Abstract
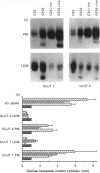
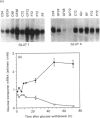
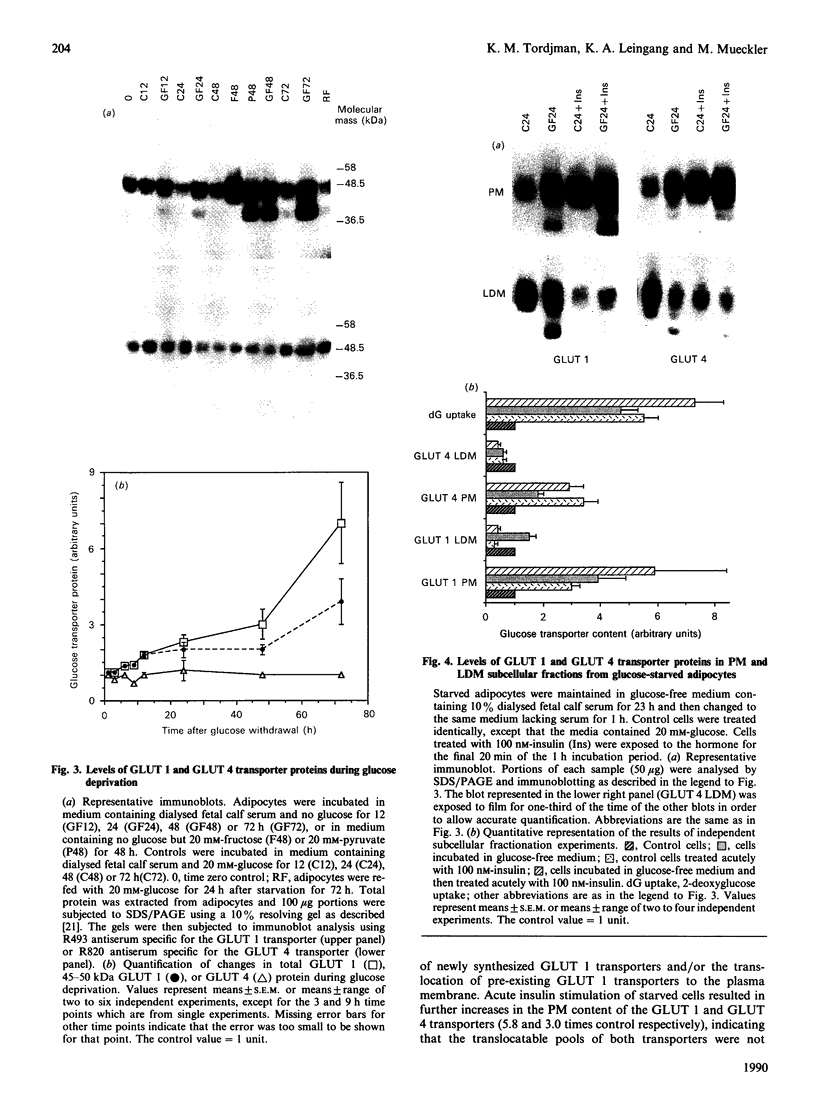
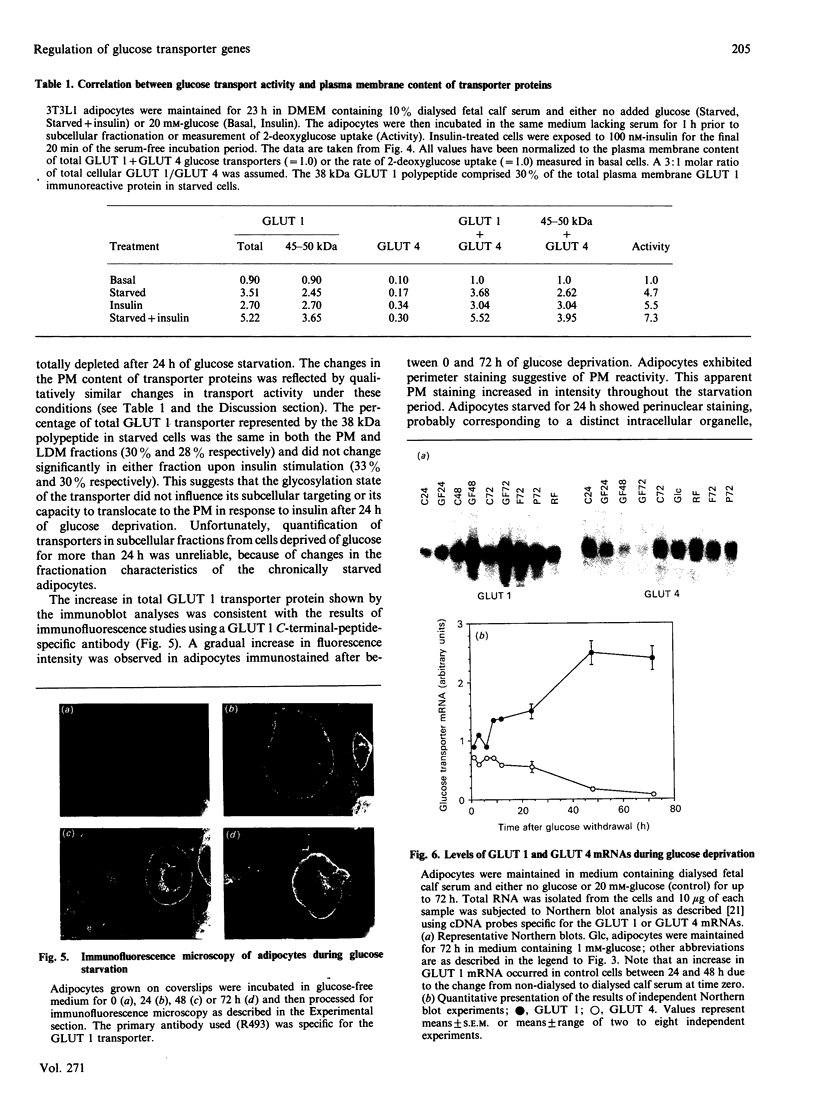
Glucose transport in 3T3L1 adipocytes is mediated by two facilitated diffusion transport systems. We examined the effect of chronic glucose deprivation on transport activity and on the expression of the HepG2 (GLUT 1) and adipocyte/muscle (GLUT 4) glucose transporter gene products in this insulin-sensitive cell line. Glucose deprivation resulted in a maximal increase in 2-deoxyglucose uptake of 3.6-fold by 24 h. Transport activity declined thereafter but was still 2.4-fold greater than the control by 72 h. GLUT 1 mRNA and protein increased progressively during starvation to values respectively 2.4- and 7.0-fold greater than the control by 72 h. Much of the increase in total immunoreactive GLUT 1 protein observed later in starvation was the result of the accumulation of a non-functional or mistargeted 38 kDa polypeptide. Immunofluorescence microscopy indicated that increases in GLUT 1 protein occurred in presumptive plasma membrane (PM) and Golgi-like compartments during prolonged starvation. The steady-state level of GLUT 4 protein did not change during 72 h of glucose deprivation despite a greater than 10-fold decrease in the mRNA. Subcellular fractionation experiments indicated that the increased transport activity observed after 24 h of starvation was principally the result of an increase in the 45-50 kDa GLUT 1 transporter protein in the PM. The level of the GLUT 1 transporter in the PM and low-density microsomes (LDM) was increased by 3.9- and 1.4-fold respectively, and the GLUT 4 transporter content of the PM and LDM was 1.7- and 0.6-fold respectively greater than that of the control after 24 h of glucose deprivation. These data indicate that newly synthesized GLUT 1 transporters are selectively shuttled to the PM and that GLUT 4 transporters undergo translocation from an intracellular compartment to the PM during 24 h of glucose starvation. Thus glucose starvation results in an increase in glucose transport in 3T3L1 adipocytes via a complex series of events involving increased biosynthesis, decreased turnover and subcellular redistribution of transporter proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Murray J. C., Nakamura Y., Kayano T., Eddy R. L., Fan Y. S., Byers M. G., Shows T. B. Polymorphic human insulin-responsive glucose-transporter gene on chromosome 17p13. Diabetes. 1989 Aug;38(8):1072–1075. doi: 10.2337/diab.38.8.1072. [DOI] [PubMed] [Google Scholar]
- Berger J., Biswas C., Vicario P. P., Strout H. V., Saperstein R., Pilch P. F. Decreased expression of the insulin-responsive glucose transporter in diabetes and fasting. Nature. 1989 Jul 6;340(6228):70–72. doi: 10.1038/340070a0. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum M. J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989 Apr 21;57(2):305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Blok J., Gibbs E. M., Lienhard G. E., Slot J. W., Geuze H. J. Insulin-induced translocation of glucose transporters from post-Golgi compartments to the plasma membrane of 3T3-L1 adipocytes. J Cell Biol. 1988 Jan;106(1):69–76. doi: 10.1083/jcb.106.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron M. J., Brosius F. C., 3rd, Alper S. L., Lodish H. F. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2535–2539. doi: 10.1073/pnas.86.8.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Fukumoto H., Kayano T., Buse J. B., Edwards Y., Pilch P. F., Bell G. I., Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989 May 15;264(14):7776–7779. [PubMed] [Google Scholar]
- Garvey W. T., Huecksteadt T. P., Birnbaum M. J. Pretranslational suppression of an insulin-responsive glucose transporter in rats with diabetes mellitus. Science. 1989 Jul 7;245(4913):60–63. doi: 10.1126/science.2662408. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Derechin V., James D. E., Tordjman K., Ahern S., Gibbs E. M., Lienhard G. E., Mueckler M. Insulin-stimulated translocation of the HepG2/erythrocyte-type glucose transporter expressed in 3T3-L1 adipocytes. J Biol Chem. 1989 Feb 5;264(4):2180–2184. [PubMed] [Google Scholar]
- Haspel H. C., Wilk E. W., Birnbaum M. J., Cushman S. W., Rosen O. M. Glucose deprivation and hexose transporter polypeptides of murine fibroblasts. J Biol Chem. 1986 May 25;261(15):6778–6789. [PubMed] [Google Scholar]
- James D. E., Brown R., Navarro J., Pilch P. F. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988 May 12;333(6169):183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., McLenithan J. C., Braiterman L. T., Cornelius P., Pekala P. H., Lane M. D. Sequence, tissue distribution, and differential expression of mRNA for a putative insulin-responsive glucose transporter in mouse 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1989 May;86(9):3150–3154. doi: 10.1073/pnas.86.9.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B., Charron M. J., Lodish H. F., Cushman S. W., Flier J. S. Differential regulation of two glucose transporters in adipose cells from diabetic and insulin-treated diabetic rats. J Clin Invest. 1989 Aug;84(2):404–411. doi: 10.1172/JCI114180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Mueckler M. Family of glucose-transporter genes. Implications for glucose homeostasis and diabetes. Diabetes. 1990 Jan;39(1):6–11. doi: 10.2337/diacare.39.1.6. [DOI] [PubMed] [Google Scholar]
- Shows T. B., Eddy R. L., Byers M. G., Fukushima Y., Dehaven C. R., Murray J. C., Bell G. I. Polymorphic human glucose transporter gene (GLUT) is on chromosome 1p31.3----p35. Diabetes. 1987 Apr;36(4):546–549. doi: 10.2337/diab.36.4.546. [DOI] [PubMed] [Google Scholar]
- Simpson I. A., Yver D. R., Hissin P. J., Wardzala L. J., Karnieli E., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transporters in the isolated rat adipose cells: characterization of subcellular fractions. Biochim Biophys Acta. 1983 Dec 19;763(4):393–407. doi: 10.1016/0167-4889(83)90101-5. [DOI] [PubMed] [Google Scholar]
- Sivitz W. I., DeSautel S. L., Kayano T., Bell G. I., Pessin J. E. Regulation of glucose transporter messenger RNA in insulin-deficient states. Nature. 1989 Jul 6;340(6228):72–74. doi: 10.1038/340072a0. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman K. M., Leingang K. A., James D. E., Mueckler M. M. Differential regulation of two distinct glucose transporter species expressed in 3T3-L1 adipocytes: effect of chronic insulin and tolbutamide treatment. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7761–7765. doi: 10.1073/pnas.86.20.7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. S., Donovan J. A., Van Ness B. G., Fellows R. E., Pessin J. E. Glucose-dependent regulation of glucose transport activity, protein, and mRNA in primary cultures of rat brain glial cells. J Biol Chem. 1988 Oct 25;263(30):15594–15601. [PubMed] [Google Scholar]
- Walker P. S., Ramlal T., Donovan J. A., Doering T. P., Sandra A., Klip A., Pessin J. E. Insulin and glucose-dependent regulation of the glucose transport system in the rat L6 skeletal muscle cell line. J Biol Chem. 1989 Apr 15;264(11):6587–6595. [PubMed] [Google Scholar]
- Zorzano A., Wilkinson W., Kotliar N., Thoidis G., Wadzinkski B. E., Ruoho A. E., Pilch P. F. Insulin-regulated glucose uptake in rat adipocytes is mediated by two transporter isoforms present in at least two vesicle populations. J Biol Chem. 1989 Jul 25;264(21):12358–12363. [PubMed] [Google Scholar]
- van Putten J. P., Krans H. M. Glucose as a regulator of insulin-sensitive hexose uptake in 3T3 adipocytes. J Biol Chem. 1985 Jul 5;260(13):7996–8001. [PubMed] [Google Scholar]