and risk of allergic diseases in early life. Pediatr Res. (2017) 81:851–6. doi: 10.1038/pr.2017.20 [DOI] [PubMed] [Google Scholar]
- 82. Wang Y, Cao Z, Zhao H, Ren Y, Hao L, Gu Z. Bisphenol A exacerbates allergic inflammation in an ovalbumin-induced mouse model of allergic rhinitis. J Immunol Res. (2020) 2020:7573103. doi: 10.1155/2020/7573103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xu J, Huang G, Guo TL. Developmental bisphenol A exposure modulates immune-related diseases. Toxics. (2016) 4:23. doi: 10.3390/toxics4040023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Aljadeff G, Longhi E, Yehuda Shoenfeld Y. Bisphenol A: A notorious player in the mosaic of autoimmunity. Autoimmunity. (2018) 51:370–7. doi: 10.1080/08916934.2018.1551374 [DOI] [PubMed] [Google Scholar]
- 85. Buck Louis GM, Sundaram R, Sweeney AM, Schisterman EF, Maisog J, Kannan K. Urinary bisphenol A, phthalates, and couple fecundity: the Longitudinal Investigation of Fertility and the Environment (LIFE) Study. Fertil Steril. (2014) 101:1359–66. doi: 10.1016/j.fertnstert.2014.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Stavridis K, Triantafyllidou O, Pasimiri M, Vlahos N. Bisphenol-A and female fertility: An update of existing epidemiological studies. J Clin Med. (2022) 11:7227. doi: 10.3390/jcm11237227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kechagias KS, Semertzidou AA, Antonios PM, Kyrgiou M. Bisphenol-A and polycystic ovary syndrome: a review of the literature. Rev Environ Health. (2020) 35:323–31. doi: 10.1515/reveh-2020-0032 [DOI] [PubMed] [Google Scholar]
- 88. Keri RA, Ho SM, Hunt PA, Knudsen KE, Soto AM, Prins GS. An evaluation of evidence for the carcinogenic activity of bisphenol A. Reprod Toxicol. (2007) 24:240–52. doi: 10.1016/j.reprotox.2007.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lozada KW, Keri RA. Bisphenol A increases mammary cancer risk in two distinct mouse models of breast cancer. Biol Reprod. (2011) 85:490–7. doi: 10.1095/biolreprod.110.090431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Seachrist DD, Bonk KW, Ho SM, Prins GS, Soto AM, Keri RA. A review of the carcinogenic potential of bisphenol A. Reprod Toxicol. (2016) 59:167–82. doi: 10.1016/j.reprotox.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Khan NG, Correia J, Adiga D, Rai PS, Dsouza HS, Chakrabarty S, et al. A comprehensive review on the carcinogenic potential of bisphenol A: clues and evidence. Environ Sci pollut Res Int. (2021) 28:19643–63. doi: 10.1007/s11356-021-13071-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Perrone P, Lettieri G, Marinaro C, Longo V, Capone S, Forleo A, et al. Molecular alterations and severe abnormalities in spermatozoa of young men living in the "Valley of sacco river" (Latium, Italy): A preliminary study. Int J Environ Res Public Health. (2022) 19:11023. doi: 10.3390/ijerph191711023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ferrero G, Festa R, Follia L, Lettieri G, Tarallo S, Notari T, et al. Small noncoding RNAs and sperm nuclear basic proteins reflect the environmental impact on germ cells. Mol Med. (2024) 30:12. doi: 10.1186/s10020-023-00776-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mazza A, Piscitelli P, Falco A, Santoro ML, Colangelo M, Imbriani G, et al. Heavy environmental pressure in campania and other italian regions: A short review of available evidence. Int J Environ Res Public Health. (2018) 15:105. doi: 10.3390/ijerph15010105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gao H, Yang BJ, Li N, Feng LM, Shi SY, Zhai WH, et al. Bisphenol A and hormone-associated cancers: current progress and perspectives. Med (Baltimore). (2015) 94:e211. doi: 10.1097/MD.0000000000000211 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Boxplots of numerical variables that resulted with significant differences in means, stratified per Age and BMI per zone (two tailed Wilcoxon test shown in Table 2 ).
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.