Abstract
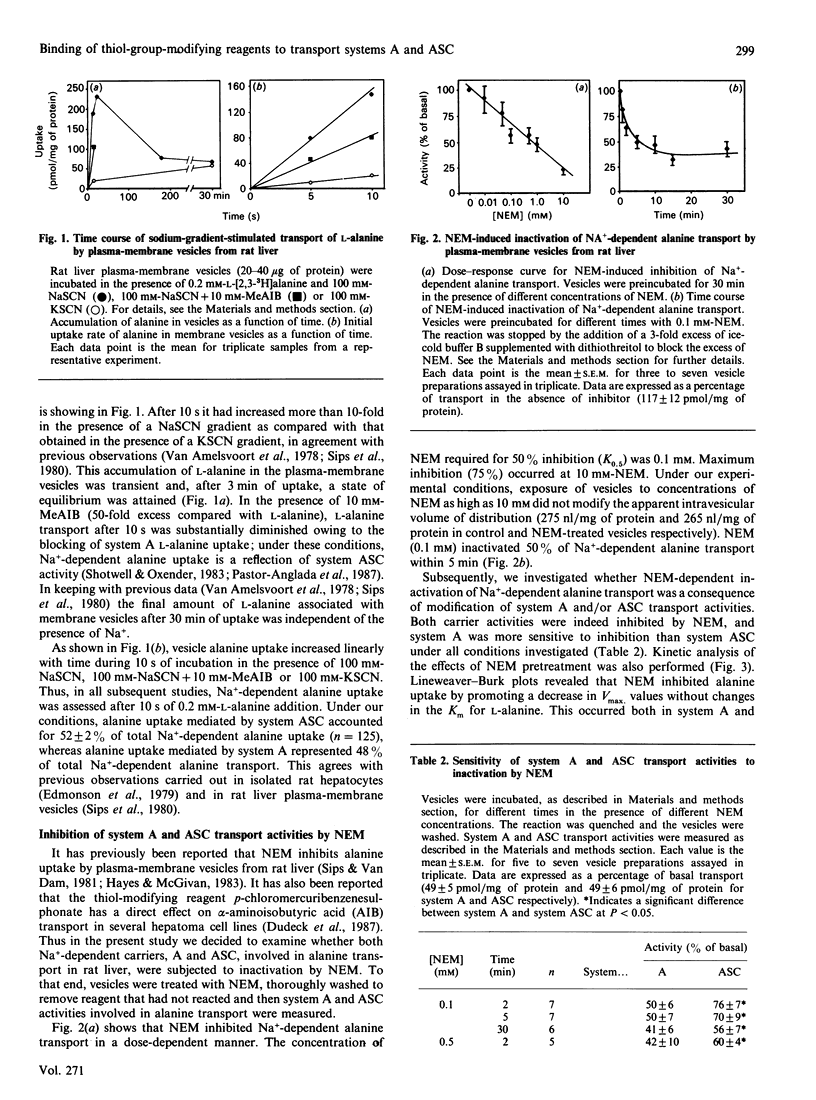
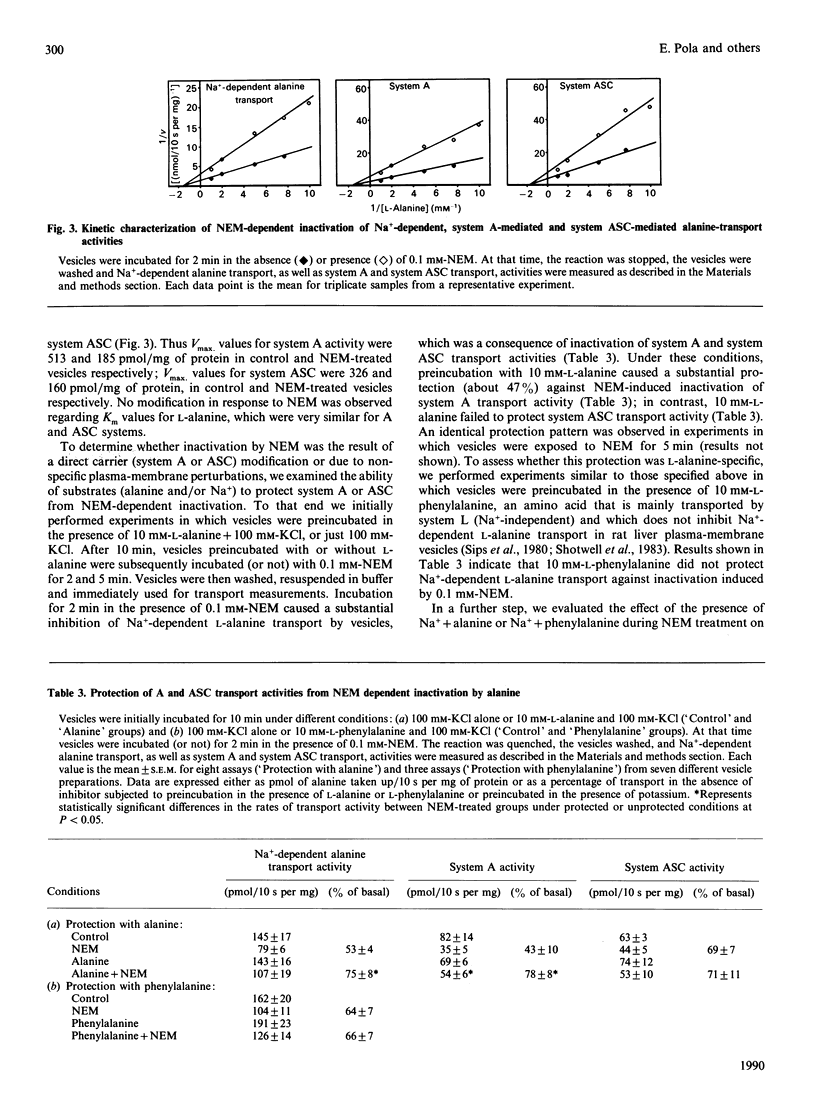
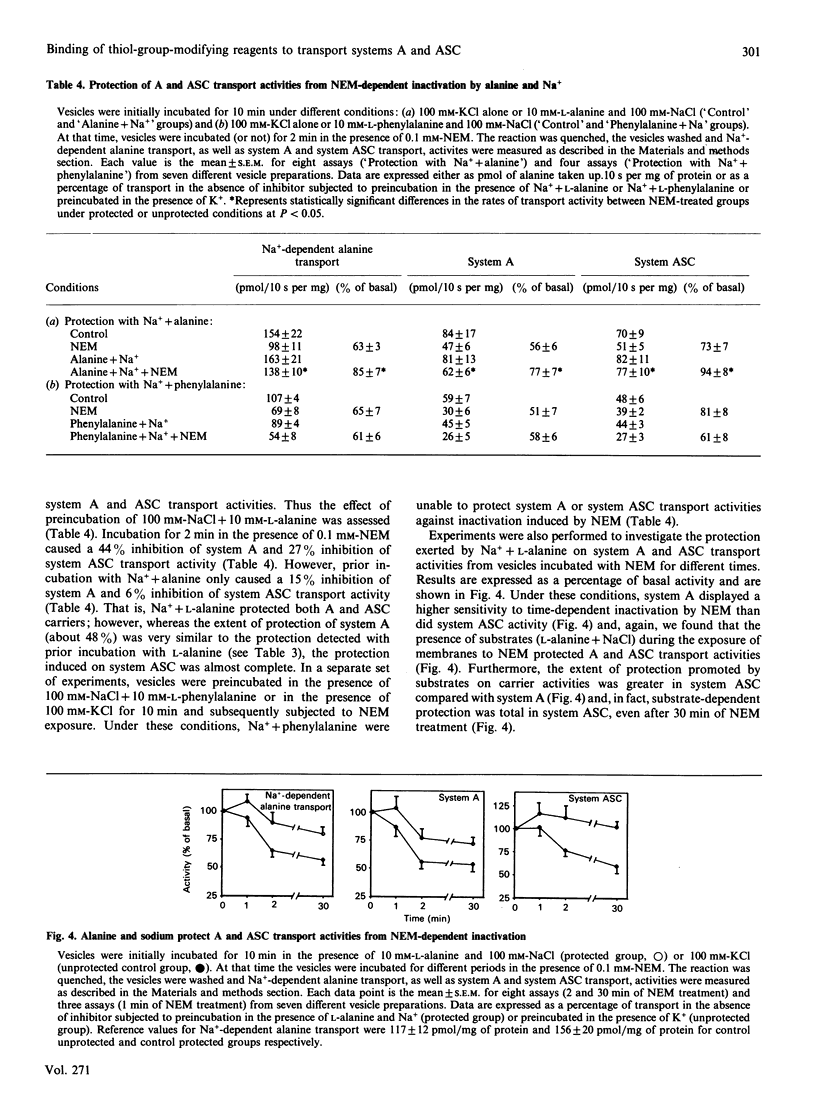
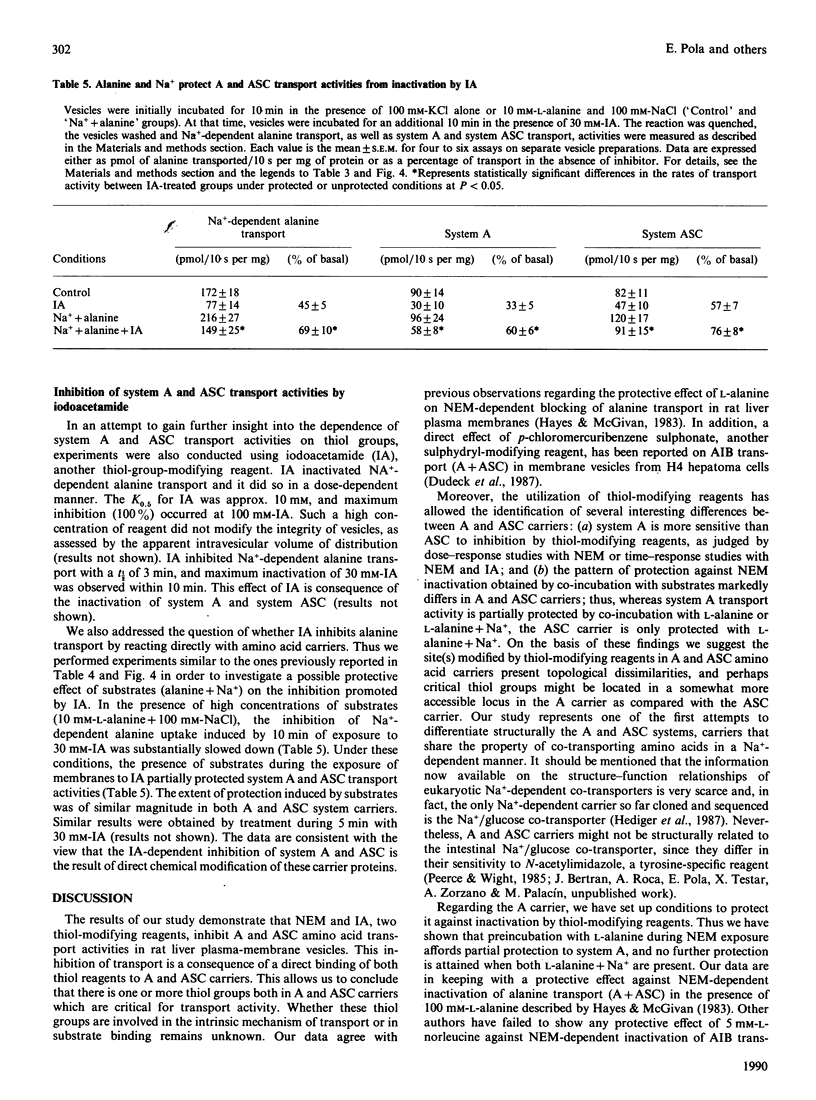
1. In the present study we have examined the sensitivity of A and ASC amino-acid-carrier activities in rat liver plasma-membrane vesicles to the thiol-group modifying reagents N-ethylmaleimide (NEM) and iodoacetamide (IA). To this end, the different Na(+)-dependent entities involved in alanine transport were assessed. 2. NEM inactivated Na(+)-dependent alanine transport as a result of the inhibition of both system A and ASC transport activities. The functional sensitivity of system A to NEM was greater than that of system ASC. 3. The presence of L-alanine (10 mM) during the exposure of vesicles to NEM afforded partial protection to system A, but not to the ASC, carrier. This effect was specific, since the presence of L-phenylalanine (10 mM) did not cause any protection. 4. Na+ did not protect A or ASC carriers against NEM inactivation; however, the presence of Na+ (100 mM-NaCl) and L-alanine (10 mM) during the exposure of the vesicles to NEM protected against inactivation of system A and ASC transport activities. The extent of protection was greater in the case of the system ASC transport activity than in the case of the A carrier. 5. IA also diminished Na(+)-dependent alanine transport by inhibition of A and ASC transport activities. Sodium and L-alanine afforded protection to both A and ASC transport activities from the inhibitory action of IA. The extent of protection induced by substrates was similar for both carriers. 6. It is concluded that there is one, or several, free thiol groups in A and ASC carriers, the integrity of which is essential for transport activity. Sensitivity to thiol-group-specific reagents and the pattern of protection with substrates against inactivation is different in A and ASC carriers. That suggests the existence of topological dissimilarities regarding the thiol-group containing site(s) in A and ASC amino acid carriers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson N. N., Jr, Touster O. Isolation of rat liver plasma membrane fragments in isotonic sucrose. Methods Enzymol. 1974;31:90–102. doi: 10.1016/0076-6879(74)31009-9. [DOI] [PubMed] [Google Scholar]
- Barber E. F., Handlogten M. E., Kilberg M. S. Induction of amino acid transport system A in rat hepatocytes is blocked by tunicamycin. J Biol Chem. 1983 Oct 10;258(19):11851–11855. [PubMed] [Google Scholar]
- Bracy D. S., Handlogten M. E., Barber E. F., Han H. P., Kilberg M. S. Cis-inhibition, trans-inhibition, and repression of hepatic amino acid transport mediated by System A. Substrate specificity and other properties. J Biol Chem. 1986 Feb 5;261(4):1514–1520. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carroll M. Characterization of proteins structurally related to human N-acetyl-beta-D-glucosaminidase. Biochem J. 1978 Jul 1;173(1):191–196. doi: 10.1042/bj1730191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charalambous B. M., Wheeler K. P. Sodium ions protect a membrane transport protein from proteolysis. Biochim Biophys Acta. 1987 Oct 16;903(3):441–448. doi: 10.1016/0005-2736(87)90051-4. [DOI] [PubMed] [Google Scholar]
- Charalambous B. M., Wheeler K. P. Sodium-induced conformation changes in membrane transport proteins. FEBS Lett. 1985 Sep 23;189(2):163–166. doi: 10.1016/0014-5793(85)81016-4. [DOI] [PubMed] [Google Scholar]
- Chiles T. C., Dudeck-Collart K. L., Kilberg M. S. Inactivation of amino acid transport in rat hepatocytes and hepatoma cells by PCMBS. Am J Physiol. 1988 Sep;255(3 Pt 1):C340–C345. doi: 10.1152/ajpcell.1988.255.3.C340. [DOI] [PubMed] [Google Scholar]
- Christensen H. N., Liang M., Archer E. G. A distinct Na+-requiring transport system for alanine, serine, cysteine, and similar amino acids. J Biol Chem. 1967 Nov 25;242(22):5237–5246. [PubMed] [Google Scholar]
- Dudeck K. L., Dudenhausen E. E., Chiles T. C., Fafournoux P., Kilberg M. S. Evidence for inherent differences in the system A carrier from normal and transformed liver tissue. Differential inactivation and substrate protection in membrane vesicles and reconstituted proteoliposomes. J Biol Chem. 1987 Sep 15;262(26):12565–12569. [PubMed] [Google Scholar]
- Edmondson J. W., Lumeng L., Li T. K. Comparative studies of alanine and alpha-aminoisobutyric acid uptake by freshly isolated rat liver cells. J Biol Chem. 1979 Mar 10;254(5):1653–1658. [PubMed] [Google Scholar]
- Fehlmann M., Le Cam A., Freychet P. Insulin and glucagon stimulation of amino acid transport in isolated rat hepatocytes. Synthesis of a high affinity component of transport. J Biol Chem. 1979 Oct 25;254(20):10431–10437. [PubMed] [Google Scholar]
- Guidotti G. G., Borghetti A. F., Gazzola G. C. The regulation of amino acid transport in animal cells. Biochim Biophys Acta. 1978 Dec 15;515(4):329–366. doi: 10.1016/0304-4157(78)90009-6. [DOI] [PubMed] [Google Scholar]
- Gumà A., Testar X., Palacín M., Zorzano A. Insulin-stimulated alpha-(methyl)aminoisobutyric acid uptake in skeletal muscle. Evidence for a short-term activation of uptake independent of Na+ electrochemical gradient and protein synthesis. Biochem J. 1988 Aug 1;253(3):625–629. doi: 10.1042/bj2530625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handlogten M. E., Kilberg M. S. Induction and decay of amino acid transport in the liver. Turnover of transport activity in isolated hepatocytes after stimulation by diabetes or glucagon. J Biol Chem. 1984 Mar 25;259(6):3519–3525. [PubMed] [Google Scholar]
- Hayes M. R., McGivan J. D. Comparison of the effects of certain thiol reagents on alanine transport in plasma membrane vesicles from rat liver and their use in identifying the alanine carrier. Biochem J. 1983 Aug 15;214(2):489–495. doi: 10.1042/bj2140489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hediger M. A., Coady M. J., Ikeda T. S., Wright E. M. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. 1987 Nov 26-Dec 2Nature. 330(6146):379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S. Amino acid transport in isolated rat hepatocytes. J Membr Biol. 1982;69(1):1–12. doi: 10.1007/BF01871236. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S., Han H. P., Barber E. F., Chiles T. C. Adaptive regulation of neutral amino acid transport System A in rat H4 hepatoma cells. J Cell Physiol. 1985 Feb;122(2):290–298. doi: 10.1002/jcp.1041220219. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S., Handlogten M. E., Christensen H. N. Characteristics of an amino acid transport system in rat liver for glutamine, asparagine, histidine, and closely related analogs. J Biol Chem. 1980 May 10;255(9):4011–4019. [PubMed] [Google Scholar]
- Kilberg M. S., Handlogten M. E., Christensen H. N. Characteristics of system ASC for transport of neutral amino acids in the isolated rat hepatocyte. J Biol Chem. 1981 Apr 10;256(7):3304–3312. [PubMed] [Google Scholar]
- McCormick J. I., Johnstone R. M. Simple and effective purification of a Na+-dependent amino acid transport system from Ehrlich ascites cell plasma membrane. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7877–7881. doi: 10.1073/pnas.85.21.7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OXENDER D. L., CHRISTENSEN H. N. DISTINCT MEDIATING SYSTEMS FOR THE TRANSPORT OF NEUTRAL AMINO ACIDS BY THE EHRLICH CELL. J Biol Chem. 1963 Nov;238:3686–3699. [PubMed] [Google Scholar]
- Pastor-Anglada M., Remesar X., Bourdel G. Alanine uptake by liver at midpregnancy in rats. Am J Physiol. 1987 Mar;252(3 Pt 1):E408–E413. doi: 10.1152/ajpendo.1987.252.3.E408. [DOI] [PubMed] [Google Scholar]
- Peerce B. E., Wright E. M. Conformational changes in the intestinal brush border sodium-glucose cotransporter labeled with fluorescein isothiocyanate. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2223–2226. doi: 10.1073/pnas.81.7.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerce B. E., Wright E. M. Evidence for tyrosyl residues at the Na+ site on the intestinal Na+/glucose cotransporter. J Biol Chem. 1985 May 25;260(10):6026–6031. [PubMed] [Google Scholar]
- Peerce B. E., Wright E. M. Sodium-induced conformational changes in the glucose transporter of intestinal brush borders. J Biol Chem. 1984 Nov 25;259(22):14105–14112. [PubMed] [Google Scholar]
- Quesada A. R., McGivan J. D. A rapid method for the functional reconstitution of amino acid transport systems from rat liver plasma membranes. Partial purification of System A. Biochem J. 1988 Nov 1;255(3):963–969. doi: 10.1042/bj2550963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Daniels G. A., Boerner P., Lin J. Neutral amino acid transport systems in animal cells: potential targets of oncogene action and regulators of cellular growth. J Membr Biol. 1988 Aug;104(1):1–20. doi: 10.1007/BF01871898. [DOI] [PubMed] [Google Scholar]
- Shotwell M. A., Kilberg M. S., Oxender D. L. The regulation of neutral amino acid transport in mammalian cells. Biochim Biophys Acta. 1983 May 24;737(2):267–284. doi: 10.1016/0304-4157(83)90003-5. [DOI] [PubMed] [Google Scholar]
- Sips H. J., Van Amelsvoort J. M., Van Dam K. Amino acid transport in plasma-membrane vesicles from rat liver. Characterization of L-alanine transport. Eur J Biochem. 1980 Apr;105(2):217–224. doi: 10.1111/j.1432-1033.1980.tb04492.x. [DOI] [PubMed] [Google Scholar]
- Sips H. J., van Dam K. Amino acid-dependent sodium transport in plasma membrane vesicles from rat liver. J Membr Biol. 1981;62(3):231–237. doi: 10.1007/BF01998168. [DOI] [PubMed] [Google Scholar]
- Wright E. M., Peerce B. E. Identification and conformational changes of the intestinal proline carrier. J Biol Chem. 1984 Dec 25;259(24):14993–14996. [PubMed] [Google Scholar]
- YONETANI T. Studies on cytochrome oxidase. IV. The cytochrome oxidase activity. J Biol Chem. 1962 Feb;237:550–559. [PubMed] [Google Scholar]
- Zorzano A., Balon T. W., Garetto L. P., Goodman M. N., Ruderman N. B. Muscle alpha-aminoisobutyric acid transport after exercise: enhanced stimulation by insulin. Am J Physiol. 1985 May;248(5 Pt 1):E546–E552. doi: 10.1152/ajpendo.1985.248.5.E546. [DOI] [PubMed] [Google Scholar]
- Zorzano A., Balon T. W., Goodman M. N., Ruderman N. B. Insulin and exercise stimulate muscle alpha-aminoisobutyric acid transport by a Na+-K+-ATPase independent pathway. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1342–1349. doi: 10.1016/0006-291x(86)90397-9. [DOI] [PubMed] [Google Scholar]
- van Amelsvoort J. M., Sips H. J., van Dam K. Sodium-dependent alanine transport in plasma-membrane vesicles from rat liver. Biochem J. 1978 Sep 15;174(3):1083–1086. doi: 10.1042/bj1741083. [DOI] [PMC free article] [PubMed] [Google Scholar]


