Abstract
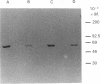
Bacteriophage-T4 and human type I DNA ligases were found capable of self-adenylating upon exposure to both ribo- and deoxyribo-[alpha-35S]thio-ATP. However, the joining reaction does not take place in the presence of the deoxyribotriphosphates. Enzyme adenylation is reversed in all cases by an excess of PPi, but the rate of reversion is lower with thio derivatives. Therefore thio derivatives can be used to study the adenylation of DNA ligases and to search for specific inhibitors of the first step of the ligation reaction. In addition we show that thio derivatives can be used to detect DNA ligase adenylation activity covalently bound to a solid matrix.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eckstein F., Goody R. S. Synthesis and properties of diastereoisomers of adenosine 5'-(O-1-thiotriphosphate) and adenosine 5'-(O-2-thiotriphosphate). Biochemistry. 1976 Apr 20;15(8):1685–1691. doi: 10.1021/bi00653a015. [DOI] [PubMed] [Google Scholar]
- Harvey C. L., Gabriel T. F., Wilt E. M., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. IX. Synthesis and properties of the deoxyribonucleic acid adenylate in the phage T4 ligase reaction. J Biol Chem. 1971 Jul 25;246(14):4523–4530. [PubMed] [Google Scholar]
- Lehman I. R. DNA ligase: structure, mechanism, and function. Science. 1974 Nov 29;186(4166):790–797. doi: 10.1126/science.186.4166.790. [DOI] [PubMed] [Google Scholar]
- Modrich P., Lehman I. R. Enzymatic joining of polynucleotides. IX. A simple and rapid assay of polynucleotide joining (ligase) activity by measurement of circle formation from linear deoxyadenylate-deoxythymidylate copolymer. J Biol Chem. 1970 Jul 25;245(14):3626–3631. [PubMed] [Google Scholar]
- Montecucco A., Ciarrocchi G. AMP-dependent DNA relaxation catalyzed by DNA ligase occurs by a nicking-closing mechanism. Nucleic Acids Res. 1988 Aug 11;16(15):7369–7381. doi: 10.1093/nar/16.15.7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco A., Pedrali-Noy G., Spadari S., Ciarrocchi G. Multiple roles of DNA ligase at the replication fork. Biochim Biophys Acta. 1988 Dec 20;951(2-3):330–334. doi: 10.1016/0167-4781(88)90103-0. [DOI] [PubMed] [Google Scholar]
- Montecucco A., Pedrali-Noy G., Spadari S., Lestingi M., Ciarrocchi G. Effects of DNA-binding drugs on T4 DNA ligase. Biochem J. 1990 Mar 1;266(2):379–384. doi: 10.1042/bj2660379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco A., Pedrali-Noy G., Spadari S., Zanolin E., Chiesa M. T., Ciarrocchi G. DNA ligases are specifically inhibited by anthracyclines with a free 3'-amino group. J Chemother. 1989 Jul;1(4 Suppl):1138–1139. [PubMed] [Google Scholar]
- Montecucco A., Pedrali-Noy G., Spadari S., Zanolin E., Ciarrocchi G. DNA unwinding and inhibition of T4 DNA ligase by anthracyclines. Nucleic Acids Res. 1988 May 11;16(9):3907–3918. doi: 10.1093/nar/16.9.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. H., Hunter W. N., d'Estaintot B. L., Kennard O. DNA-drug interactions. The crystal structure of d(CGATCG) complexed with daunomycin. J Mol Biol. 1989 Apr 20;206(4):693–705. doi: 10.1016/0022-2836(89)90577-9. [DOI] [PubMed] [Google Scholar]
- Spadari S., Ciarrocchi G., Falaschi A. Purification and properties of a polynucleotide ligase from human cell cultures. Eur J Biochem. 1971 Sep 13;22(1):75–78. doi: 10.1111/j.1432-1033.1971.tb01516.x. [DOI] [PubMed] [Google Scholar]
- Teraoka H., Sawai M., Tsukada K. Kinetic studies on the reaction catalyzed by DNA ligase from calf thymus. Biochim Biophys Acta. 1983 Sep 14;747(1-2):117–122. doi: 10.1016/0167-4838(83)90129-2. [DOI] [PubMed] [Google Scholar]