ABSTRACT
Patients with chronic kidney disease (CKD) have a high incidence and prevalence of atrial fibrillation (AF). While general treatment strategies for AF may largely be transferred to patients with mild to moderate CKD, patients with advanced CKD—particularly hemodialysis (HD) patients—with AF pose substantial therapeutical challenges to cardiologists and nephrologists. The arguably greatest dilemma is the very limited evidence on appropriate strategies for prevention of stroke and systemic embolism in HD patients with AF, since the risk for both thromboembolic events without oral anticoagulation and severe bleeding events with oral anticoagulation are substantially increased in advanced CKD, compared with the general population. Thus, the benefit to risk ratio of either vitamin K antagonists or direct oral anticoagulants is less evident in HD than in non-CKD patients with AF.
As a multidisciplinary panel of clinicians, we here propose 10 tips that may help our colleagues to navigate between the risk of undertreatment—exposing CKD patients with AF to a high stroke risk—and overtreatment—exposing the very same patients to a prohibitively high bleeding risk. These tips include ideas on alternative risk stratification strategies and novel treatment approaches that are currently in clinical studies—such as factor XI inhibitors or left atrial appendage closure—and may become game-changers for HD patients with AF.
Keywords: atrial fibrillation, FXI inhibition, hemodialysis, oral anticoagulation, risk stratification
INTRODUCTION
Oral anticoagulation (OAC) for stroke prevention in atrial fibrillation (AF) is particularly challenging in patients on maintenance hemodialysis (HD). Evidence obtained from studies on patients without severe kidney dysfunction cannot be extrapolated uncritically to HD patients, since they have many particularities that profoundly alter the risk–benefit ratio of OAC. Studies dedicated to the HD population are limited to three small and underpowered randomized controlled trials (RCTs) [1–4] and observational studies flawed by indication bias as well as healthy user bias. As a consequence, guidance for nephrologists and cardiologists remains largely opinion-based. In the present communication, we provide practical tips to help clinicians manage the conundrum of OAC in HD patients with AF.
TIP 1: DO NOT UNCRITICALLY APPLY THE CHA2DS2-VASc SCORE BUT CONSIDER USING THE DIALYSIS RISK SCORE FOR RISK STRATIFICATION IN HD PATIENTS WITH AF OR “NO OAC” AS A STRATEGY
The decision to use OAC in AF involves weighing the risk of a thromboembolic event without therapy against the risk of a hemorrhagic event on therapy. The CHA2DS2-VASc score is widely employed to discriminate patients who will derive a net benefit from OAC from those who will suffer a net harm. The components of the CHA2DS2-VASc score are so prevalent in HD patients with AF that most qualify for OAC if the guidelines are applied. The Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) score added markers of kidney disease to most of the components of the CHA2DS2-VASc score [5]. In the large UK Clinical Practice Research Datalink AF cohort, the ATRIA score identified low-risk patients better than the CHA2DS2-VASc score, in whom it may prevent overuse of OAC [6]. However, ATRIA is not widely used in clinical practice.
The relationship between AF and stroke is not as straightforward in HD patients as in the general population. Although several (but not all) studies identify AF as a risk factor for ischemic stroke in HD, it remains unclear to what extent AF is an effector of cardioembolic events rather than a surrogate marker of cardiovascular disease. In addition, a recent systematic review revealed that, while ischemic stroke remains the most common type of stroke among patients with chronic kidney disease (CKD), hemorrhagic stroke becomes more frequent with worsening kidney function, such that the incidence of hemorrhagic stroke approaches the incidence of ischemic stroke in the dialysis population [7]. Even in HD patients not taking antiplatelet agents or OAC, the risk of bleeding requiring hospitalization is disproportionately high [8].
In our opinion, this altered risk–benefit ratio warrants a more restrictive use of OAC in HD. In an attempt to define a subpopulation of HD patients at high risk of thromboembolic events offsetting the risk of major bleeding, the Dialysis Risk Score was developed (Fig. 1). Other approaches to predicting bleeding in dialysis patients have been suggested, including the recently proposed BLEED-HD score [9]. Of note, BLEED-HD does not specifically focus upon patients with AF. Additionally, it does not aim to trade-off the risks of drug-induced bleeding against the benefits of stroke prevention in dialysis patients with AF who are considered candidates for oral anticoagulation; instead, it provides absolute estimates of bleeding risk in individual dialysis patients. An even more conservative approach is to eliminate OAC altogether, a strategy that is currently explored in five RCTs comparing OAC with no OAC in patients with advanced CKD and AF (Table 1). Pending results from these trials, we strongly advise involving the individual patient in shared decision-making discussions about whether or not to treat with OAC.
Figure 1:
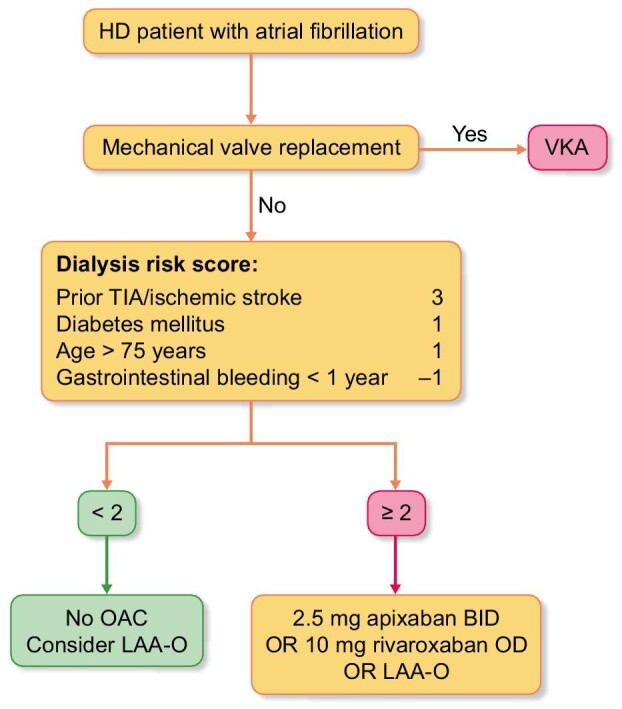
Dialysis Risk Score. The Dialysis Risk Score assigns points to the factors that were significantly associated with subsequent stroke (previous stroke, diabetes and older age) and ignores the factors that were not (hypertension and heart failure) in the Dialysis Outcomes and Practice Patterns Study (DOPPS) [70]. The increased bleeding risk in HD patients is accounted for by including history of gastrointestinal bleeding during the previous year, since this is predictive of subsequent bleeding [8]. Most weight is given to a history of transient ischemic attack or ischemic stroke, since in our view these patients should receive OAC even if they experienced a major bleeding episode. The Dialysis Risk Score is an attempt to restrict OAC to HD patients with a favorable benefit–risk ratio but has not been validated in this population.
Table 1:
Recently completed, ongoing and planned RCT of OAC versus no OAC in advanced CKD with AF.
| Study | Number and selection of patients | Intervention | Duration | Primary Outcome |
|---|---|---|---|---|
| VISIONAIRE | ∼1500 patients CKD5, CHA2DS2-VASc ≥2 | Randomized 1:1:2 to edoxaban 30 mg qd, VKA (INR 2–3), or no OAC | N/A | Composite of stroke or systemic embolism; major and clinically relevant non-major bleeding (ISTH definition) |
| SACK, NCT05679024 | 1400 CKD5 or CKD5D, CHA2DS2-VASc ≥2 for men or ≥3 for women | Apixaban 2.5 mg bid vs no OAC | 72 months or when 247 primary events have been reached, whichever comes first | Time to first thromboembolic event; time to dialysis access thrombosis; time to kidney replacement therapy; delayed graft function; thrombosis of renal artery or vein in patients undergoing kidney transplantation; time to major bleeding (ISTH definition) |
| AVKDIAL, NCT02886962 | 855 prevalent HD, CHA2DS2-VASc ≥2 | VKA (INR 2–3) vs no OAC | 2 years | Severe bleeding and thrombosis |
| DANWARD, NCT03862859 | 718 prevalent dialysis; incident AF only with CHA2DS2-VASc ≥2 | VKA (INR 2–3) vs no OAC | ≤4 years | TIA, ischemic or unspecific stroke; major bleeding (ISTH definition) |
| SAFE-Db, NCT03987711 | 151 prevalent HD/PD, CHADS-65 criteria: age ≥65 or age <65 years with one of: hypertension, diabetes, congestive heart failure, stroke/TIA or peripheral embolism | VKA (INR 2–3) vs apixaban 5 mg bida vs no OAC | 26 weeks | Pilot to test feasibility of larger trial: recruitment of target population within 2 years; ≥80% participants remain in study and on allocated treatment after Week 26 |
a2.5 mg bid in patients meeting the criteria for reduced dose.
bAs of March 2024, the study is completed, but results are not yet published in a peer-reviewed journal.
TIA: transient ischemic attack; ISTH: International Society on Thrombosis and Hemostasis.
TIP 2: CONSIDER INCLUDING AF BURDEN (TIME IN AF) AS AN ADDITIONAL RISK FACTOR
AF is typically categorized clinically according to AF burden or the proportion of time that the patients spend in AF: paroxysmal AF (<7 days), persistent AF (>7 days), longstanding persistent AF (>12 months) and permanent AF (accepted by patient and physician). The concept that AF burden proportionally affects stroke risk has been brought up previously [10]. AF burden gained particular interest when the ASSERT (ASymptomatic atrial fibrillation and Stroke Evaluation in pacemaker patients and the atrial fibrillation Reduction atrial pacing Trial) study demonstrated higher rates of clinical overt AF as well as strokes in patients with subclinical AF, defined as atrial high rate episodes (AHRE) only detected by implanted devices (mostly pacemakers and implantable cardioverter defibrillators) [11]. Predominantly longer (>24 h) AHRE episodes contributed to increased stroke risk [12]. A statement of the American Heart Association (AHA) elaborated on the relevance of subclinical AF for stroke risk, suggesting that clinically overt AF episodes may only be the “tip of the iceberg” [13]. Recently, two interventional trials suggested that OAC in high-risk patients with subclinical AF (CHA2DS2-VASc 3.9 and 4, mean AHRE duration ∼3 h) allow a modest reduction of stroke and systemic embolism at the price of an increased bleeding rate, compared with placebo NOAH-AFNET6 (Non-vitamin K antagonist oral anticoagulants in patients with Atrial High rate episodes Atrial Fibrillation NETwork) [14] or with aspirin ARTESIA (Apixaban for the Reduction of Thrombo-Embolism in patients with device-detected subclinical atrial fibrillation trial) [15]. Both trials excluded patients with severe kidney disease; mean creatinine clearance was 66 mL/min in NOAH-AFNET6 and 71 mL/min in ARTESIA. In a recent metaanalysis of both trials [16], increased bleeding risk was shown to occur early, while the stroke-preventive effect accrued over time. As life expectancy is far lower in dialysis patients with AF than in the non-dialysis participants of NOAH-AFNET6 and ARTESIA, and as drug adherence is particularly short in dialysis patients on OAC, this metaanalysis further supports our reluctancy to initiate OAC in dialysis patients, who may particularly suffer early bleeding events (given their highly increased bleeding risk), but potentially not witness the potential longer term benefits of stroke prevention (given the poor drug adherence and high mortality).
Whether the association of AF burden with stroke risk is consistent in patients on dialysis has not been studied. The issue is further complicated by the high prevalence of self-limiting bouts of AF occurring during dialysis as a result of fluid and electrolyte shifts [17, 18] when the patient is already heparinized.
In summary, while AF burden may be considered for individual therapeutic decisions, more evidence is needed before it can be integrated into routine clinical pathways for dialysis patients.
TIP 3: REGULARLY RE-ESTIMATE INDICATION FOR OAC AND BLEEDING RISK IN HD PATIENTS
There is compelling evidence in the non-dialysis population that risk stratification with common bleeding and stroke risk scores such as CHA2DS2-VASc and HAS-BLED results in a net clinical benefit that strongly supports initiation of indefinite OAC in most patients. Guidelines generally endorse risk assessment approximately every year in stable patients [19, 20].
However, while the absolute risk of thromboembolism generally by far exceeds that of life-threatening bleeding risk in the non-dialysis population, risk stratification is more challenging in advanced CKD, since dialysis patients with AF have a markedly increased risk not only of thromboembolic events, but also of severe bleeding events, including intracranial hemorrhage [21].
The RENAL-AF (Renal Hemodialysis Patients Allocated Apixaban Versus Warfarin in Atrial Fibrillation) trial illustrates the complexity of standard risk assessment in this population [1]. Patients with AF receiving HD with a CHA2DS2-VASc score ≥2, the current threshold of stroke risk supporting initiation of OAC, were randomized to apixaban or warfarin. With a median follow-up <1 year, the occurrence of major or clinically relevant non-major bleeding was 22%–26% accompanied by a mortality rate of 18%–26%, both of which were markedly higher than the 1%–3% incidence of ischemic stroke [22]. These data underscore that although anticoagulation is effective in reducing AF-related stroke, the absolute benefit in this population is modest for many patients in contrast to the markedly higher risk of bleeding and the high mortality rate that may in large part not be thrombosis mediated and therefore not modifiable by anticoagulation.
Given the differential risks in the dialysis population, current stroke and bleeding risk stratification tools do not accurately identify patients with a net clinical benefit of anticoagulation. In addition, the short-term risk of mortality must be incorporated as the limited life-expectancy of many patients precludes a meaningful benefit of anticoagulation. As the AF patient on dialysis is more complex with highly variable clinical course, more regular risk reassessment, every 3–6 months, is recommended.
In patients with advanced CKD, the European Heart Rhythm Association recommends to discontinue apixaban, edoxaban and rivaroxaban before elective procedures with low and high bleeding risk for 36 and 48 h, respectively [23]. Of note, these recommendations—which have subsequently been endorsed by KDIGO [24]—formally only apply to patients with creatinine clearance of 15–30 mL/min, and deliberately exclude CKD G5 patients. Notably, pre-operative bridging with heparin is not recommended in direct oral anticoagulant (DOAC)-treated patients, as the predictable waning of the anticoagulation effect allows for properly timed short-term cessation of DOAC before surgery [23]. Full-dose DOAC should be resumed 24 h after low-risk and 48–72 h after high-risk interventions, and prophylactic dosage(s) of heparin may be considered 6–8 h after surgery, until re-initiation of full-dose DOAC.
TIP 4: DO NOT ROUTINELY USE VITAMIN K ANTAGONISTS IN HD PATIENTS
Numerous epidemiological studies have suggested that vitamin K antagonists (VKA) may not reduce the number of ischemic strokes in HD patients with AF, whereas they expose these patients to a high risk of major bleeding—including hemorrhagic strokes [25]. Pending the results of RCT comparing VKA with no anticoagulation in those patients (Table 1), the apparent lack of efficiency of VKA for prevention of ischemic strokes may be explained by a number of observations.
Firstly, as discussed before, it is still unclear how far AF is an effector of cardioembolic events—including ischemic strokes—rather than a mere surrogate marker of cardiovascular disease. In the latter case, VKA are less efficient for prevention of ischemic strokes. Secondly, effective anticoagulation with VKA is challenging in HD patients, who spend alarmingly little time in the target international normalized ratio (INR) range. Vice versa, HD patients without OAC still require intermittent heparinization during each dialysis session. As such, patients with and without VKA differ less distinctively with respect to the degree of anticoagulation. Thirdly, data from non-dialysis patients suggest that OAC with VKA may exert more nephrotoxic effects than DOAC [26, 27]. If similar effects also occur in HD patients with residual renal function, faster progression towards anuria and more profound uremia with VKA treatment might accelerate the progression of atherosclerotic vascular disease. Admittedly, clinical evidence for protective renal effects of DOAC in dialysis patients are outstanding. Fourthly, VKA may have procalcific effects by inactivating vitamin K–dependent proteins that inhibit vascular calcification, which will further increase the risk of atherosclerotic events, including ischemic stroke [28].
While the clinical relevance of some of these pathophysiological pathways is disputed, results from the three published RCT that compared VKA and DOAC in HD patients with AF suggest in aggregate a prohibitively high bleeding risk with VKA, which is not offset by a better efficiency for the prevention of ischemic strokes (Table 2).
Table 2:
RCT of DOAC vs VKA in dialysis patients with AF.
| Study [Ref] | Design | Patients | Age | Intervention | Duration | Primary endpoint | Further endpoints (selection) | Mortality |
|---|---|---|---|---|---|---|---|---|
| VALKYRIE, 2020–2021 [2, 3] | Open label, N = 132 | Incident or prevalent, HD pat., CHA2DS2-VASc ≥2 (median 5) | 80 years (median) | VKA (INR 2–3; TTR 48% in Months 1–6) vs rivaroxaban 10 mg vs rivaroxaban 10 mg plus Menaquinone-7 (‘Riva + Vit K2’) | Median 1.88 years | Cardiovascular events including stroke: 63.8/100 PY (VKA), 26.2/100 PY (Riva), 21.4/100 PY (Riva + Vit K2)d | Life-threatening/severe bleeding, VKA: 17 pat. (30 events), Riva: 8 pat. (11 events), Riva + Vit K2: 9 pat. (12 events) | 33.7/100 PY (VKA), 28.3/100 PY (Riva), 30.2/100 PY (Riva + Vit K2) |
| RENAL-AF, 2022, [1] | Open label, N = 154 | Prevalent HD pat.c, CHA2DS2-VASc ≥2 | Median (IQR) 68 (61–75) years | VKA (INR 2–3; TTR 44 %) vs apixaban 5 mg bida | Median 330 days (apixaban) 340 days (VKA) | Major bleeding or CRNMB (ISTH definition), 31.5 1-year incidence (apixaban), 25.5 1-year incidence (VKA) (including intracranial bleeding: 1 pat. in both groups) | Ischemic stroke/systemic embolism, apixaban: 1 (1%), VKA: 2 (3%) | Apixaban: 21 (26%), VKA: 13 (18%) |
| AXADIA, 2023 [4] | Open label, N = 97 | Prevalent HD pat.b, CHA2DS2-VASc ≥2 (median 4.5) | 74.7 ± 7.9 years | VKA (INR 2–3; TTR 50.7%) vs apixaban 2.5 mg bid | Median 429 days (apixaban) 506 days (VKA) | All-cause death, major bleeding, CRNMB (ISTH definition), 36.1/100 PY (apixaban), 36.6/100 PY (VKA) | Thromboembolic events (AMI, ischemic stroke, all-cause death, VTE), apixaban 16.4/100 PY, VKA 22.0 PY | Apixaban 14.8/100 PY, VKA 17.6/100 PY |
Two times 2.5 mg for patients with target weight ≤60 kg and/or age ≥80 years.
bInitial target: 222 patients.
cInitial target: 760 patients.
In initial publication: primary endpoint: “Change of coronary artery calcification, thoracic aorta calcification, and PWV over 18 months.”
AMI: acute myocardial infarction; CRNMB: clinically relevant nonmajor bleeding; IQR: interquartile range; ISTH: International Society on Thrombosis and Hemostasis; PY: patient years; Riva: rivaroxaban; TIA: transient ischemic attack; TTR: time in therapeutic range; VALKYRIE: Safety and Efficacy of Vitamin K Antagonists Versus Rivaroxaban in Hemodialysis Patients With Atrial Fibrillation: a Multicenter Randomized Controlled Trial; VTE: venous thromboembolism (deep vein thrombosis, pulmonary embolism).
We acknowledge that VKA remain mandatory in patients after mechanical valve replacement, and that data from RCTs in the general population suggest their superiority over DOAC in rheumatic moderate or severe mitral stenosis [29], as well as in patients with antiphospholipid-antibody syndrome [30–32].
TIP 5: PREFER DOSE-ADJUSTED APIXABAN OR RIVAROXABAN OVER DABIGATRAN OR EDOXABAN IN HD PATIENTS
In an individual patient-level network meta-analysis comprising data of 71 683 patients from the key efficacy trials comparing DOAC with VKA, DOAC (dose adjusted based on age, weight and kidney function, as indicated in study protocols) had beneficial effects with respect to protection against thromboembolic events that rose with declining renal function [33]. In contrast, DOAC dosage lowering that went beyond these protocol-specified adjustments for age, weight and kidney function increased the incidence of thromboembolic events [33]. However, these data only apply to patients with CKD down to a creatinine clearance of 25 mL/min, who were included into these key trials.
Prescribing DOAC in HD patients is complicated by renal clearance requiring dose adjustments and potential removal by dialysis. Dosing recommendations are supported by limited pharmacokinetic and clinical data. Dabigatran is 80% renally eliminated and a regular HD session removes 50%–60% of the dose. The risk of accumulation and the unpredictable effect of dialysis rule out dabigatran for use in HD patients. Edoxaban is minimally affected by dialysis, but exhibits substantial renal elimination (50%) requiring a dose reduction to avoid accumulation. To date, no study has evaluated the efficacy and safety of a reduced dose of edoxaban in HD. Rivaroxaban is also significantly eliminated by the kidneys (35%) and not removed by dialysis [34]. Low-dose rivaroxaban (10 mg) in HD results in similar exposure as regular dose (20 mg) in healthy volunteers [34] and compares favorably to VKA with respect to safety and efficacy in the setting of a RCT [2, 3]. Apixaban has the lowest renal elimination (27%) and is not removed by dialysis. The official dosing recommendations from the US Food and Drug Administration (FDA; 5 mg bid or 2.5 mg bid if body weight ≤60 kg or age ≥80 years) have given rise to substantial controversy. A pharmacokinetic study demonstrated that apixaban 2.5 mg bid in HD patients resulted in comparable drug exposure as the standard dose in patients without renal function impairment, while 5 mg bid was associated with supratherapeutic levels [35]. In contrast, the pharmacokinetic data collected during the RENAL-AF trial revealed that apixaban exposure in dialysis patients taking 5 mg was similar to that of patients with estimated creatinine clearance of 30–44 and 45–59 mL/min [1]. In a large observational study of 4313 apixaban new users with AF and advanced CKD, use of 5 mg was associated with a higher risk of bleeding compared with 2.5 mg, with no difference in the risk of stroke, systemic embolism or death [36], thus challenging the official FDA dosing recommendations.
Concomitant administration of low molecular weight heparin (LMWH) during dialysis may further compound the bleeding risk in patients on DOAC.
Taken together, we prefer the use of low-dose rivaroxaban (10 mg qd) or apixaban (2.5 mg bid) in HD patients, acknowledging that this suggestion is based on pharmacokinetic studies, and more clinical evidence on the clinical efficiency of low-dose rivaroxaban or apixaban for thromboembolic risk reduction is needed.
Moreover, it should be noted that, in contradistinction to the FDA, the European Medicines Agency does not recommend the use of rivaroxaban and apixaban in patients with estimated glomerular filtration rate (eGFR) <15 mL/min.
TIP 6: ADJUST LMWH DOSAGE IN PATIENTS ON OAC
To prevent intradialytic extracorporeal clotting, the vast majority of HD patients receive systemic anticoagulation during each dialysis session—in most cases either unfractionated heparin (UFH) or LMWHs. Even in HD patients without concomitant OAC there is no consensus on whether to prefer intradialytic UFH or LMWH with regard to safety or efficiency, nor if and how to monitor their anticoagulatory effects, even though many centers use either activated clotting time (ACT) or activated partial thromboplastin time (aPTT) for UFH, and anti-factor Xa activity for LMWH, respectively [37, 38]. No specific targets for ACT, aPTT or anti-factor Xa activity have been defined and prospectively validated in multicenter trials for patients without OAC.
From a pharmacodynamic point of view, patients with AF who receive DOAC or VKA should require either no or only low-dose heparin intradialytically. Few prospective studies tested whether intradialytic heparin for prevention of dialysis circuit clotting can be omitted in patients with OAC, and these yielded inconsistent results [39–41]. Similarly, few data exist on how far the heparin dosage can be reduced, whether to prefer UFH or LMWH, and how to monitor intradialytic anticoagulation in HD patients on OAC. In the absence of solid evidence, we maintain our previous recommendation of an individualized and empirical step-wise dose reduction of heparin in patients on OAC [42]; dialysis filters should be checked regularly for clotting.
We do not recommend LMWH for prevention of ischemic stroke or systemic embolism in HD patients with AF. No single LMWH regimen (usually the combination of a single intravenous dose on dialysis days and a single subcutaneous dose on non-dialysis days) has been tested for efficacy and safety in an adequately sized clinical study. Instead, safety concerns exist, which include the risk of hyperlipidemia, osteoporosis, heparin-induced thrombocytopenia and hyperkalaemia. Next, use of LMWH in HD patients bears the risk of under- or overdosage, as LMWH may accumulate substantially in advanced CKD. Regular measurements of anti-factor Xa activity are cumbersome (given the need for sampling at defined time points after LMWH dosing) and of uncertain clinical relevance. Finally, it has been demonstrated that a majority of patients (and care providers) prefer oral medication over subcutaneous administration [43].
TIP 7: ESTIMATE OVERALL CARDIOVASCULAR RISK, CONSIDER USING LOW-DOSE DOAC AS “VASCULOPROTECTION” ALSO IN PATIENTS WITHOUT AF
More than three-quarters of patients on HD are suffering from cardiovascular events [44] with (i) an almost 6-fold increase in ischemic stroke with concomitant AF [21], (ii) an increased risk for arterial events independent of AF [45] and (iii) a >4-fold elevated risk of venous thromboembolism [46]. Thus, it is crucial to address the entire spectrum of cardiovascular risk in these patients. The vasculoprotective effects of DOAC beyond their anticoagulant properties have recently garnered attention. In patients with vascular disease and renal dysfunction (eGFR <60 mL/min/1.73 m2, excluding HD) low-dose DOAC (rivaroxaban 2.5 mg bid) reduced the composite of cardiovascular death, myocardial infarction or stroke from 8.4% to 6.4%, but increased major bleeding from 2.7% to 3.9% in the COMPASS trial [47]. A very high risk for major adverse cardiac events (MACE) and major adverse limb events (MALE) exists after revascularization for peripheral arterial disease. The VOYAGER PAD (Efficacy and Safety of Rivaroxaban in Reducing the Risk of Major Thrombotic Vascular Events in Subjects With Symptomatic Peripheral Artery Disease Undergoing Peripheral Revascularization Procedures of the Lower Extremities) trial revealed that in patients with eGFR <45 mL/min/1.73 m2, rivaroxaban 2.5 mg bid versus placebo reduced MACE and MALE from 12.7 to 11.0 per 100 patient years, while the risk for major bleeding was increased from 0.8 to 2.5 [48]. The absolute net clinical benefit was greatest in polyvascular patients, those with mild or moderate heart failure, kidney disease, diabetes and after peripheral intervention, and in patients taking four or more concomitant cardiovascular medications, as is frequently the case in renal insufficiency [49]. Data on vasculo-protection of DOAC in HD patients are still missing. Currently, the placebo-controlled TRACK (Treatment of cardiovascular disease with low dose Rivaroxaban in Advanced Chronic Kidney disease) trial (ClinicalTrials.gov Identifier NCT03969953) examines the effect of low-dose rivaroxaban (2.5 mg bid, as in [47]) on major adverse cardiovascular events (a composite of cardiovascular death, non-fatal myocardial infarction, stroke and peripheral artery disease) and major bleeding, and targets to recruit 2000 patients with advanced CKD (CKD G4–5, including peritoneal dialysis and HD patients) with elevated cardiovascular risk who do not have a conventional indication for OAC.
TIP 8: CONSIDER IMPLANTATION OF LEFT ATRIAL APPENDAGE OCCLUDERS FOR STROKE PREVENTION IN HD PATIENTS WITH AF
Transcatheter interventional left atrial appendage occlusion (LAAO) targeting thromboembolism from the left atrial appendage (LAA) has recently emerged as an alternative to OAC, by demonstrating that endocardial LAA closure provides similar protection against stroke, systemic embolism and cardiovascular mortality as VKA [50–52] and DOAC [53, 54], and by extension providing proof of concept of LAA closure [55]. Following successful LAAO, dual antiplatelet therapy (DAPT) is limited to 3 months and followed by long-term aspirin monotherapy. According to current evidence and guidelines, LAAO is a feasible alternative to OAC for stroke prevention in AF patients with bleeding history or contraindications for long-term anticoagulant treatment (class IIb B recommendation in European 2020 AF guidelines and IIa B recommendation in 2023 ACC/AHA/ACCP/HRS AF guidelines) [19, 20].
LAA closure could be especially attractive in HD patients with high stroke and bleeding risk. Unfortunately, HD patients were excluded or underrepresented in all LAAO RCTs and large registries [50–54]. Evidence from relatively small-sized registries [56, 57] and a meta-analysis [58] demonstrated comparable procedural safety and efficacy of LAAO in patients with advanced CKD as compared with patients with normal kidney function. One Italian multicenter registry analyzed HD patients undergoing LAAO and compared procedural and clinical outcomes with HD patients either receiving OAC or no antithrombotic therapy [57]. Few periprocedural complications were reported. After LAAO implantation, over a median follow-up of 5 years, thromboembolism, bleeding events and death occurred less frequently than in patients on warfarin. Compared with patients who did not undergo LAAO implantation and who received no OAC, patients after LAAO implantation had fewer thromboembolic events.
RCTs are needed to address the status of interventional LAAO in HD patients with AF. The ongoing European-wide ‘Left Atrial Appendage closure in patients with non-valvular AF and end stage chronic KIDNEY disease (LAA-KIDNEY)’ trial (NCT05204212) is the first RCT to systematically examine the clinical benefit of interventional LAAO versus best medical care in AF patients with kidney failure (eGFR <15 mL/min/1.73 m²). The primary endpoint is a combined net clinical benefit endpoint, defined as time to first stroke (including ischemic or hemorrhagic strokes), systemic embolism, cardiovascular or unexplained death or major bleeding. As of June 2024, more than 100 of 430 planned patients (23%) have been randomized in this event-driven trial. The LAA-KIDNEY trial has the potential to fill an important evidence gap and help to determine the best treatment for stroke prevention in AF patients with kidney failure. Of note, two other RCTs had been initiated before LAA-KIDNEY (Watch-AFIB (Left Atrial Appendage Occlusion vs. Usual Care in Patients With Atrial Fibrillation and Severe Chronic Kidney Disease), NCT02039167 and STOP-HARM (The Strategy to Prevent Hemorrhage Associated With Anticoagulation in Renal Disease Management Trial), NCT02885545), but were both prematurely terminated due to a slow recruitment rate.
Thus, until LAA-KIDNEY data become available, careful patient selection, management and surveillance involving multidisciplinary teams are essential for selecting HD patients who may benefit from LAAO. Outside of clinical studies, LAAO implantation should be currently restricted to HD patients with bleeding history or high bleeding risk, to ensure maximum clinical safety and benefit.
TIP 9: DO NOT ROUTINELY COMBINE OAC AND APT OR USE DAPT IN HD PATIENTS FOR A PROLONGED PERIOD OF TIME
Combining OAC and antiplatelet therapy (APT) is generally required for a limited period of time after percutaneous coronary interventions (PCI) and/or acute coronary syndrome (ACS) in patients with AF. Current cardiological guidelines recommend dual therapy with P2Y12-inhibition and DOAC, rather than triple therapy with VKA, P2Y12 inhibition and aspirin [59].
Importantly, 12 months after PCI, OAC monotherapy is superior to a combination with APT [60], rendering 1 year the maximum recommended combination therapy period in patients with AF and PCI, regardless of renal function.
In patients not on OAC, recent data indicate safety and efficacy of shorter durations of DAPT following PCI. This is particularly the case in patients with a “high bleeding risk” (HBR) profile, to which CKD substantially contributes. A metaanalysis on n = 25 960 patients demonstrated a reduced bleeding risk and no increase in ischemic cardiovascular events if DAPT is reduced to a ticagrelor monotherapy after 1–3 months, whereas data on early clopidogrel monotherary are less convincing [61]. Of note, as most of the data available, CKD patients were scarce in the studies, with 15% of patients of the metaanalysis were reported to have had a ‘history of renal disease’, and the mean creatinine clearance being 83 and 90 mL/min/1.73 m² in the ticagrelor and clopidogrel comparisons, respectively.
The prospective trials on shortened DAPT durations following PCI in HBR had selected patients and conditions (such as biodegradable polymer stents) and may not be generalized. Particularly, they generally excluded patients with severe kidney disease, including HD patients. The most recent ESC guidelines on ACS therefore only state “no information available” for clopidogrel and “not recommended” for ticagrelor and prasugrel in patients with CKD G5 [59]. A detailed discussion of platelet dysfunction in advanced CKD and the effects of different APT agents—which is beyond the scope of the present manuscript—have been provided recently [62].
Given their high bleeding risk, HD patients may particularly benefit from minimizing the duration of DAPT and of combined OAC and APT. In HD patients on OAC, one antiplatelet drug (preferentially clopidogrel) should be continued for at least 6 months following PCI. In HD patients with AF not on OAC, DAPT following PCI should generally be restricted in time to 3–6 months, but antiplatelet monotherapy (generally aspirin, but clopidogrel is also possible) must be continued lifelong.
TIP 10: INCLUDE HD PATIENTS IN CLINICAL STUDIES ON FACTOR XI INHIBITORS, WHENEVER POSSIBLE
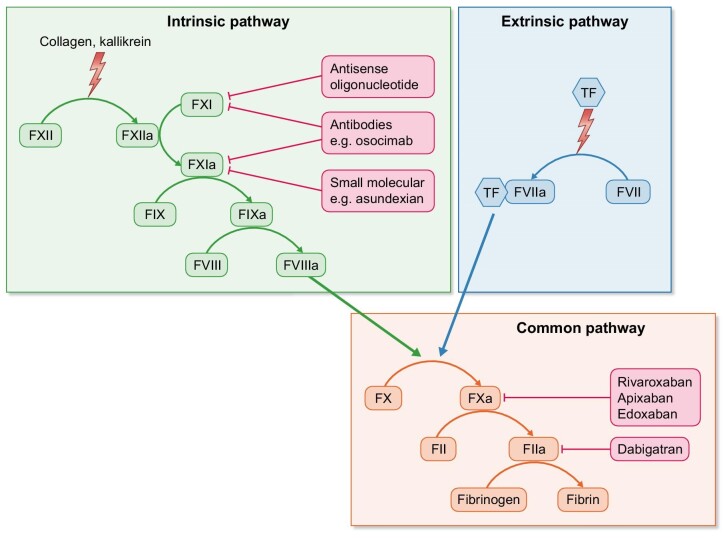
The efficacy and safety of anticoagulation therapy might be improved by the introduction of factor XI (FXI) inhibitors into clinical medicine (Fig. 2). Inhibition of FXI is thought to prevent pathological thrombus formation and growth without interfering with normal hemostasis after tissue injury and may thus allow clinically effective anticoagulation with a lower bleeding risk compared with LMWH, VKA and DOAC [63].
Figure 2:
FXI/XIa inhibition and DOAC in coagulation cascade. Simplified overview over the human coagulation system and site of action for novel FXI inhibition strategies and for DOAC. Figure adapted from Nopp et al. [71].
A large number of phase 2 trials conducted in the setting of orthopedic surgery, AF, stroke and myocardial infarction have evaluated the safety profile of the FXI inhibitors. Also in HD, the feasibility of safe anticoagulation by inhibition of FXI was demonstrated [64].
The CONVERT (Study to Investigate the Safety of a Drug Called Osocimab at Low and High Doses in Adult Patients With Kidney Failure Requiring Regular Hemodialysis) study randomized 686 HD patients to receive the FXI inhibitor osocimab (either at lower or higher dose) or placebo for a maximum of 18 months [65]. Major and clinically relevant nonmajor bleeding did not occur more often with osocimab than with placebo, and no relevant risk signals emerged. As only 46 patients had AF at baseline, the study was not powered to discern the efficiency of osocimab to reduce embolic (or other cardiovascular) events. Similarly, the RE-THINC (Factor XI LICA to Reduce Events Such as Heart Attack and Stroke in Patients Whose Kidneys Are no Longer Able to Work as They Should and Require Treatment to Filter Wastes From the Blood: Focus is on the Safety of BAY2976217 and the Way the Body Absorbs, Distributes and Removes the Study Drug) study revealed that the bleeding risk—predefined as primary safety endpoint—associated with fesomersen was not different from that of placebo [66]. Of note, major atherosclerotic events were defined as one out of several “other endpoints of interest.” Given the limited number of patients included (n = 307), and the short follow-up period (6–12 months), only four major atherosclerotic events occurred, which were equally distributed across study groups. Thus, RE-THINC could not demonstrate a reduction in atherothrombotic events with fesomersen compared with placebo. Other trials in HD have recently been completed or are currently ongoing (Table 3).
Table 3:
Trials evaluating the effects of FXI inhibition in kidney failure.
| Study | Population | Intervention | Number of participants | Primary outcome | Status |
|---|---|---|---|---|---|
| CS4, NCT02553889 (Phase 2) | Kidney failure treated with hemodialysis | Fesomersen (FXI antisense oligonucleotid) vs placebo | 49 | Safety and tolerability | Completed and published [72] |
| EMERALD, NCT03358030 (Phase 2) | Kidney failure treated with hemodialysis | IS 416858 (FXI antisense oligonucleotid) vs placebo | 213 | Number of participants with major bleeding or clinically relevant non major bleeding | Completed, results on clinicaltrials.gov, not yet published |
| RE-THINc ESRD, NCT04534114 (Phase 2b) | Kidney failure treated with hemodialysis | Fesomersen (FXI antisense oligonucelotid) vs placebo | 307 | Incidence of major bleeding or clinically relevant non major bleeding | Completed and published [66] |
| FXI Hemodialysis Study, NCT05027074 (Phase 2) | Kidney failure treated with hemodialysis via arteriovenous graft | MK-2060 (anti-FXI monoclonal antibody) vs placebo | 489 | Time to first arteriovenous graft thrombosis | Active, not yet recruiting |
| AB023, NCT03612856 (Phase 2) | Kidney failure treated with hemodialysis | AB023 (Xisomab, anti-FXI monoclonal antibody) vs placebo | 27 | Number of treatment-related adverse events | Completed and published [73] |
| CONVERT, NCT04523220 (Phase 2b) | Kidney failure treated with hemodialysis | Osocimab (anti-FXI monoclonal antibody) vs placebo | 704 | Cumulative incidence of risk of the first major bleeding or clinically relevant non major bleeding | Completed and published [65] |
Thus, the safety of FXI inhibitors in hemodialysis patients appears favourable. However, their efficacy still needs to be demonstrated. This is underlined by the phase 3 OCEANIC AF (A Study to Learn How Well the Study Treatment Asundexian Works and How Safe it is Compared to Apixaban to Prevent Stroke or Systemic Embolism in People With Irregular and Often Rapid Heartbeat (Atrial Fibrillation), and at Risk for Stroke) study (ClinicalTrials.gov Identifier NCT 05643573), which—outside the core field of nephrology—was planned to test the efficacy and safety of the FXI inhibitor asundexian in 18 000 AF patients. As communicated recently, OCEANIC AF was terminated prematurely, since asundexian had lower efficacy compared with standard treatment with apixaban [67].
Therefore, while the safety of FXI inhibitors appears promising for the time being, more data on its efficacy (prevention of ischemic stroke and systemic embolism) are needed before the first FXI inhibitor may ultimately be licensed.
FUTURE OUTLOOK
The complexities of anticoagulation management in patients with HD and AF, as outlined above, underscore the urgent need to generate reliable evidence in this population. Even sophisticated analytical methods applied to large observational data are unable to guarantee elimination of moderate systemic biases [68]. Well designed and large RCT remain the best way to evaluate the efficacy and safety of different anticoagulation strategies.
Unfortunately, in the last two decades, representation of patients with advanced CKD in cardiovascular RCTs has not improved for a variety of reasons [69], and trials that aimed to selectively include HD patients often had been terminated prematurely because of poor recruitment [e.g. Watch-AFIB (NCT02039167) and STOP-HARM (NCT02885545)] or recruited fewer patients than planned (RENAL-AF [1], AXADIA (A Safety Study Assessing Oral Anticoagulation with Apixaban versus Vitamin-K Antagonists in Patients with Atrial Fibrillation (AF) and End-Stage Kidney Disease (ESKD) on Chronic Hemodialysis Treatment) [4]). It is therefore critical that the nephrological community makes every effort to include patients in these trials.
Contributor Information
Gunnar H Heine, Saarland University Medical Center, Internal Medicine IV, Department of Nephrology and Hypertension, Homburg, Germany; Agaplesion Markus Hospital, Medical Clinic II, Department of Nephrology and Hypertension, Frankfurt am Main, Germany.
Carolin Schneppe, Saarland University Medical Center, Internal Medicine IV, Department of Nephrology and Hypertension, Homburg, Germany; Agaplesion Markus Hospital, Medical Clinic II, Department of Nephrology and Hypertension, Frankfurt am Main, Germany.
Rupert Bauersachs, Cardioangiologic Center Bethanien, CCB, Gefäßzentrum, Frankfurt am Main, Germany.
Ingo Eitel, University Hospital Schleswig-Holstein, University Heart Center Lübeck, Department of Cardiology, Angiology and Intensive Care Medicine, Lübeck, Germany.
Brendon L Neuen, University of New South Wales, Faculty of Medicine and Health, The George Institute for Global Health, Sydney, Australia.
Christian T Ruff, TIMI Study Group, Cardiovascular Division, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
Stephan H Schirmer, Kardiopraxis Schirmer, Am Altenhof 8, Kaiserslautern, Germany.
An De Vriese, Division of Nephrology and Infectious Diseases, AZ Sint-Jan Brugge, Brugge, and Department of Internal Medicine, Ghent University, Ghent, Belgium.
DATA AVAILABILITY STATEMENT
No new data were generated or analyzed in support of this research.
CONFLICT OF INTEREST STATEMENT
G.H.H. and A.D.V. have no conflicts of interests related to this publication.
C.S. has received honoraria as speaker from AstraZeneca and support for meeting and traveling from Boehringer Ingelheim.
R.B. has received honoraria from Bayer, Bristol Meyers Squibb, LEO-Pharma and Pfizer.
I.E. has received grants from ABBOTT, consulting fees from Daiichi-Sanyko and Boehringer Ingelheim, speaker honoraria from ABBOTT, Boston Scientific, Bayer, Bristo-Myers Squibb, Daiichi-Sankyo and Boehringer-Ingelheim, and received support for traveling and meetings from ABBOTT, Boston Scientific, Bayer, Bristo-Myers Squibb, Daiichi-Sankyo and Boehringer-Ingelheim.
B.N. has received fees for advisory boards, scientific presentations, continuing education, steering committee roles and travel from AstraZeneca, Alexion, Bayer, Boehringer Ingelheim, Cambridge Healthcare, Cornerstone Medical Education, the Limbic, Medscape, Novo Nordisk and Travere Pharmaceuticals, with all honoraria paid to his institution.
C.T.R. has received research grants through institution from Athos, AstraZeneca, Daiichi Sankyo, Janssen and Novartis, and honoraria for scientific ad boards and consulting from Anthos, Bayer, Bristol Myers Squibb, Daiichi Sankyo, Janssen and Pfizer.
S.H.S. has received personal consulting fees from Boehringer Ingelheim, Bristol Myers Squibb (BMS) and Daiichi-Sankyo, lecture fees from Boehringer Ingelheim, BMS, Daiichi-Sankyo, Bayer, AstraZeneca, and support for meeting and travels from Boehringer Ingelheim, BMS, Daiichi-Sankyo, Bayer and AstraZeneca. He has stocks from Bayer, FMC and possible others through indirect stakeholding via funds.
REFERENCES
- 1. Pokorney SD, Chertow GM, Al-Khalidi HR et al. Apixaban for patients with atrial fibrillation on hemodialysis: a multicenter randomized controlled trial. Circulation 2022;146:1735–45. 10.1161/CIRCULATIONAHA.121.054990 [DOI] [PubMed] [Google Scholar]
- 2. De Vriese AS, Caluwé R, Pyfferoen L et al. Multicenter randomized controlled trial of vitamin K antagonist replacement by rivaroxaban with or without vitamin K2 in hemodialysis patients with atrial fibrillation: the Valkyrie Study. J Am Soc Nephrol 2020;31:186–96. 10.1681/ASN.2019060579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Vriese AS, Caluwé R, Van Der Meersch H et al. Safety and efficacy of vitamin K antagonists versus rivaroxaban in hemodialysis patients with atrial fibrillation: a multicenter randomized controlled trial. J Am Soc Nephrol 2021;32:1474–83. 10.1681/ASN.2020111566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reinecke H, Engelbertz C, Bauersachs R et al. A randomized controlled trial comparing apixaban with the vitamin K antagonist phenprocoumon in patients on chronic hemodialysis: the AXADIA-AFNET 8 study. Circulation 2023;147:296–309. 10.1161/CIRCULATIONAHA.122.062779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singer DE, Chang Y, Borowsky LH et al. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc 2013;2:e000250. 10.1161/JAHA.113.000250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van den Ham HA, Klungel OH, Singer DE et al. Comparative performance of ATRIA, CHADS2, and CHA2DS2-VASc risk scores predicting stroke in patients with atrial fibrillation. J Am Coll Cardiol 2015;66:1851–9. 10.1016/j.jacc.2015.08.033 [DOI] [PubMed] [Google Scholar]
- 7. Zamberg I, Assouline-Reinmann M, Carrera E et al. Epidemiology, thrombolytic management, and outcomes of acute stroke among patients with chronic kidney disease: a systematic review and meta-analysis. Nephrol Dial Transplant 2022;37:1289–301. 10.1093/ndt/gfab197 [DOI] [PubMed] [Google Scholar]
- 8. Sood MM, Larkina M, Thumma JR et al. Major bleeding events and risk stratification of antithrombotic agents in hemodialysis: results from the DOPPS. Kidney Int 2013;84:600–8. 10.1038/ki.2013.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Madken M, Mallick R, Rhodes E et al. Development and validation of a predictive risk algorithm for bleeding in individuals on long-term hemodialysis: an international prospective cohort study (BLEED-HD). Can J Kidney Health Dis 2023;10:20543581231169610. 10.1177/20543581231169610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tiver KD, Quah J, Lahiri A et al. Atrial fibrillation burden: an update—the need for a CHA2DS2-VASc-AFBurden score. Europace 2021;23:665–73. 10.1093/europace/euaa287 [DOI] [PubMed] [Google Scholar]
- 11. Healey JS, Connolly SJ, Gold MR et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–9. 10.1056/NEJMoa1105575 [DOI] [PubMed] [Google Scholar]
- 12. Van Gelder IC, Healey JS, Crijns HJGM et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J 2017;38:1339–44. 10.1093/eurheartj/ehx042 [DOI] [PubMed] [Google Scholar]
- 13. Noseworthy PA, Kaufman ES, Chen LY et al. Subclinical and device-detected atrial fibrillation: pondering the knowledge gap: a scientific statement from the American Heart Association. Circulation 2019;140: e944–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirchhof P, Toennis T, Goette A et al. Anticoagulation with edoxaban in patients with atrial high-rate episodes. N Engl J Med 2023;389:1167–79. 10.1056/NEJMoa2303062 [DOI] [PubMed] [Google Scholar]
- 15. Healey JS, Lopes RD, Granger CB et al. Apixaban for stroke prevention in subclinical atrial fibrillation. N Engl J Med 2024;390:107–17. 10.1056/NEJMoa2310234 [DOI] [PubMed] [Google Scholar]
- 16. Huang C, Li L, Liu W et al. Time to benefit and harm of direct oral anticoagulants in device-detected atrial fibrillation: a pooled analysis of the NOAH-AFNET 6 and ARTESiA trials. Heart Rhythm 2024. 10.1016/j.hrthm.2024.06.038. [DOI] [PubMed] [Google Scholar]
- 17. Buiten MS, de Bie MK, Rotmans JI et al. The dialysis procedure as a trigger for atrial fibrillation: new insights in the development of atrial fibrillation in dialysis patients. Heart 2014;100:685–90. 10.1136/heartjnl-2013-305417 [DOI] [PubMed] [Google Scholar]
- 18. Vincenti A, Passini E, Fabbrini P et al. Recurrent intradialytic paroxysmal atrial fibrillation: hypotheses on onset mechanisms based on clinical data and computational analysis. Europace 2014;16:396–404. 10.1093/europace/eut346 [DOI] [PubMed] [Google Scholar]
- 19. Joglar JA, Chung MK, Armbruster AL et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024;149:e1–e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hindricks G, Potpara T, Dagres N et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 21. Reinecke H, Brand E, Mesters R et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol 2009;20:705–11. 10.1681/ASN.2007111207 [DOI] [PubMed] [Google Scholar]
- 22. Benz AP, Eikelboom JW. Apixaban compared with warfarin in patients with atrial fibrillation and end-stage renal disease: lessons learned. Circulation 2022;146:1746–8. 10.1161/CIRCULATIONAHA.122.061647 [DOI] [PubMed] [Google Scholar]
- 23. Steffel J, Collins R, Antz M et al. 2021 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace 2021;23:1612–76. 10.1093/europace/euab065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stevens PE, Ahmed SB, Carrero JJ et al. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2024;105:S117–314. 10.1016/j.kint.2023.10.018 [DOI] [PubMed] [Google Scholar]
- 25. Van Der Meersch H, De Bacquer D, De Vriese AS. Vitamin K antagonists for stroke prevention in hemodialysis patients with atrial fibrillation: a systematic review and meta-analysis. Am Heart J 2017;184:37–46. 10.1016/j.ahj.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 26. Sitticharoenchai P, Takkavatakarn K, Boonyaratavej S et al. Non-vitamin K antagonist oral anticoagulants provide less adverse renal outcomes than warfarin in non-valvular atrial fibrillation: a systematic review and metaanalysis. J Am Heart Assoc 2021;10:e019609. 10.1161/JAHA.120.019609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kreutz R, Deray G, Floege J et al. Rivaroxaban vs vitamin K antagonist in patients with atrial fibrillation and advanced chronic kidney disease. JACC Adv 2024;3:100813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kosciuszek ND, Kalta D, Singh M et al. Vitamin K antagonists and cardiovascular calcification: a systematic review and meta-analysis. Front Cardiovasc Med 2022;9:938567. 10.3389/fcvm.2022.938567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Connolly SJ, Karthikeyan G, Ntsekhe M et al. Rivaroxaban in rheumatic heart disease–associated atrial fibrillation. N Engl J Med 2022;387:978–88. 10.1056/NEJMoa2209051 [DOI] [PubMed] [Google Scholar]
- 30. Pengo V, Denas G, Zoppellaro G et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood 2018;132:1365–71. 10.1182/blood-2018-04-848333 [DOI] [PubMed] [Google Scholar]
- 31. Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M et al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome. Ann Intern Med 2019;171:685. 10.7326/M19-0291 [DOI] [PubMed] [Google Scholar]
- 32. Woller SC, Stevens SM, Kaplan D et al. Apixaban compared with warfarin to prevent thrombosis in thrombotic antiphospholipid syndrome: a randomized trial. Blood Adv 2022;6:1661–70. 10.1182/bloodadvances.2021005808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harrington J, Carnicelli AP, Hua K et al. Direct oral anticoagulants versus warfarin across the spectrum of kidney function: patient-level network meta-analyses from COMBINE AF. Circulation 2023;147:1748–57. 10.1161/CIRCULATIONAHA.122.062752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Vriese AS, Caluwé R, Bailleul E et al. Dose-finding study of rivaroxaban in hemodialysis patients. Am J Kidney Dis 2015;66:91–8. 10.1053/j.ajkd.2015.01.022 [DOI] [PubMed] [Google Scholar]
- 35. Mavrakanas TA, Samer CF, Nessim SJ et al. Apixaban pharmacokinetics at steady state in hemodialysis patients. J Am Soc Nephrol 2017;28:2241–8. 10.1681/ASN.2016090980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu Y, Chang AR, Inker LA et al. Associations of apixaban dose with safety and effectiveness outcomes in patients with atrial fibrillation and severe chronic kidney disease. Circulation 2023;148:1445–54. 10.1161/CIRCULATIONAHA.123.065614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Natale P, Palmer SC, Ruospo M et al. Anticoagulation for people receiving long-term haemodialysis. Cochrane Database Syst Rev 2024;1:CD011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lazrak HH, René É, Elftouh N et al. Safety of low-molecular-weight heparin compared to unfractionated heparin in hemodialysis: a systematic review and meta-analysis. BMC Nephrol 2017;18:187. 10.1186/s12882-017-0596-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ziai F, Benesch T, Kodras K et al. The effect of oral anticoagulation on clotting during hemodialysis. Kidney Int 2005;68:862–6. 10.1111/j.1523-1755.2005.00468.x [DOI] [PubMed] [Google Scholar]
- 40. Kodras K, Benesch T, Neumann I et al. Comparison of two dialysers (AN69ST vs. FX100) for heparin-free dialysis in patients with oral anticoagulation. Blood Purif 2008;26:226–30. 10.1159/000118846 [DOI] [PubMed] [Google Scholar]
- 41. Krummel T, Scheidt E, Borni-Duval C et al. Haemodialysis in patients treated with oral anticoagulant: should we heparinize? Nephrol Dial Transplant 2014;29:906–13. 10.1093/ndt/gft522 [DOI] [PubMed] [Google Scholar]
- 42. De Vriese AS, Heine G. Anticoagulation management in haemodialysis patients with atrial fibrillation: evidence and opinion. Nephrol Dial Transplant 2022;37:2072–9. 10.1093/ndt/gfab060 [DOI] [PubMed] [Google Scholar]
- 43. McBane RD, Wysokinski WE, Le-Rademacher JG et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: the ADAM VTE trial. J Thromb Haemost 2020;18:411–21. 10.1111/jth.14662 [DOI] [PubMed] [Google Scholar]
- 44. Johansen KL, Chertow GM, Foley RN et al. US Renal Data System 2020 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2021;77:A7–8. 10.1053/j.ajkd.2021.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gupta R, Woo K, Yi JA. Epidemiology of end-stage kidney disease. Semin Vasc Surg 2021;34:71–8. 10.1053/j.semvascsurg.2021.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Molnar AO, Bota SE, McArthur E et al. Risk and complications of venous thromboembolism in dialysis patients. Nephrol Dial Transplant 2018;33:874–80. 10.1093/ndt/gfx212. [DOI] [PubMed] [Google Scholar]
- 47. Fox KAA, Eikelboom JW, Shestakovska O et al. Rivaroxaban plus aspirin in patients with vascular disease and renal dysfunction. J Am Coll Cardiol 2019;73:2243–50. 10.1016/j.jacc.2019.02.048 [DOI] [PubMed] [Google Scholar]
- 48. Hsia J, Szarek M, Anand S et al. Rivaroxaban in patients with recent peripheral artery revascularization and renal impairment. J Am Coll Cardiol 2021;78:757–9. 10.1016/j.jacc.2021.06.021 [DOI] [PubMed] [Google Scholar]
- 49. Vanassche T, Verhamme P, Anand SS et al. Low-dose rivaroxaban plus aspirin in patients with polypharmacy and multimorbidity: an analysis from the COMPASS trial. Eur Heart J Cardiovasc Pharmacother 2022;8:462–73. 10.1093/ehjcvp/pvab050 [DOI] [PubMed] [Google Scholar]
- 50. Holmes DR, Reddy VY, Turi ZG et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet North Am Ed 2009;374:534–42. 10.1016/S0140-6736(09)61343-X [DOI] [PubMed] [Google Scholar]
- 51. Holmes DR, Doshi SK, Kar S et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation. J Am Coll Cardiol 2015;65:2614–23. 10.1016/j.jacc.2015.04.025 [DOI] [PubMed] [Google Scholar]
- 52. Holmes DR, Kar S, Price MJ et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure Device in Patients With Atrial Fibrillation Versus Long-Term Warfarin Therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1–12. 10.1016/j.jacc.2014.04.029 [DOI] [PubMed] [Google Scholar]
- 53. Osmancik P, Herman D, Neuzil P et al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol 2020;75:3122–35. 10.1016/j.jacc.2020.04.067 [DOI] [PubMed] [Google Scholar]
- 54. Osmancik P, Herman D, Neuzil P et al. 4-Year outcomes after left atrial appendage closure versus nonwarfarin oral anticoagulation for atrial fibrillation. J Am Coll Cardiol 2022;79:1–14. 10.1016/j.jacc.2021.10.023 [DOI] [PubMed] [Google Scholar]
- 55. Whitlock RP, Belley-Cote EP, Paparella D et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med 2021;384:2081–91. 10.1056/NEJMoa2101897 [DOI] [PubMed] [Google Scholar]
- 56. Fink T, Paitazoglou C, Bergmann MW et al. Left atrial appendage closure in end-stage renal disease and hemodialysis: data from a German multicenter registry. Catheter Cardiovasc Interv 2023;101:610–9. 10.1002/ccd.30559 [DOI] [PubMed] [Google Scholar]
- 57. Genovesi S, Porcu L, Rebora P et al. Long-term safety and efficacy of left atrial appendage occlusion in dialysis patients with atrial fibrillation: a multi-center, prospective, open label, observational study. Clin Kidney J 2023;16:2683–92. 10.1093/ckj/sfad221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Flores-Umanzor E, Asghar A, Cepas-Guillén PL et al. Transcatheter left atrial appendage occlusion in patients with chronic kidney disease: a systematic review and meta-analysis. Clin Res Cardiol 2023. 10.1007/s00392-023-02359-1. [DOI] [PubMed] [Google Scholar]
- 59. Byrne RA, Rossello X, Coughlan JJ et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J 2023;44:3720–826. 10.1093/eurheartj/ehad191 [DOI] [PubMed] [Google Scholar]
- 60. Yasuda S, Kaikita K, Akao M et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med 2019;381:1103–13. 10.1056/NEJMoa1904143 [DOI] [PubMed] [Google Scholar]
- 61. Valgimigli M, Gragnano F, Branca M et al. Ticagrelor or clopidogrel monotherapy vs dual antiplatelet therapy after percutaneous coronary intervention. JAMA Cardiol 2024;9:437. 10.1001/jamacardio.2024.0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Baaten CCFMJ, Schröer JR, Floege J et al. Platelet abnormalities in CKD and their implications for antiplatelet therapy. Clin J Am Soc Nephrol 2022;17:155–70. 10.2215/CJN.04100321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Eikelboom J, Floege J, Thadhani R et al. Anticoagulation in patients with kidney failure on dialysis: factor XI as a therapeutic target. Kidney Int 2021;100:1199–207. 10.1016/j.kint.2021.08.028 [DOI] [PubMed] [Google Scholar]
- 64. De Vriese AS, Ledó N. Factor XI antagonists: the discovery of the philosopher's stone? Clin Kidney J 2024;17:sfae106. 10.1093/ckj/sfae106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Weitz JI, Tankó LB, Floege J et al. Anticoagulation with osocimab in patients with kidney failure undergoing hemodialysis: a randomized phase 2 trial. Nat Med 2024;30:435–42. 10.1038/s41591-023-02794-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Winkelmayer WC, Lensing AWA, Thadhani RI et al. A phase II randomized controlled trial evaluated antithrombotic treatment with fesomersen in patients with kidney failure on hemodialysis. Kidney Int 2024;106:145–53. 10.1016/j.kint.2024.02.024 [DOI] [PubMed] [Google Scholar]
- 67. Piccini JP, Patel MR, Steffel J et al. Asundexian versus apixaban in patients with atrial fibrillation. N Engl J Med 2024. https://www.nejm.org/doi/abs/10.1056/NEJMoa2407105 [DOI] [PubMed] [Google Scholar]
- 68. Herrington WG, Staplin N, Haynes R. Kidney disease trials for the 21st century: innovations in design and conduct. Nat Rev Nephrol 2020;16:173–85. 10.1038/s41581-019-0212-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Colombijn JMT, Idema DL, van Beem S et al. Representation of patients with chronic kidney disease in clinical trials of cardiovascular disease medications. JAMA Netw Open 2024;7:e240427. 10.1001/jamanetworkopen.2024.0427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wizemann V, Tong L, Satayathum S et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int 2010;77:1098–106. 10.1038/ki.2009.477 [DOI] [PubMed] [Google Scholar]
- 71. Nopp S, Kraemmer D, Ay C. Factor XI inhibitors for prevention and treatment of venous thromboembolism: a review on the rationale and update on current evidence. Front Cardiovasc Med 2022;9:903029. 10.3389/fcvm.2022.903029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Walsh M, Bethune C, Smyth A et al. Phase 2 study of the factor XI antisense inhibitor IONIS-FXIRx in patients with ESRD. Kidney Int Rep 2022;7:200–9. 10.1016/j.ekir.2021.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lorentz CU, Tucker EI, Verbout NG et al. The contact activation inhibitor AB023 in heparin-free hemodialysis: results of a randomized phase 2 clinical trial. Blood 2021;138:2173–84. 10.1182/blood.2021011725 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.