Abstract
Objective:
To compare the diagnostic accuracy of Contrast Enhanced Computed Tomography (CECT) compared to conventional imaging modalities and histopathological investigation in cervical lymph node metastasis in adults through a novel meta- analysis.
Method:
The review protocol is registered under PROSPERO(CRD42021225704) and performed in accordance to Preferred Reporting Items for Systematic Reviews and Meta-Analysis – Diagnostic Test Accuracy (PRISMA- DTA) checklist. Databases like PubMed, Google Scholar, EBSCOhost were searched from 2000 to 2023 to identify the diagnostic potential of CECT in cervical lymph node metastasis of oral carcinoma. True-positive, false-positive, true-negative, false-negative, sensitivity, specificity values were extracted or calculated if not present for each study. Quality of selected studies was evaluated based on Quality assessment of diagnostic accuracy studies (QUADAS)- 2 tool. Meta-analysis was performed in Meta-Disc 1.4 software and Review Manager 5.3 using a bivariate model parameter for the pooled sensitivity and pooled specificity. Additional analysis was performed in terms of positive likelihood ratio (+LR), negative likelihood ratio (-LR), diagnostic odds ratio (DOR) and summary receiver operating characteristics (SROC) with Area Under Curve (AUC) and p<0.05 as statistically significant.
Results:
Six studies were included for qualitative synthesis and as well as for meta-analysis. Two studies had high risk of bias while four studies had low risk of bias. 651 patients underwent CECT and were taken for meta-analysis. The meta-analysis revealed that CECT for diagnosing cervical lymph node metastasis had a pooled sensitivity of 71%, pooled specificity of 14% with 60% Area Under Curve (AUC). +LR of 0.84, -LR of 1.36 and DOR of 0.59.
Conclusion:
CECT has an overall fair diagnostic ability and is a valid and reliable tool in diagnosing the target condition overcoming high reliance on master specialized capacity for their execution and understanding like other conventional imaging techniques. CECT can be concluded for secondary level of prevention for cervical node metastasis of oral carcinoma under early diagnosis and prompt treatment. However, further standardized accuracy studies are indicated to improve the overall diagnostic accuracy of CECT.
Key Words: Accuracy, computed tomography, diagnosis, meta-analysis, oral cancer
Introduction
Oral carcinoma is considered as one of the most common head and neck carcinomas, and more than 90% of oral carcinomas are squamous cell carcinomas [1]. Lymph node metastasis is a prevalent condition in the oral cavity. Approximately 50% of oral cancer patients have squamous cell carcinoma, and 20% to 30% of patients have occult lymph node metastasis in early-stage (cT1-2N0) oral squamous cell carcinoma. Studies have indicated that the prognosis of the oral cancer was closely related to the cervical lymph node status [2].
Metastatic tumours increase the risk of advanced disease and may impair survival. However, there are no conventional therapies for neck lymph nodes in early-stage oral cancer. Therefore, assessment of lymph node metastasis is very important for the treatment and prognosis of oral cancer [3].
Lymph node metastasis is a common event in oral cancer. About 50% of oral cancer patients have metastatic lymph nodes and 20–30% patients have occult lymph node metastases in early-stage (cT1-2N0) oral squamous cell carcinoma. Studies have shown that the prognosis of the oral cancer was closely related to the cervical lymph node status. The presence of metastatic lymph nodes commits patients to an advanced-stage disease category and can reduce the survival rate. Treatment of oral cancer is surgical resection of the primary tumor with or without postoperative adjuvant therapy (radiation or chemoradiation therapy). But there are no standard treatments of neck lymph nodes for early-stage oral cancer. Unnecessary prophylactic neck dissection does harm to patients without metastasis, while the selection of observation may result in the metastasis of lymph node. Therefore, evaluation of lymph node metastasis is extremely essential for the treatment and prognostication of oral cancer.
Lymph node status is one of the major predictors of prognosis in patients with cancer. Furthermore, correctly diagnosing the enlarged lymph nodes in patients with or without primary tumors is essential to allow selection of an appropriate treatment strategy. A large number of modalities may be used to characterize lymph nodes, such as computed tomography, magnetic resonance imaging and gray scale ultrasound; these depend mainly on morphological characteristics for the identification of enlarged lymph nodes.
The most accurate method for preliminary evaluation of lymph nodes is fine needle aspiration. However, fine needle aspiration is an invasive method and is not suitable for every lymph node biopsy [4]. The information provided by biopsy is not sufficient to reach certainty, which is important for determining treatment strategies and improving the prognosis of the disease. Therefore, imaging plays an important role in the diagnosis of lymph node metastasis. Some imaging techniques, such as computed tomography (CT) and magnetic resonance imaging (MRI), can detect large lymph nodes through morphological changes and increase in size; however, small or early lymph node metastases might be misdiagnosed with these imaging techniques. Therefore, a non-invasive tool with good diagnostic value is highly demanded [5].
Available imaging methods include computed tomography (CT), contrast-enhanced computed tomography (CECT), positron tomography computed tomography (PET-CT), magnetic resonance imaging (MRI) and ultrasound (US). For the last three treatments, diagnoses depend largely on the experience of the practitioner, making them poor [6]. Therefore, there remains the need for an accurate, safe, non-invasive, and inexpensive imaging technology. CECT is currently regarded as the first-line examination, and the accuracy of nodal staging has been improved over clinical palpation with the use of this modality. While remarkable achievements have been made with CECT, they are associated with unavoidable radiation exposure. However, because none of the imaging diagnostic tools has had 100% accuracy, the method of choice remains controversial [7].
Understanding the correct diagnosis will help clinicians to make the correct diagnosis and choose the best treatment. Diagnostic accuracy includes sensitivity, specificity, and summary receiver operating characteristic (SROC) analysis [8]. Sensitivity and specificity describe the test’s ability to accurately identify patients and non-patients, respectively. These are independent of the prevalence of the disease, the pathway of disease in the population at a given time, and summary of receiver operating characteristics (SROC) analysis is used to assess diagnostic power [8].
There have been already few reviews published on CECT as a diagnostic tool for various malignant tumours [9] but till date, no studies have provided a comprehensive, quantitative and diagnostic analysis of CECT in cervical lymph node metastasis of oral carcinoma. Therefore, we updated our research for existing scientific evidences and conducted this review with the aim to compare the diagnostic accuracy of CECT compared to conventional imaging modalities and histopathological investigation in cervical lymph node metastasis in adults through a meta- analysis.
Materials and Methods
Protocol and Registration
The systematic review and meta-analysis protocol was registered at the international prospective register of systematic reviews (PROSPERO- CRD42021225704) and performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis – Diagnostic Test Accuracy (PRISMA- DTA) checklist [10].
Study Design
The following focused research question in the Participants (P), Index test (I), reference standard (R) and target condition (T) format was proposed “Is there a difference in the diagnostic accuracy of CECT (Index Test) compared to conventional imaging modalities and histopathological investigation (gold standard) for the cervical lymph node metastasis in oral carcinoma?
Eligibility Criteria: studies were selected based on following criteria’s
Inclusion Criteria
The following criteria were included:
(1) Study Design: In-vivo studies, Observational studies, retrospective study, prospective study, cross-sectional study comparing the diagnostic accuracy of CECT with conventional techniques.
(2) Participant characteristics: patients diagnosed with oral squamous cell carcinoma aged 18 years and up.
(3) Outcome measurements: Diagnostic accuracy including sensitivity, specificity, accuracy, determined using different methods irrespective of the methods of quantifying the outcomes.
(4) Articles written in English language
(5) Articles from 2000 – 2023 and available as free full text
Exclusion Criteria
The exclusion criteria were as follows:
(1) Non-clinical studies, in-vitro studies, and animal studies.
(2) Studies reporting about a single diagnostic tool were also excluded.
(3) Studies done on individuals less than 18 years of age.
(4) Studies not fully available as full text article
(5) Article reporting only abstracts were also excluded.
(6) Studies not reporting primary outcomes of accuracy, sensitivity, and specificity as well as where primary outcomes are not possible to calculate from the given raw data.
Search protocol and study selection
A comprehensive electronic search was performed till May 2023 for the studies published within the last 23 years (from 2000 to 2023) using the following databases: PubMed and EBSCOhost to retrieve articles in the English language. The searches in the clinical trials database, cross-referencing and grey literature were conducted using Google Scholar, Greylist, and OpenGrey. In addition to the electronic search, a hand search was also made, and reference lists of the selected articles were screened.
Search Strategy
Appropriate key words and Medical Subject Heading (MeSH) terms were selected and combined with Boolean operators like AND. The search strategy used was as follows: (contrast enhanced computed tomography AND sensitivity AND specificity AND oral cancer), (cervical lymph nodes AND oral cancer AND diagnosis).
Two review authors did the search and screening, in accordance with the previously agreed process. The article selection process was divided into two phases. During phase one, two reviewers examined the titles and abstracts of each paper. Articles that did not met the inclusion criteria were excluded. In Phase 2, selected entire articles were independently evaluated and screened by the same reviewers. Any disagreements were settled through conversation. When two reviewers could not reach an agreement, a third reviewer was consulted to make the ultimate decision. All three authors agreed on the final selection.
Data extraction
For all included studies, following descriptive study details were extracted by two independent reviewing authors using pilot-tested customized data extraction forms: authors, study year, sample size, study design, index test/ reference standard used, sensitivity, specificity and conclusion. Quantitative data of sensitivity and specificity were compiled from each study and using these quantitative data, values like true positive, true negative, false positive and false negatives were calculated manually for the studies using the below formula’s where the data was not provided by authors [8]. The corresponding authors were contacted via email where further information was needed.
a) False positive = (1-specificity) x (1- diseased cases/ total sample)
b) True negative = specificity x (1- diseased cases/total sample)
c) True positive = sensitivity x diseased cases/ total sample
d) False negative = (1- sensitivity) x diseased cases/total sample
Assessment of methodological quality
The systemic quality or the risk of bias was assessed involving Quality Evaluation for Indicative Exactness Studies - 2 (QUADAS-2) apparatus [11]. The QUADAS-2 is a modified instrument created to evaluate nature of symptomatic examinations through its four domains: patient determination, index test, reference standard, flow and timing of members. Every domain had flagging inquiries with choices of “Yes”, “No” or “Unclear”. The general risk of bias was evaluated as high: whenever responded to ‘No’ to any question, Low: whenever addressed ‘Yes’ to all inquiries and Unclear: whenever addressed ‘Unclear’ to all inquiries or joined by any ‘Yes’. Risk of bias summary and applicability concern was graphically plotted using Review Manager (RevMan) software version 5.3.
Statistical analysis and data synthesis
Crude information was utilized to work out responsiveness and explicitness for each biomarker with their assessment technique. For by and large exactness, we determined pooled responsiveness, pooled explicitness with 95% certainty stretch, region under outline recipient working trademark. (Understanding of AUC values were as per the following: esteem above 80% were considered as brilliant, somewhere in the range of 70% and 80% as great, somewhere in the range of 60% and 69% as fair and beneath 60% as unfortunate results for a symptomatic test [12]. To evaluate the effect of heterogeneity, Higgins I2 test was utilized. This test addresses the extent of fluctuation because of heterogeneity instead of because of inspecting blunder [13]. As per I2 test measurement the heterogeneity could be low (I2 <50%) or high (I2 >50%). Subgroup examination was additionally completed. Results were introduced graphically as coupled woodland plot for each salivary biomarker with their assessment strategy utilizing Meta-Circle 1.4 programming.
Additional analysis
Additional analysis was performed with positive likelihood ratio (PLR) and negative likelihood ratio (NLR) using DerSimonian-Laird’s estimator considering random effect model. Positive likelihood ratio (PLR) in range of 2-5, 5-10 and >10 represents small, moderate and large increase in probability of disease when test is positive while Negative likelihood ratio (NLR) in range of 0.2-0.5, 0.2-0.1 and <0.1 represents small, moderate and large decrease in probability of disease when test is negative.
Results
Study Selection
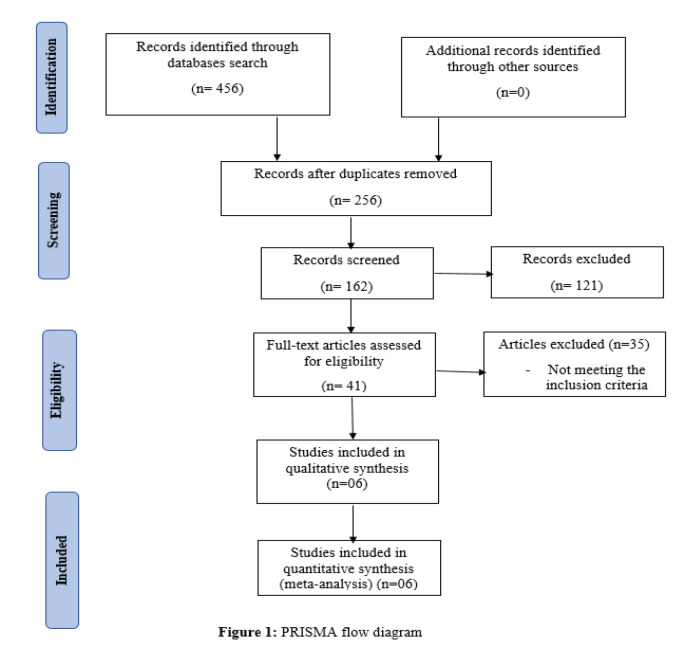
After copies evacuation, reference rundown of all included examinations was screened. Of which 121 examinations were barred. After this full text articles were evaluated for qualification and articles that didn’t meet consideration rules were barred. Just six examinations satisfied qualification models and were remembered for subjective combination as well as for meta-analysis as shown in Figure 1 below.
Figure 1.
PRISMA Flow Diagram
Study Characteristics
A summary of descriptive characteristics of all included six studies is provided in (Table 1). Data were reviewed from six studies from aggregate of 651 patients diagnosed with oral carcinoma. Among the included studies, two studies [14, 15] were from Pakistan, two studies [1, 2] were from China, one study [16] was from Japan and one study [3] was from Germany. Four studies [16, 15, 2, 3] had retrospective study design, one study [1] had prospective study design and one study [14] had cross-sectional study design. For cervical lymph node metastasis, the index test used was CECT compared to the reference standard (conventional imaging, contrast enhanced ultrasound (CEUS), computed tomography (CT), histopathologic evaluation). All the included studies had an overall sensitivity ranging from 45 – 84% with mean sensitivity of 74% while overall specificity ranged from 62 – 98% with mean specificity being 83%. It was concluded that CECT overall had a greater diagnostic accuracy compared to conventional modalities and could be used as reliable and valid diagnostic tool with good sensitivity and specificity.
Table 1.
Descriptive Study Details of Included Studies
| Authors, year of study | Country | Sample size | Study Design | Index test/ reference standard | Sensitivity (%) | Specificity (%) | Conclusion |
|---|---|---|---|---|---|---|---|
| Ando et al., 2020 | Japan | 97 | Retrospective study | CECT/conventional imaging | 0.75 | 0.02 | CECT can be used as diagnostic tool |
| Ding et al., 2020 | China | 48 | Prospective study | CECT/CEUS | 0.59 | 0.11 | CEUS was deemed to have high diagnostic accuracy due to high sensitivity |
| Noor et al., 2020 | Pakistan | 125 | Cross-sectional study | CECT/histopathologic evaluation | 0.78 | 0.41 | CECT had an acceptable accuracy |
| Qureshi et al., 2021 | Pakistan | 100 | Retrospective study | CECT/ histopathologic evaluation | 0.59 | 0.2 | CECT was accepted as a successful diagnostic tool |
| Ding et al., 2022 | China | 42 | Retrospective study | CECT/ conventional Ultrasound | 0.7 | 0 | CECT overall has better accuracy |
| Struckmeier et al., 2023 | Germany | 239 | Retrospective study | CECT/CT | 0.84 | 0.02 | CECT can be used as diagnostic tool |
Risk of Bias within Studies
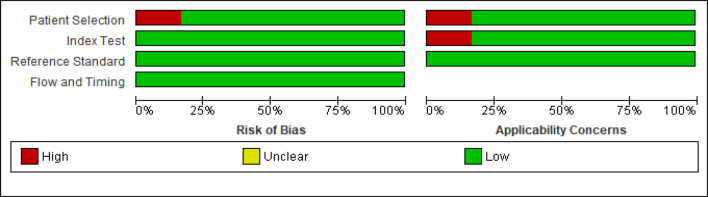
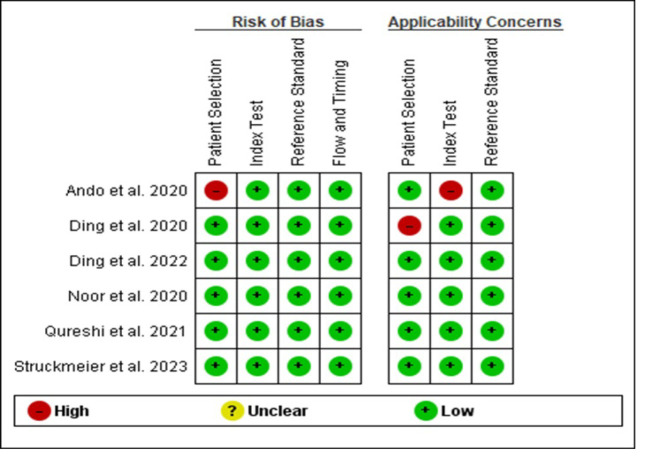
Although four studies [1, 14, 15, 2, 3] were evaluated as low risk of bias for all four domains. Patient selection was considered as high risk of bias in one study [16], which was mainly due to method of patient enrollment, the nature of the study design and implementing inappropriate exclusion.
The index test was considered to be at low risk of bias in all included studies. High risk of bias was reported with respect to index test domain in one study [16] due to insufficient details reported as to whether results of index test were interpreted without prior knowledge of reference standard results, lack of pre-specified test-positive threshold and disclosure of conflict of interest. Similarly, the reference standard and flow and timing domain was considered at low risk in all studies. The risk of bias and applicability concern summary and graph is depicted in Figure 2 and 3.
Figure 2.
Risk of Bias and Applicability Concerns Summary: Review Authors' judgements about Each Domain for Each Included Study
Figure 3.
Risk of Bias and Applicability Concerns Graph: Review Authors' Judgements about each Domain Presented as Percentages Across Included Studies
Synthesis of Results
The meta-analysis was conducted for evaluating the overall diagnostic accuracy of CECT for cervical lymph nodes metastasis in patients with OSCC. Summary statistics measure was calculated in terms of pooled sensitivity, specificity, positive and negative likelihood ratio (PLR & NLR), diagnostic odd’s ratio (DOR) and area under the curve (AUC).
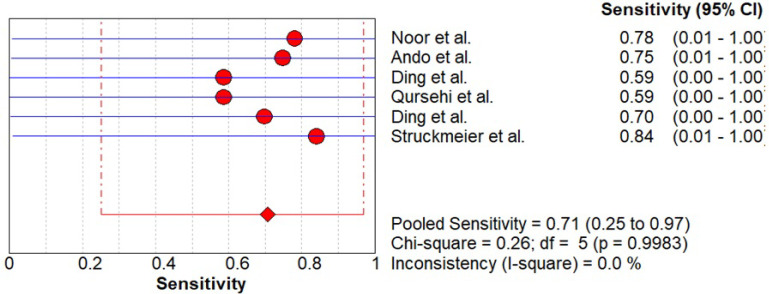
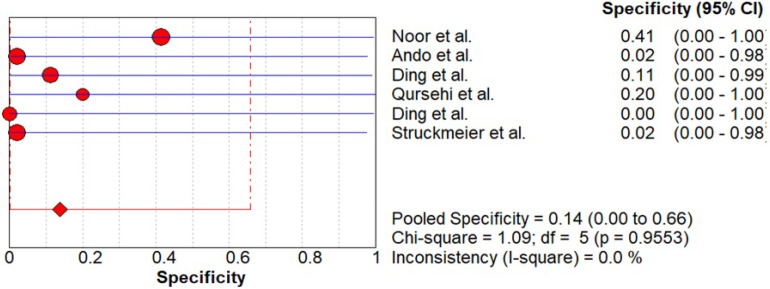
As shown in Figure 4-5, data was evaluated from six studies investigating the overall diagnostic accuracy. The pooled sensitivity was 0.71 (CI 0.25- 0.97) and pooled specificity was 0.14 (CI 0.00- 0.66) with I2 being 0%.
Figure 4.
Pooled Sensitivity of CECT for Cervical Node Metastasis in Patients with OSCC
Figure 5.
Pooled Sensitivity of CECT for Cervical Node Metastasis in Patients with OSCC
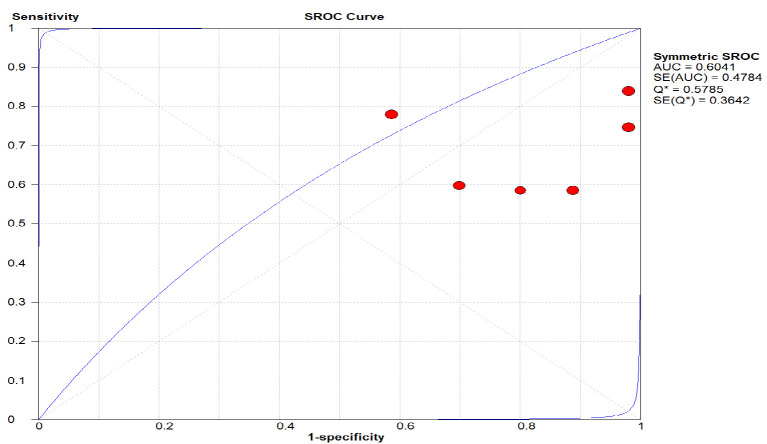
As shown in Figure 6. the area under the curve (AUC) was plotted with sensitivity and 1-specificity and standard error. An overall accuracy of (AUC) 0.60 was seen for CECT indicating that the CBCT had an overall fair diagnostic efficacy in diagnosing the condition.
Figure 6.
The Area under the Curve (AUC) was Plotted with Sensitivity and 1-Specificity and Standard Error. An overall accuracy of (AUC) 0.60 was seen for CECT indicating that the CBCT had an overall fair diagnostic efficacy in diagnosing the condition.
Additional analysis
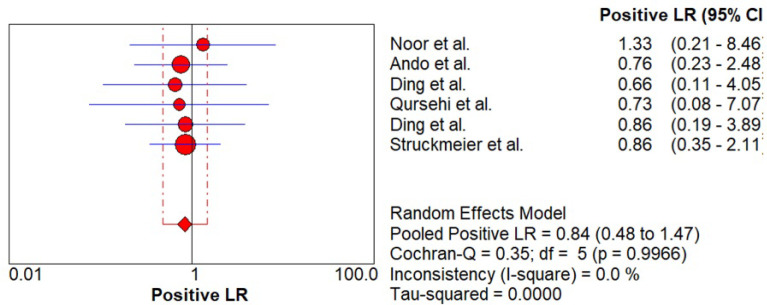
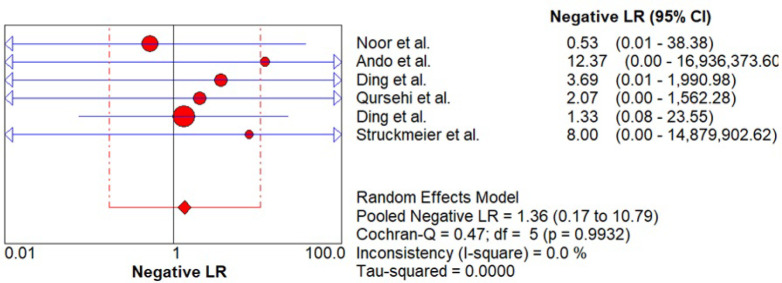
Likelihood ratio was estimated which signifies the ability of the index test to predict the test results (positive / negative) when the disease condition in actual is present or absent. As shown in Figure 7 - 8, pooled positive likelihood ratio (PLR) 0.84 (0.48 – 1.47) and negative likelihood ratio (NLR) 1.36 (0.17 – 10.79) was estimated. Pooled +PLR suggested that CECT is 0.84 times more likely to have a positive detection of cervical lymph node metastasis than someone without cervical lymph node metastasis while pooled -NLR suggested that CECT is 1.36 times as likely to have a negative cervical lymph node metastasis detection as someone without nodal metastasis.
Figure 7.
Pooled +LR of CECT for Cervical Node Metastasis in Patients with OSCC
Figure 8.
Pooled -LR of CECT for Cervical Node Metastasis in Patients with OSCC
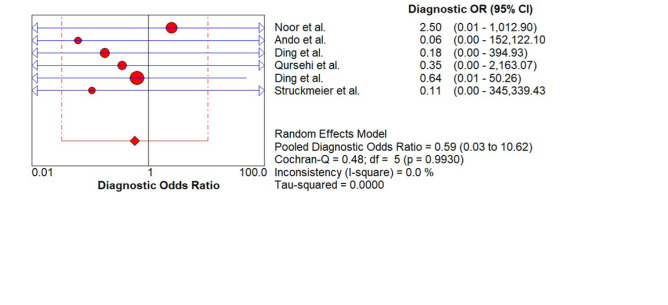
As shown in Figure 9. the pooled Diagnostic Odds Ratio (DOR) is 0.59 (0.03 – 10.62) suggesting that overall ability of index test in correctly diagnosing the target condition is fair to average.
Figure 9.
Pooled (DOR) of CECT for Cervical Node Metastasis in Patients with OSCC
Discussion
Going through existing literature evidences, no systematic review has analysed the diagnostic ability of CECT for cervical node metastasis. Therefore, this systematic review and meta-analysis was conducted to provide a comprehensive review on the diagnostic ability of CECT for cervical lymph node metastasis in patients with oral cancer.
Electronic databases were searched for past 23 years which yielded six studies fulfilling our eligibility criteria’s. All the included studies had retrospective, prospective and cross-sectional study design evaluating the diagnostic ability of CECT as an index test against reference standards. Quality assessment revealed presence of moderate to low risk of bias. Meta-analysis revealed sensitivity and specificity of 71% and 14% with 60% AUC. However, although a fair overall diagnostic accuracy was seen, very low specificity was observed which can be a major drawback for a diagnostic tool. However, to overcome low specificity and to reduce the number of false positives, further more diagnostic accuracy studies or cross-sectional studies should be conducted with larger sample size emphasizing on increasing true positive and true negative and reducing the number of false positive and false negatives. Also, furthermore databases search should be done with the use of other Boolean operators (OR, NOT) and various truncations, so as in order to fetch more articles.
Li et al. [17] conducted a systematic review to assess the diagnostic accuracy of computed tomography in differentiating the mandibular invasion (including bone cortex and bone marrow invasion) and mandibular medullary alone (bone marrow invasion) caused by head and neck cancer and compared it with CECT. Cohort studies were taken into consideration and histopathologic investigation as the reference standard. 30 studies with a total of 1257 scans were taken for meta-analysis. Meta-analysis revealed CT for the diagnosis of mandibular invasion had a pooled sensitivity of 0.72, specificity of 0.90, +LR of 5.33, -LR of 0.36, DOR of 21.41 and AUC being 0.9022 while the CT findings for mandibular medullar invasion had a sensitivity of 0.81, specificity of 0.85, +LR of 4.76, -LR of 0.24, DOR of 29.49, AUC of 0.9240. It was concluded that CT had an overall acceptable diagnostic accuracy compared to CECT in detecting mandibular invasion.
Kong et al. [9] in 2023 conducted a systematic review to assess the diagnostic accuracy of CECT in detecting metastatic lymph nodes in patients with malignant tumours like papillary thyroid carcinoma, colorectal cancer, oesophageal cancer, hepatocellular carcinoma, squamous cell carcinoma of the oropharynx and lung cancer. Retrospective and prospective studies were taken into consideration. Sixteen studies with 984 patients having (2,577 lymph nodes) involvement were taken for analysis. Meta-analysis revealed a sensitivity and specificity of 94% and 74% with AUC being 0.94 suggesting of CECT being a good diagnostic tool with presence of minimal heterogeneity.
This study is limited by overall quality of included studies for patient’s selection and index test. Further standardised diagnostic test accuracy studies that minimises potential sources of bias through rigorous design, conduct and reporting are needed. Future research must focus on the accuracy of CECT in detection of cervical node metastasis of OSCC with clear and robust methodology. Included studies were at high risk of selection bias arising from use of a ‘case-control’ study design. Furthermore, patient sampling and/or recruitment into studies were insufficiently reported. Among the included studies, apart from two studies [16, 1] other studies had sufficiently reported patient selection process. All studies used conventional imaging modalities (conventional imaging, contrast enhanced ultrasound (CEUS), computed tomography (CT), histopathologic evaluation) as reference standard and CECT as index test. However, insufficient detail and lack of clarity in reporting studies made it difficult to assess risk of bias. Therefore, use of STARD [18] checklist in reporting primary studies could have facilitated the quality appraisal. Reporting guidelines for primary diagnostic studies should be followed strictly and studies should address all potential source of bias and applicability concern as indicated in QUADAS-2 tool [11].
Adherence to the PRISMA guidelines, the thorough unrestricted literature search, utilization of reliable methodology with regard to the qualitative synthesis of data, the quality assessment of evidence with the QUADAS -2 tool strengthens this review. The quality assessment of all the included studies showed low-moderate risk of bias whereas overall quality was high, specifying lack of potential and inevitable sources of bias with limited variability and reporting deficiencies.
A systematic review is a transparent and repeatable procedure for identifying, selecting and critically assessing published or unpublished data to address a well-defined research question. Meta-analyses, a statistical analysis that incorporates numerical data from related studies, are frequently paired with systematic reviews. The best evidence is generally regarded as systematic reviews and meta-analyses. However, the calibre of the included studies has an impact on how strong the evidence is from a systematic review and meta-analysis. In the current systematic review, sufficient studies with a brief observation period and a known risk of bias were included. As a result, the presently available evidence is sufficient to make therapeutic recommendations in response to the current systematic review’s focus question.
In conclusion, Despite of some limitations, it was found that CECT has an overall fair diagnostic ability and is a valid and reliable tool in diagnosing the target condition overcoming high dependence on expert technical ability for their execution and interpretation like other conventional imaging techniques. Our findings provide evidence on ability of CECT on cervical lymph node metastasis of oral carcinoma for early screening and diagnosis. Thus, we can conclude CECT for secondary level of prevention for cervical node metastasis of oral carcinoma under early diagnosis and prompt treatment. However, further standardized accuracy studies are indicated to improve the overall diagnostic accuracy of CECT.
Acknowledgements
Novelty Expression File
Oral carcinoma is considered as one of the most common head and neck carcinomas, and more than 90% of oral carcinomas are squamous cell carcinomas. Lymph node metastasis is a prevalent condition in the oral cavity. Approximately 50% of oral cancer patients have squamous cell carcinoma, and 20% to 30% of patients have occult lymph node metastasis in early-stage (cT1-2N0) oral squamous cell carcinoma. Studies have indicated that the prognosis of the oral cancer was closely related to the cervical lymph node status.
The most accurate method for preliminary evaluation of lymph nodes is fine needle aspiration. However, fine needle aspiration is an invasive method and is not suitable for every lymph node biopsy. The information provided by biopsy is not sufficient to reach certainty, which is important for determining treatment strategies and improving the prognosis of the disease. Therefore, imaging plays an important role in the diagnosis of lymph node metastasis.
CECT is currently regarded as the first-line examination, and the accuracy of nodal staging has been improved over clinical palpation with the use of this modality. While remarkable achievements have been made with CECT, they are associated with unavoidable radiation exposure.
Author Contribution Statement
All authors contributed equally in this study.
References
- 1.Ding Z, Deng C, Wang Z, Liu L, Ma X, Huang J, et al. Comparison of contrast-enhanced ultrasound and contrast-enhanced computed tomography for the diagnosis of cervical lymph node metastasis in squamous cell carcinoma of the oral cavity. Int J Oral Maxillofac Surg. 2021;50(3):294–301. doi: 10.1016/j.ijom.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Ding S, Xiong P, Zuo J. Value of contrast-enhanced ultrasound in predicting early lymph-node metastasis in oral cancer. Dentomaxillofac Radiol. 2022;51(3):20210293. doi: 10.1259/dmfr.20210293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Struckmeier AK, Yekta E, Agaimy A, Kopp M, Buchbender M, Moest T, et al. Diagnostic accuracy of contrast-enhanced computed tomography in assessing cervical lymph node status in patients with oral squamous cell carcinoma. J Cancer Res Clin Oncol. 2023;149(19):17437–50. doi: 10.1007/s00432-023-05470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abd El-Hafez YG, Chen CC, Ng SH, Lin CY, Wang HM, Chan SC, et al. Comparison of pet/ct and mri for the detection of bone marrow invasion in patients with squamous cell carcinoma of the oral cavity. Oral Oncol. 2011;47(4):288–95. doi: 10.1016/j.oraloncology.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Hendrikx AW, Maal T, Dieleman F, Van Cann EM, Merkx MA. Cone-beam ct in the assessment of mandibular invasion by oral squamous cell carcinoma: Results of the preliminary study. Int J Oral Maxillofac Surg. 2010;39(5):436–9. doi: 10.1016/j.ijom.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Huang SH, Chien CY, Lin WC, Fang FM, Wang PW, Lui CC, et al. A comparative study of fused fdg pet/mri, pet/ct, mri, and ct imaging for assessing surrounding tissue invasion of advanced buccal squamous cell carcinoma. Clin Nucl Med. 2011;36(7):518–25. doi: 10.1097/RLU.0b013e318217566f. [DOI] [PubMed] [Google Scholar]
- 7.Momin MA, Okochi K, Watanabe H, Imaizumi A, Omura K, Amagasa T, et al. Diagnostic accuracy of cone-beam ct in the assessment of mandibular invasion of lower gingival carcinoma: Comparison with conventional panoramic radiography. Eur J Radiol. 2009;72(1):75–81. doi: 10.1016/j.ejrad.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Shaw AK, Garcha V, Shetty V, Vinay V, Bhor K, Ambildhok K, et al. Diagnostic accuracy of salivary biomarkers in detecting early oral squamous cell carcinoma: A systematic review and meta-analysis. Asian Pac J Cancer Prev. 2022;23(5):1483–95. doi: 10.31557/APJCP.2022.23.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong D, Chen X, Gao P, Zhao K, Zheng C, Zhou H. Diagnostic accuracy of contrast-enhanced dual-energy computed tomography for detecting metastatic lymph nodes in patients with malignant tumors: A systematic review and meta-analysis. Quant Imaging Med Surg. 2023;13(5):3050–65. doi: 10.21037/qims-22-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salameh JP, Bossuyt PM, McGrath TA, Thombs BD, Hyde CJ, Macaskill P, et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (prisma-dta): Explanation, elaboration, and checklist. Bmj. 2020;370:m2632. doi: 10.1136/bmj.m2632. [DOI] [PubMed] [Google Scholar]
- 11.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. Quadas-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 12.Jones CM, Athanasiou T. Summary receiver operating characteristic curve analysis techniques in the evaluation of diagnostic tests. Ann Thorac Surg. 2005;79(1):16–20. doi: 10.1016/j.athoracsur.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 13.Lijmer JG, Bossuyt PM, Heisterkamp SH. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med. 2002;21(11):1525–37. doi: 10.1002/sim.1185. [DOI] [PubMed] [Google Scholar]
- 14.Noor M, Ishaq Y, Mehmood M, Sarfraz A, Bhutta U, Manji S, et al. Diagnostic role of contrast enhanced computed tomography (cect) in detecting nodal metastasis in patients with oral squamous cell carcinoma taking histopathology as gold standard. 2020:13412051. [Google Scholar]
- 15.Qureshi TA, Wasif M, Awan MS, Muhammad AY, Mughal A, Ameen A. Role of contrast enhanced computed tomography in assessing cervical lymph node metastases in oral cavity squamous cell carcinoma. J Pak Med Assoc. 2021;71(3):826–9. doi: 10.47391/JPMA.594. [DOI] [PubMed] [Google Scholar]
- 16.Ando T, Kato H, Kawaguchi M, Tanahashi Y, Aoki M, Kuze B, et al. Diagnostic ability of contrast-enhanced computed tomography for metastatic cervical nodes in head and neck squamous cell carcinomas: Significance of additional coronal reconstruction images. Pol J Radiol. 2020;85:e1–e7. doi: 10.5114/pjr.2020.92668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, Men Y, Yang W, Pan J, Sun J, Li L. Computed tomography for the diagnosis of mandibular invasion caused by head and neck cancer: A systematic review comparing contrast-enhanced and plain computed tomography. J Oral Maxillofac Surg. 2014;72(8):1601–15. doi: 10.1016/j.joms.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, et al. Stard 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open. 2016;6(11):e012799 . doi: 10.1136/bmjopen-2016-012799. [DOI] [PMC free article] [PubMed] [Google Scholar]